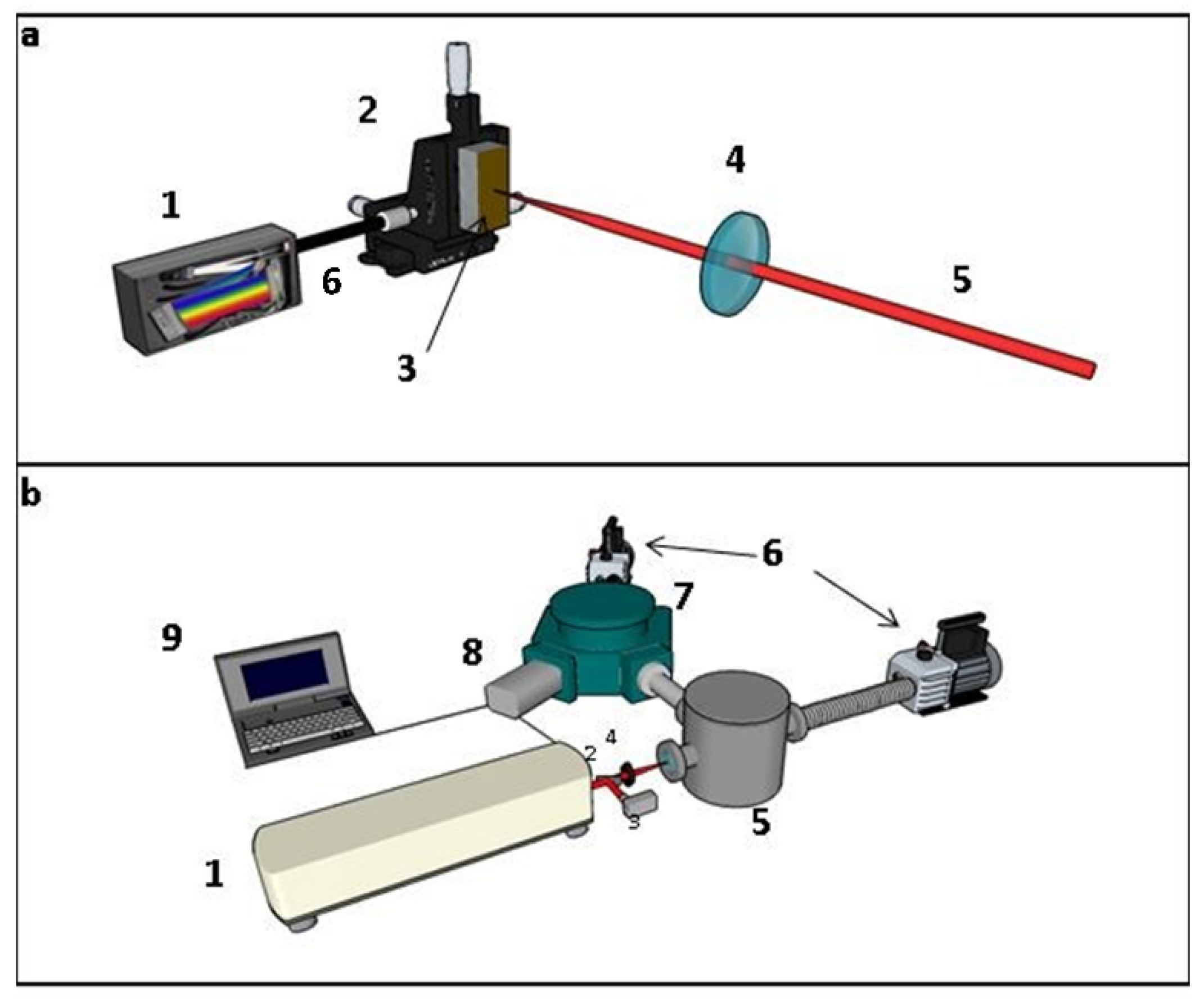
3.1. LIBS Investigations
In order to determine the sulfur spectral lines which can be observed in a soil organic matrix and to select the most suitable ones for quantification purposes, both the VUV (175–200 nm) and the UV-Vis-NIR (200–1200 nm) spectral regions were searched. Initially, the presence of sulfur lines previously reported in other studies was examined. Such studies referred to the elemental analysis of minerals [
16], the detection and quantification of sulfur in petroleum derivatives [
17,
18], and the detection of sulfur in environmental samples [
19,
20]. In all these investigations, the sulfur spectral lines that were considered for analytical purposes lay in the UV and NIR spectral regions. In the present work, several LIBS experiments were performed on samples consisting of organic soil (purchased from the local market) enriched up to ~30% wt.% with sulfur and using different experimental conditions (e.g., laser energy, laser focusing conditions, etc.). In all cases and for all the experimental conditions used, the spectral lines of sulfur lying in the UV-ViS-NIR spectral region were found to be inadequate for quantitative analysis purposes, either because they were too weak to ensure a reasonable signal-to-noise ratio, or because, in most of the cases, they were found to overlap with spectral lines of other elements present in the organic soil matrix or in air. Interestingly, some sulfur emission lines were clearly observed as lying just below 200 nm. These lines were intense enough and relatively free from spectral interferences from other elements. So, they were selected to be used for qualitative and quantitative LIBS measurements.
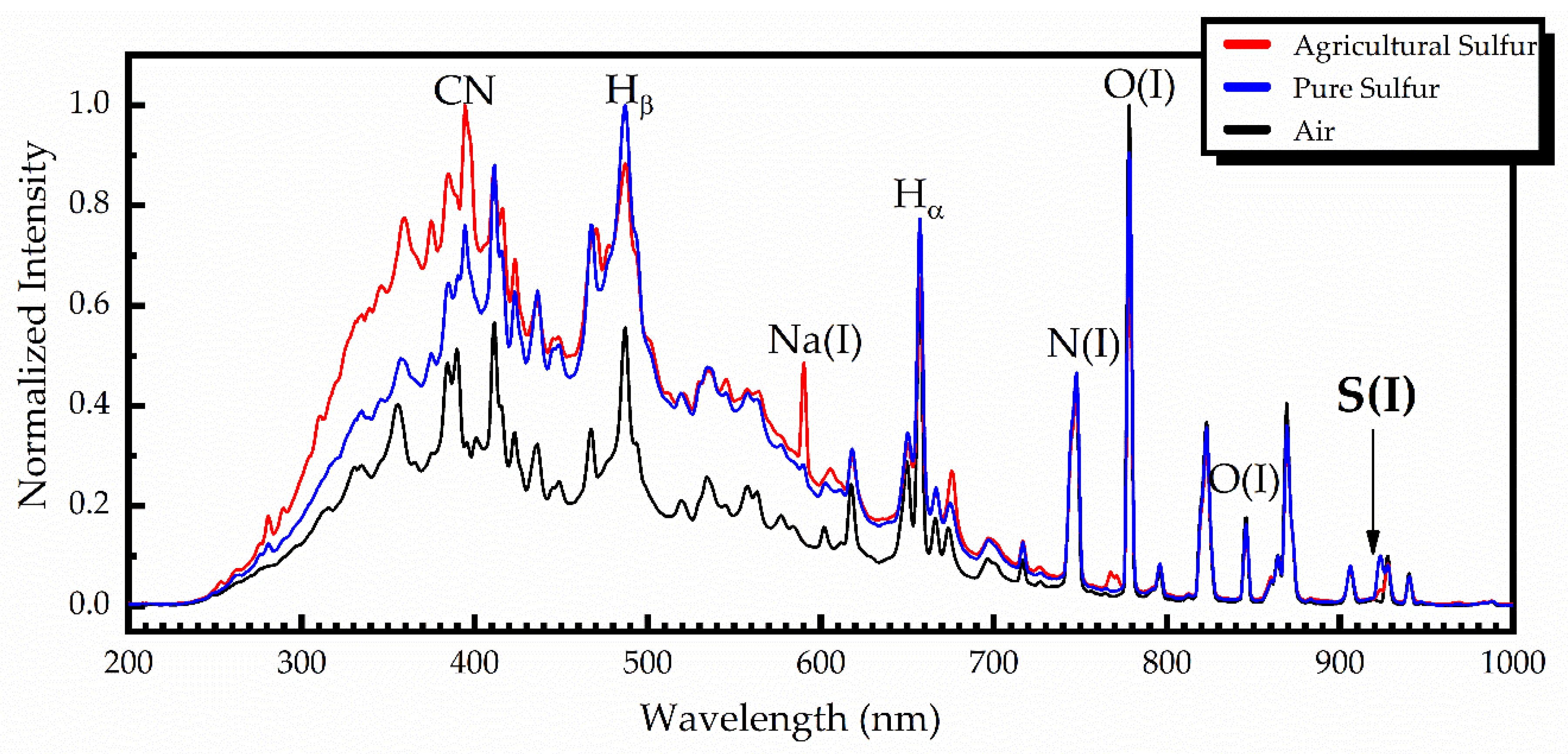
For comparison purposes, some characteristic LIBS spectra concerning the UV-ViS-NIR spectral region, obtained from pure and agricultural sulfur samples, are shown in
Figure 3, together with a LIBS spectrum obtained in air. All these spectra were obtained using a time delay of 1.28 μs and an integration time of 1.05 ms. To facilitate comparison, the shown spectra are normalized.
As can be seen from this figure, the LIBS spectra of pure and agricultural sulfur samples were very similar, exhibiting only minor differences, arising from the impurities contained in the agricultural sulfur samples. As an example, some of the observed differences are zoomed into and are shown in
Figure 4. As shown, the sodium spectral lines Na(I) at 589.0 and 589.6 nm, and the potassium spectral lines K(I) at 766.5 and 769.9 nm are clearly observed in the agricultural sulfur LIBS spectra, while they are absent in the pure sulfur spectrum [
21,
22].
Similarly, in
Figure 5, the LIBS spectra of pure and agricultural sulfur samples, together with the LIBS spectrum of air, in the NIR region between 900 and 950 nm are presented. In this spectral region, three spectral lines of sulfur are expected to appear, namely at 921.3 nm, 922.8 nm and 923.8 nm. However, due to the limited resolution of the spectrograph, the broadening of the lines, and their partial overlapping with the neighboring oxygen O(I) emission at 926.6 nm, they are not clearly observed. So, it was concluded that these NIR spectral lines of sulfur cannot be employed for quantitative purposes.
Next, the effect of the signal gating conditions on the LIBS spectra and more importantly on the strength of the sulfur spectral lines of the samples consisting from commercially packaged organic soil mixed with agricultural sulfur was studied. In the case of the UV-ViS-NIR measurements, the detector of the spectrograph could attain a minimum gate width (t
w) and time delay (t
d) of 1.05 ms and 1.28 μs, respectively. So, the effect of the time delay on the sulfur emissions was investigated for several t
d values, greater than 1.28 μs, keeping the integration time constant, i.e., at 1.05 ms. The obtained LIBS spectra are presented in
Figure 6a. As it can be seen from the intensity dependence plots shown in
Figure 6b,c, the spectral lines of S(I) and O(I) exhibit an exponential-like decay, decreasing rapidly within 2–3 μs. More precisely, for t
d ≥ 3.28 μs, both the S(I) and O(I) lines were observed to decrease significantly, resulting in a very low signal to noise ratio. This finding suggests that it is rather unfavorable to perform measurements for higher time delays. Based on this experimental evidence, and the relatively weak background observed (see also
Figure 6c), it was concluded that the use of t
d lower than 3.28 μs was more appropriate for performing measurements. In addition, it is evident that the only suitable spectral line of sulfur for analytical use in these soil samples would be most probably the three S(I) lines at 921.3, 922.8, and 923.8 nm. However, these lines were found to remain weak under all experimental conditions tried.
Due to the aforementioned problems and limitations met while studying the LIBS spectra of the UV-VIS-NIR region, sulfur emissions in the near VUV spectral region were searched. Since in this case the samples were placed under vacuum conditions (i.e., in the vacuum chamber), where it was difficult to move them to avoid the problem of crater formation, the soil-sulfur samples were mixed with starch or lactose to improve their cohesivity. As a result, the crater formation was reduced significantly, improving the reproducibility of the measurements and greatly reducing the shot-to-shot variations of the plasma light intensity.
Some representative LIBS spectra of sulfur-soil samples with starch and lactose are illustrated in
Figure 7. As can been seen in the LIBS spectra of
Figure 7, three sulfur lines, namely the S(I) at 180.7 nm, S(I) at 182.1 nm, and S(I) at 182.6 nm, along with a carbon line C(I) at 193.1 nm, are clearly observed, while some weaker emissions at 185.6 and 186.3 nm, are tentatively attributed to different Fe (II) transitions. The spectroscopic information on the observed atomic transitions of interest was found from the NIST database [
21] and is presented in
Table 2.
In order to investigate possible contributions and/or other matrix effects arising from the addition of starch or lactose, LIBS measurements were performed on the samples containing starch and lactose under identical experimental conditions. As shown in
Figure 7, the samples with starch as the binding material present higher intensities than the samples with lactose. In particular, in the case of lower sulfur concentration (see e.g., black lines), the emissions of S(I) from the lactose-sulfur sample almost vanished. These observations were confirmed by repeating the experiments using starch and lactose from different suppliers.
To further explore the issue of the most suitable binder for the experiments, the PCA algorithm was implemented [
22]. In
Figure 8, the score plot of PCA for the mixed samples with starch and lactose, having identical concentrations with those shown of
Table 1, is presented. As can be seen, two PCs explain approximately 98% (i.e., PC1 explains 96.2% and PC2 explains 1.8%) of the original data variance. The red dots correspond to the spectra of lactose-containing samples, while the black squares correspond to the starch-containing samples. From the score plot investigation, some patterns are clearly observable. At first, it seems that the starch-containing samples (black arrow) are mainly scattered at the PC2 axis, while the lactose-containing samples (red arrow) are scattered through the PC1 axes. In addition, both the lactose- and starch-containing samples exhibit the same behavior for low sulfur concentrations, since they have negative scores for both the PC1 and PC2 axes, while for a higher concentration, the lactose-containing samples have positive PC1 scores, in contrast with the starch-containing samples which have positive PC2 scores. Although the scores show an increasing behavior, it should be noted that, for lactose-containing samples, the dispersion of the scores becomes larger as sulfur’s concentration increases, while the scores of the starch-containing samples exhibit a more linear behavior and much smaller dispersion. These findings are strong evidence that the matrix effects in the presence of starch are much weaker than those related to the presence of lactose. As a result, we decided to use starch for increasing the cohesivity of the samples and to perform the following quantitative analyses.