Prey Capturing Dynamics and Nanomechanically Graded Cutting Apparatus of Dragonfly Nymph
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Specimen Collection and Videography
2.2. Microscopy
2.3. Microindentation and Nanoindentation
3. Results and Discussion
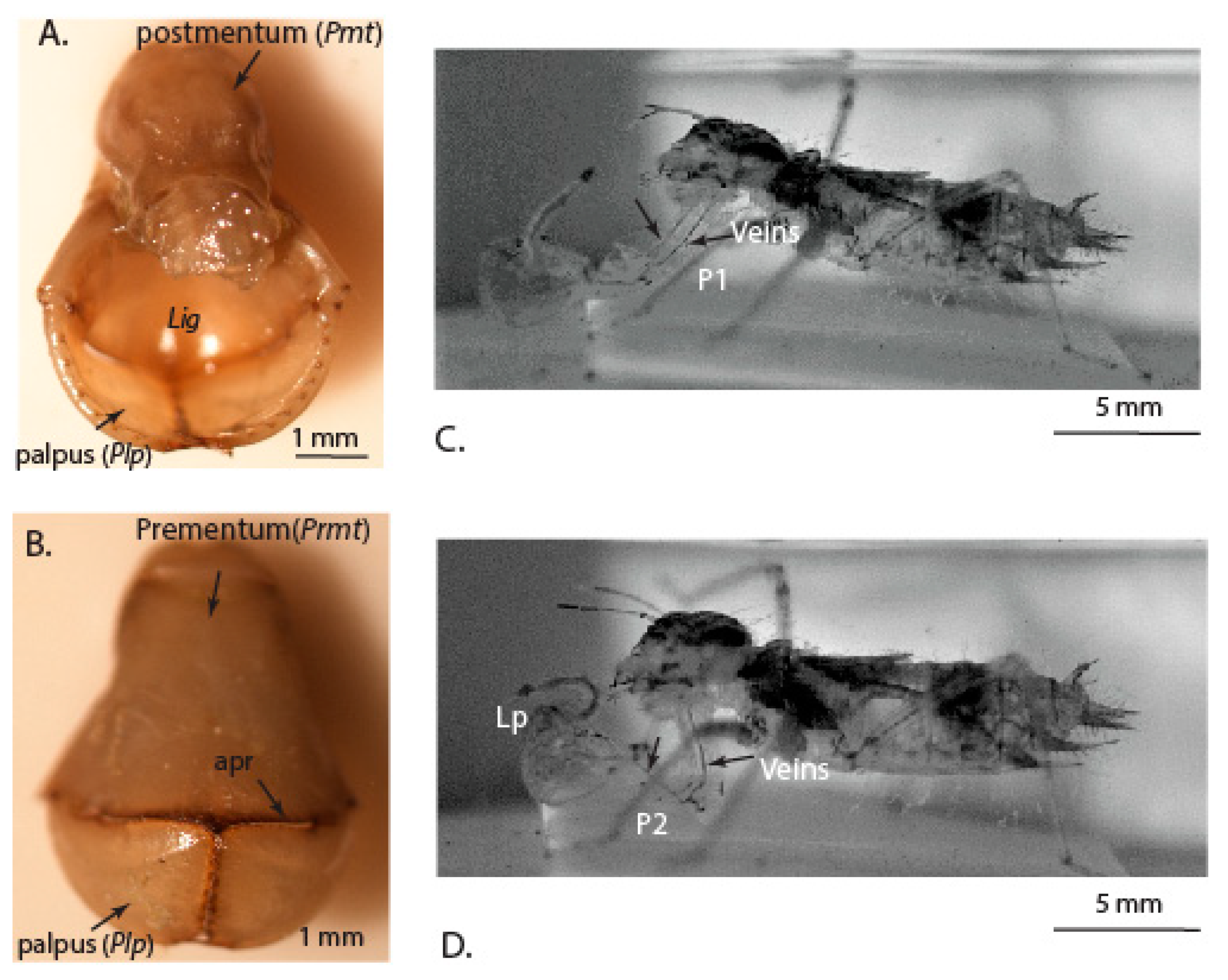
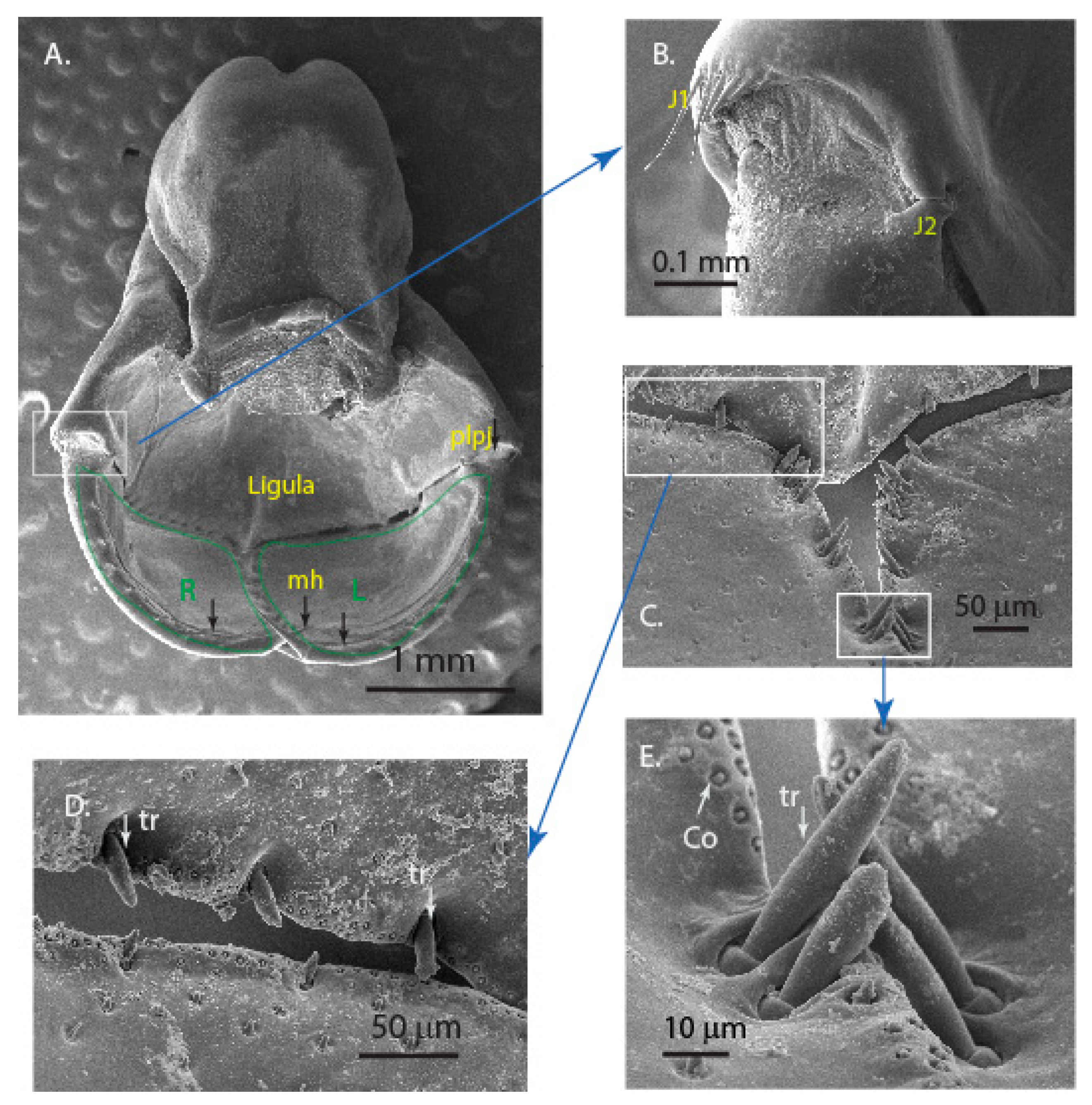
3.1. Microstructure of the Mouthparts
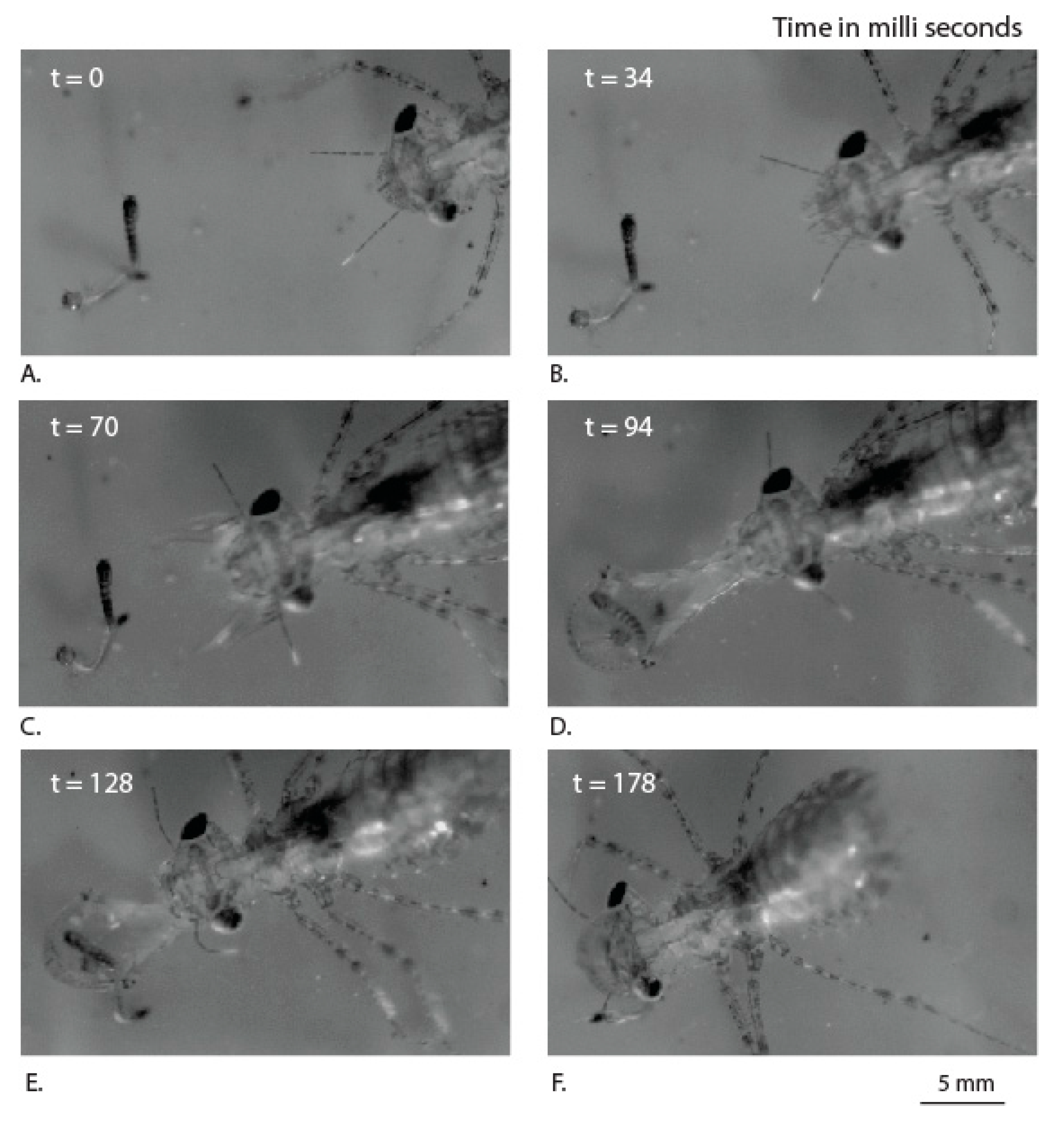
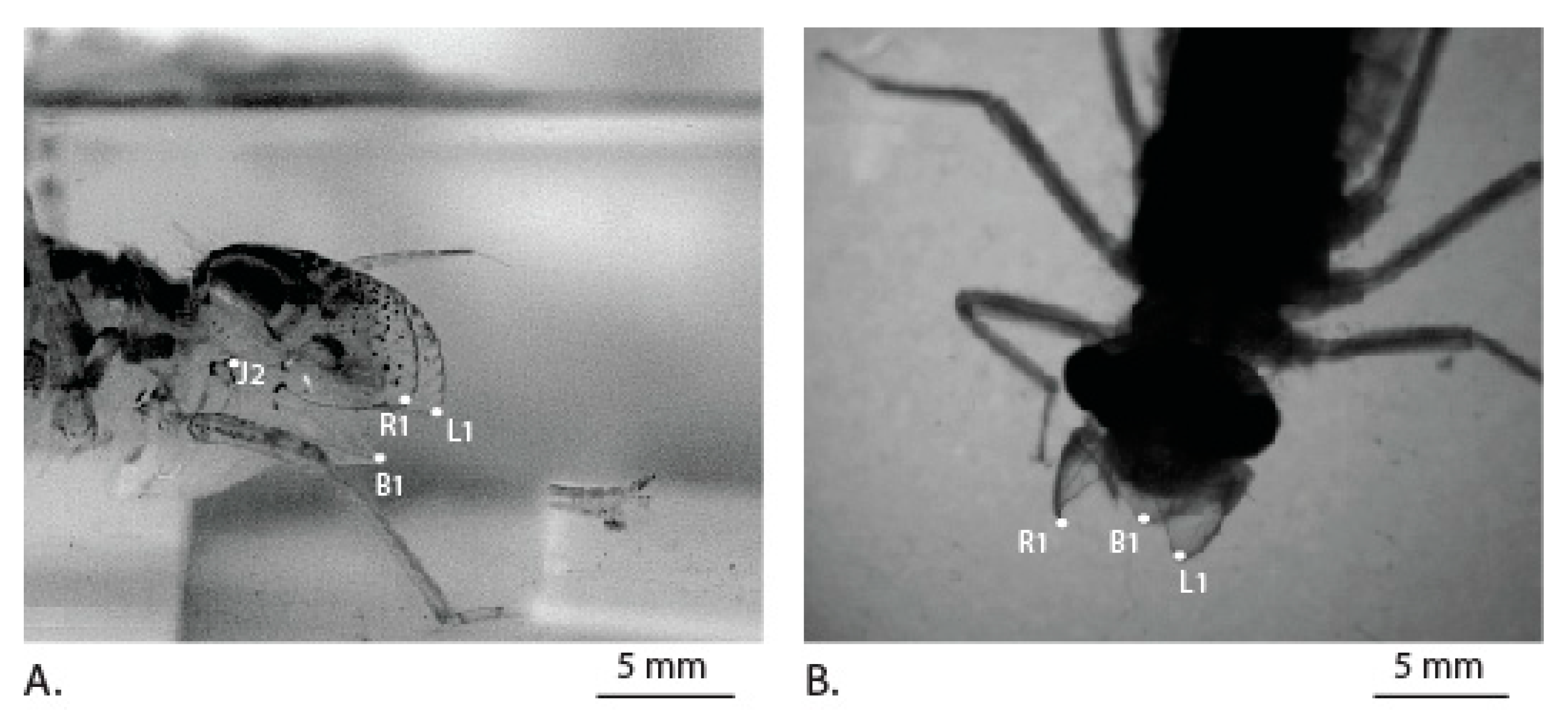
3.2. Labial Movement during Prey Capture
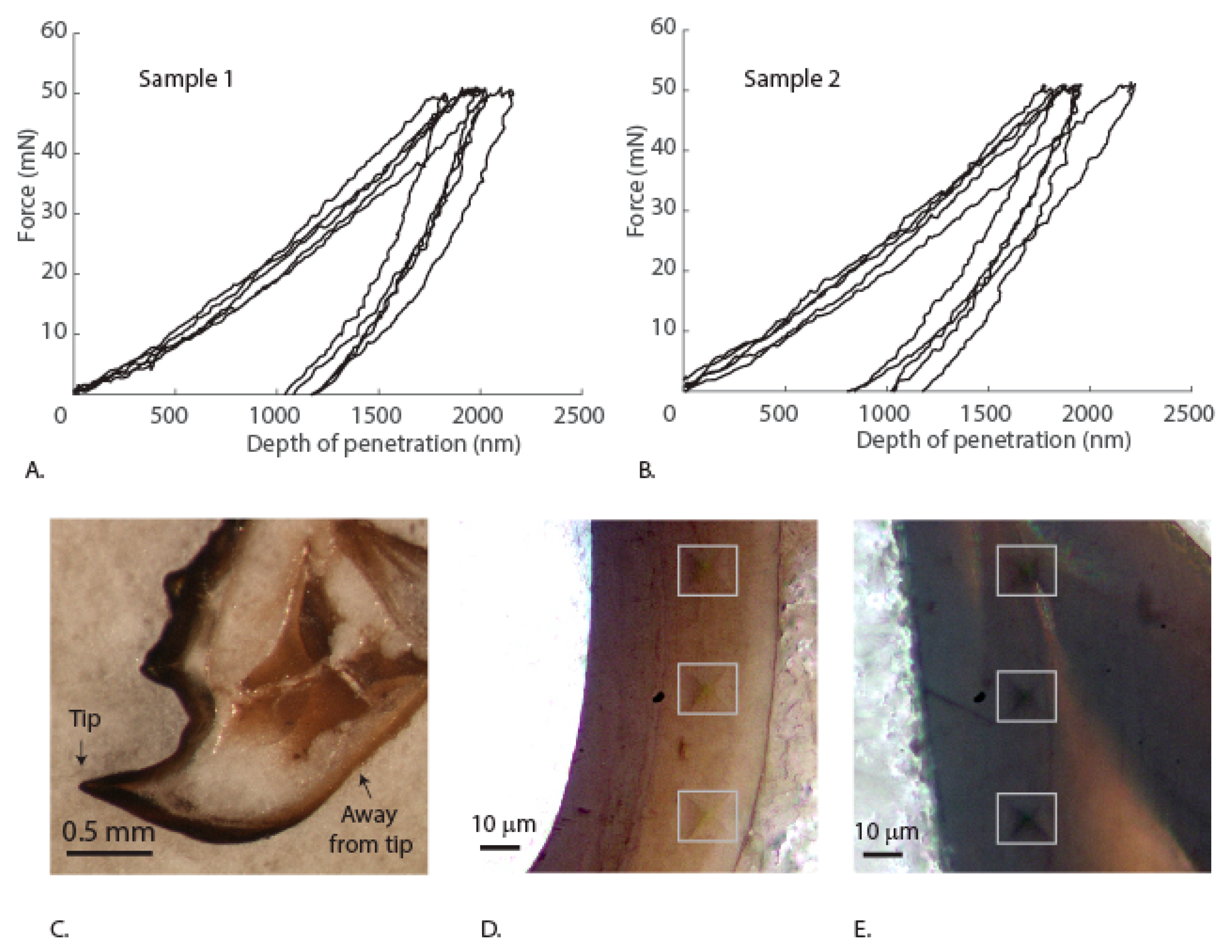
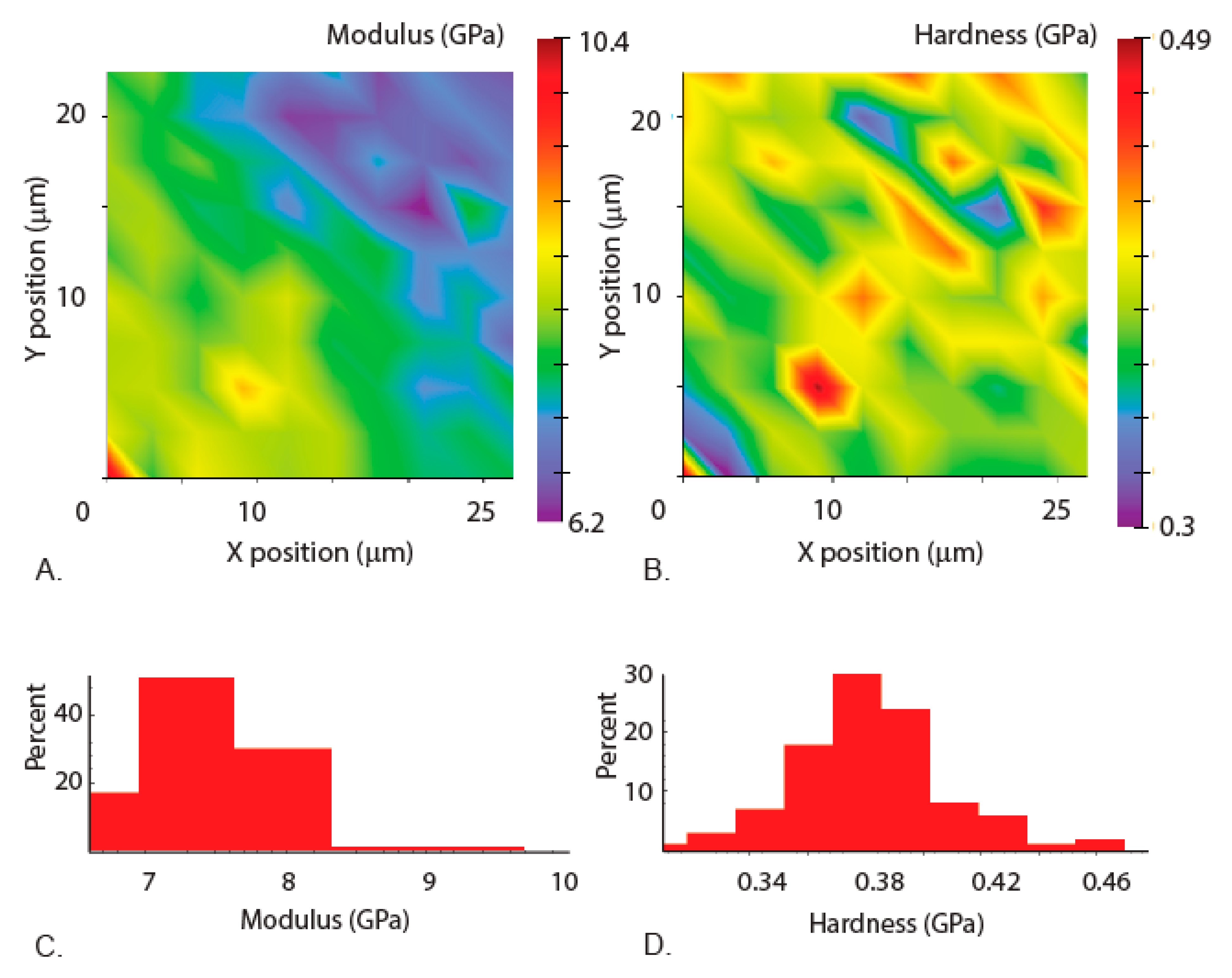
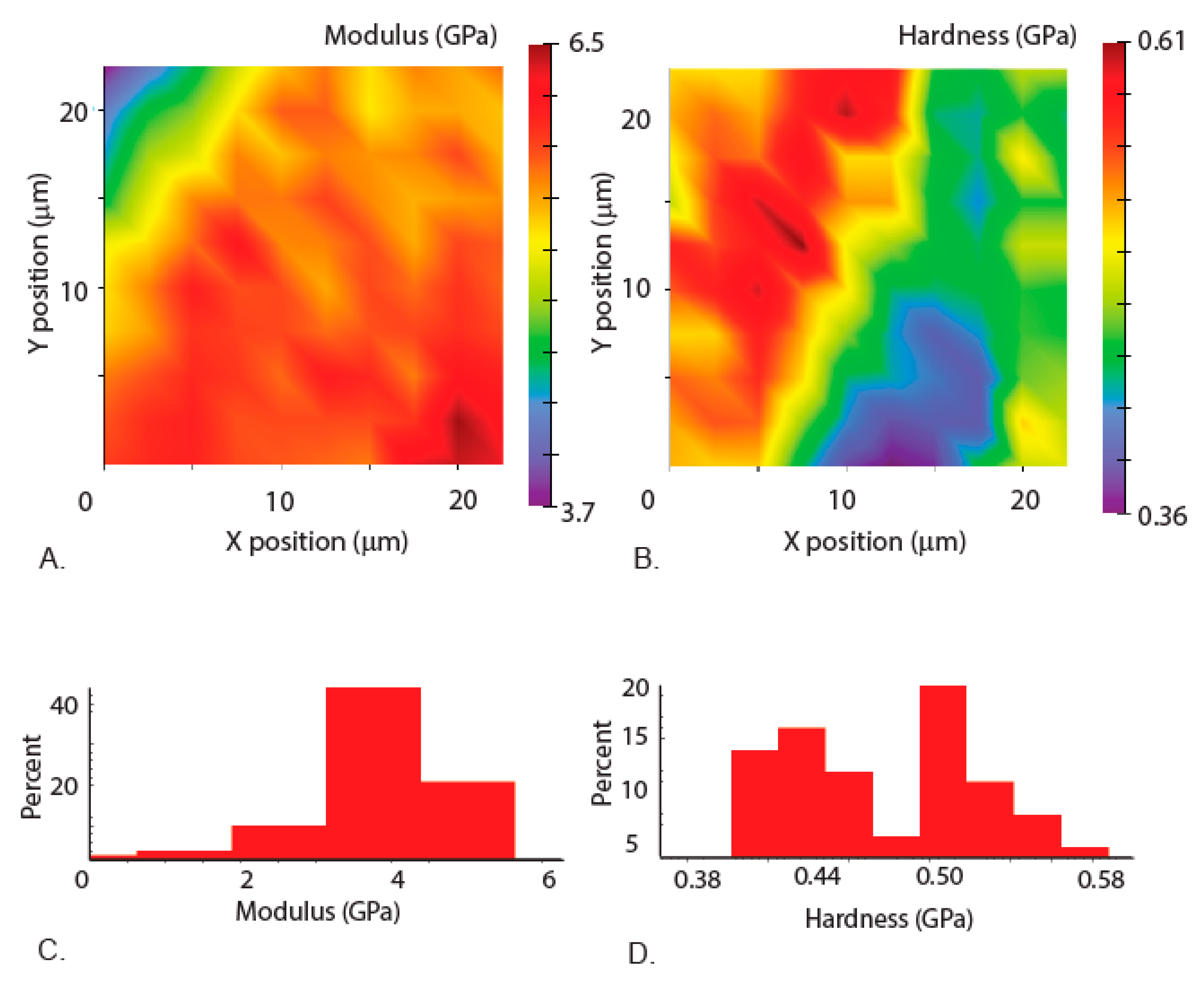
3.3. Mechanical Properties of the Mandibles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klecka, J.; Boukal, D.S. Who eats whom in a pool? A comparative study of prey selectivity by predatory aquatic insects. PLoS ONE 2012, 7, e37741. [Google Scholar] [CrossRef] [PubMed]
- Gronenberg, W. Fast actions in small animals: Springs and click mechanisms. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 1996, 178, 727–734. [Google Scholar] [CrossRef]
- Burrows, M.; Hoyle, G. Neuromuscular Physiology of the Strike Mechanism of the Mantis Shrimp, Hemisquilla. J. Exp. Zool. 1972, 179, 379–394. [Google Scholar] [CrossRef]
- Olesen, J. Prey capture in dragon fly nymphs (ODONATA, INSECTA): Labila protraction by means of a multi-purpose abdominal pump. Vidensk. Meddr Dansk Baturh. Foren. 1979, 141, 81–96. [Google Scholar]
- Saha, N.; Aditya, G.; Banerjee, S.; Saha, G.K. Predation potential of odonates on mosquito larvae: Implications for biological control. Biol. Control. 2012, 63, 1–8. [Google Scholar] [CrossRef]
- Rebora, M.; Piersanti, S.; Gaino, E. Visual and mechanical cues used for prey detection by the larva of libellula depressa (Odonata Libellulidae). Ethol. Ecol. Evol. 2004, 16, 133–144. [Google Scholar] [CrossRef]
- Büsse, S.; Hörnschemeyer, T.; Gorb, S.N. The head morphology of Pyrrhosoma nymphula larvae (Odonata: Zygoptera) focusing on functional aspects of the mouthparts. Front. Zool. 2017, 14, 1–13. [Google Scholar] [CrossRef]
- Olesen, J. The hydraulic mechanism of labial extension and jet propulsion in dragonfly nymphs. J. Comp. Physiol. 1972, 81, 53–55. [Google Scholar] [CrossRef]
- Parry, D.A. Labial extension in the dragonfly larva Anax imperator. J. Exp. Biol. 1983, 107, 495–499. [Google Scholar]
- Tanaka, Y.; Hisada, M. The hydraulic mechanism of the predatory strike in dragonfly larvae. J. Exp. Biol. 1980, 88, 1–19. [Google Scholar]
- Gordon, P. Further observations on the functional morphology of the head and mouthparts of dragonfly larvae (Odonata). Quaest. Entomol. 1976, 1, 89–114. [Google Scholar]
- Snodgrass, R. The dragon fly larva. Smithson. Misc. Collect. 1942, 114, 85. [Google Scholar]
- Antonelli, G. Underwater Robots-2nd Edition-Motion and Force Control of Vehicle-Manipulator Systems; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Rubin, C.; Krishnamurthy, N.; Capilouto, E.; Yi, H. Clinical Science: Stress analysis of the Human Tooth Using a Three-dimensional Finite Element Model. J. Dent. Res. 1983, 62, 1408–1411. [Google Scholar] [CrossRef] [PubMed]
- Ache, J.M.; Matheson, T. Passive joint forces are tuned to limb use in insects and drive movements without motor activity. Curr. Biol. 2013, 23, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Büsse, S.; Gorb, S.N. Material composition of the mouthpart cuticle in a damselfly larva (Insecta: Odonata) and its biomechanical significance. R. Soc. Open Sci. 2018, 5, 172117. [Google Scholar] [CrossRef] [PubMed]
- Bassemir, U.; Hansen, K. Single-pore sensilla of damselfly-larvae: Representatives of phylogenetically old contact chemoreceptors? Cell Tissue Res. 1980, 207, 307–320. [Google Scholar] [CrossRef]
- Gupta, A.; Dey, S.; Gupta, S. Cuticular structures on the labium of the larva of Crocothemis servilia (Drury) (Anisoptera: Libellulidae). Odonatologica 1992, 21, 97–101. [Google Scholar]
- Gaino, E.; Rebora, M. Apical antennal sensilla in nymphs of LibelZulu depressa (Odonata: Libellulidae). Invertebr. Biol. 2001, 120, 162–169. [Google Scholar] [CrossRef]
- Song, L.M.; Wang, X.M.; Huang, J.P.; Zhu, F.; Jiang, X.; Zhang, S.G.; Ban, L.P. Ultrastructure and morphology of antennal sensilla of the adult diving beetle Cybister japonicus Sharp. PLoS ONE 2017, 12, e0174643. [Google Scholar] [CrossRef]
- Corrette, B.J. Prey capture in the praying mantis Tenodera aridifolia sinensis: Coordination of the capture sequence and strike movements. Co. Biol. Ltd. 1990, 148, 147–180. [Google Scholar]
- Cheng, J.-Y.; Davison, I.G.; Demont, M.E. Dynamics and energetics of scallop locomotion. J. Exp. Biol. 1996, 199, 1931–1946. [Google Scholar] [PubMed]
- Burrows, M. Morphology and action of the hind leg joints controlling jumping in froghopper insects. J. Exp. Biol. 2006, 209, 4622–4637. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, S.A. Mechanical Design in Organisms; Princeton University Press: Princeton, NJ, USA, 2010. [Google Scholar]
- Cribb, B.W.; Stewart, A.; Huang, H.; Truss, R.; Noller, B.; Rasch, R.; Zalucki, M.P. Insect mandibles--comparative mechanical properties and links with metal incorporation. Naturwissenschaften 2008, 95, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Schofield, R.M.S.; Nesson, M.H.; Richardson, K.A. Tooth hardness increases with zinc-content in mandibles of young adult leaf-cutter ants. Naturwissenschaften 2002, 89, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Broomell, C.C.; Mattoni, M.A.; Zok, F.W.; Waite, J.H. Critical role of zinc in hardening of Nereis jaws. J. Exp. Biol. 2006, 209, 3219–3225. [Google Scholar] [CrossRef]
- Kundanati, L.; Gundiah, N. Biomechanics of substrate boring by fig wasps. J. Exp. Biol. 2014, 217, 1946–1954. [Google Scholar] [CrossRef]
- Politi, Y.; Priewasser, M.; Pippel, E.; Zaslansky, P.; Hartmann, J.; Siegel, S.; Li, C.; Barth, F.G.; Fratzl, P. A spider’s fang: How to design an injection needle using chitin-based composite material. Adv. Funct. Mater. 2012, 22, 2519–2528. [Google Scholar] [CrossRef]
- Kundanati, L.; Chahare, N.R.; Jaddivada, S.; Karkisaval, A.G.; Sridhar, R.; Pugno, N.M.; Gundiah, N. Cutting mechanics of wood by beetle larval mandibles. J. Mech. Behav. Biomed. Mater. 2020, 112, 104027. [Google Scholar] [CrossRef]
- Rus, D.; Tolley, M.T. Design, fabrication and control of soft robots. Nature 2015, 521, 467–475. [Google Scholar] [CrossRef]

| Sample | Time for Protraction (ms) | Total Time for Capture (ms) |
|---|---|---|
| 1 | 60 | 225 |
| 2 | 36 | 114 |
| 3 | 40 | 124 |
| 4 | 78 | 210 |
| 5 | 64 | 240 |
| 6 | 100 | 208 |
| Average | 63 ± 24 | 187 ± 54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kundanati, L.; Das, P.; Pugno, N.M. Prey Capturing Dynamics and Nanomechanically Graded Cutting Apparatus of Dragonfly Nymph. Materials 2021, 14, 559. https://doi.org/10.3390/ma14030559
Kundanati L, Das P, Pugno NM. Prey Capturing Dynamics and Nanomechanically Graded Cutting Apparatus of Dragonfly Nymph. Materials. 2021; 14(3):559. https://doi.org/10.3390/ma14030559
Chicago/Turabian StyleKundanati, Lakshminath, Prashant Das, and Nicola M. Pugno. 2021. "Prey Capturing Dynamics and Nanomechanically Graded Cutting Apparatus of Dragonfly Nymph" Materials 14, no. 3: 559. https://doi.org/10.3390/ma14030559
APA StyleKundanati, L., Das, P., & Pugno, N. M. (2021). Prey Capturing Dynamics and Nanomechanically Graded Cutting Apparatus of Dragonfly Nymph. Materials, 14(3), 559. https://doi.org/10.3390/ma14030559







