Quantitative Non-Linear Effect of High Ambient Temperature on Chloride Threshold Value for Steel Reinforcement Corrosion in Concrete under Extreme Boundary Conditions
Abstract
:1. Introduction
2. Research Significance
3. Experimental Program
3.1. Materials and Mix Proportions
3.2. Specimen Preparation and Curing Conditions
3.3. Corrosion Measurement
3.4. Chloride Content
4. Results and Discussion
4.1. Chloride Content Analysis
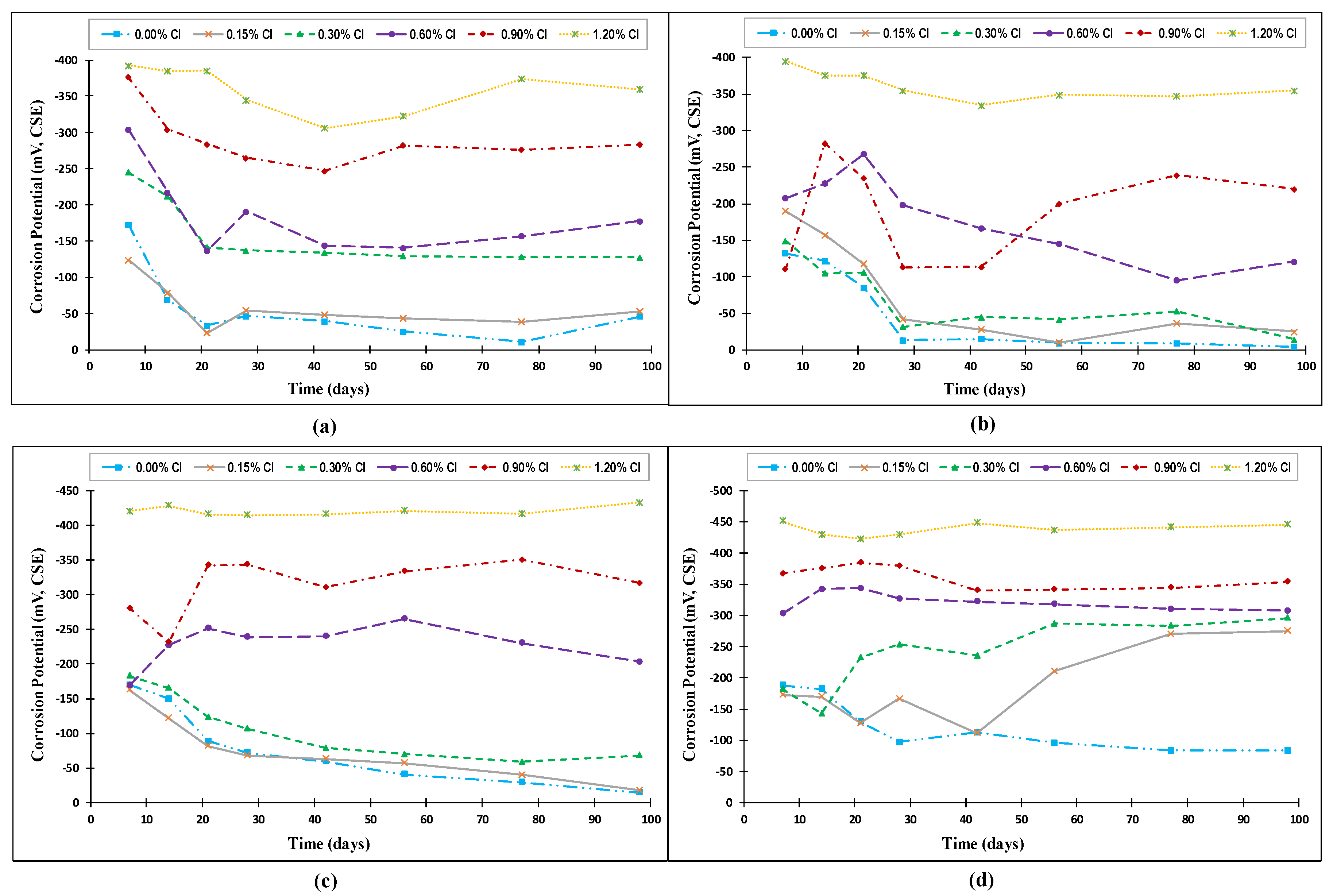
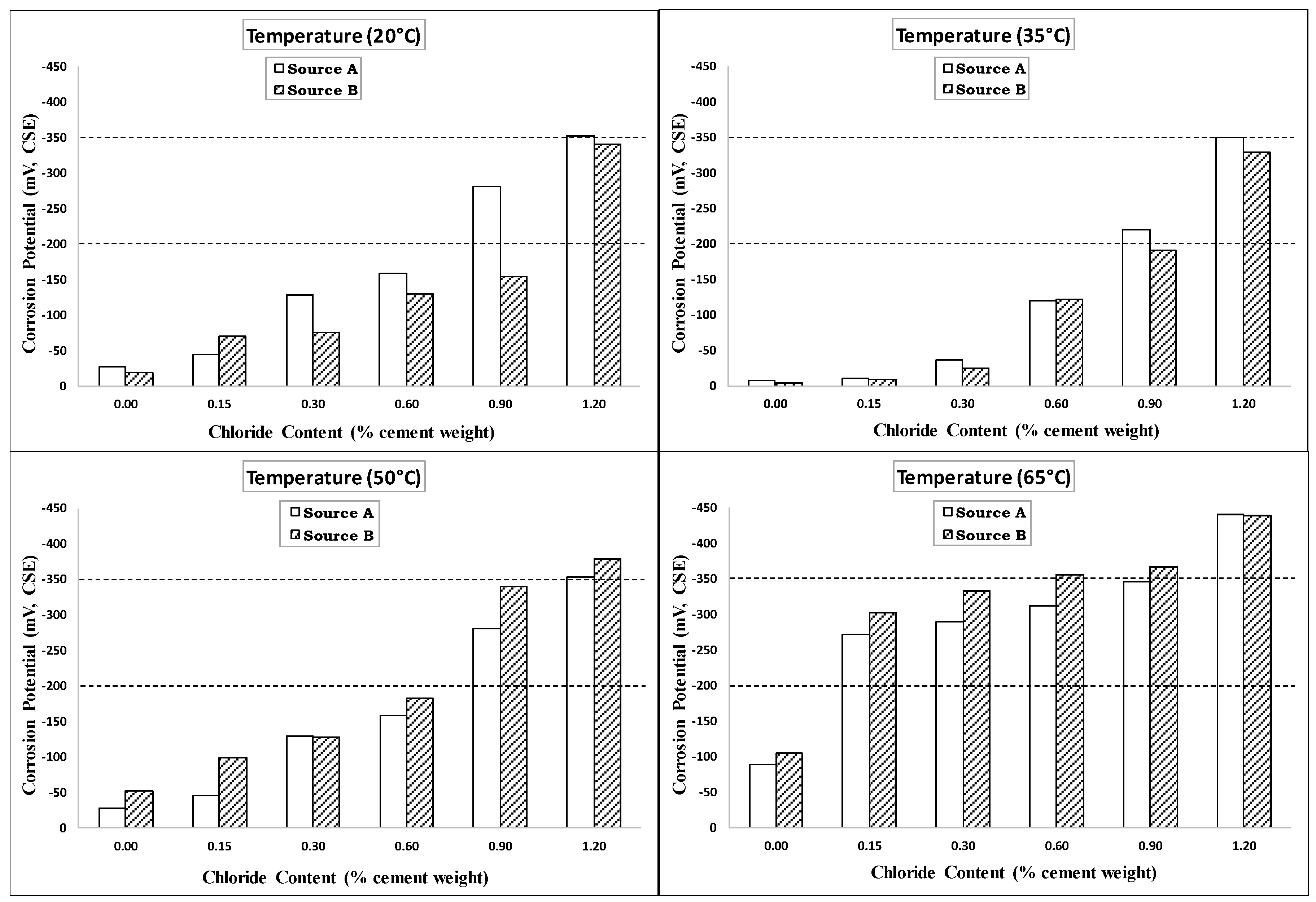
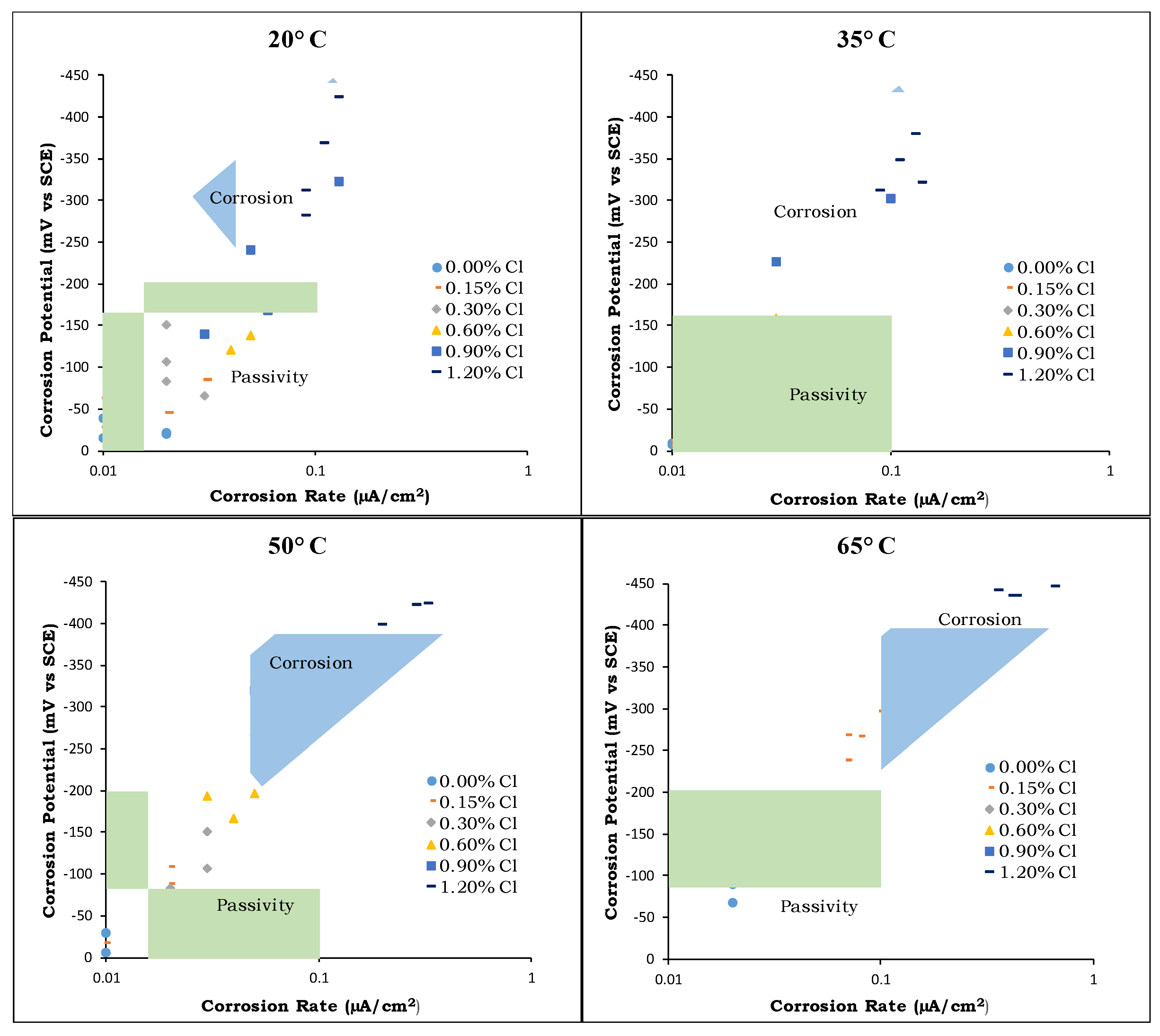
4.2. Corrosion Potential
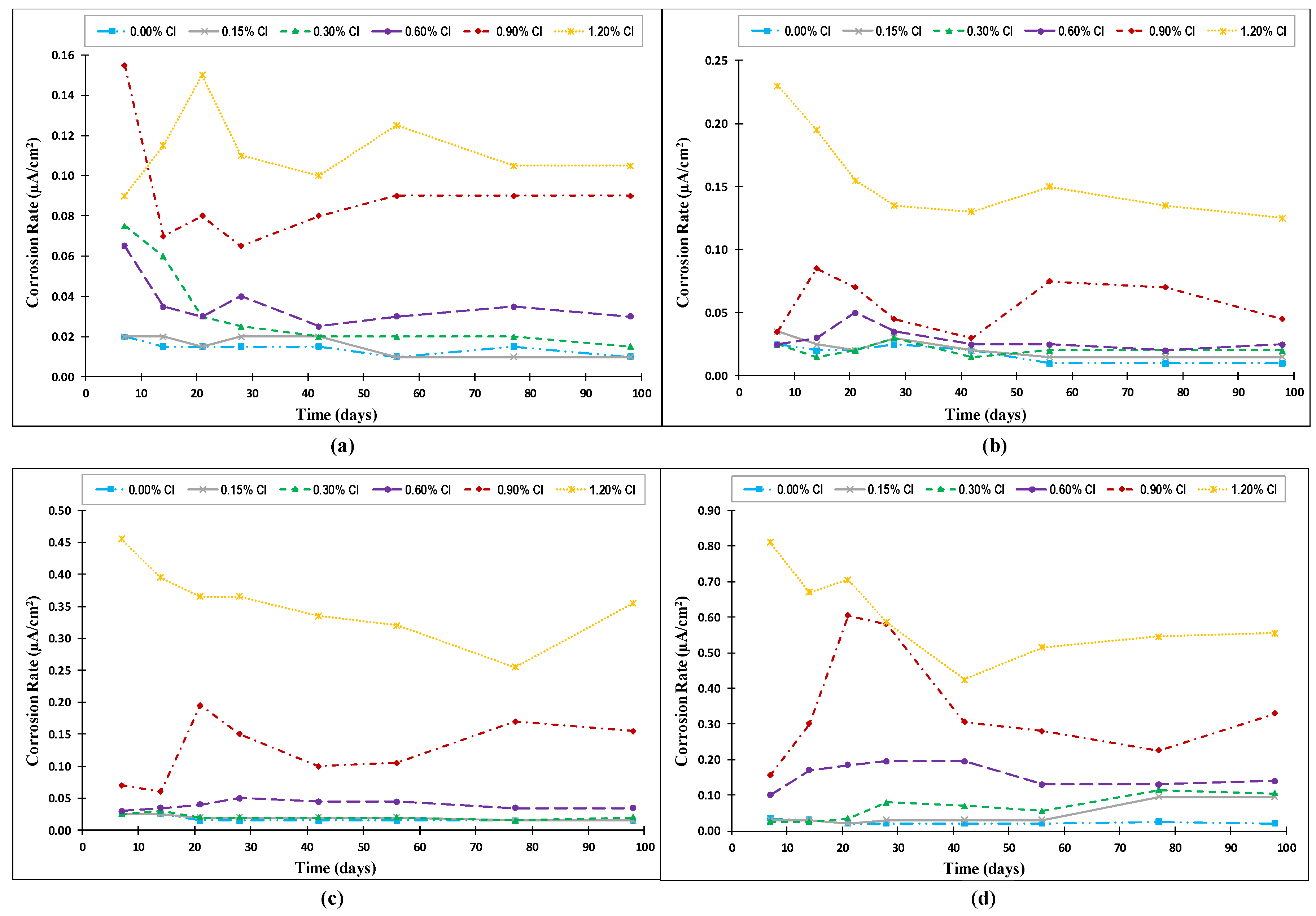
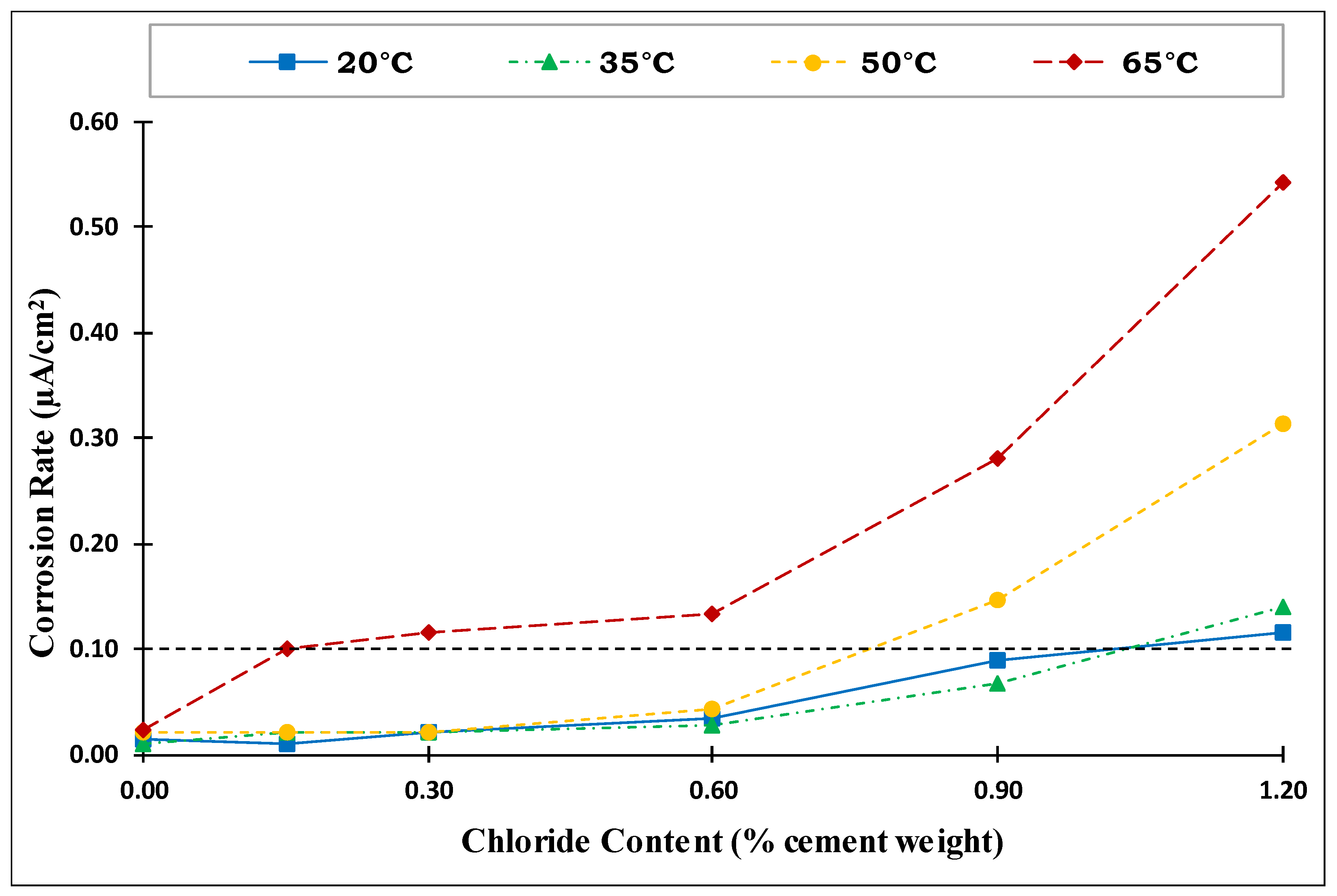
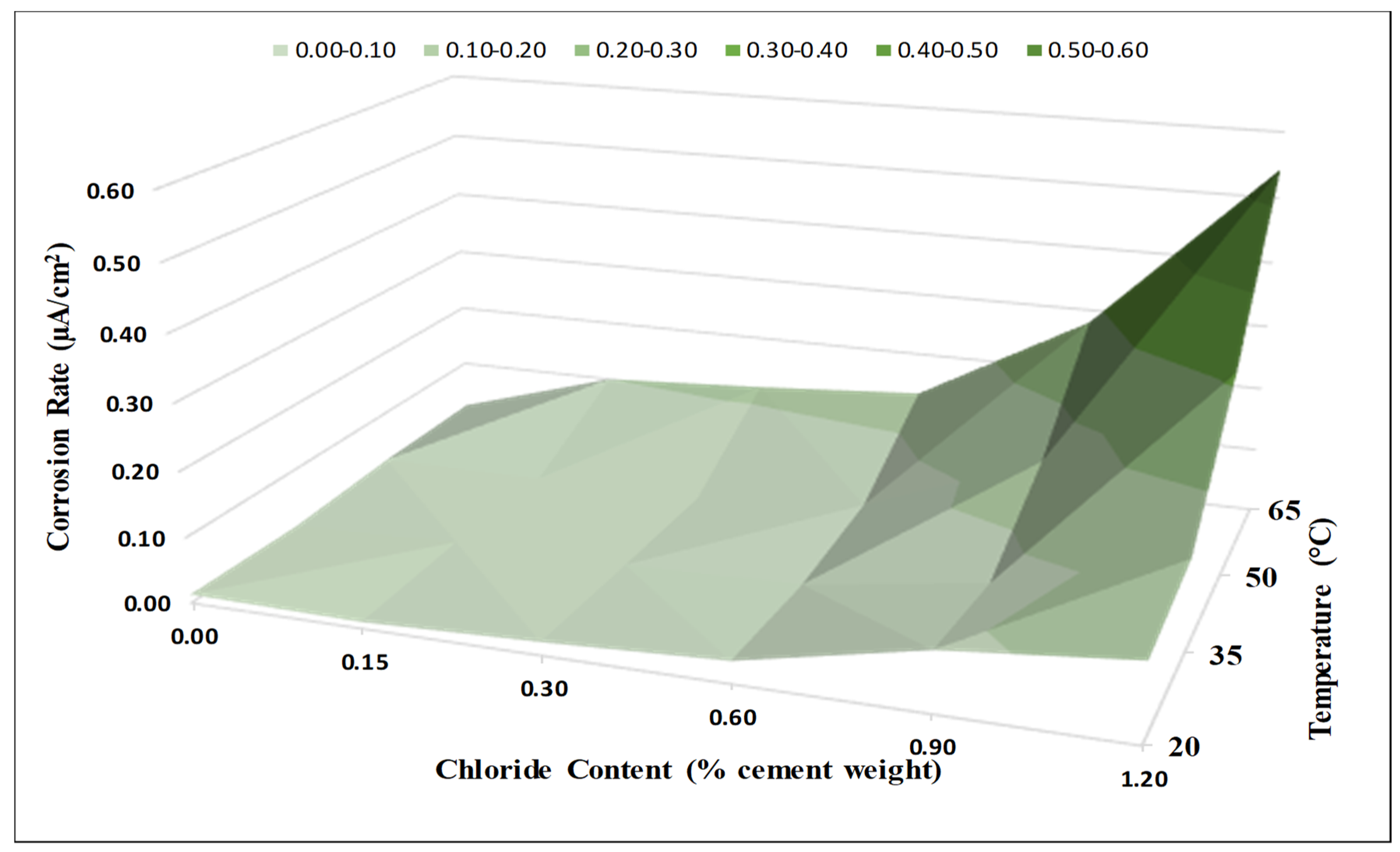
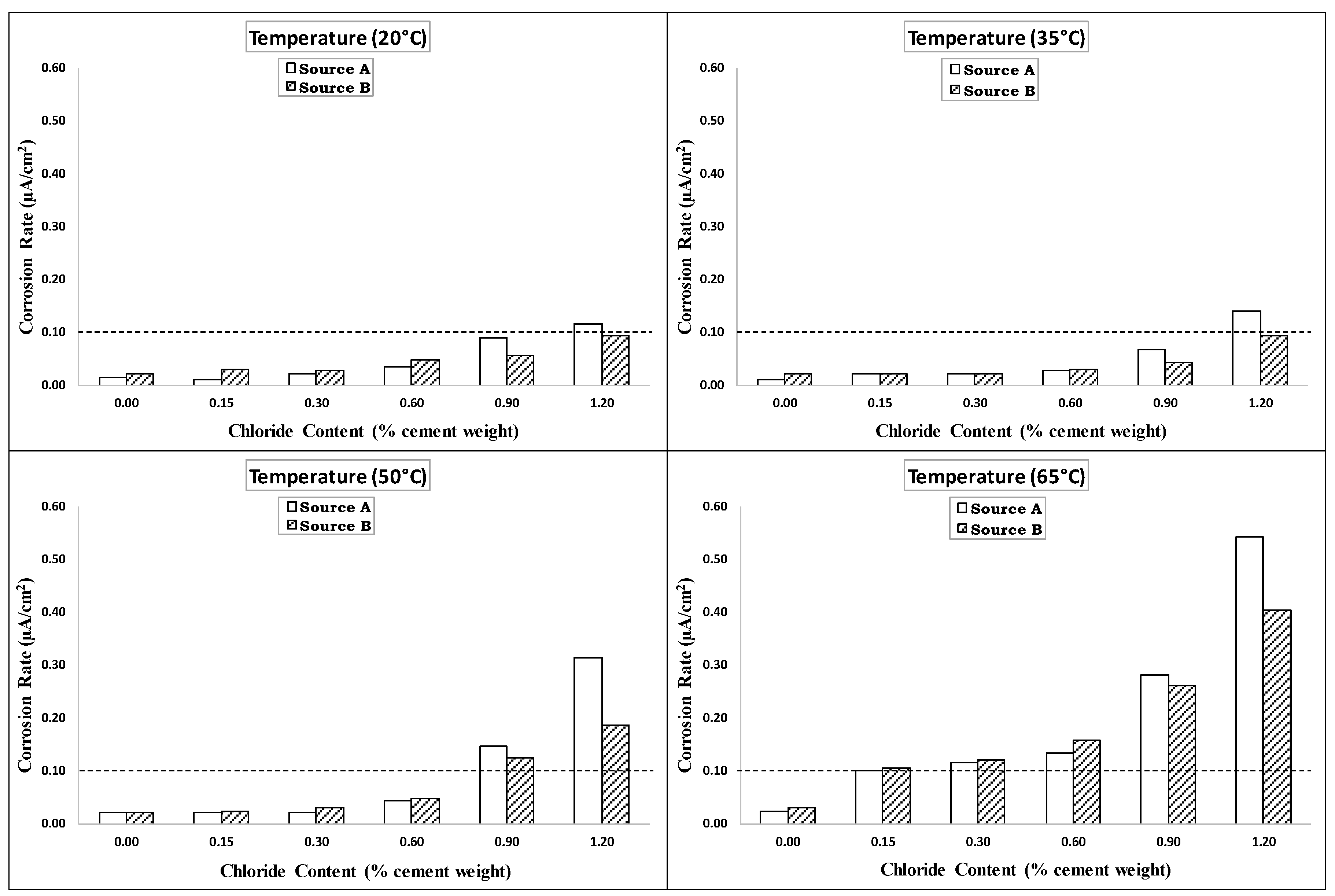
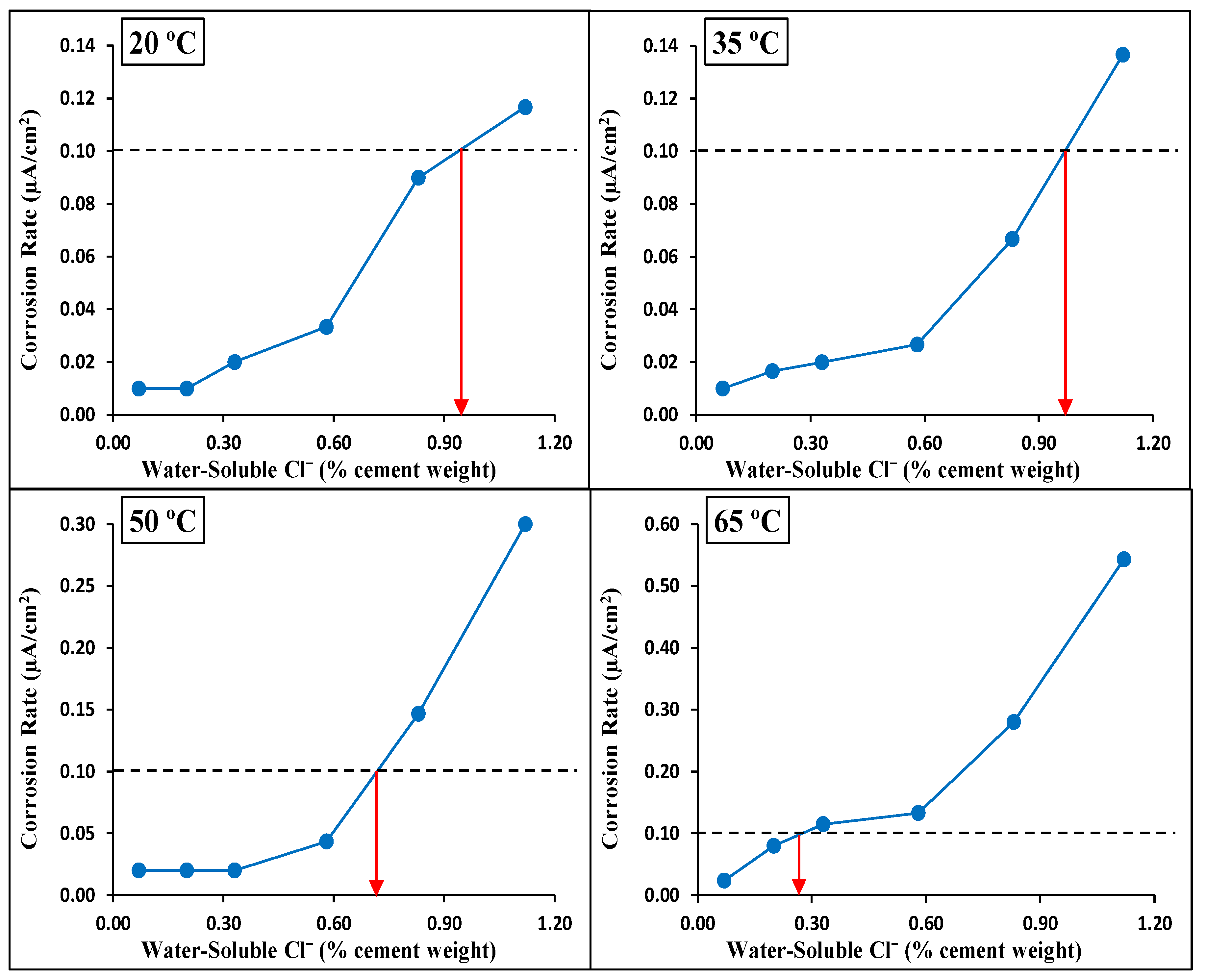
4.3. Corrosion Rate
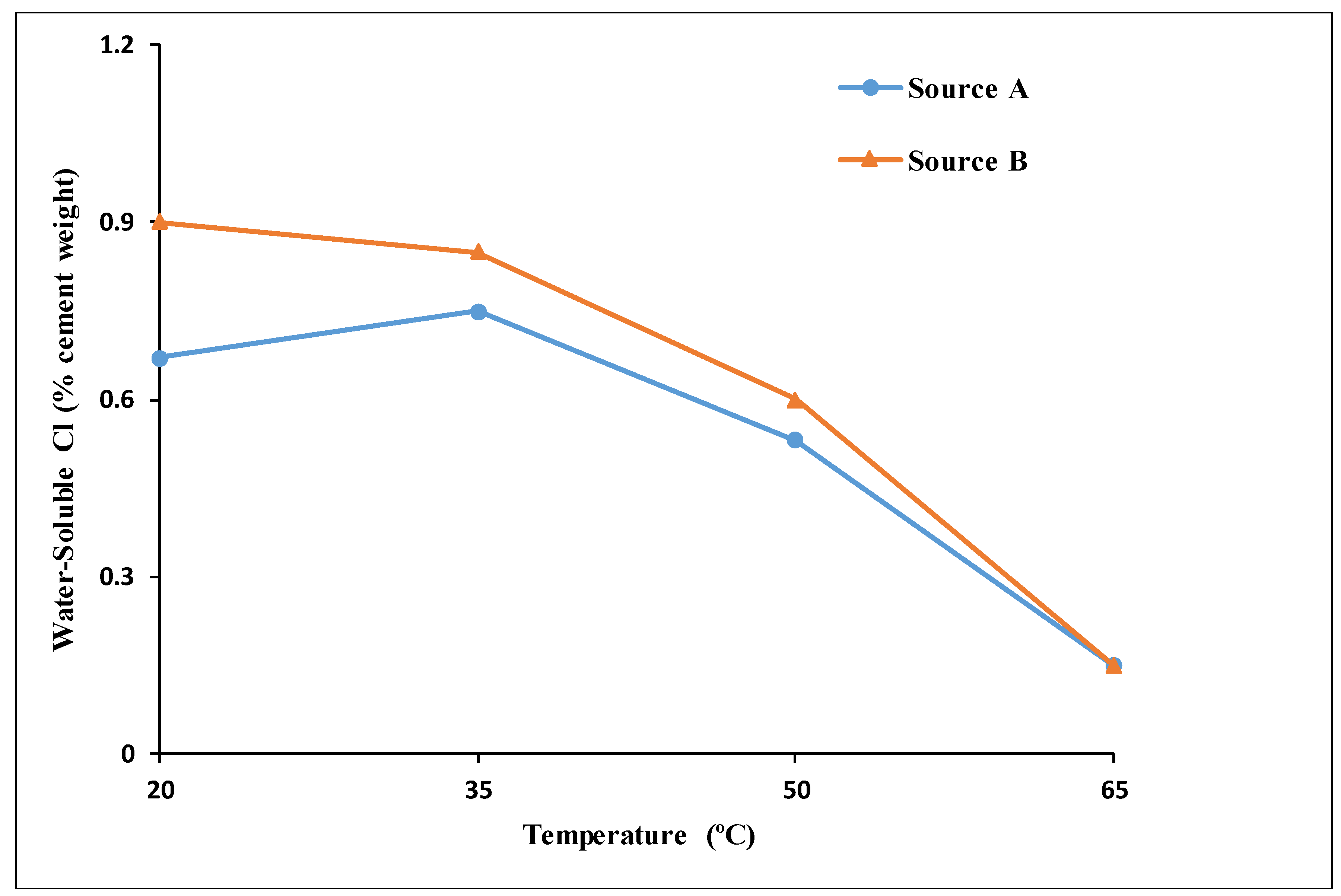
4.4. Effect of Rebar Sources
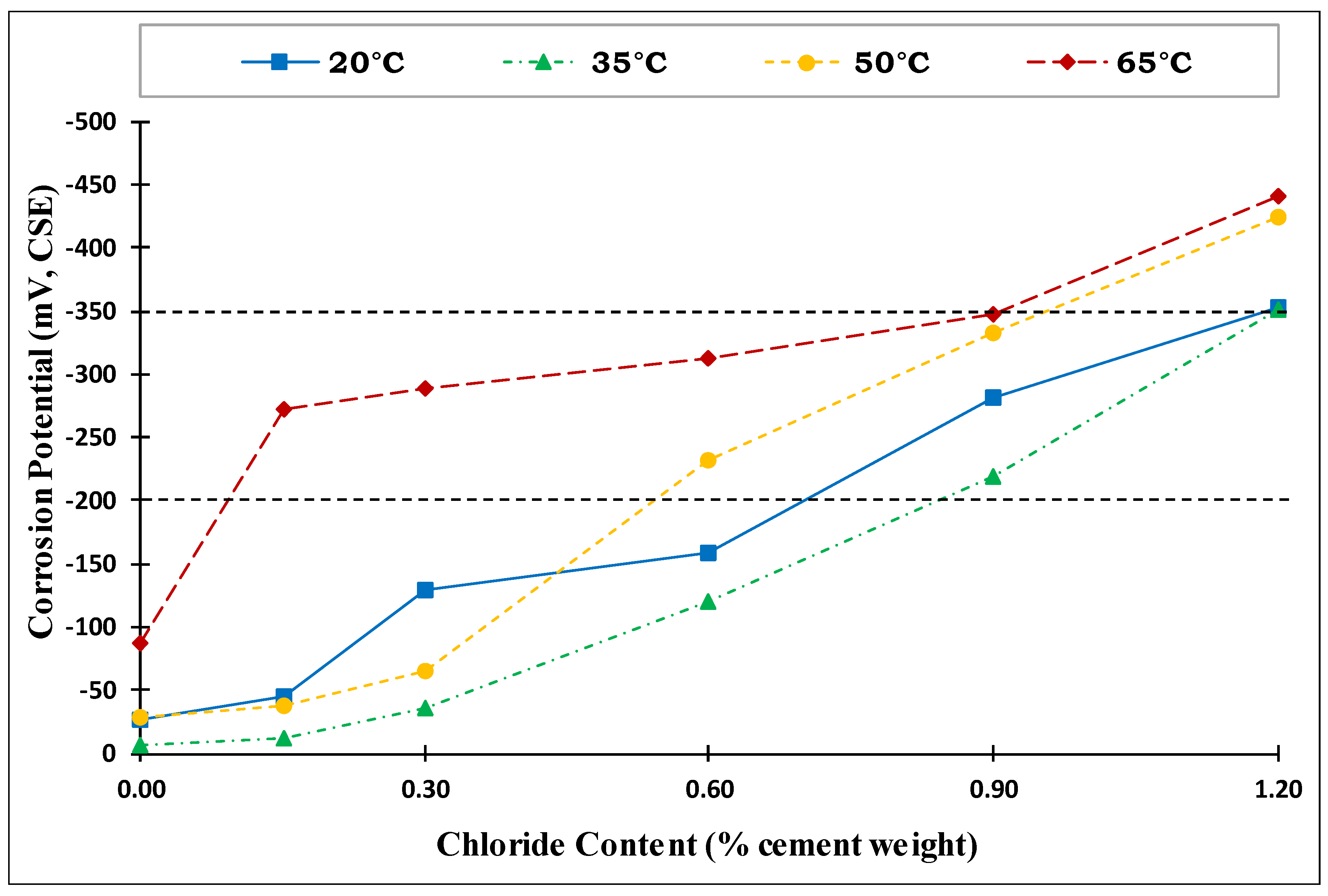
4.5. CT Values
5. Conclusions
- The corrosion potential and corrosion rate measurements clearly showed a strong coupled effect of the chloride content and temperature on the de-passivation of steel rebars in concrete, especially at high exposure temperatures.
- The CT values determined based on a corrosion rate of 0.1 µA/cm2 were consistently within the range of the corresponding CT values obtained from the half-cell potential based on the ASTM C876 criteria.
- The behaviors and trends shown for specimens from Source A and Source B under increasing temperature and chloride levels indicated limited influence of the rebar source on the de-passivation and CT values of carbon steel rebars.
- Temperature had a clear and significant non-linear influence on the CT values. The CT values (water-soluble chlorides by weight of cement) obtained for Source A based on the corrosion rate for the four investigated temperatures were as follows:
- ○
- At temperatures of 20 °C (68 °F) and 35 °C (95 °F), the CT values were almost identical at 0.95% and 96%, respectively.
- ○
- At 50 °C (122 °F), the CT value decreased to 0.71%.
- ○
- At 65 °C (149 °F), the CT value dropped dramatically to 0.26%.
- The effect of increased temperature on the CT value was limited up to a temperature of 35 °C (95 °F). However, a dramatic drop to approximately 1/4 of the CT value at 20 °C (68 °F) was observed at an exposure temperature of 65 °C (149 °F), highlighting the crucial influence of high temperature on the de-passivation of carbon steel rebars in the presence of chlorides.
- The low CT value for carbon steel at an exposure temperature of 65 °C (149 °F) should be a major concern when selecting and specifying durability requirements for RC structures to prevent chloride-induced corrosion under tropical conditions.
- The relevant code provisions for the durability of RC construction in tropical climates need to be assessed considering the exposure class and the effect of high temperature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Page, C.L.; Treadaway, K.W. Aspects of the electrochemistry of steel in concrete. Nature 1982, 297, 109–115. [Google Scholar] [CrossRef]
- Schiessl, P.; Raupach, M. Influence of concrete composition and microclimate on the critical chloride content in concrete. Corros. Reinf. Concr. 1990, 49–58. [Google Scholar]
- Angst, U.; Elsener, B.; Larsen, C.; Vennesland, Ø. Critical chloride content in reinforced concrete-A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Frederiksen, J.M. On the need for more precise threshold values for chloride initiated corrosion. Mater. Corros. 2009, 60, 597–601. [Google Scholar] [CrossRef]
- Glass, G.; Buenfeld, N.R. The Presentation of the chloride level for corrosion of steel in concrete. Corros. Sci. 1997, 39, 1001–1013. [Google Scholar] [CrossRef]
- Hausmann, D.A. Steel corrosion in concrete; How does it occur? Mater. Prot. 1967, 6, 19–23. [Google Scholar]
- Kenny, A.; Katz, A. Steel-concrete interface influence on chloride threshold for corrosion—Empirical reinforcement to theory. Constr. Build. Mater. 2020, 244, 118376. [Google Scholar] [CrossRef]
- Alonso, M.; Moreno, M. Analysis of the variability of chloride threshold values in the literature. Mater. Corros. 2009, 60, 631–637. [Google Scholar] [CrossRef]
- ACI Committee 318. Building Code Requirments for Structural Concrete (ACI 318-19); American Concrete Inistitute: Farmington Hills, MI, USA, 2019. [Google Scholar]
- British Standard 8110: Part 1, Structural Use of Concrete—Code of Practice for Design and Construction; British Standards Institute: London, UK, 1985.
- Schiessl, P. Corrosion of Steel in Concrete: Report of the Technical Committee 60 CSC, RILEM (the International Union of Testing and Research Laboratories for Materials and Structures); Chapman and Hall: New York, NY, USA; London, UK, 1988. [Google Scholar]
- DuraCrete. Modelling of Degradation : DuraCrete, Probabilistic Performance Based Durability Design of Concrete Structures; DuraCrete Project Document No. BE95-1347/R45; CUR: Gouda, The Netherlands, 1998. [Google Scholar]
- LIFE-365. ACI-Committee-365, Service Life Prediction Model, Computer Program for Predicting the Service Life and Life-Cycle Costs of Reinforced Concrete Exposed to Chlorides; American Concrete Institute: Farmington Hills, MI, USA, 2005. [Google Scholar]
- Hooton. Challenges in predicting service-life of concrete structures exposed to chlorides. In Proceedings of the 2nd International Symposium on Service Life Design of Infrastructure, Delft, The Netherlands, 4–6 October 2010; RILEM Publications SARL: Paris, France; pp. 3–16. [Google Scholar]
- Hooton, R.; Geiker, M.; Bentz, E. Effects of curing on chloride ingress and implications on service life. ACI Mater. J. 2002, 99, 201–206. [Google Scholar]
- Liu, Y.; Weyer, R. Time to cracking for chloride-induced corrosion in reinforced concrete. Corros. Reinf. Concr. Constr. 1996, 183, 88–104. [Google Scholar]
- Funahashi, M. Predicting corrosion free service life of a concrete structure in a chloride environment. ACI Mater. J. 1990, 87, 581–587. [Google Scholar]
- Clear, K. Time to Corrosion of Reinforcing Steel in Concrete Slab; Federal Highway Administration: Washington, DC, USA, 1976. [Google Scholar]
- West, R.; Hime, W. Chloride profiles in salty concrete. Mater. Perform. 1985, 24, 29–36. [Google Scholar]
- Neville, A.M. Properties of Concrete, 4th ed.; Longman Group Ltd.: London, UK, 1995. [Google Scholar]
- Tuutti, K. Corrosion of Steel in Concrete; Swedish Cement and Concrete Research Institute: Stockholm, Sweden, 1982. [Google Scholar]
- Sharifi-Asl, S.; Mao, F.; Lu, P.; Kursten, B.; MacDonald, D. Exploration of the effect of chloride ion concentration and temperature on pitting corrosion of carbon steel in saturated Ca(OH)2 solution. Corros. Sci. 2015, 98, 708–715. [Google Scholar] [CrossRef]
- Lu, P.; Sharifi-Asl, S.; Kursten, B.; Macdonald, D. The Irreversibility of the Passive State of Carbon Steel in the Alkaline Concrete Pore Solution under Simulated Anoxic Conditions. J. Electrochem. Soc. 2015, 162, 572–581. [Google Scholar] [CrossRef]
- Hussain, S.; Rasheeduzzafar; Al-Musallam, A.; Al-Gahtani, A. Factors affecting threshold chloride for reinforcement corrosion in concrete. Cem. Concr. Res. 1995, 25, 1543–1555. [Google Scholar] [CrossRef]
- Matsumura, T.; Shiraib, K.; Saegusa, T. Verification method for durability of reinforced concrete structures subjected to salt attack under high temperature conditions. Nucl. Eng. Des. 2008, 238, 1181–1188. [Google Scholar] [CrossRef]
- Gastaldi, M.; Bertolini, L. Effect of temperature on the corrosion behaviour of low-nickel duplex stainless steel bars in concrete. Cem. Concr. Res. 2014, 56, 52–60. [Google Scholar] [CrossRef]
- Uomoto, T. Non-Destructive Testing in Civil Engineering 2000; Elsevier Science: Tokyo, Japan, 2000; pp. 671–678. [Google Scholar]
- Cigna, R.; Andrade, C.; Nürnberger, U.; Polder, R.; Weydert, R.; Seitz, E. Corrosion of Steel in Reinforced Concrete Structures; COST 521: Luxembourg, 2002. [Google Scholar]
- Stern, M.; Geary, A. Electrochemical Polarization: I. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Gecor 8 Corrosion Rate Meter: Operator’s Instruction Manual; James Instruments Inc.: Chicago, IL, USA, 2016.
- ASTM C1218-15. Standard Test Method for Water-Soluble Chloride in Mortar and Concrete; American Society for Testing and Materials: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM C1152-04. Standard Test Method for Acid-Soluble Chloride in Mortar and Concrete; American Society for Testing and Materials: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Whiting, D.A. Origins of Chloride Limits for Reinforced Concrete; Portland Cement Association: Skokie, IL, USA, 1997. [Google Scholar]
- Whiting, D.A.; Taylor, P.C.; Nagi, M.A. Chloride Limits in Reinforced Concrete; Portland Cement Association: Skokie, IL, USA, 2002. [Google Scholar]
- Taylor, P.C.; Nagi, M.A.; Whiting, D.A. Threshold Chloride Content for Corrosion of Steel in Concrete: A Literature Review, PCA R&D Serial No. 2169; Portland Cement Association: Skokie, IL, USA, 1999. [Google Scholar]
- ASTM C876. Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete; American Society for Testing and Materials: West Conshohocken, PA, USA, 2015. [Google Scholar]
- RILEM TC154-EMC by Andrade & Alonso. Electrochemical Techniques for Measuring Metallic Corrosion: Test methods for on-site corrosion rate measurement of steel reinforcement in concrete by means of the polarization resistance method. Mater. Struct. 2004, 37, 623–643. [Google Scholar]
- Gonzalez, J.; Andrade, C. Effect of carbonation and relative ambient humidity on the corrosion of galvanised rebars embedded in concrete. Br. Corros. J. 1982, 17, 21–28. [Google Scholar] [CrossRef]
- Alonso, C.; Andrade, C.; Castellote, M.; Castro, P. Chloride threshold values to depassivate reinforcing bars embedded in a standardized OPC mortar. Cem. Concr. Res. 2000, 30, 1047–1055. [Google Scholar] [CrossRef]
- UNE 83992-2 (2012). Durability of Concrete, Test Methods, Chloride Penetration Tests on Concrete. Part 2: Integral Accelerated Method; AENOR: Madrid, Spain, 2012; p. 15. [Google Scholar]
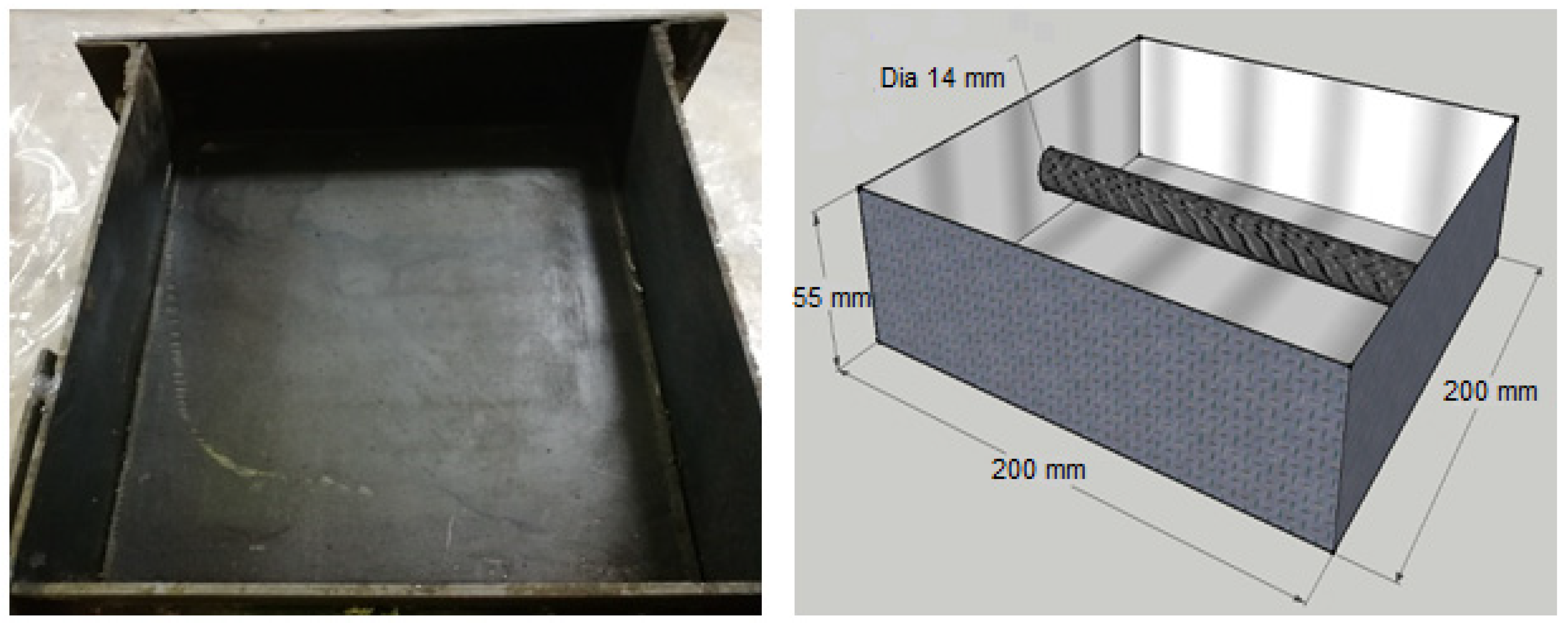


| Materials | Proportions (kg/m3) | Proportions (lb/ft3) |
|---|---|---|
| Cement | 350 | 21.8 |
| Water | 175 | 10.9 |
| Coarse Aggregate | 1040 | 64.9 |
| Crushed Sand | 210 | 13.1 |
| White Sand | 490 | 30.6 |
| Element (%) | C | Mn | Si | Cu | Cr | Ni | Ti | Fe |
|---|---|---|---|---|---|---|---|---|
| Source A | 0.22 | 0.59 | 0.17 | - | - | - | - | balance |
| Source B | 0.12 | 0.85 | 0.21 | 0.26 | 0.07 | 0.10 | 0.02 | balance |
| Added Cl (% wt of Cement) | Water-Soluble Cl (% wt of Cement) | Acid-Soluble Cl (% wt of Cement) | Bound Chloride Percentage |
|---|---|---|---|
| 0.00 | 0.07 | 0.10 | 30.0 |
| 0.15 | 0.20 | 0.25 | 20.0 |
| 0.30 | 0.33 | 0.40 | 17.5 |
| 0.60 | 0.58 | 0.69 | 15.9 |
| 0.90 | 0.83 | 0.97 | 14.4 |
| 1.20 | 1.12 | 1.28 | 12.5 |
| Corrosion Condition | Icorr |
|---|---|
| Passive condition | <0.1 μA/cm2 |
| Low–moderate | 0.1 to 0.5 μA/cm2 |
| Intermediate–high | 0.5 to 1 μA/cm2 |
| Very high | >1 μA/cm2 |
| Exposure Temperature | CT Value (% by Weight of Cement) | |||
|---|---|---|---|---|
| Half-Cell Potential | Corrosion Rate | |||
| Source A | Source B | Source A | Source B | |
| 20 °C (68 °F) | 0.67–1.10 | 0.90–1.13 | 0.95 | 1.12 |
| 35 °C (95 °F) | 0.75–1.08 | 0.85–1.15 | 0.96 | 1.12 |
| 50 °C (122 °F) | 0.53–0.88 | 0.60–0.90 | 0.71 | 0.75 |
| 65 °C (149 °F) | 0.15–0.82 | 0.15–0.55 | 0.26 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhozaimy, A.M.; Ahmed, M.; Hussain, R.R.; Al-Negheimish, A. Quantitative Non-Linear Effect of High Ambient Temperature on Chloride Threshold Value for Steel Reinforcement Corrosion in Concrete under Extreme Boundary Conditions. Materials 2021, 14, 7595. https://doi.org/10.3390/ma14247595
Alhozaimy AM, Ahmed M, Hussain RR, Al-Negheimish A. Quantitative Non-Linear Effect of High Ambient Temperature on Chloride Threshold Value for Steel Reinforcement Corrosion in Concrete under Extreme Boundary Conditions. Materials. 2021; 14(24):7595. https://doi.org/10.3390/ma14247595
Chicago/Turabian StyleAlhozaimy, Abdulrahman M., Mshtaq Ahmed, Raja Rizwan Hussain, and Abdulaziz Al-Negheimish. 2021. "Quantitative Non-Linear Effect of High Ambient Temperature on Chloride Threshold Value for Steel Reinforcement Corrosion in Concrete under Extreme Boundary Conditions" Materials 14, no. 24: 7595. https://doi.org/10.3390/ma14247595
APA StyleAlhozaimy, A. M., Ahmed, M., Hussain, R. R., & Al-Negheimish, A. (2021). Quantitative Non-Linear Effect of High Ambient Temperature on Chloride Threshold Value for Steel Reinforcement Corrosion in Concrete under Extreme Boundary Conditions. Materials, 14(24), 7595. https://doi.org/10.3390/ma14247595






