Pitting Corrosion Behavior and Surface Microstructure of Copper Strips When Rolled with Oil-in-Water Emulsions
Abstract
:1. Introduction
2. Materials and Methods
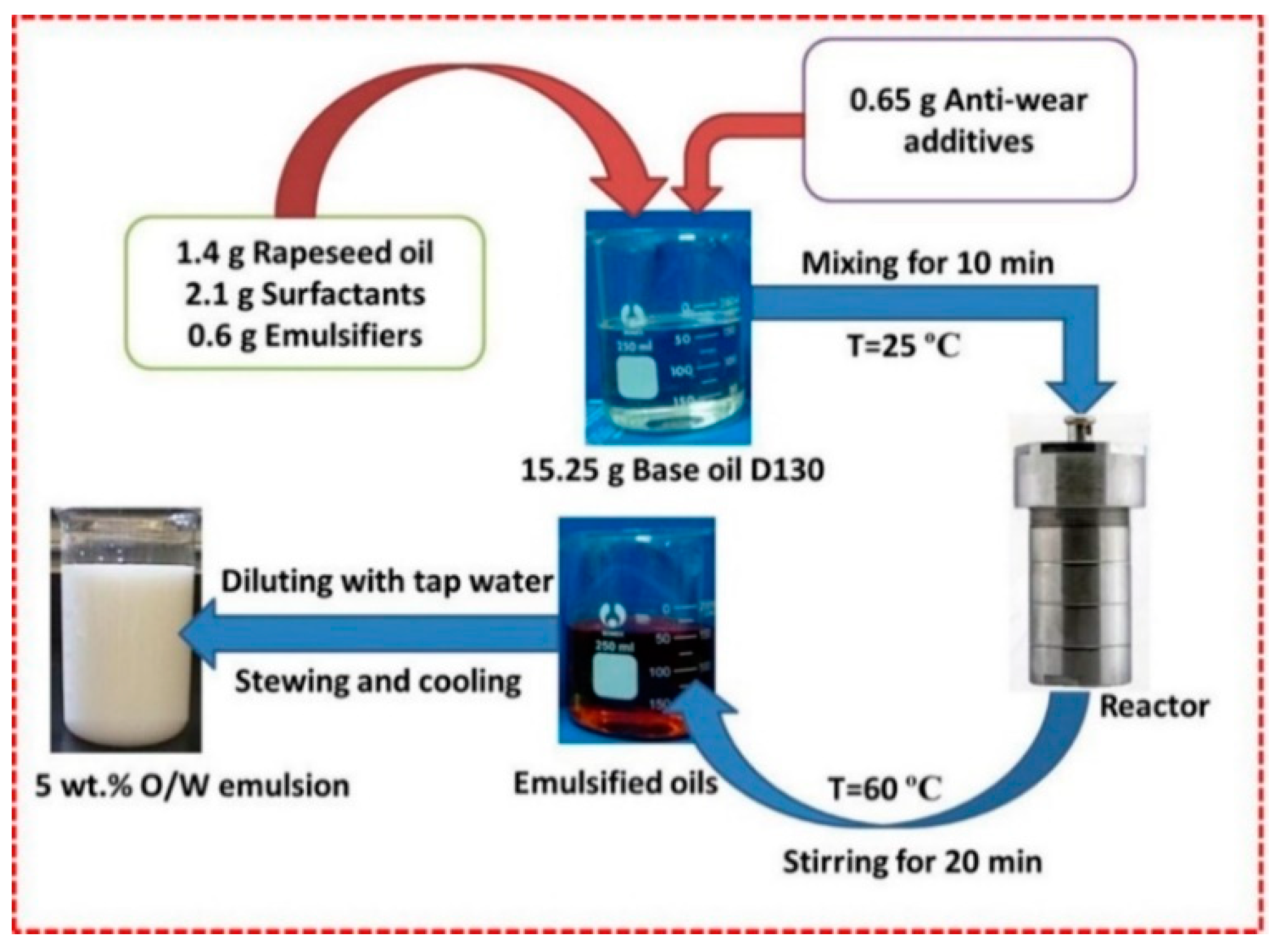
2.1. Materials and O/W Emulsions Preparation
2.2. Cold Rolling Tests
2.3. Electrochemical Measurement
2.4. Surface Observations
2.5. EBSD Characterizations
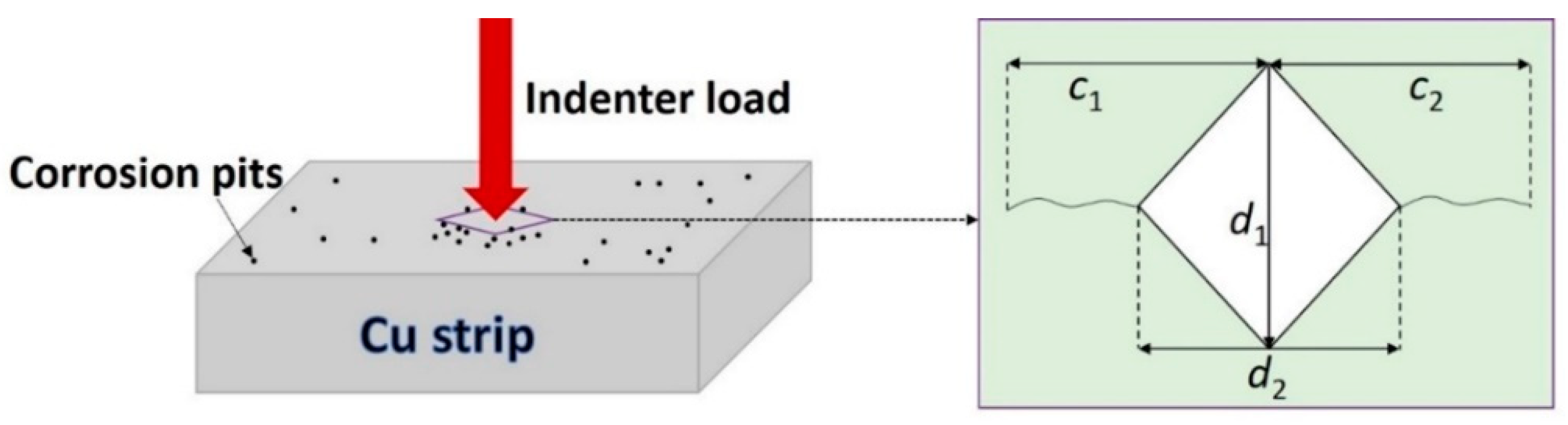
2.6. Mechanical Properties Tests
3. Results and Discussion
3.1. Electrochemical Corrosion Properties
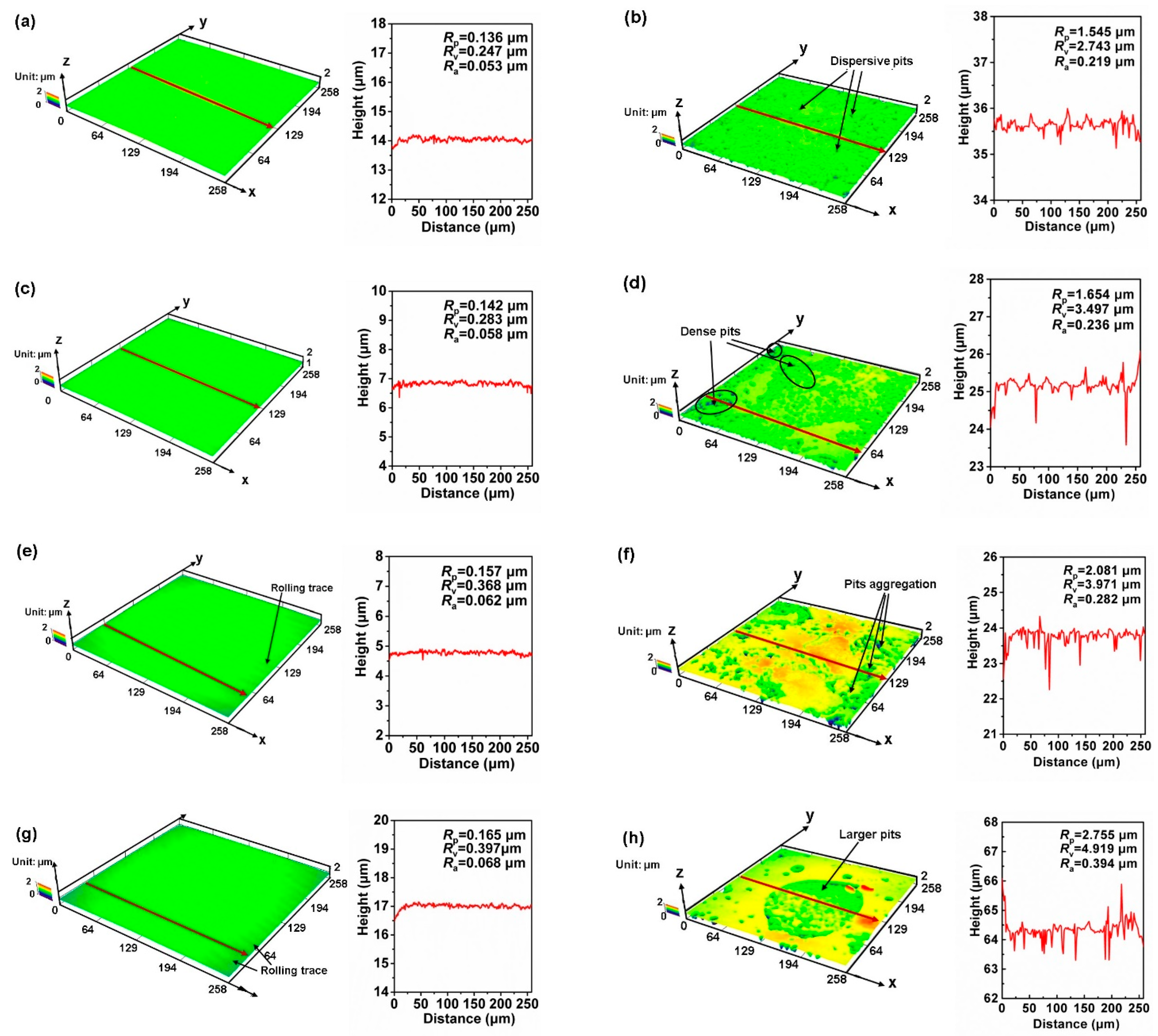
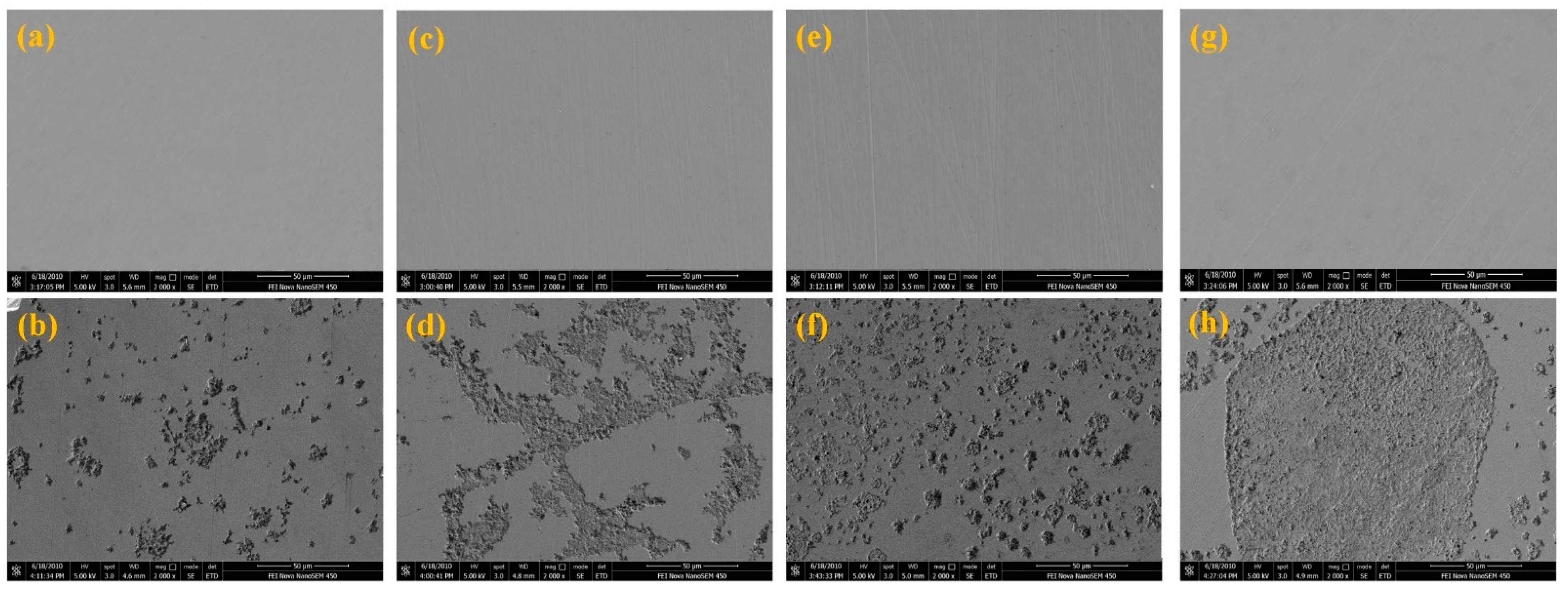
3.2. Surface Analysis
3.3. Microstructure and Mechanical Properties
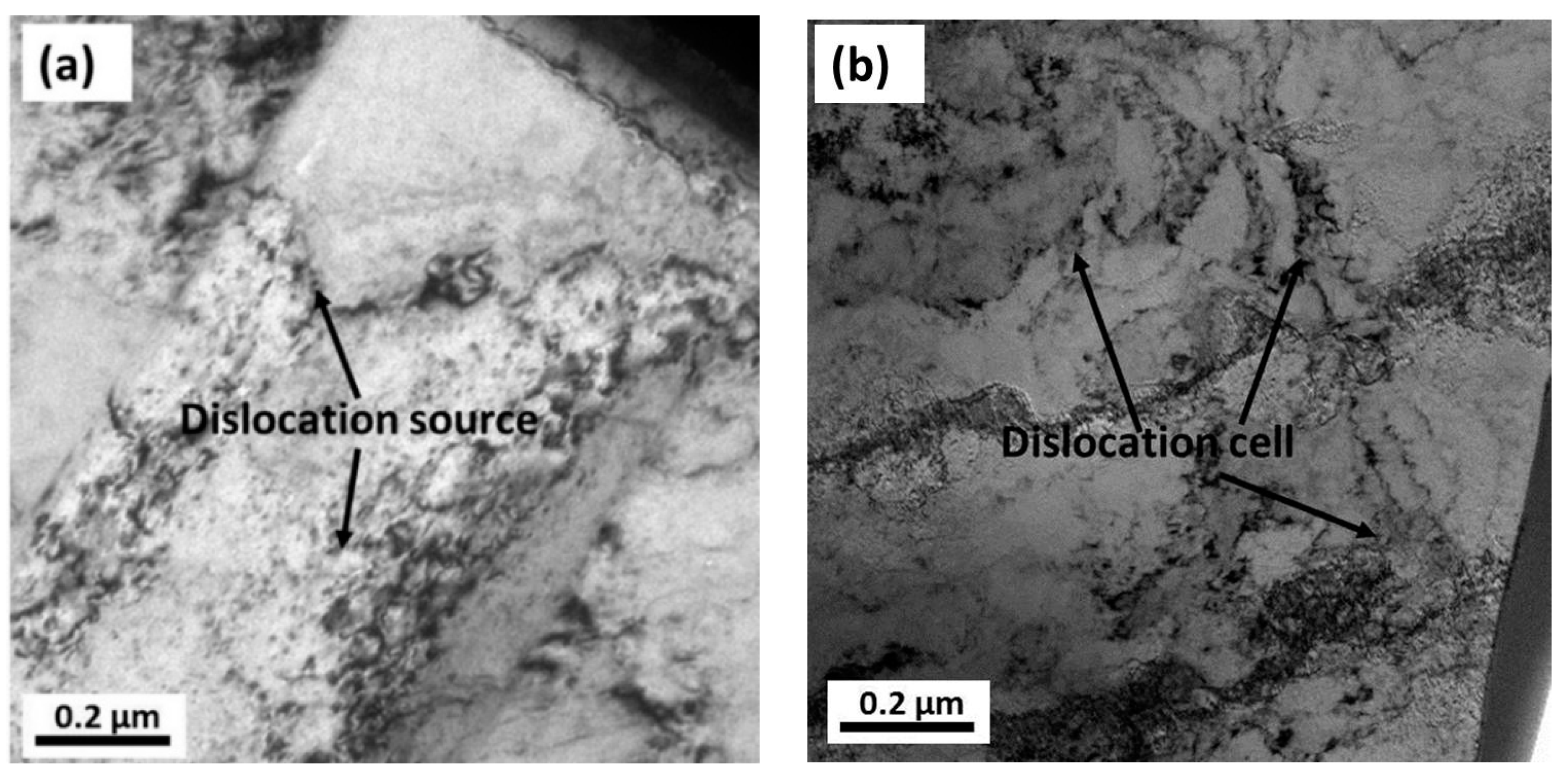
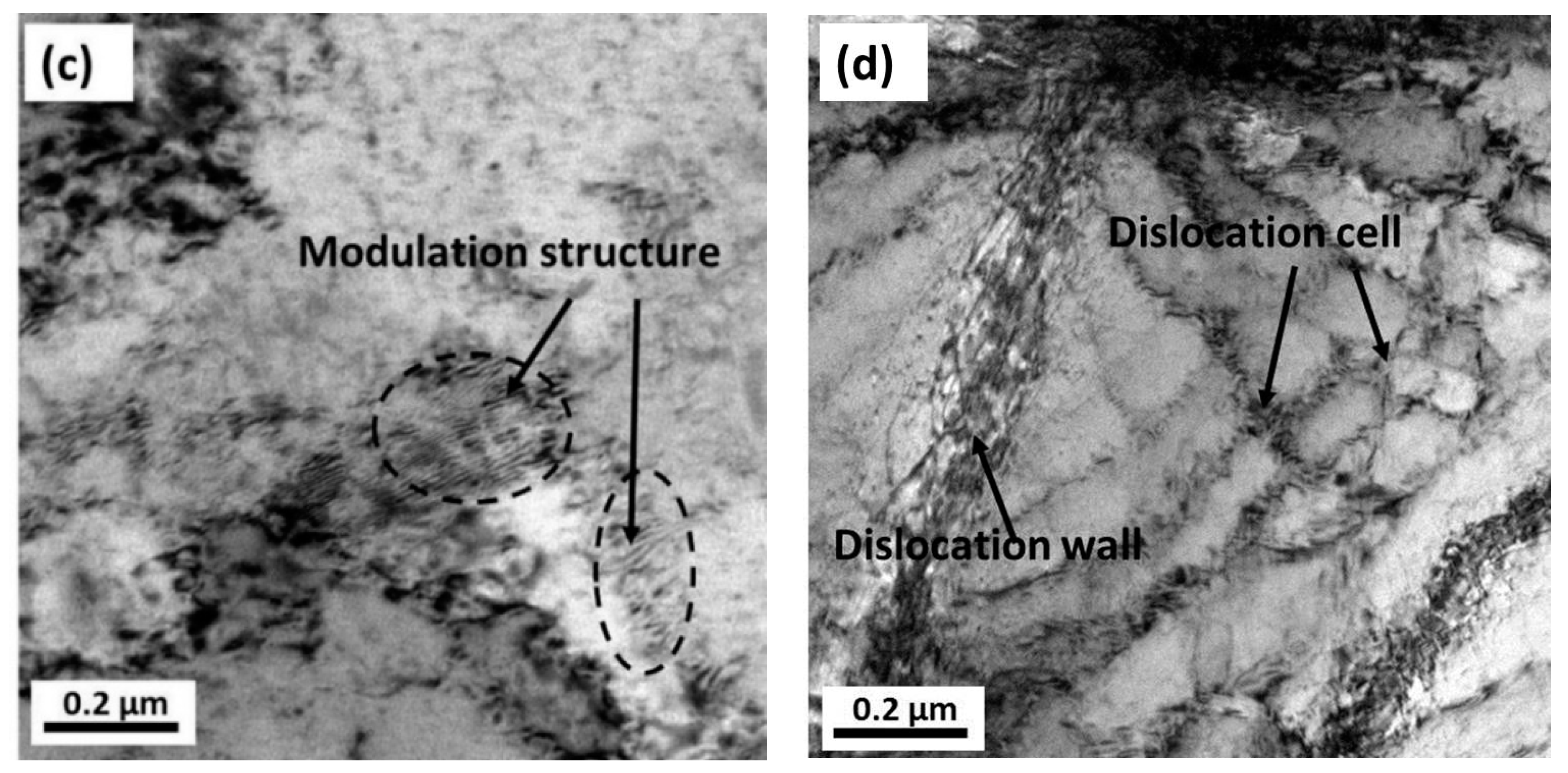
3.4. FIB and TEM Results
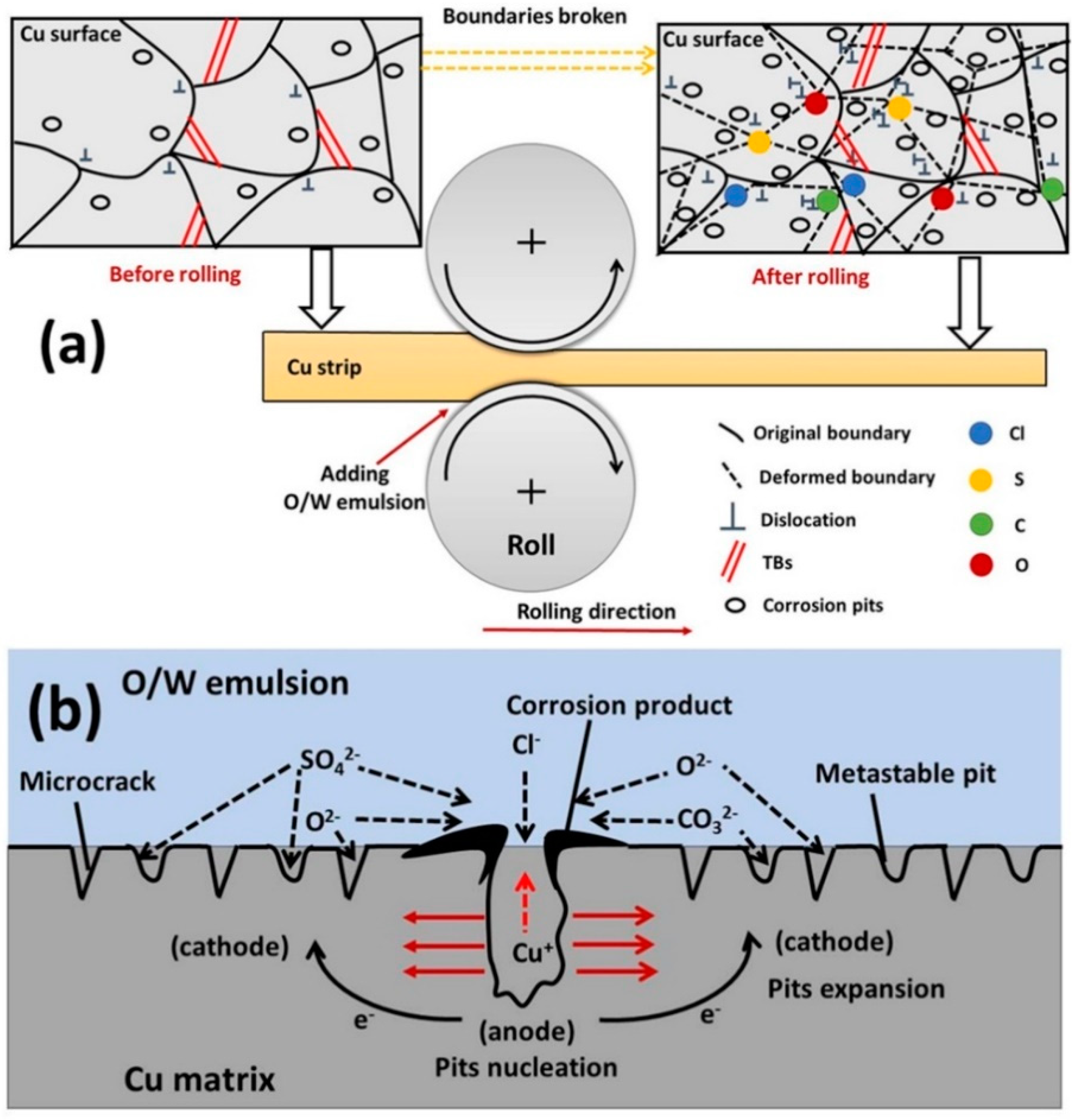
3.5. Discussions
4. Conclusions
- (1)
- The electrochemical results show the corrosion current densities of Cu strips in the O/W emulsions increased with accumulated reduction, while the corrosion potentials shifted towards being more negative in the anodic region. The pitting potentials and corrosion resistances were both decreased with the processing of cold rolling. These phenomena demonstrated that the corrosive tendency of Cu strips under different rolling reduction (ε) followed the order of ε0% < ε20.7% < ε50.6% < ε77.3%.
- (2)
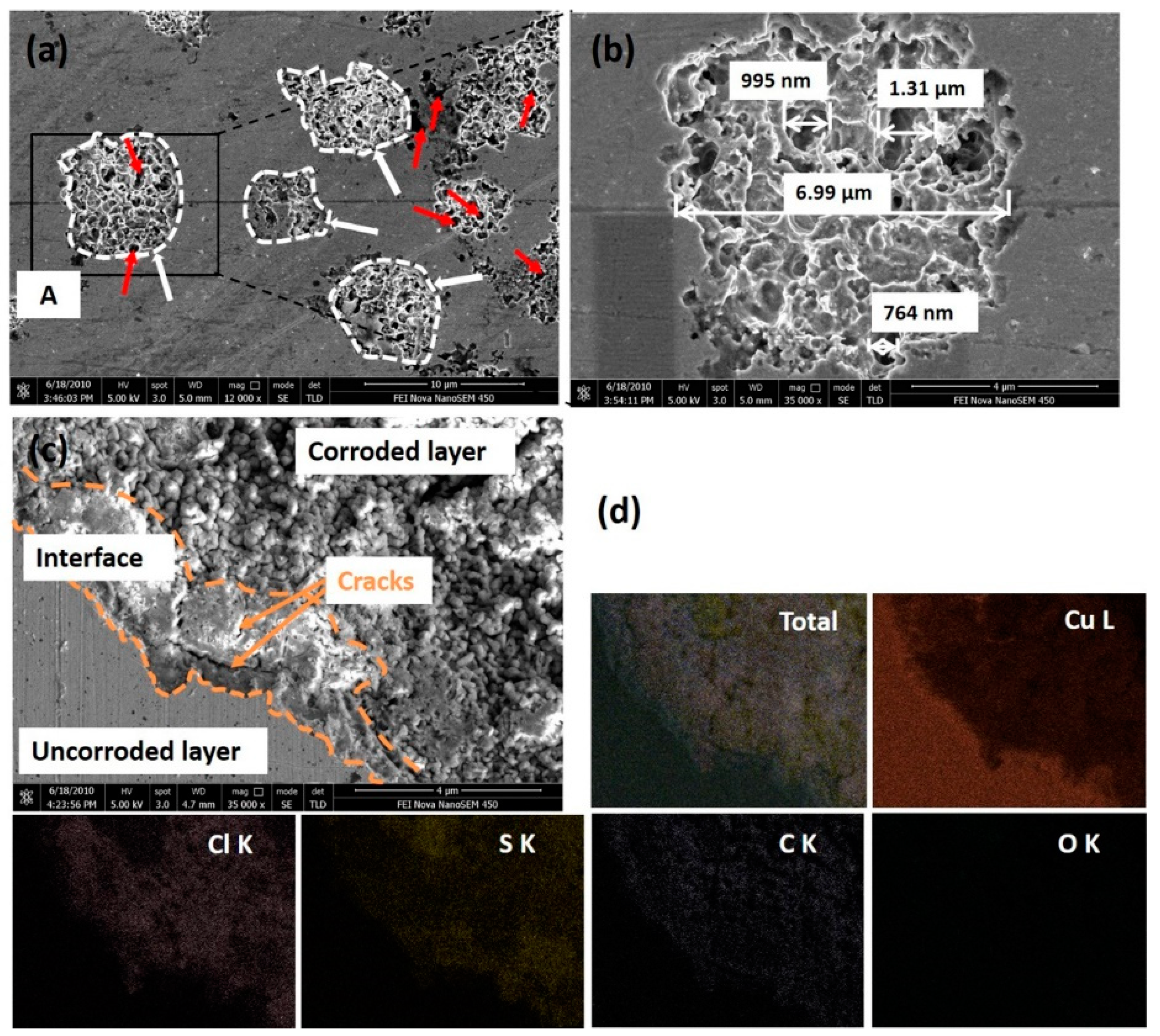
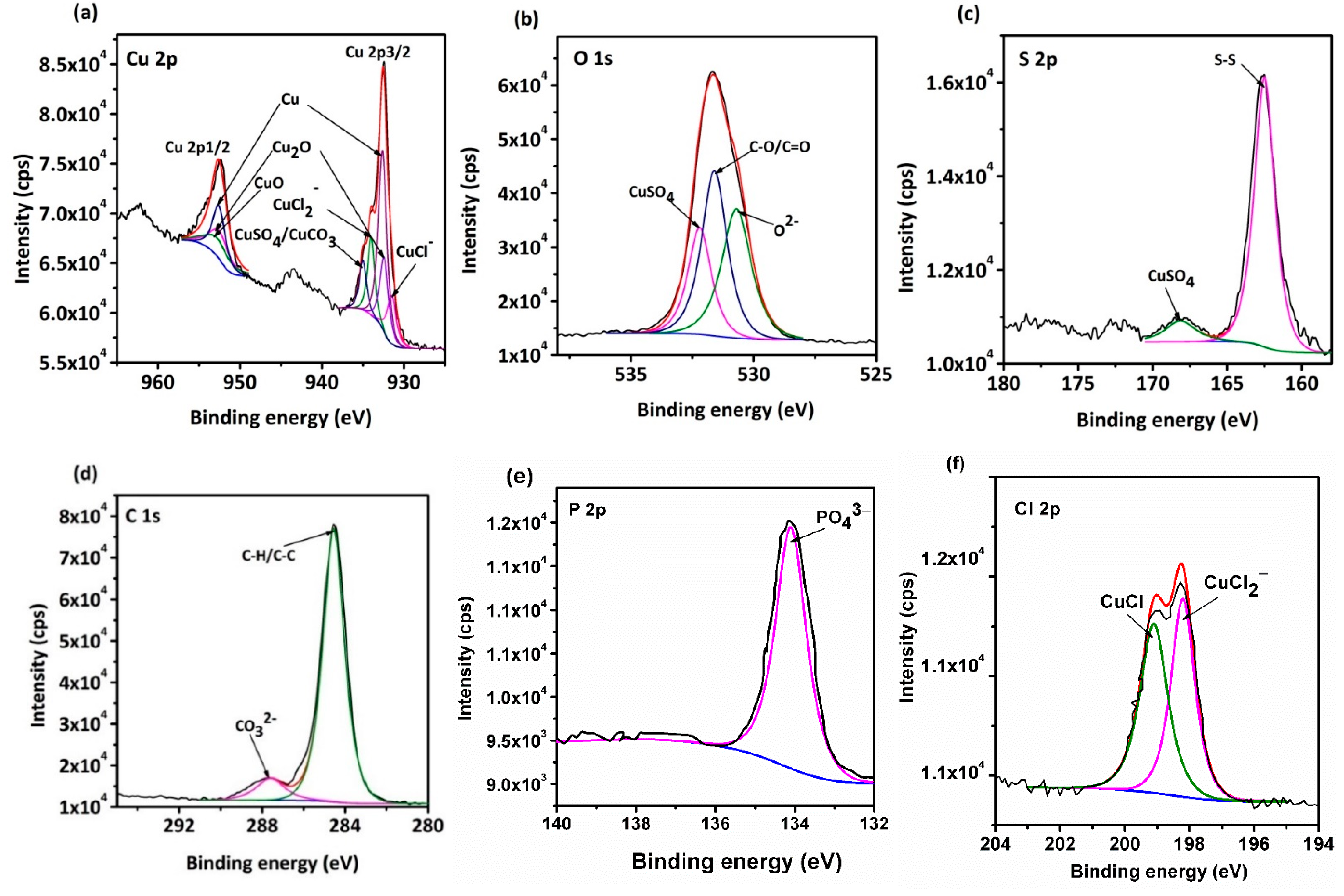
- Surface observations indicated that there were different degrees of pit expansions that occurred on the rolled Cu surfaces. These pits became denser and the surface became rougher with the increase of rolling reduction. Some metastable pits existed in the larger pits. Cu reacted easily with chlorine, sulfur, and carbon components from the O/W emulsions, Then, the corrosion products, i.e., CuCl, , Cu2O, CuCO3, and CuSO4 were generated, which appeared in the form of pitting corrosion on the Cu surface.
- (3)
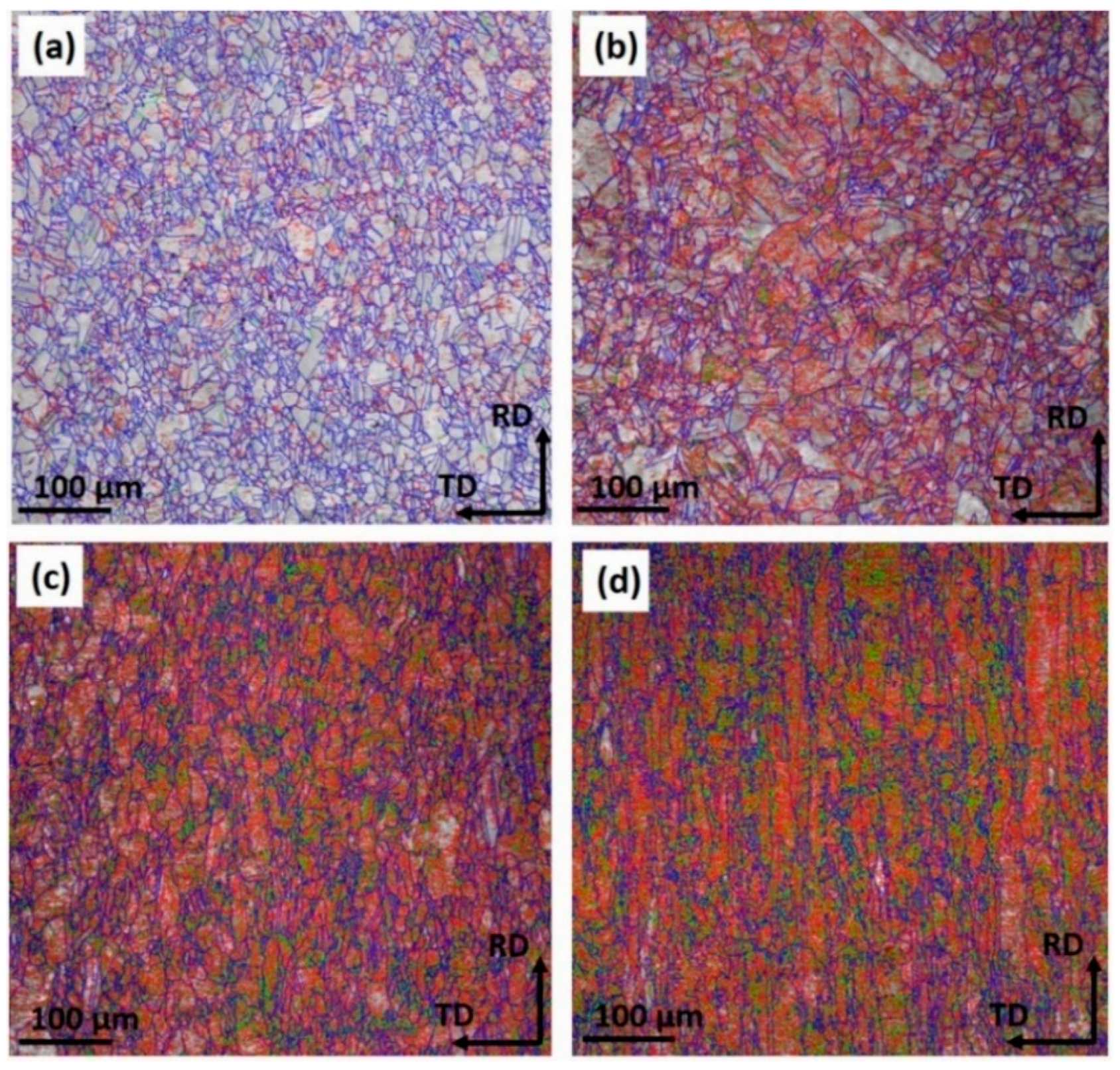
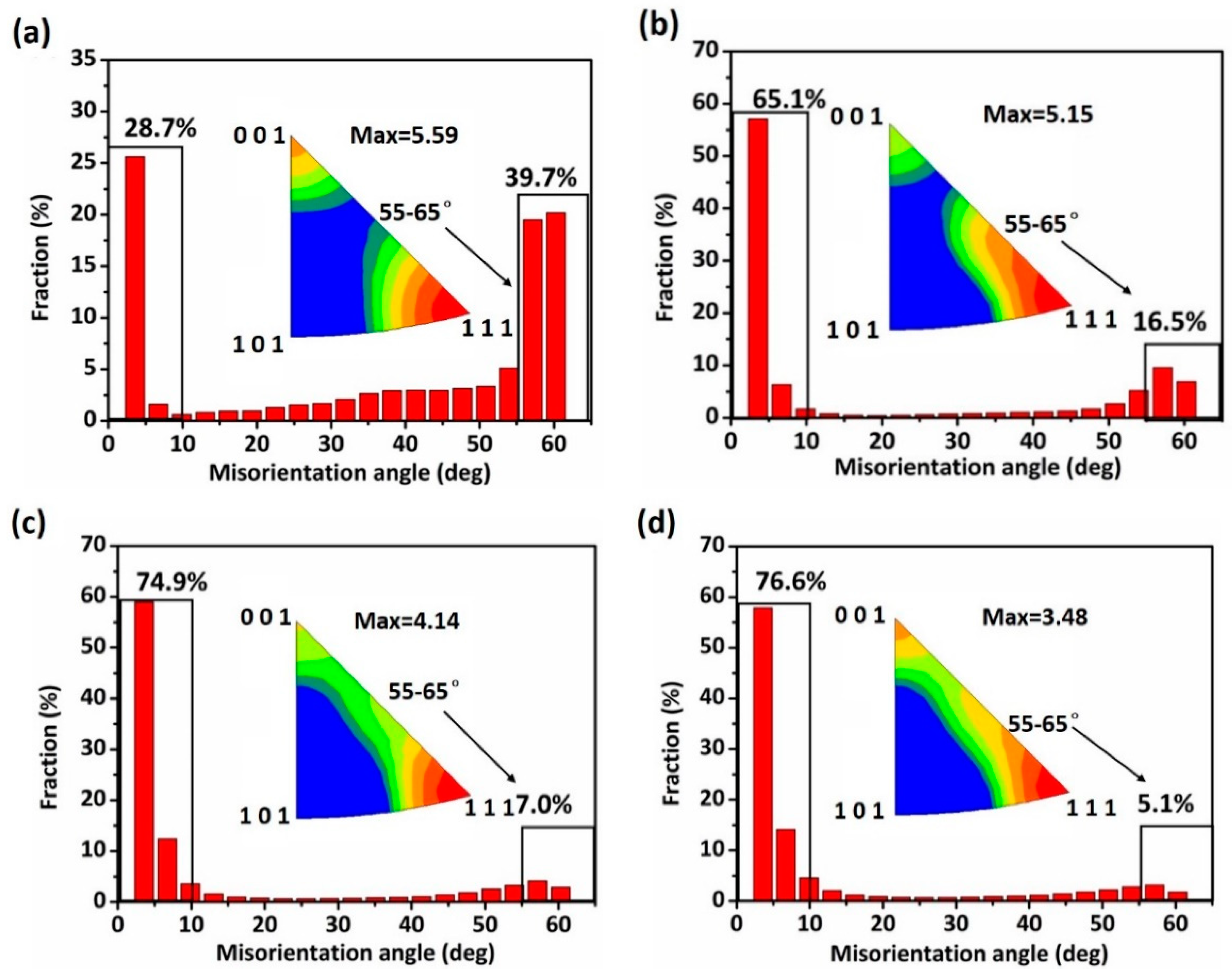
- EBSD mappings demonstrated the average grain size of Cu strips refined from 5.21 μm to 2.42 μm with the accumulated reduction. The fraction of low-angle boundaries increased, while that of high-angle boundaries decreased. This was due to the accumulation of residual stress, which induced stress corrosion in the material interior, further increased the corrosive tendency, and contributed to the expansion of the pitting corrosion.
- (4)
- From the TEM and FIB characterizations, it is concluded that corrosion pits expanded along the rolling direction under the interactive effect of pitting corrosion and stress corrosion. Meanwhile, anions aggregated in the surface defects, such as dislocations, metastable pits, and microcracks, which thereby accelerated the pitting corrosion of the surface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solmaz, R.; Şahin, E.A.; Döner, A.; Kardaş, G. The investigation of synergistic inhibition effect of rhodanine and iodide ion on the corrosion of copper in sulphuric acid solution. Corros. Sci. 2011, 53, 3231–3240. [Google Scholar] [CrossRef]
- Ghelichkhaha, Z.; Sharifi-Aslb, S.; Farhadia, K.; Banisaieda, S.; Ahmadic, S.; Macdonald, D.D. L-cysteine/polydopamine nanoparticle-coatings for copper corrosion protection. Corros. Sci. 2015, 91, 129–139. [Google Scholar] [CrossRef]
- Gelman, D.; Starosvetsky, D.; Ein-Eli, Y. Copper corrosion mitigation by binary inhibitor compositions of potassium sorbate and benzotriazole. Corros. Sci. 2014, 718, 610–619. [Google Scholar] [CrossRef]
- Sherif, E.M.; Park, S.M. Effects of 2-amino-5-ethylthio-1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in aerated acidic pickling solutions. Electrochim. Acta 2006, 51, 6556–6562. [Google Scholar] [CrossRef]
- Liu, T.; Yin, Y.; Chen, S.; Chang, X.; Cheng, S. Super-hydrophobic surfaces improve corrosion resistance of copper in seawater. Electrochim. Acta 2007, 52, 3709–3713. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, L.; Zhou, G. Inhibition of copper corrosion by bis-(1-benzotriazolymethylene)-(2,5-thiadiazoly)-disulfide in chloride media. Appl. Surf. Sci. 2004, 225, 287–293. [Google Scholar] [CrossRef]
- Yan, J.; Zeng, X.; Ren, T.; Heide, E. Boundary lubrication of stainless steel and CoCrMo alloy based on phosphorous and boron compounds in oil-in-water emulsion. Appl. Surf. Sci. 2014, 315, 415–424. [Google Scholar] [CrossRef]
- Yan, X.; Sun, J.; Xiong, S.; Hou, Y. Insights into the sliding wear behavior of a copper-steel pair with oils containing extreme-pressure additives. Wear 2017, 386–387, 211–217. [Google Scholar] [CrossRef]
- Dong, C.; Sun, J.; Cheng, Z.; Hou, Y.Q. Preparation and tribological properties of a microemulsion for magnesium alloy warm rolling. Ind. Lubr. Tribol. 2019, 71, 74–82. [Google Scholar] [CrossRef]
- Xiong, S.; Si, J.; Sun, J.; Wu, H.; Dong, H.; Zhang, C. Experimental and computational studies on heterocyclic organic compounds as corrosion inhibitors for copper immersed in O/W emulsion medium. Anti-Corros. Method Mater. 2020, 2, 214–227. [Google Scholar] [CrossRef]
- Yan, X.; Sun, J.; Meng, Y. Experimental insight into the chemical corrosion mechanism of copper with an oil-in-water emulsion solution. RSC Adv. 2018, 8, 9833–9840. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Sun, J. Effect of water hardness and pH value on the corrosion behavior of copper in an emulsion. Int. J. Electrochem. Sci. 2017, 12, 11580–11593. [Google Scholar] [CrossRef]
- Deng, H.; Yi, J.; Xia, C.; Yi, Y. Improving the mechanical properties of carbon nanotube-reinforced pure copper matrix composites by spark plasma sintering and hot rolling. Mater. Lett. 2018, 210, 177–181. [Google Scholar] [CrossRef]
- Robin, A.; Martinez, G.A.S.; Suzuki, P.A. Effect of cold-working process on corrosion behavior of copper. Mater. Des. 2012, 34, 319–324. [Google Scholar] [CrossRef]
- Lapeire, L.; Lombardia, E.M.; De Graeve, I.; Terryn, H.; Verbeken, K. Influence of grain size on the electrochemical behavior of pure copper. J. Mater. Sci. 2017, 52, 1501–1510. [Google Scholar] [CrossRef]
- Deng, W.; Lin, P.; Li, Q.; Mo, G. Ultrafine-grained copper produced by machining and its unusual electrochemical corrosion resistance in acidic chloride pickling solutions. Corros. Sci. 2013, 74, 44–49. [Google Scholar] [CrossRef]
- Nikfahm, A.; Danaee, I.; Ashrafi, A.; Toroghinejad, M.R. Effect of grain size changes on corrosion behavior of copper produced by accumulative roll bonding process. Mater. Sci. Mater. Res.-Ibero-Am. J. Mater. 2013, 16, 1379–1386. [Google Scholar] [CrossRef] [Green Version]
- Xiong, S.; Sun, J.L.; Xu, Y.; Yan, X.D. Effect of Lubricants and Annealing Treatment on the Electrical Conductivity and Microstructure of Rolled Copper Foil. J. Electron. Mater. 2015, 44, 2432–2439. [Google Scholar] [CrossRef]
- Brian, R.L.; Edwin, R.F. Measurement of thin-layer surface stresses by indentation fracture. J. Mater. Sci. 1984, 19, 4061–4067. [Google Scholar]
- Diard, J.P.; Le Canut, M.L.; Le Gorrec, B.; Montella, C. Copper electrodissolution in 1 M HCl at low current densities, I. General steady-state study. Electrochim. Acta 1998, 43, 2469–2483. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Xu, S.; Yin, L. The effect of 5-nitroindazole as an inhibitor for the corrosion of copper in a 3.0% NaCl solution. RSC Adv. 2015, 5, 63866–63873. [Google Scholar] [CrossRef]
- Gholami, M.; Hoseinpoor, M.; Moayed, M.H. A statistical study on the effect of annealing temperature on pitting corrosion resistance of 2205 duplex stainless steel. Corros. Sci. 2015, 94, 156–164. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Yan, S.; Zou, X.; Chen, S. Three indazole derivatives as corrosion inhibitors of copper in a neutral chloride solutions. Corros. Sci. 2017, 126, 295–304. [Google Scholar] [CrossRef]
- Wang, D.; Xiang, B.; Liang, Y.P.; Song, S.; Liu, C. Corrosion control of copper in 3.5wt.% NaCl Solution by Domperidone: Experimental and Theoretical Study. Corros. Sci. 2014, 85, 77–86. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Guo, L.; Zheng, X.; Xiang, B.; Chen, S. Experimental and theoretical studies of four allyl imidazolium-basedionic liquids as green inhibitors for copper corrosion in sulfuric acid. Corros. Sci. 2017, 119, 68–78. [Google Scholar] [CrossRef]
- Mansour, A.N. Characterization of LiNiO2 by XPS. Surf. Sci. Spectra 1994, 202, 279–286. [Google Scholar] [CrossRef]
- Jolley, J.G.; Geesey, G.G.; Hankins, M.R.; Wright, R.B.; Wichlacz, P.L. Auger electron and X-ray photoelectron spectroscopic study of the biocorrosion of copper by alginic acid polysaccharide. Appl. Surf. Sci. 1989, 37, 469–480. [Google Scholar] [CrossRef]
- Hussain, Z.; Salim, M.A.; Khan, M.A.; Khawaja, E.E. X-ray photoelectron and auger spectroscopy study of copper-sodium-germanate glasses. J. Non-Cryst. Solids 1989, 110, 44–52. [Google Scholar] [CrossRef]
- Nefedov, V.I.; Firsov, M.N.; Shaplygin, I.S. Electronic structures of MRhO2, MRh2O4, RhMO4 and Rh2MO6 on the basis of X-ray spectroscopy and ESCA data. J. Electron Spectrosc. Relat. Phenom. 1982, 26, 65–78. [Google Scholar] [CrossRef]
- Fazal, M.A.; Haseeb, A.S.M.A.; Masjuki, H.H. Corrosion mechanism of copper in palm biodiesel. Corros. Sci. 2013, 67, 50–59. [Google Scholar] [CrossRef]
- Dake, L.S.; Baer, D.R.; Friedrich, D.M. Auger parameter measurements of phosphorus compounds for characterization of phosphazenes. J. Vac. Sci. Technol. A 1989, 7, 1634–1638. [Google Scholar] [CrossRef]
- Hashemi, T.; Hogarth, C.A. The mechanism of corrosion inhibition of copper in NaCl solution by benzotriazole studied by electron spectroscopy. Electrochim. Acta 1988, 33, 1123–1127. [Google Scholar] [CrossRef]
- Klein, J.C.; Li, C.P.; Hercules, D.M.; Black, J.F. Decomposition of copper compounds in X-ray Photoelectron Spectrometers. Appl. Spectrosc. 1984, 38, 729–734. [Google Scholar] [CrossRef]
- Ding, Y.; Lv, Y.; Chen, K.; Zhao, B.; Han, Y.; Wang, L. Effects of microstructure on the stress corrosion cracking behavior of nickel-aluminum bronze alloy in 3.5% NaCl solution. Mater. Sci. Eng. A 2018, 733, 361–373. [Google Scholar] [CrossRef]
- Gertsman, V.Y.; Bruemmer, S.M. Study of grain boundary character along intergranular stress corrosion crack paths in austenitic alloys. Acta Mater. 2001, 49, 1589–1598. [Google Scholar] [CrossRef]
- Konkova, T.; Mironov, S.; Korznikov, A. Microstructure instability in cryogenically deformed copper. Scripta Mater. 2010, 63, 921–924. [Google Scholar] [CrossRef]
- Guo, N.; Song, B.; Yu, H.; Xin, R.; Wang, B.; Liu, T. Enhancing tensile strength of Cu by introducing gradient microstructures via a simple torsion deformation. Mater. Des. 2016, 90, 545–555. [Google Scholar] [CrossRef]
- Kumar, B.R.; Singh, R.; Mahato, B. Effect of texture on corrosion behavior of AISI 304L stainless steel. Mater. Charact. 2005, 54, 141–147. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of grain size on corrosion: A review. Corrosion 2010, 66, 075005–075005-13. [Google Scholar] [CrossRef]
- Van Boven, G.; Chen, W.; Rogge, R. The role of residual stress in neutral pH stress corrosion cracking of pipeline steels. Part I: Pitting and cracking occurrence. Acta Mater. 2007, 55, 29–42. [Google Scholar] [CrossRef]
- Hong, S.; Chen, W.; Luo, H.Q.; Li, B.N. Inhibition effect of 4-amino-antipyrine on the Corrosion of copper in 3 wt.% NaCl solution. Corros. Sci. 2012, 57, 270–278. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, D.; Zhang, D.; Wang, Y.; Gao, L. Anti-corrosion film formed on HAl77-2 copper alloy surface by aliphatic polyamine in 3 wt.% NaCl solution. Appl. Surf. Sci. 2017, 392, 768–776. [Google Scholar] [CrossRef]
- Chen, W.; Hong, S.; Li, H.B.; Luo, H.Q.; Li, M.; Li, B.N. Protection of copper corrosion in 0.5 M Nacl solution by modification of 5-mercapto-3-phenyl-1,3,4-thiadiazole-2-thione potassium self-assembled monolayer. Corros. Sci. 2012, 61, 53–62. [Google Scholar] [CrossRef]
- Zhang, X.; Pehkonen, S.O.; Kocherginsky, N.; Ellis, G.A. Copper corrosion in mildly alkaline water with the disinfectant monochloramine. Corros. Sci. 2002, 44, 2507–2528. [Google Scholar] [CrossRef]
- Zelinsky, A.G.; Pirogov, B.Y. Numerical simulation of corrosion process with two-step charge transfer mechanism in the Cu/CuSO4+H2SO4 system. Corros. Sci. 2006, 48, 2867–2881. [Google Scholar] [CrossRef]
- Nazarov, A.; Thierry, D. Rate-determining reactions of atmospheric corrosion. Electrochim. Acta 2004, 49, 2717–2724. [Google Scholar] [CrossRef]



| Component | Cu | Pb | S | Cd | P | Fe |
| Wt.% | 99.9900 | 0.0003 | 0.0016 | 0.0002 | 0.0013 | 0.0009 |
| Properties | Mineral Oil D130 | Rapeseed Oil |
|---|---|---|
| Main compositions | Direct alkane, Branched alkane, Cycloalkanes | Erucic acid, Arachidic acid, Linoleic acid, linolenic acid |
| Aromatics content (wt.%) | 0.5 | - |
| Sulfur content (wt.%) | <0.1 | <0.1 |
| Phosphorous content (wt.%) | <0.1 | <0.1 |
| Viscosity for 40 °C (mm2/s) | 6.12 | 13.5~14.0 |
| Flash point (°C) | >140 | >110 |
| Suppliers | Sinopec Group Shanghai Co., Ltd., Shanghai, China | Red Oil Chengdu Ltd., Chengdu, China |
| Year of production | 2017 | 2019 |
| Ionic Types | ||||||||
| Concentrations (ppm) | 15.62 | 21.78 | 71.52 | 6.35 | - | 67.26 | 13.94 | 6.39 |
| Rolling Pass | 0 | 1 | 2 | 3 | 4 | 5 |
| Thickness (mm) | 1.98 | 1.85 | 1.57 | 1.16 | 0.82 | 0.45 |
| Reduction | Ecorr (mV) | icorr (A/cm2) | βa (V/dec) | βc (V/dec) | Ep (V) |
|---|---|---|---|---|---|
| 0% | −134 | 1.23 × 10−6 | 3.09 | −1.92 | 0.58 |
| 20.7% | −425 | 9.55 × 10−6 | 2.98 | −2.01 | 0.45 |
| 58.6% | −505 | 1.76 × 10−5 | 2.75 | −2.09 | 0.41 |
| 77.3% | −527 | 2.56 × 10−5 | 2.99 | −1.98 | 0.11 |
| Reduction | Rct (kΩ cm2) | Rf (kΩ cm2) | Rpo (kΩ cm2) | Rs (kΩ cm2) | Qf (μF cm−2) | Qdl (μF cm−2) | W (×10−2 Ω cm2 S1/2) |
|---|---|---|---|---|---|---|---|
| 0% | 107.82 | 1.12 | 108.94 | 2.81 | 12.68 | 25.64 | 18.62 |
| 20.7% | 44.08 | 0.42 | 44.50 | 2.82 | 18.86 | 38.40 | 15.64 |
| 58.6% | 28.31 | 0.38 | 28.69 | 2.81 | 24.42 | 50.42 | 31.08 |
| 77.3% | 20.27 | 0.32 | 20.59 | 2.82 | 28.82 | 340.64 | 40.12 |
| Reduction | 0% | 20.7% | 58.6% | 77.3% |
| Dadv (μm) | 5.21 | 5.14 | 3.08 | 2.42 |
| Number of GBs | 285,646 | 463,615 | 971,524 | 13,543,64 |
| Number of LAGBs | 82,266 | 305,522 | 745,159 | 10,672,39 |
| Fraction of LAGBs (%) | 28.8 | 65.9 | 76.7 | 78.8 |
| Number of HAGBs | 203,380 | 158,093 | 226,365 | 287,125 |
| Fraction of HAGBs (%) | 71.2 | 34.1 | 23.3 | 21.2 |
| ε | D/μm | H/MPa | C/μm | KIC/MPa·m1/2 | σγ/MPa |
|---|---|---|---|---|---|
| 0% | 165.79 | 221.76 | 69.48 | 15.38 | −35.69 |
| 20.7% | 1297.67 | 251.64 | 106.76 | 11.25 | −238.43 |
| 58.6% | 356.36 | 286.67 | 139.47 | 8.78 | −296.45 |
| 77.3% | 386.37 | 357.73 | 156.98 | 3.21 | −598.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Sun, J. Pitting Corrosion Behavior and Surface Microstructure of Copper Strips When Rolled with Oil-in-Water Emulsions. Materials 2021, 14, 7911. https://doi.org/10.3390/ma14247911
Yan X, Sun J. Pitting Corrosion Behavior and Surface Microstructure of Copper Strips When Rolled with Oil-in-Water Emulsions. Materials. 2021; 14(24):7911. https://doi.org/10.3390/ma14247911
Chicago/Turabian StyleYan, Xudong, and Jianlin Sun. 2021. "Pitting Corrosion Behavior and Surface Microstructure of Copper Strips When Rolled with Oil-in-Water Emulsions" Materials 14, no. 24: 7911. https://doi.org/10.3390/ma14247911
APA StyleYan, X., & Sun, J. (2021). Pitting Corrosion Behavior and Surface Microstructure of Copper Strips When Rolled with Oil-in-Water Emulsions. Materials, 14(24), 7911. https://doi.org/10.3390/ma14247911





