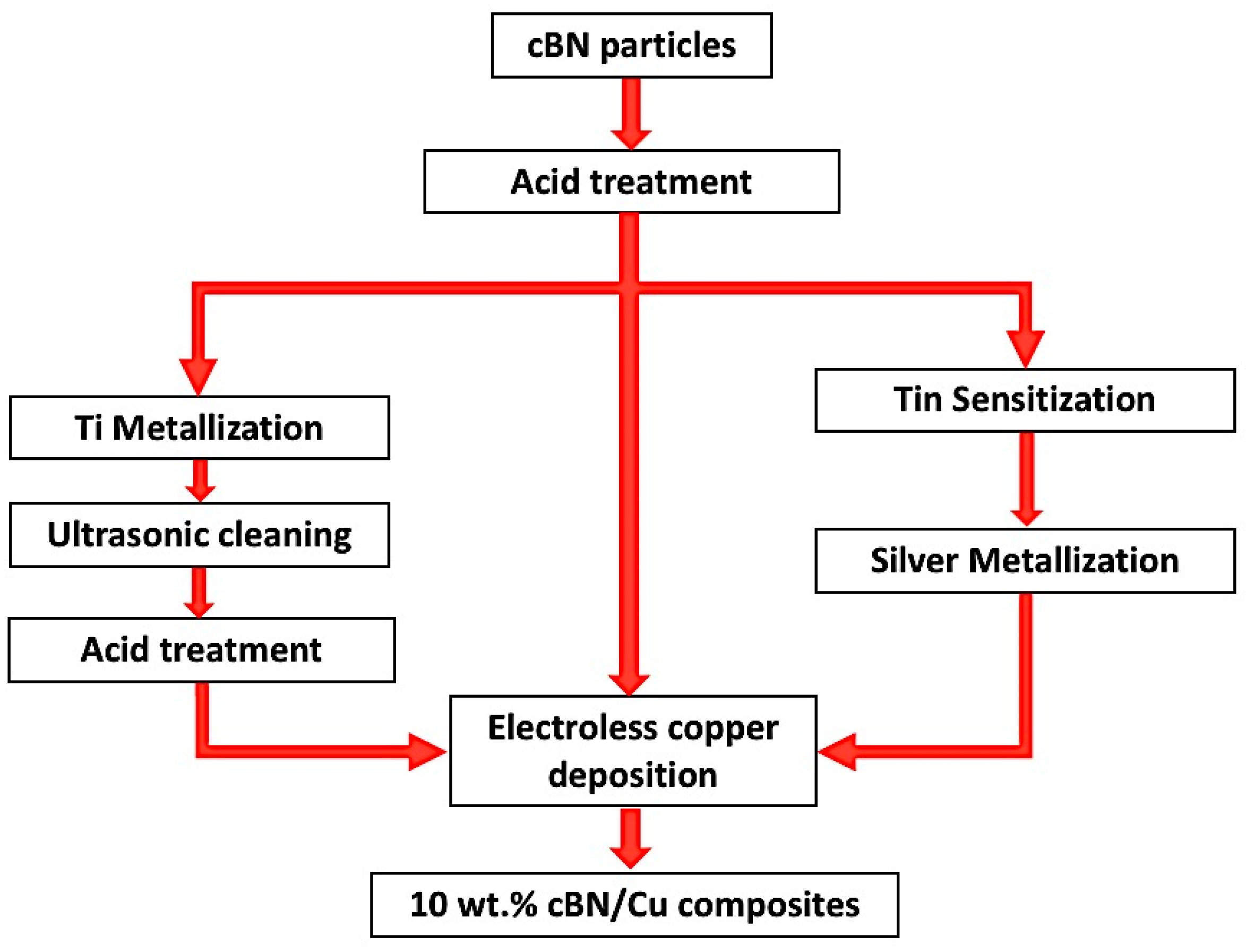
3.2.1. Ti Metallization
The surface of the treated polycrystalline cBN abrasive particles was metallized by depositing a conductive metallic Ti nanolayer. The Ti molten salt process was used to introduce an autocatalytic promoter layer to enhance the electroless copper deposition of the nanosized copper particles on the nonconductive ceramic surfaces of the cBN abrasive particles. In the molten salt reaction process, Ti metal was deposited on the surface of the cBN abrasive particles by heating the constituent of the molten salts chemical reaction under an inert atmosphere of Argon at 900 °C for 1 h.
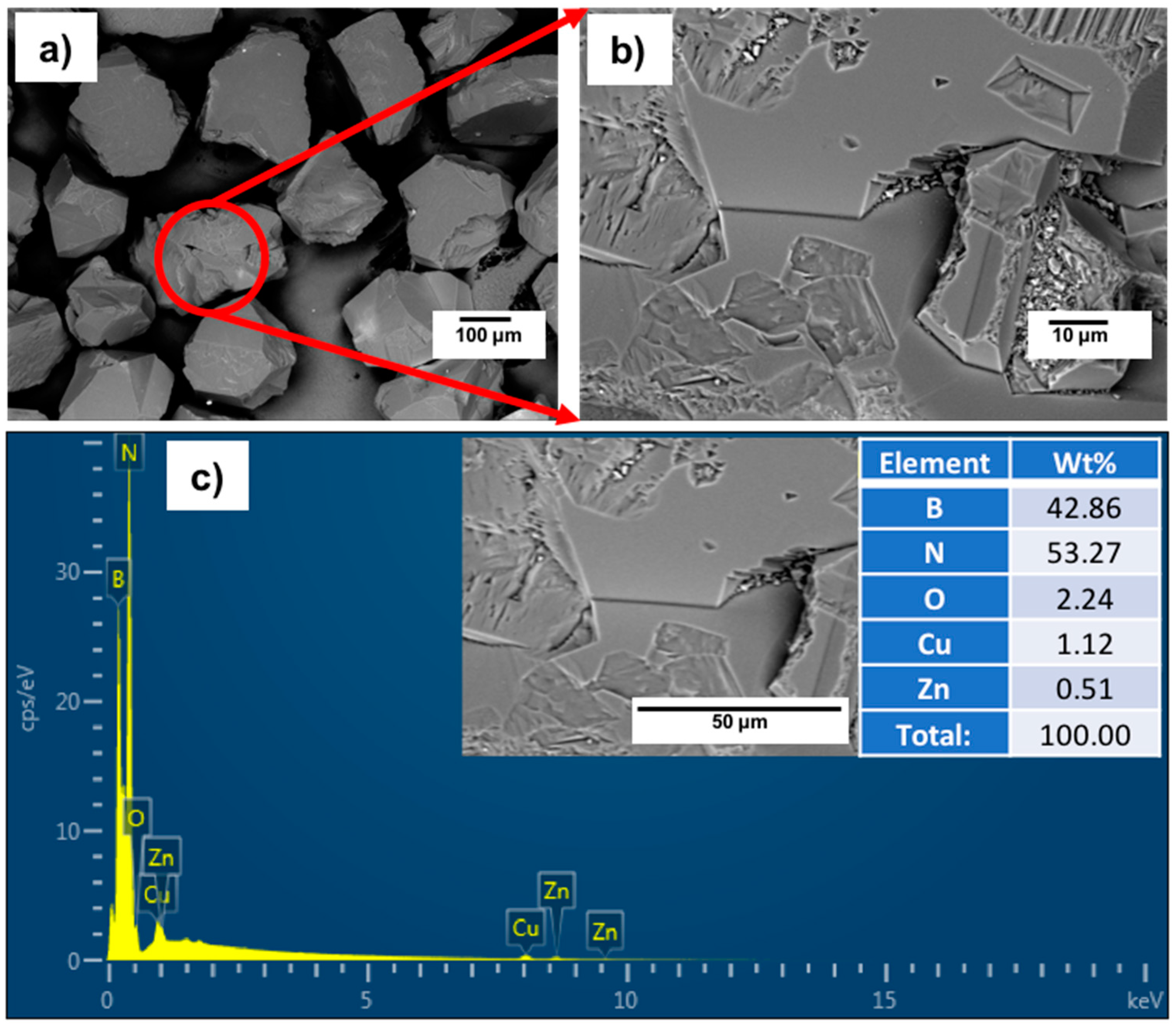
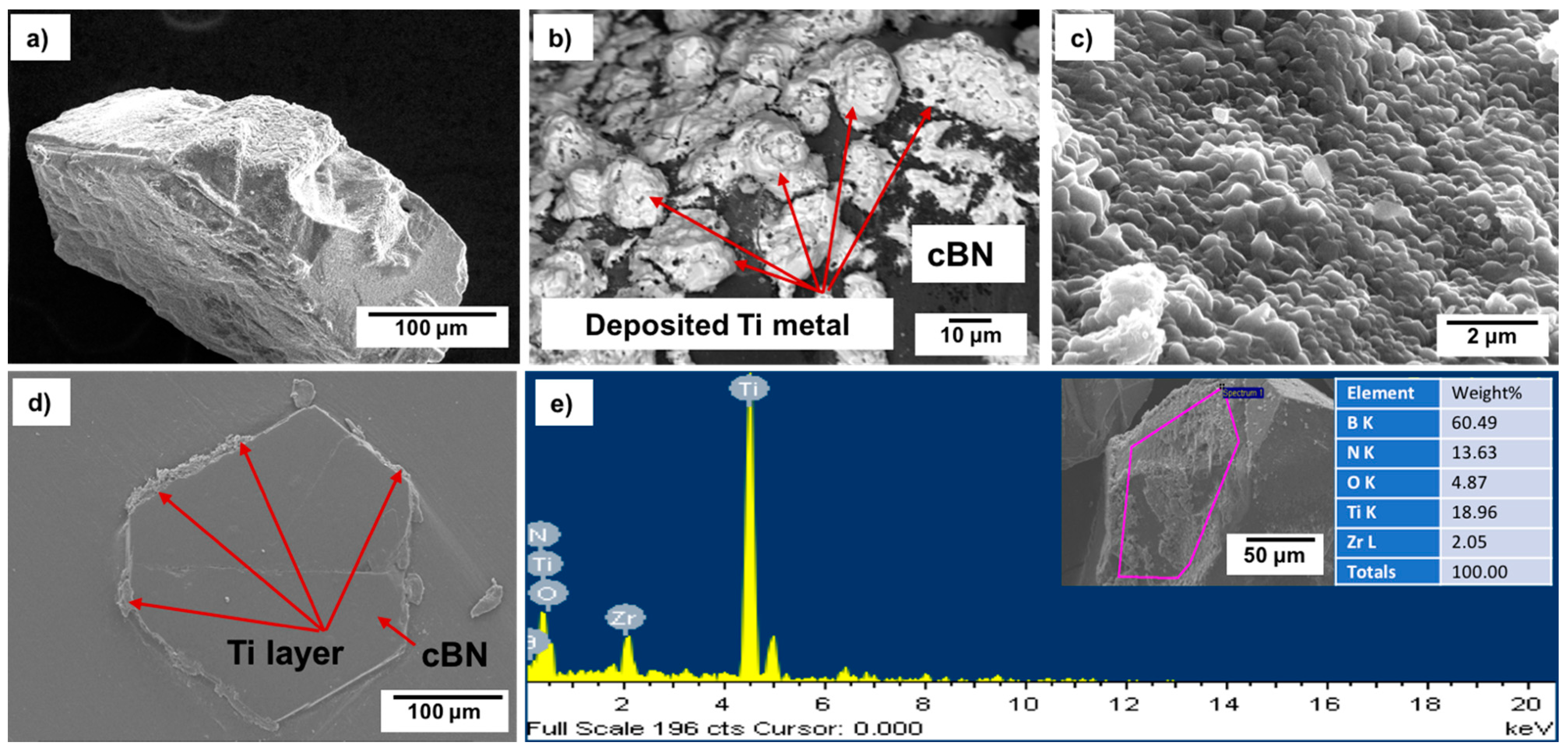
Figure 5a−d shows FESEM images with different magnifications of the surface morphologies, particle size and shape, as well as the cross-sectional area of the deposited Ti layer on the cBN abrasive particles after surface cleaning with sonication using HCl solution. From the preliminary studies, it was determined that the remaining molten salt complex layer that adhered to the cBN particles could be dissolved in 10 vol.% HCl solution within 30 s under the effect of the ultrasonic waves at a frequency 40 kHz. Cleaned composite particles composed of Ti-metallized cBN abrasive particles were obtained. It was further observed that metallic Ti deposited on the surface of the cBN abrasive particles via the molten salt method; the cBN particles surfaces were successfully covered with a very fine and dense nano-sized metallic Ti layer. Although, as shown from the surface morphology, some agglomerated Ti particles of particle size in the microscale were deposited on the surface of the cBN particles (see
Figure 5b), the deposited Ti metal is mainly in the form of nanosized Ti nanoparticles covering the surfaces of the cBN abrasive particles as a primary layer (see
Figure 5c). The EDAX compositional analysis of the deposited layer, shown in
Figure 5e, indicated that the uniform layer was composed mainly of metallic Ti that metalized the surface of the cBN abrasive particles. Two main peaks were observed, the first one for the elemental nitrogen and the second for the elemental titanium. Low intense peaks also appeared, indicating the presence of some trace element, such as zirconium, contaminating the metallic Ti coating layer due to the side chemical reaction that takes place between the molten salts reactants and the crucible of the heating furnace [
22].
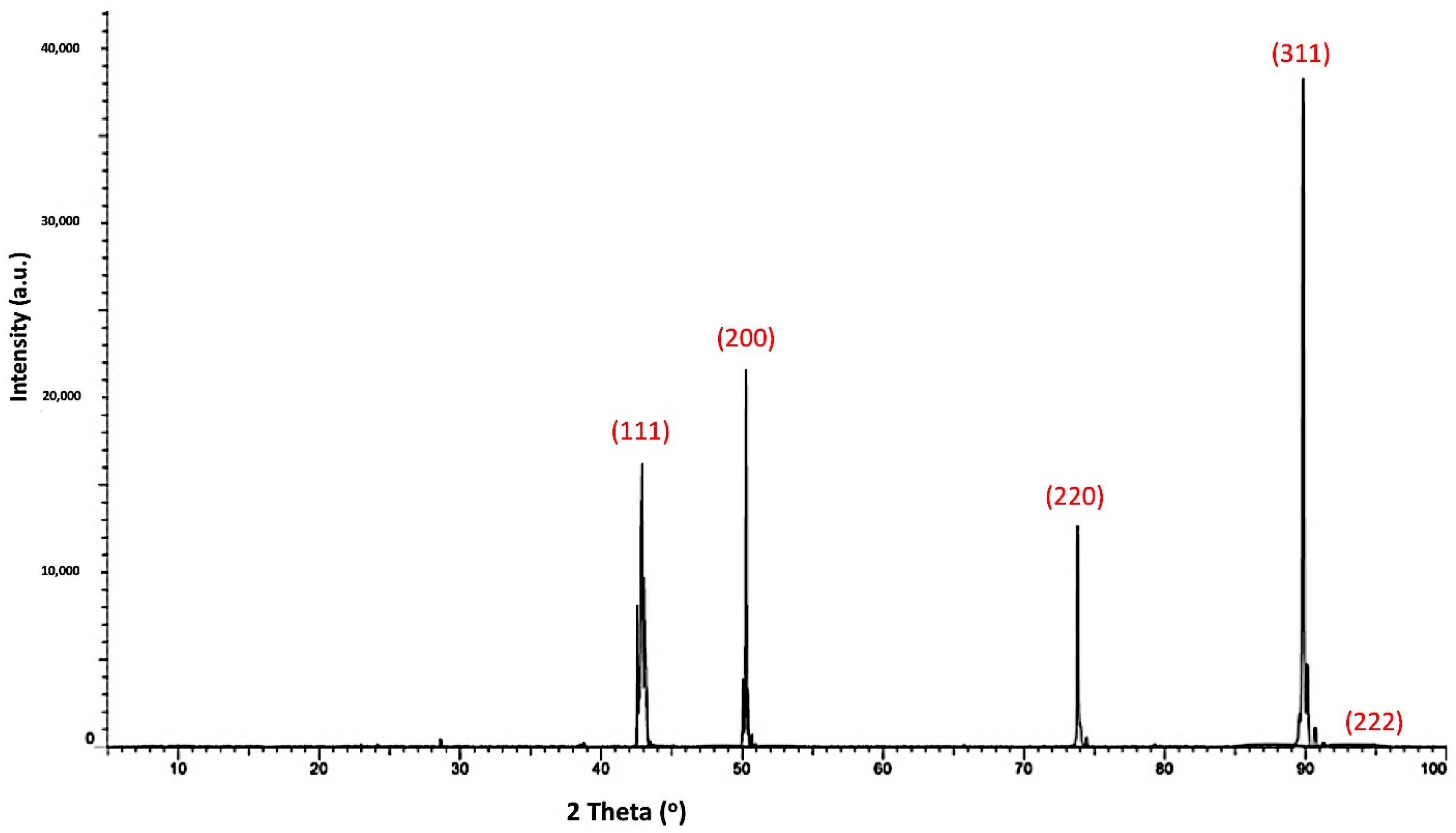
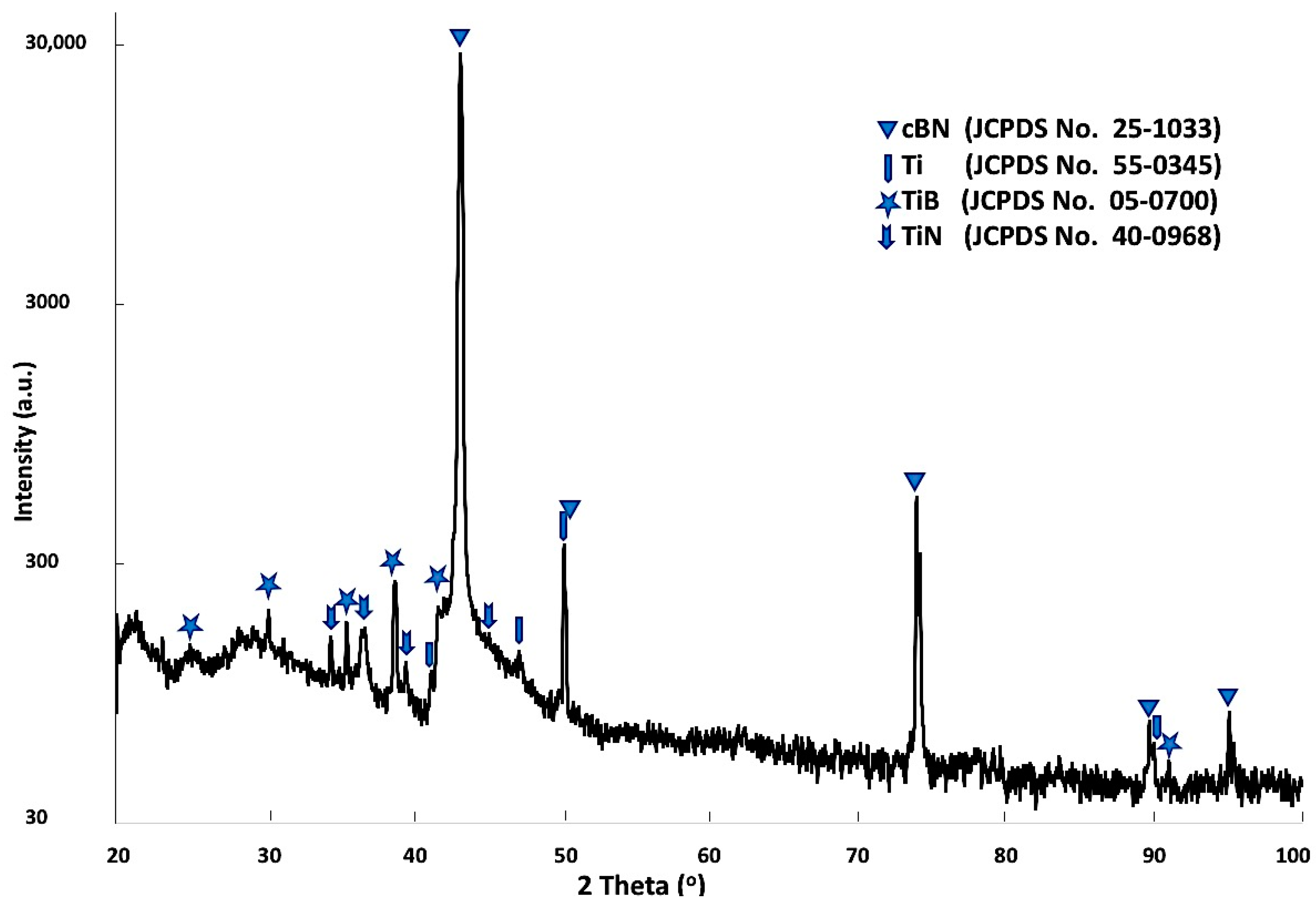
Figure 6 shows X-ray diffraction patterns of the Ti-metallized cBN particles by the molten salt reaction. It was observed from the results that four types of peaks were detected. The first type of peak, which is the main type of peak, was due to the presence of the cBN. The intensity of the (311) peak at 2θ = 90 ° was significantly decreased compared with the corresponding peak of the uncoated cBN particles (see
Figure 4). This may be due to the effect of the deposited Ti layer on the surface of the cBN, which can decrease the diffraction intensity by changing the orientation of the incident X-ray beam. In addition, broadening of the peaks could also be due to the micro-strain enhanced in the cBN particles by heating during the reaction temperature of the molten salt process. The second type of peak corresponds to the JCPDS card number 55-0345 and appeared due to the formation of the deposited Ti metallic layer on the surface of the cBN abrasive particles. The third type of peak corresponds to the JCPDS card number 05-0700 of the TiB phase. The forth peaks corresponds to the JCPDS card number 40-0968 of the Ti
3N
2-x phase. It was revealed from the results that an interaction took place between the surface of the cBN particles and the deposited Ti-layer under the molten salt reaction conditions. The formation of the two new compounds of TiB and Ti
3N
2-x provide good evidence for the formation of an interface layer between the deposited titanium layer and the surface of the cBN particles. This interface layer is responsible for the binding and the enhancement of the adhesion between the surface of the cBN particles and the deposited layer of titanium [
22].
In the Ti metallization process of the cBN particles by molten salt reactions process at a high reaction temperature of 900 °C, K
2TiF
6 dissolved into the reaction mixture of NaCl/KCl molten salts and the Ti
4+ cation was produced. K
2TiF
6 was added to the molten salt mixture to enhance the Ti atoms and reacted with Ti
4+ cations from the decomposed K
2TiF
6 and produce Ti
4+. Then, the interaction of K
2TiF
6 with the NaCl/KCl molten salt mixture helped in the formation of titanium cations. The coating process began with the deposition of Ti
4+ from the molten salt onto the surface of the cBN abrasive particles. This was followed by a disproportionation reaction on the surface of the cBN abrasive particles, whereby Ti
2+ was both reduced to produce Ti and oxidized to produce Ti
4+. It was supposed that the Ti deposition occurs in the molten salt bath is a polystep process and the slowest step is the rate determining step. The deposition rate of Ti metal on the cBN particle surface at certain temperature indicates that the deposition process may not be controlled by the diffusion process. It is reasonable to suppose that the disproportionation reaction of titanium may be the rate-determining step of the whole process; thus, sponge metallic titanium is required to add in the molten reaction in order to produce Ti
3+ cation, which is needed for enhancing and continuing the molten salt reaction [
22].
3.2.3. Electroless Copper Coating of Polycrystalline cBN Abrasive Particles
The un-metallized, Ti-metallized and tin/silver-metallized polycrystalline cBN particles underwent an autocatalytic coating by the electroless deposition of copper on its surface by the autocatalytic effect in the alkaline tartrate copper sulphate bath. The Ti metallization and the tin/silver metallization processes improve the surface chemistry and morphology of the cBN particles. It was expected to improve the coating process of the cBN abrasive particles by imparting a conductive Ti or Ag layer on the surface of the cBN particles, which ensures the subsequent deposition of a uniform copper layer.
Chemical deposition of the coating layer composed of fine copper particles in the nanoscale has been widely studied in relation to the solution composition and process kinetics [
18]. The minimum components of a solution are a soluble salt as a source of the ions of the deposited metal and a reducing agent used to reduce the ions to the metallic atoms. The rate of copper deposition can be affected by the reaction conditions, such as copper ion concentration, temperature, hydrogen ion concentration, and the type of complexing agent used to prevent the copper hydroxide deposition in the alkaline media. The source of copper could be a simple copper salt, such as copper sulfate, chloride, or nitrate. Various common reducing agents have also been suggested for use in the baths of the chemical precipitation, namely formaldehyde, hypophosphite, hydrazine, sugar, and dithionite compounds. When formaldehyde solution is utilized as a reducing agent, the pH of the solution should be adjusted above 12~13 at room temperature and pressure. Because simple copper salts are insoluble at pH above 4, the use of a complexing or chelating agent becomes necessary. The complexing agent could be one of the following groups of compounds: Tartrate salts, Alkanol amines, or EDTA (ethylendiamine, tetra-acetic acid) [
19,
20].
In the present investigations, a mixture of two chemical solutions A and B was used. The first solution was made by dissolving the equivalent amount of the CuSO4·5H2O with the tartrate chelating agent in aqueous media composed of potassium sodium tartrates alkaline solution and the pH was obtained by adjusting at 12.5 by NaOH solution. The second solution used formaldehyde as a reducing agent of the copper ions from the copper sulphate solution to copper metal. As five parts of solution A were added to one part of solution B at ambient temperature, the formaldehyde solution was dissociated in the alkaline media, forming nascent hydrogen atoms, which are responsible for the reduction of the copper ions to metallic copper atoms; the reaction started and completed within 10~15 min. The precipitation reaction of copper on the surface of cBN abrasive particles was almost completed, and the solution color changed from blue to colorless. The precipitation reaction depends mainly on the pH of the solution and the amount of formaldehyde. Once the pH of the solution reaches 12.5, copper precipitates on the surface of the cBN particles in the solution. However, in case of the Ti and tin/silver-metallized cBN abrasive particles, the reaction rate occurred faster than the reaction on the surface of un-metallized cBN abrasive particles. This is due to the fact that both the titanium as well as the silver layer enhanced the autocatalytic reaction of the electroless copper deposition on the Ti-metallized and silver-metallized cBN particles, respectively.
The Cu-precipitation is believed to proceed according to the following reaction:
By changing the pH of the bath, it was found that the rate of Cu-precipitation was increased by increasing the pH to a maximum at ~12.5 and then decreased further on. It was reported that the Cu-coating chemical process of a similar composition that was used in this investigation is considered as an autocatalytic process [
20,
21]. However, it was found that Cu-coating is deposited on any surface found in the solution once its pH value becomes above 12.5. It is believed that the Cu-coating process used in this investigation is a chemical reduction process for Cu-ions by formaldehyde, which served as a reducing agent. As a result, both Ti-metallized and tin/silver-metallized cBN abrasive particles were coated faster than the un-metallized cBN abrasive particles [
18,
20]. The deposited titanium or silver nanoparticles on the surface of the cBN abrasive particles convert the nonconductive surface to a conductive one, which can act as a promotor to enhance the autocatalytic effect of the deposition of copper nanoparticles on the surface of the cBN abrasive particles [
12,
30].
Figure 7a–c shows FESEM images with low and high magnifications of the surface of the un-metallized cBN/Cu composite particles. It was observed from the results that the cBN abrasive particles are completely encapsulated with a spongy copper layer composed of nanosized copper particles.
Figure 7d shows an EDAX compositional analysis of the deposited copper layer. It was revealed that the deposited layer is composed mainly of elemental copper. However, there were no significant peaks of boron and nitrogen due to the homogeneous encapsulation of the cBN particles with the copper layer. The appearance of the oxygen peak indicates some oxidation of some nanosized copper particles by forming copper oxide, which can be identified by the X-ray diffraction, as explained in the next section.
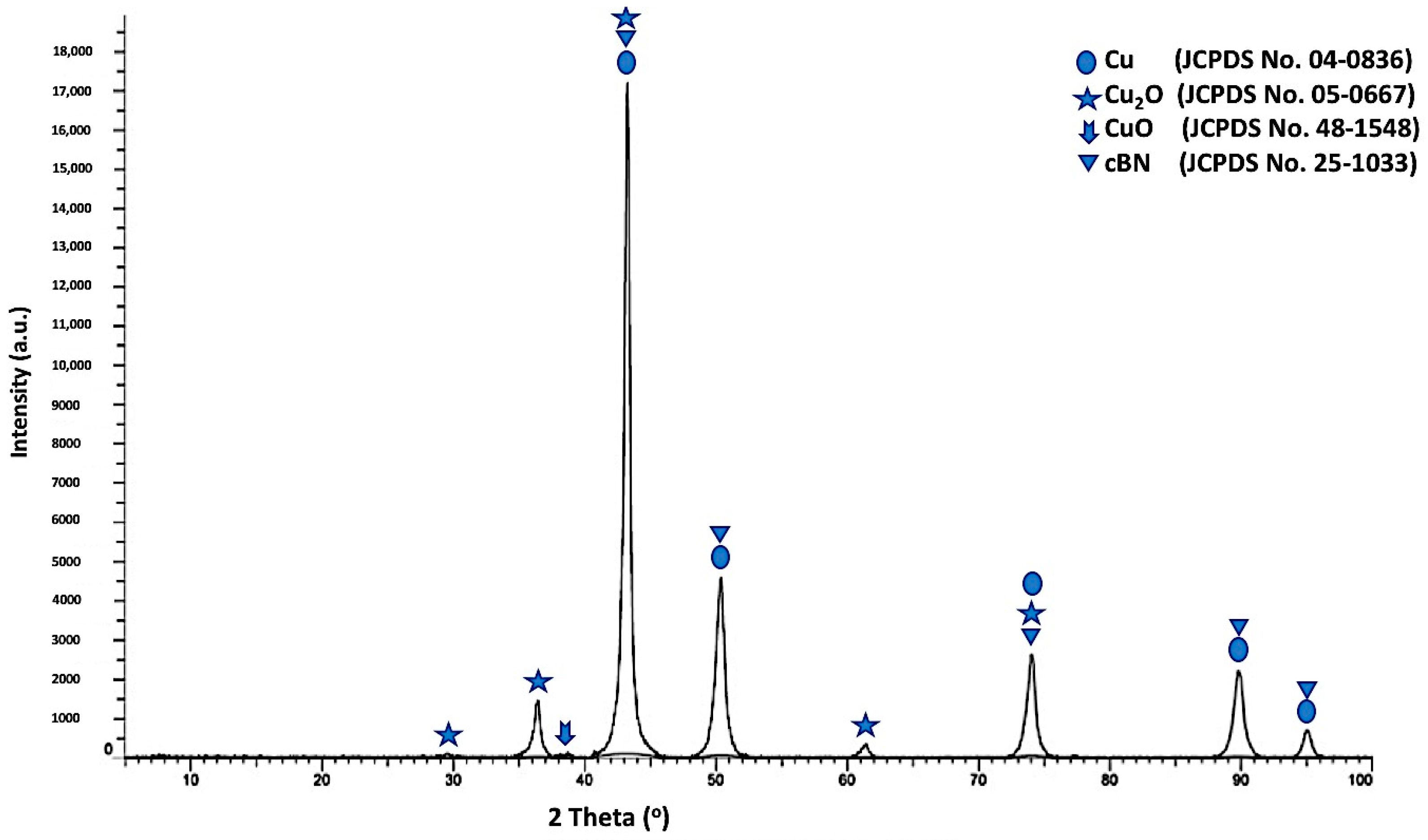
Figure 8 shows the X-ray diffraction pattern of the un- metallized cBN/Cu composite particles. It was revealed from the results that four types of peaks were detected. The first type includes five peaks—(111), (200), (220), (311) and (222)—corresponding to the JCPDS card number 04-0836 for the (fcc) copper metal as a main crystalline phase, indicating the formation of the copper phase [
19]. The second type of peak, which appeared due to the presence of the low oxidation state oxide of copper (Cu
2O) crystalline phase, includes the five peaks corresponding to the JCPDS card number 05-0667 The third type of peak, which corresponds to the JCPDS card number 48-1548, is the low intense peak, which revealed the presence of the high oxidation state oxide of copper (CuO) crystalline phase. Finally, the fourth type includes the five peaks that revealed the presence of the 10 wt. % of the polycrystalline cubic boron nitride (cBN). It was also observed that the three peaks of the (200) at (2θ = 50°), (220) at (2θ = 74°) and (311) at (2θ = 90°) for the coated cBN by copper had lower diffraction intensities than the corresponding uncoated cBN (see
Figure 4). This may be due to the effect of the layer of the deposited copper nanoparticles that covers the surface of the cBN and changes the orientation of the interacting X-ray beams with the surface of the cBN. Beside this reason, the intensity can be affected by the presence of the 10-weight percent of the cBN particles, which are coated with 90 wt.% of the copper nanoparticles. Moreover, the crystallite size of the deposited metallic copper nanoparticles on the surface of the cBN particles was calculated by the X-ray line broadening method at the highest intensity main peak of 2
θ = 43° using Scherrer’s formula (
D = 0.9 λ/
B cos
θ), and the Scherrer crystallite size was estimated at 1.8 × 10
2 Å [
20,
22].
Figure 9a–c shows FESEM images with different magnifications of the Ti-metallized cBN/Cu composite particles. It was revealed from the results that the Ti-metallized cBN particles were coated with a condensed copper layer composed of a nanosized copper particles deposited on the nanosized Ti-layer, which metallized the cBN particles.
Figure 9d shows an EDAX compositional analysis of the deposited copper layer on the Ti-metallized cBN particles. It was observed that the deposited layer on the surface of the Ti-metallized cBN abrasive particles was composed mainly of elemental copper; the presence of the Ti peaks was due to the presence of the Ti layer on the cBN particles, which revealed that the alkaline tartrate solution of the electroless copper bath does not effect the layer of the deposited Ti metal on the surface of the cBN particles. In addition, the appearance of the oxygen peak indicates the oxidation of some nanosized copper particles deposited on the surface of the cBN particles. Moreover, the disappearance of the boron and nitrogen peaks revealed that the deposited copper on the surface of the cBN was mainly composed of a thick layer of nanosized copper, which decreased the interaction between the electron beams and the encapsulated cBN particles by copper during the EDAX analysis.
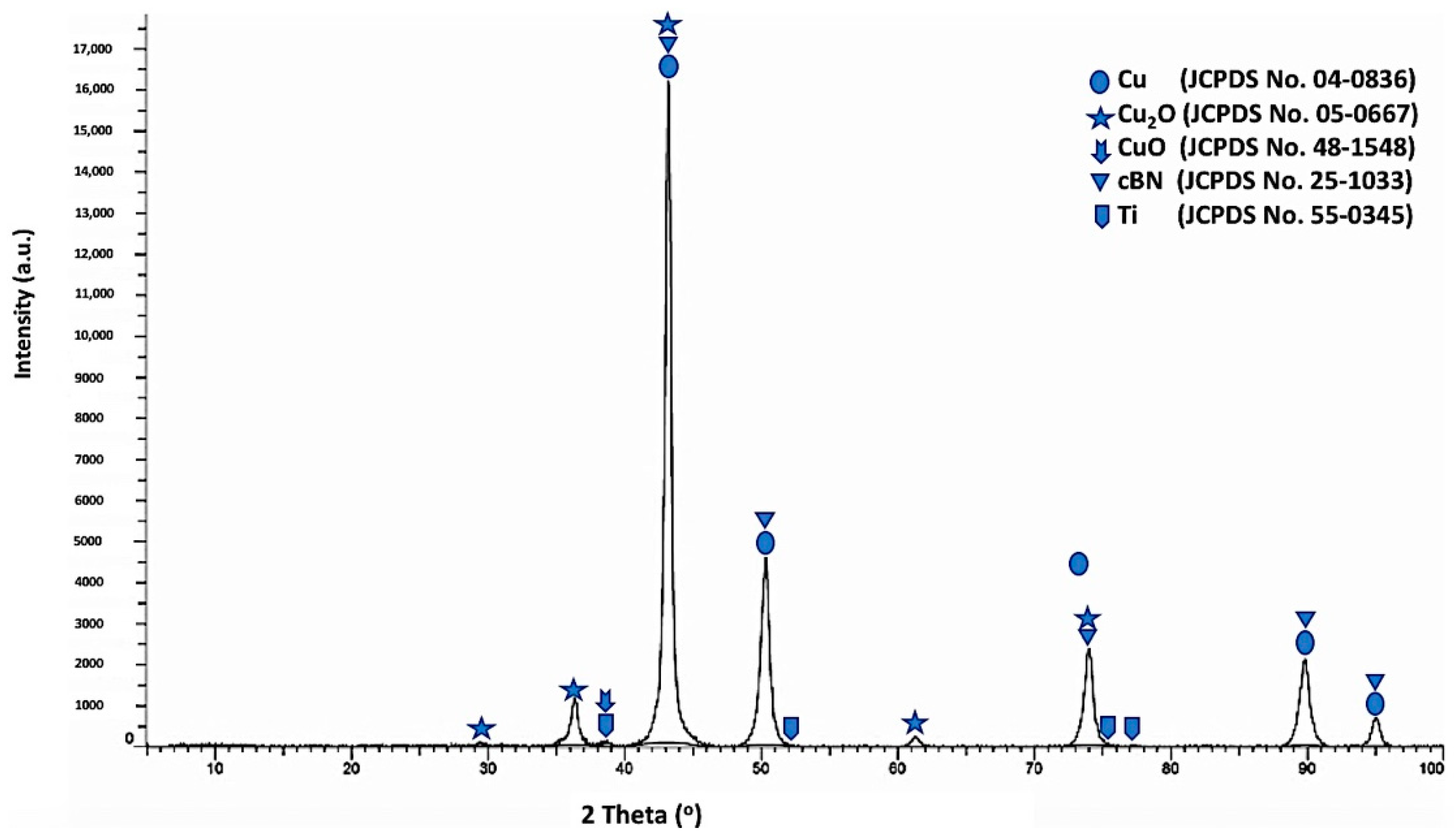
Figure 10 shows the X-ray diffraction pattern of the Ti-metallized cBN/Cu composite particles. Five types of diffraction peaks were observed from the results. The first type included five peaks (111), (200), (220), (311) and (222) for the (fcc) copper metal as a main crystalline phase, which revealed the formation of the copper phase [
20,
21,
22]. The second type of peaks is the five peaks that appeared due to the presence of the crystalline phase of the low oxidation state oxide of copper (Cu
2O). The third type of peaks is the low intense peak that revealed the presence of the crystalline phase of the high oxidation state oxide of copper (CuO). The fourth type of peaks includes the five low intense peaks that revealed the presence of the metallic Ti phase corresponding to the JCPDS card number 55-0345. However, the fifth type includes five peaks that indicated the presence of the 10 wt.% of the polycrystalline cubic boron nitride (cBN) phase. It was also observed from the results that the three peaks of the (200) at (2θ = 50°), (220) at (2θ = 74°) and (311) at (2θ = 90°) for the copper coated Ti-metallized cBN had lower diffraction intensities than the corresponding ones of the un-metallized cBN (see
Figure 4). This may be due to the effect of the Ti/Cu multilayer deposited on the surface of the cBN, which covered the surface of the cBN and changed the orientation of the incident X-ray beams with the surface of the cBN. Moreover, the crystallite size of the deposited copper on the surface of the cBN abrasive particles was calculated by the X-ray line broadening method using Scherrer’s formula (
D = 0.9 λ/
B cos
θ) at the highest intensity main peak of 2
θ = 43°, and the Scherrer crystallite size was estimated 1.8 × 10
2 Å [
22].
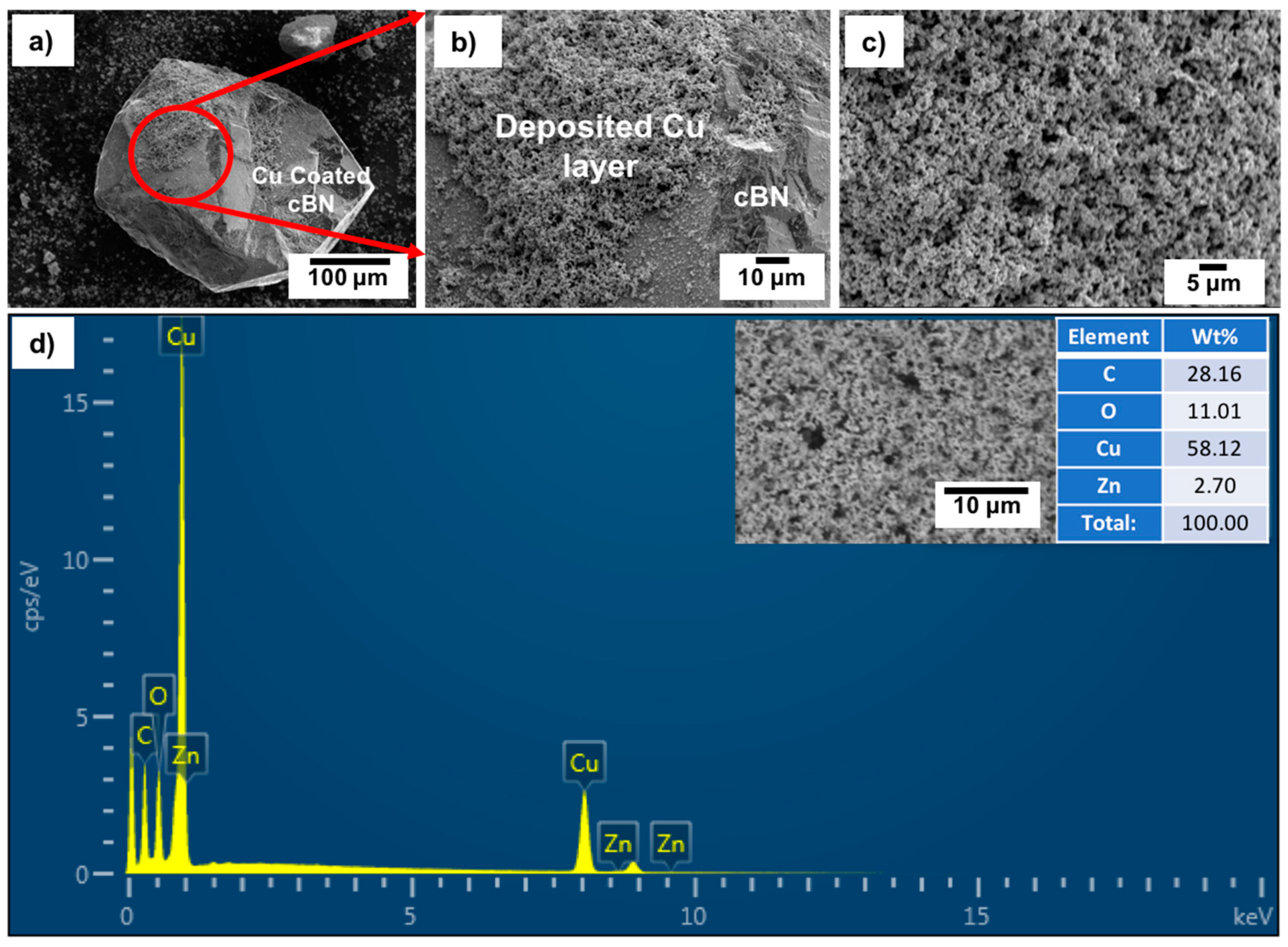
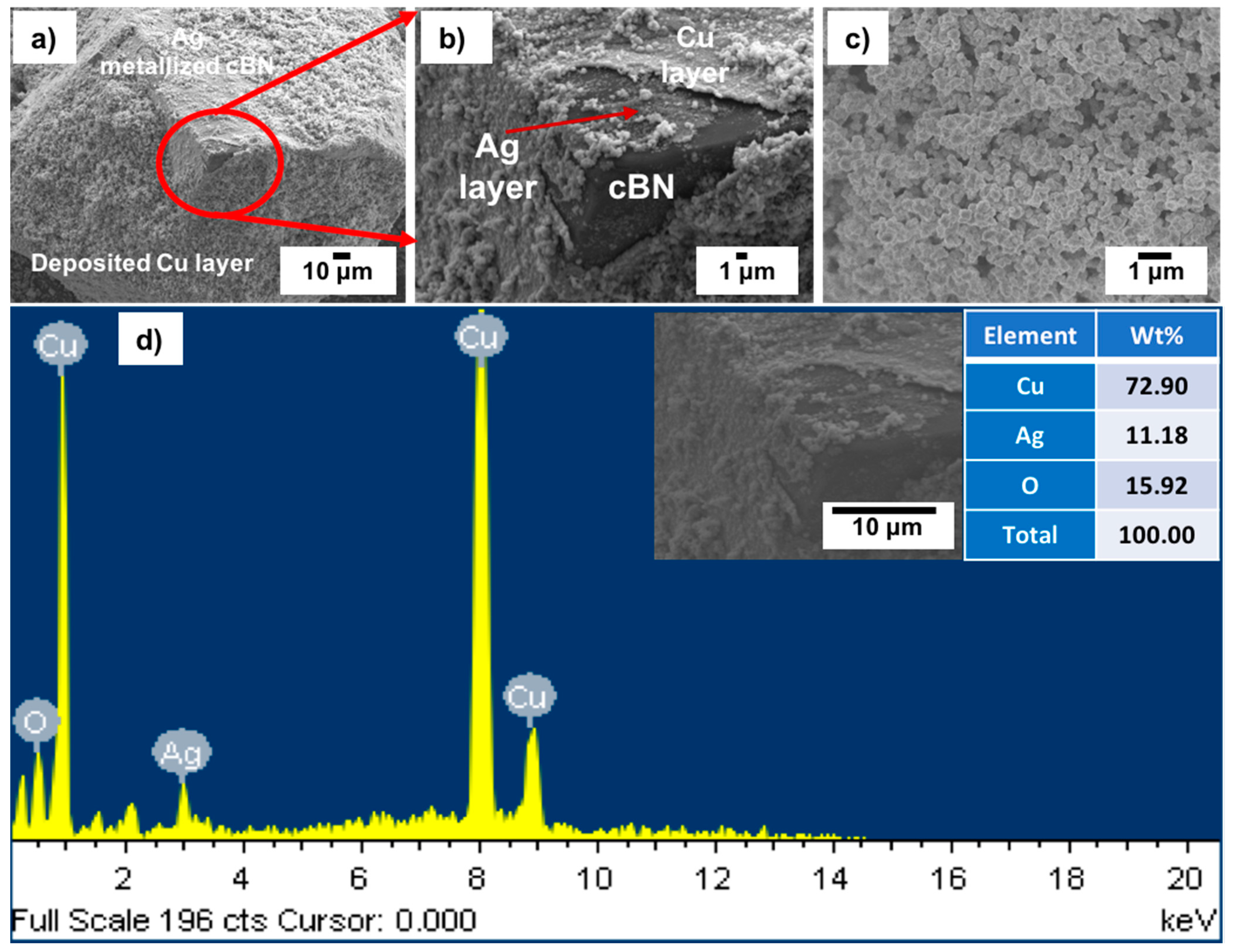
Figure 11a–c shows FESEM images with low and high magnifications of the tin/silver-metallized cBN/Cu composite particles. It was observed from the results that the cBN particles were completely encapsulated within a uniform copper layer composed mainly of copper nanoparticles.
Figure 11d shows an EDAX compositional analysis of the deposited copper layer on the tin/silver-metallized cBN abrasive particles. It was revealed that, the deposited layer on the surface of the tin/silver-metallized cBN abrasive particles was composed mainly of elemental copper. The presence of the silver peak confirmed the presence of the initial layer of the deposited Ag nano-layer on the surface of the cBN particles. It was expected that this Ag layer enhanced the autocatalytic reaction of the electroless deposition of Cu on the cBN particles. The alkaline tartrate solution of the electroless copper bath did not affect the deposited Ag layer on the surface of the cBN particles. The peaks of boron and nitrogen also disappeared, due to the effect of the deposited copper layer, which had enough thickness to cause the electron beams to reach the surface of the cBN particles during the EDAX analysis. Moreover, the presence of the oxygen peak indicated the formation of some oxides of the copper layer deposited on the surface of the cBN particles.
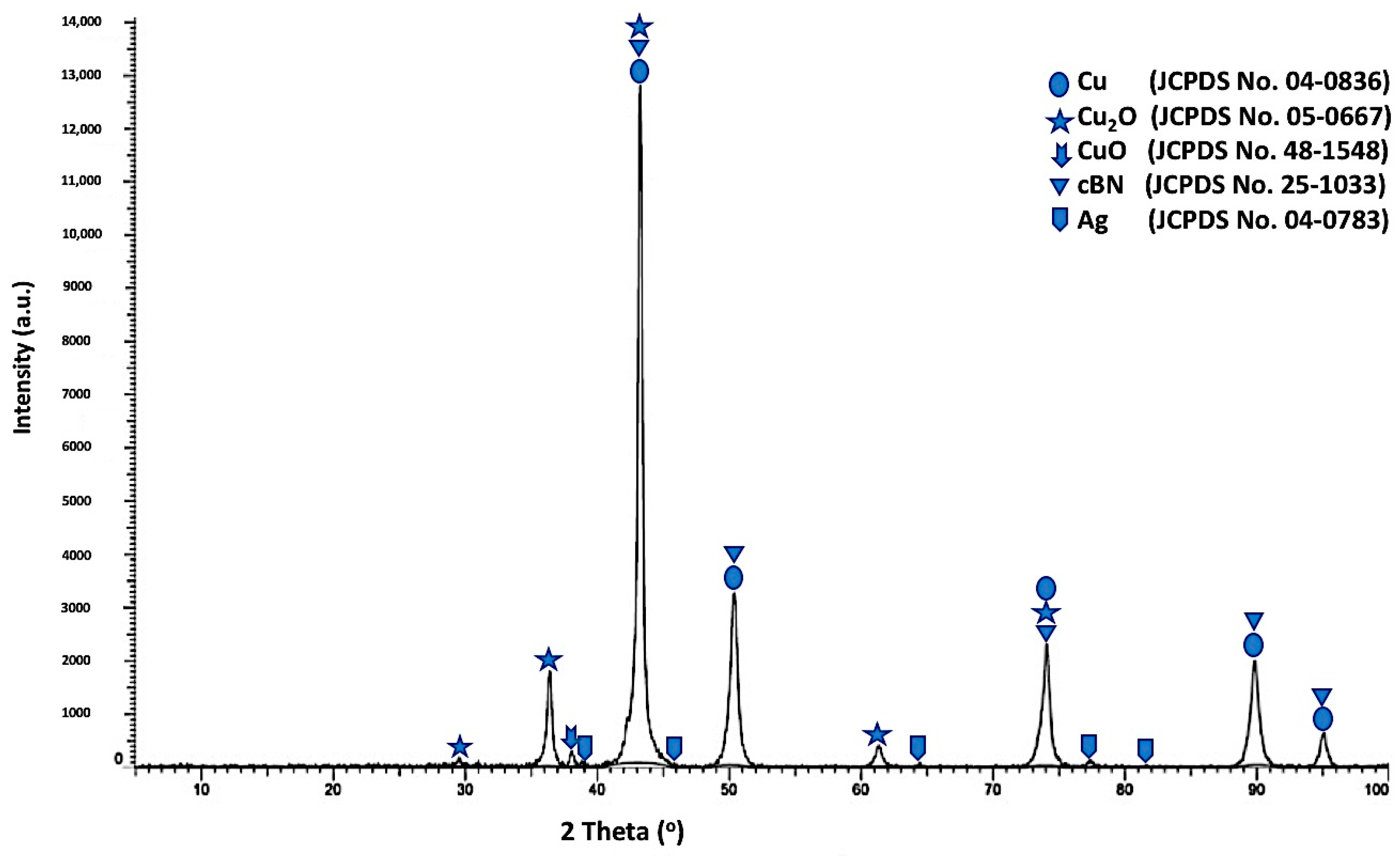
Figure 12 shows the X-ray diffraction pattern of the tin/silver-metallized cBN/Cu composite particles. Five types of diffraction peaks were detected. The first type includes five peaks, (111), (200), (220), (311) and (222) for the (fcc) copper metal as a main phase, which revealed the formation of the crystalline copper phase. The second type of peaks includes the five peaks that appeared due to the presence of the copper oxide (Cu
2O) phase [
16]. The third type of peak is the low intense peak that revealed the presence of copper oxide (CuO) phase. In addition, the fourth type includes five low intensive peaks that revealed the presence of (111), (200), (220), (311) and (222) of the silver nanolayer corresponding to the JCPDS card number 04-0783. They were initially deposited on the surface of the cBN particles during the tin/silver metallization process [
19,
20,
22,
23]. In addition, four peaks of the polycrystalline cubic boron nitride were observed. The results also showed that the three peaks of the (200) at (2θ = 50°), (220) at (2θ = 74°) and (311) at (2θ = 90°) for the coated cBN by copper have lower diffraction intensities than the corresponding uncoated cBN (see
Figure 4). This is due to the effect of the deposited silver, as well as the deposited copper multilayers, which covered the surface of the cBN by thick layers and which affected the interaction of the X-ray beams with the surface of the cBN particles. The crystallite size of the deposited copper on the surface of the tin/silver-metallized cBN/Cu composite particles was calculated at the highest intensity main peak of 2
θ = 43° by the X-ray line broadening method using Scherrer’s formula (
D = 0.9 λ/
B cos
θ), and the Scherrer crystallite size was estimated at 2.3 × 10
2 Å. The crystallite size of the deposited copper nanoparticles on the tin/silver-metallized cBN/Cu composite particles increased due to the formation of the silver nanoparticle on the interface between the cBN and the deposited copper metal. It was also observed from the diffraction pattern of the tin/silver-metallized cBN/Cu composite particles that there was not any new or foreign phase detected by the XRD that could be formed during the coating process [
21,
22,
23,
24].
It was also observed from the study of the morphology of the deposited copper layers on the metallized cBN particles by the two different methods that the tin/silver-metallized cBN particles were mostly encapsulated by a condensed copper layer. However, the un-metallized cBN particles were encapsulated by a spongy and loose copper layer bonded with the surface of the un-metallized cBN particles. On the other hand, the Ti-metallized cBN particles were coated with an adhered copper layer.