Versatile Strategy for Electrophoretic Deposition of Polyvinylidene Fluoride-Metal Oxide Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Deposition
2.3. Characterization
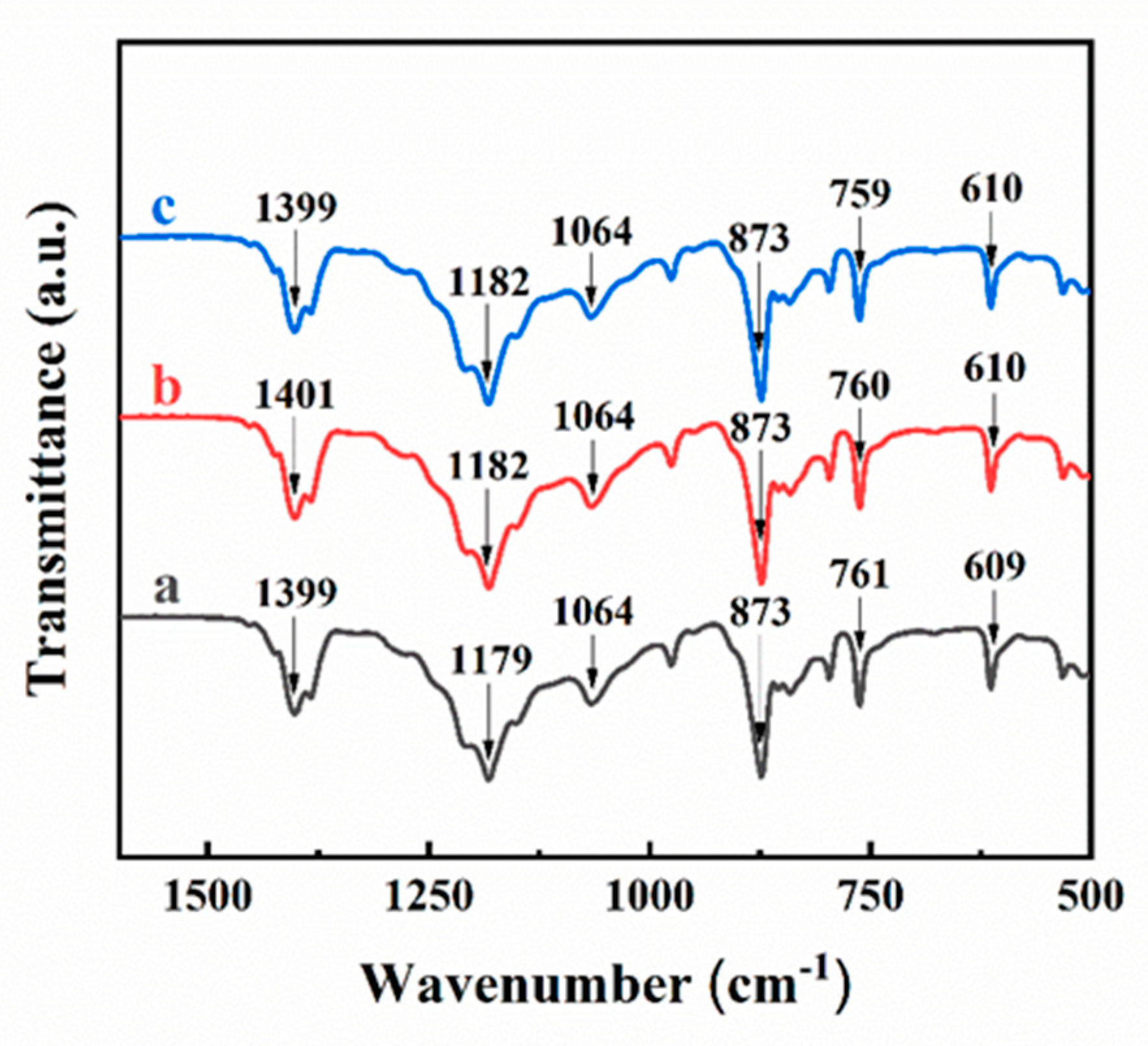
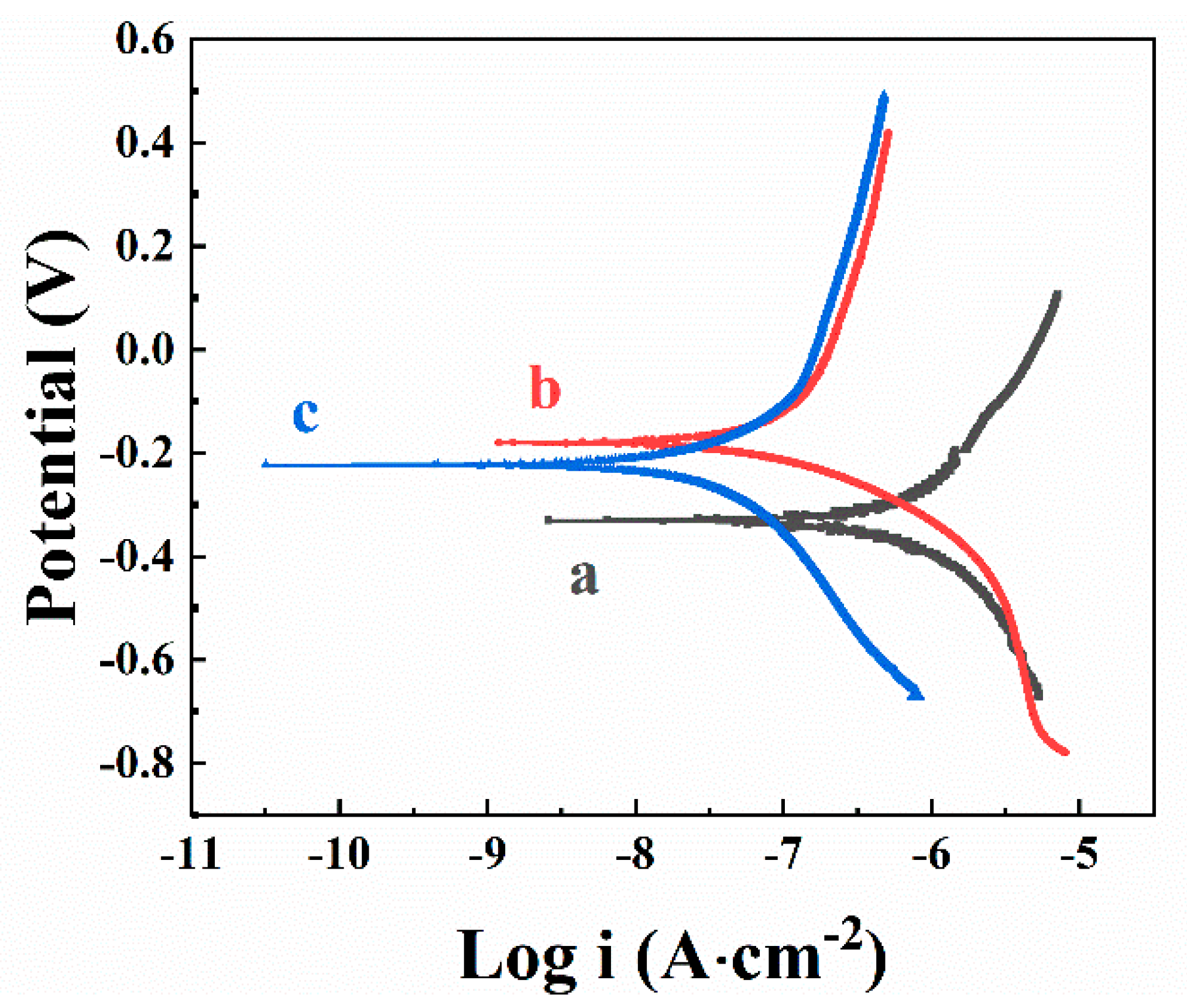
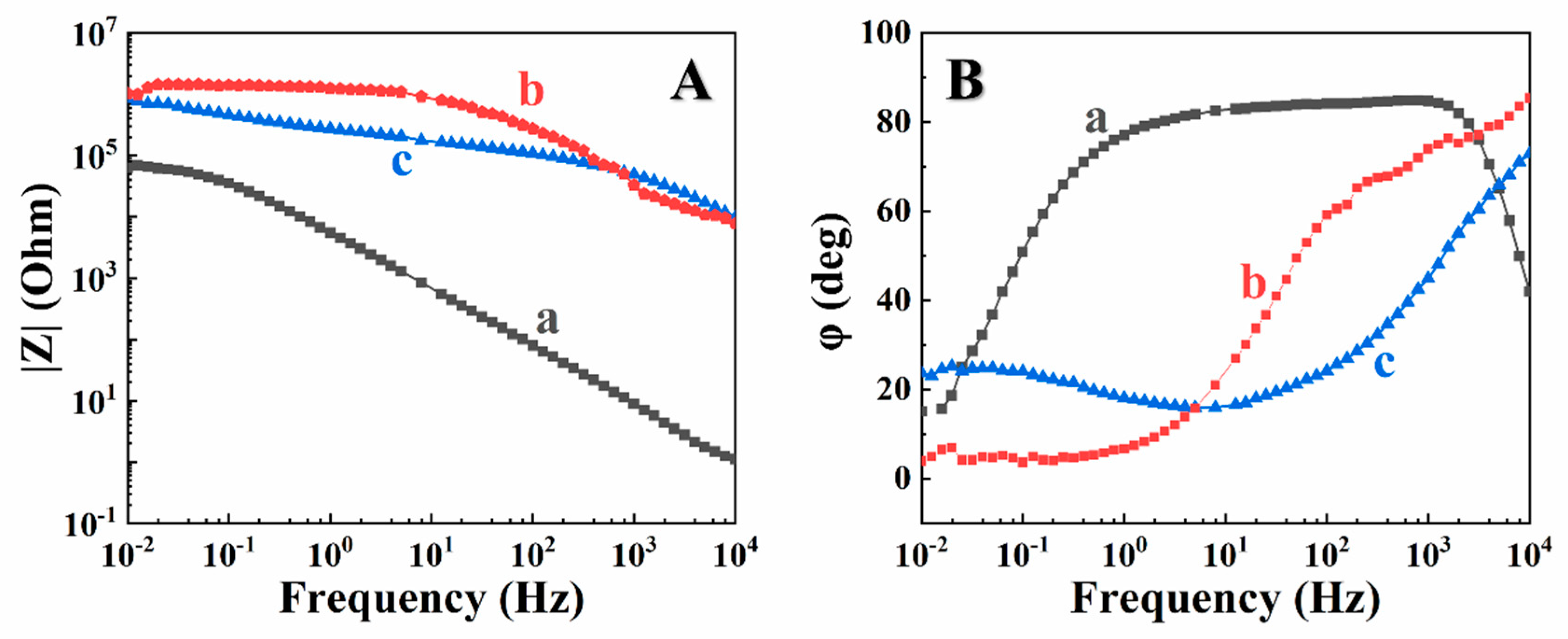
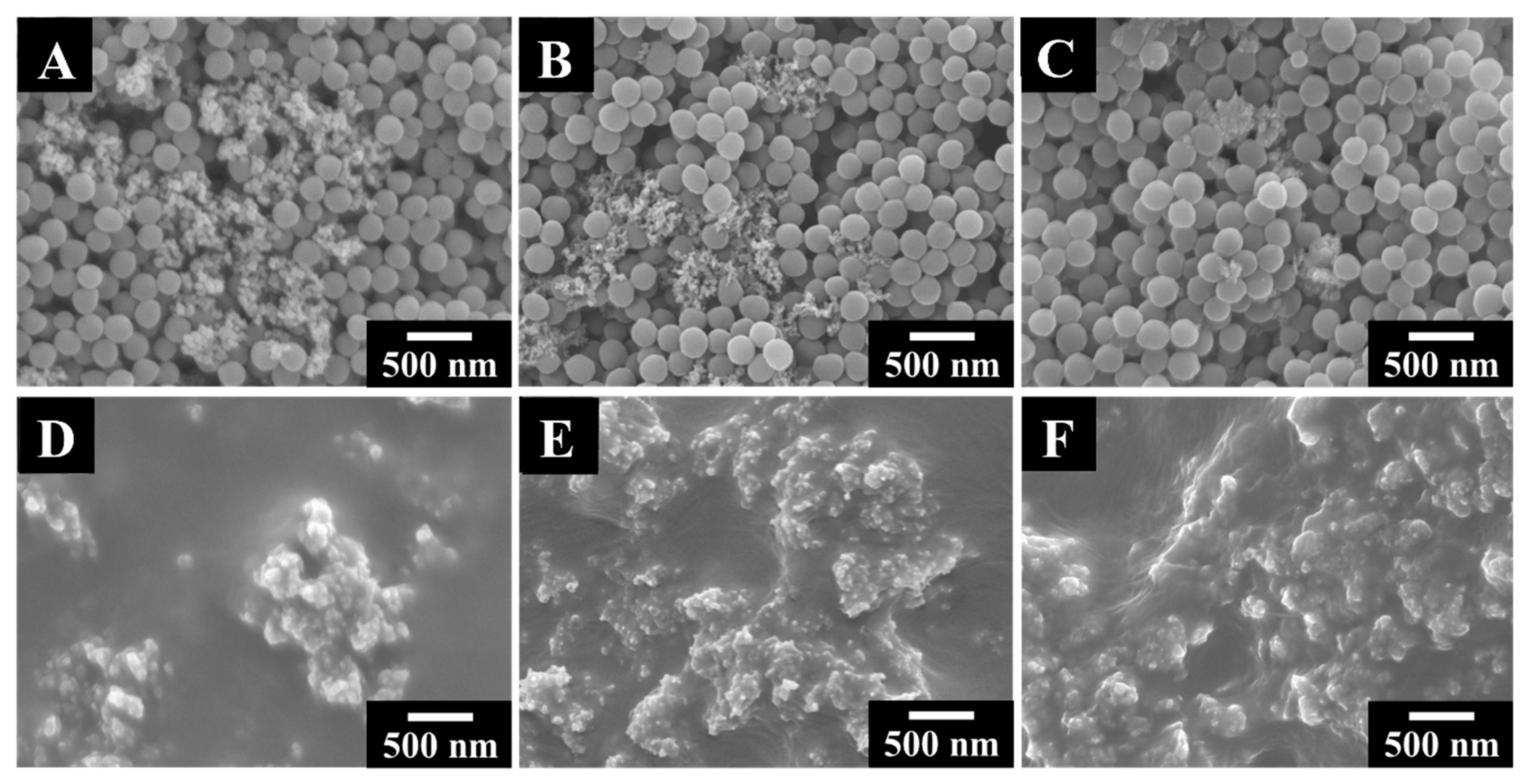
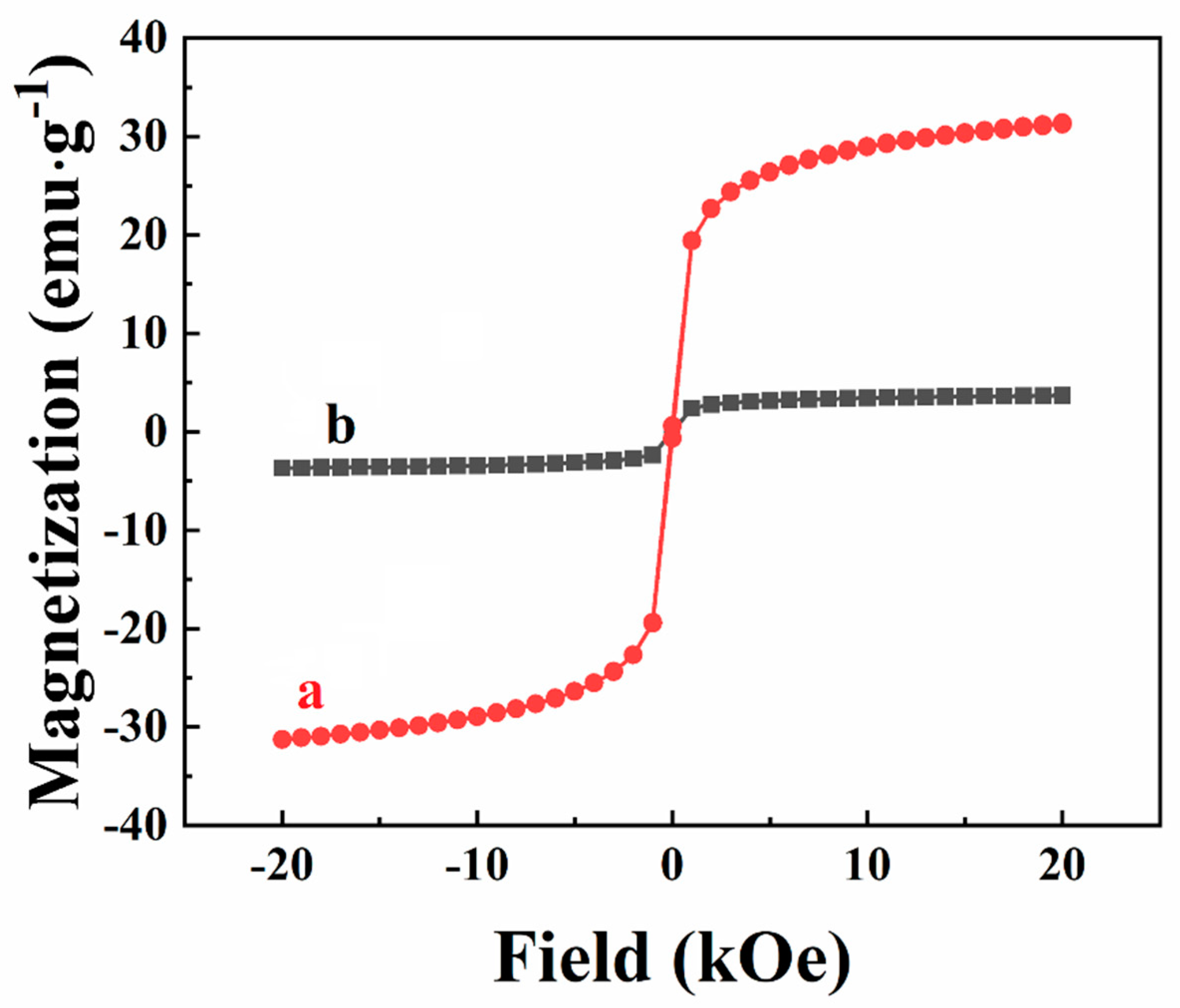
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribeiro, C.; Costa, C.M.; Correia, D.M.; Nunes-Pereira, J.; Oliveira, J.; Martins, P.; Goncalves, R.; Cardoso, V.F.; Lanceros-Mendez, S. Electroactive poly (vinylidene fluoride)-based structures for advanced applications. Nat. Protoc. 2018, 13, 681–704. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-H.; Chang, S.-H. PVDF-based ferroelectric polymers and dielectric elastomers for sensor and actuator applications: A review. Funct. Compos. Struct. 2019, 1, 012003. [Google Scholar] [CrossRef]
- Inderherbergh, J. Polyvinylidene fluoride (PVDF) appearance, general properties and processing. Ferroelectrics 1991, 115, 295–302. [Google Scholar] [CrossRef]
- Zhong, J.; Li, W.; Qian, J.; Fu, C.; Chu, H.; Xu, J.; Ran, X.; Nie, W. Modulation of the interfacial architecture enhancing the efficiency and energy density of ferroelectric nanocomposites via the irradiation method. J. Colloid Interface Sci. 2021, 586, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Ding, W.; Liu, J.; Yang, B. Flexible PVDF based piezoelectric nanogenerators. Nano Energy 2020, 586, 30–38. [Google Scholar] [CrossRef]
- Chamankar, N.; Khajavi, R.; Yousefi, A.A.; Rashidi, A.; Golestanifard, F. A flexible piezoelectric pressure sensor based on PVDF nanocomposite fibers doped with PZT particles for energy harvesting applications. Ceram. Int. 2020, 46, 19669–19681. [Google Scholar] [CrossRef]
- Ghazali, N.; Basirun, W.J.; Nor, A.M.; Johan, M.R. Super-amphiphobic coating system incorporating functionalized nano-Al2O3 in polyvinylidene fluoride (PVDF) with enhanced corrosion resistance. Coatings 2020, 10, 387. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, L.; Chen, K.; Ma, Y.; Peng, Q.; Ji, H.; Qiu, J. Flexible textured MnO2 nanorods/PVDF hybrid films with superior piezoelectric performance for energy harvesting application. Compos. Sci. Technol. 2020, 199, 108330. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, L.; Chen, K.; Ma, Y.; Ji, H.; Shen, M.; Huang, H.; He, H.; Qiu, J. Ultra-high discharged energy density in PVDF based composites through inducing MnO2 particles with optimized geometric structure. Nano Energy 2019, 65, 104007. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, N.; Lee, Y.T. Preparation and characterization of PVDF/TiO2 organic-inorganic composite membranes for fouling resistance improvement. J. Membr. Sci. 2009, 345, 13–20. [Google Scholar] [CrossRef]
- Prabhakaran, T.; Hemalatha, J. Magnetoelectric investigations on poly (vinylidene fluoride)/NiFe2O4 flexible films fabricated through a solution casting method. RSC Adv. 2016, 6, 86880–86888. [Google Scholar] [CrossRef]
- Botero, E.; Nobrega, J.; Trombini, D. Influence of NiFe2O4 on β phase formation in PVDF composites. Mater. Sci. Eng. 2020, 4, 83–86. [Google Scholar] [CrossRef]
- Prasad, P.D.; Hemalatha, J. Energy harvesting performance of magnetoelectric poly (vinylidene fluoride)/NiFe2O4 nanofiber films. J. Magn. Magn. Mater. 2021, 532, 167986. [Google Scholar] [CrossRef]
- Rehan, M.; Nada, A.A.; Khattab, T.A.; Abdelwahed, N.A.; Abou El-Kheir, A.A. Development of multifunctional polyacrylonitrile/silver nanocomposite films: Antimicrobial activity, catalytic activity, electrical conductivity, UV protection and SERS-active sensor. J. Mater. Res. Technol. 2020, 9, 9380–9394. [Google Scholar] [CrossRef]
- Huang, D.; Yan, X.; Yan, M.; Zeng, G.; Zhou, C.; Wan, J.; Cheng, M.; Xue, W. Graphitic carbon nitride-based heterojunction photoactive nanocomposites: Applications and mechanism insight. ACS Appl. Mater. Interfaces 2018, 10, 21035–21055. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Han, S.I.; Kim, D.; Hyeon, T.; Kim, D.-H. High-performance stretchable conductive nanocomposites: Materials, processes, and device applications. Chem. Soc. Rev. 2019, 48, 1566–1595. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Duan, X.; Xie, M.; Aw, K.C.; Xue, Q. Composites, fabrication and application of polyvinylidene fluoride for flexible electromechanical devices: A review. Micromachines 2020, 11, 1076. [Google Scholar] [CrossRef]
- Zhitomirsky, I.; Petric, A. Electrochemical deposition of yttrium oxide. J. Mater. Chem. 2000, 10, 1215–1218. [Google Scholar] [CrossRef]
- Zhitomirsky, I. Cathodic electrodeposition of ceramic and organoceramic materials. Fundamental aspects. Adv. Colloid Interface Sci. 2002, 97, 279–317. [Google Scholar] [CrossRef]
- Singh, A.; English, N.J.; Ryan, K.M. Highly ordered nanorod assemblies extending over device scale areas and in controlled multilayers by electrophoretic deposition. J. Phys. Chem. B 2013, 117, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Smeacetto, F.; De Miranda, A.; Polo, S.C.; Molin, S.; Boccaccini, D.; Salvo, M.; Boccaccini, A.R. Electrophoretic deposition of Mn1. 5Co1. 5O4 on metallic interconnect and interaction with glass-ceramic sealant for solid oxide fuel cells application. J. Power Sources 2015, 280, 379–386. [Google Scholar] [CrossRef]
- Djošić, M.; Mišković-Stanković, V.B.; Kačarević-Popović, Z.M.; Jokić, B.M.; Bibić, N.; Mitrić, M.; Milonjić, S.K.; Jančić-Heinemann, R.; Stojanović, J. Electrochemical synthesis of nanosized monetite powder and its electrophoretic deposition on titanium. Colloids Surf. A Physicochem. Eng. Asp. 2009, 341, 110–117. [Google Scholar] [CrossRef]
- Mayén-Mondragón, R.; Falk, G.; Clasen, R. Electrophoretic Impregnation/Deposition Complemented with Polymeric Templating for the Fabrication of Functionalized-Porosity Layered-Ceramics: A Solid-Oxide-Fuel-Cells Approach. J. Am. Ceram. Soc. 2012, 95, 593–599. [Google Scholar] [CrossRef]
- Otelaja, O.O.; Ha, D.-H.; Ly, T.; Zhang, H.; Robinson, R.D. Highly Conductive Cu2–xS Nanoparticle Films through room-temperature processing and an order of magnitude enhancement of conductivity via electrophoretic deposition. ACS Appl. Mater. Interfaces 2014, 6, 18911–18920. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhitomirsky, I. Electrophoretic deposition of graphene, carbon nanotubes and composite films using methyl violet dye as a dispersing agent. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 97–103. [Google Scholar] [CrossRef]
- Sikkema, R.; Baker, K.; Zhitomirsky, I. Electrophoretic deposition of polymers and proteins for biomedical applications. Adv. Colloid Interface Sci. 2020, 284, 102272. [Google Scholar] [CrossRef]
- Zhitomirsky, I.; Gal-Or, L. Formation of hollow fibers by electrophoretic deposition. Mater. Lett. 1999, 38, 10–17. [Google Scholar] [CrossRef]
- Lau, K.T.; Ab Razak, M.H.R.; Kok, S.L.; Zaimi, M.; Rashid, M.W.A.; Mohamad, N.; Azam, M.A. Electrophoretic Deposition and Heat Treatment of Steel-Supported PVDF-Graphite Composite Film. In Applied Mechanics and Materials; Trans Tech Publications: Bach, Switzerland, 2015; pp. 412–416. [Google Scholar]
- Lau, K.T.; Suan, M.S.M.; Zaimi, M.; Abd Razak, J.; Azam, M.; Mohamad, N. Microstructure and Phase of Poly (Vinyliden Fluoride) Films by Electrophoretic Deposition: Effect of Polymer Dispersion’s Stirring Conditions. J. Adv. Manuf. Technol. JAMT 2016, 10, 57–66. [Google Scholar]
- Yin, J.; Fukui, T.; Murata, K.; Matsuda, M.; Miyake, M.; Hirabayashi, T.; Yamamuro, S. Fabrication of protective KB/PVdF composite films on stainless steel substrates for PEFCs through electrophoretic deposition. J. Ceram. Soc. Jpn. 2008, 116, 201–204. [Google Scholar] [CrossRef][Green Version]
- Zhao, Q.; Veldhuis, S.; Mathews, R.; Zhitomirsky, I. Influence of chemical structure of bile acid dispersants on electrophoretic deposition of poly (vinylidene fluoride) and composites. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127181. [Google Scholar] [CrossRef]
- Cheong, M.; Zhitomirsky, I. Electrophoretic deposition of manganese oxide films. Surf. Eng. 2009, 25, 346–352. [Google Scholar] [CrossRef]
- Hashiba, M.; Okamoto, H.; Nurishi, Y.; Hiramatsu, K. The zeta-potential measurement for concentrated aqueous suspension by improved electrophoretic mass transport apparatus—Application to Al2O3, ZrO3 and SiC suspensions. J. Mater. Sci. 1988, 23, 2893–2896. [Google Scholar] [CrossRef]
- Ata, M.; Liu, Y.; Zhitomirsky, I. A review of new methods of surface chemical modification, dispersion and electrophoretic deposition of metal oxide particles. RSC Adv. 2014, 4, 22716–22732. [Google Scholar] [CrossRef]
- Su, Y.; Zhitomirsky, I. Electrophoretic assembly of organic molecules and composites for electrochemical supercapacitors. J. Colloid Interface Sci. 2013, 392, 247–255. [Google Scholar] [CrossRef]
- Su, Y.; Zhitomirsky, I. Cataphoretic assembly of cationic dyes and deposition of carbon nanotube and graphene films. J. Colloid Interface Sci. 2013, 399, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Tashiro, K.; Tadokoro, H. Molecular vibrations of three crystal forms of poly (vinylidene fluoride). Macromolecules 1975, 8, 158–171. [Google Scholar] [CrossRef]
- Zeng, Z.; Yu, D.; He, Z.; Liu, J.; Xiao, F.-X.; Zhang, Y.; Wang, R.; Bhattacharyya, D.; Tan, T.T.Y. Graphene oxide quantum dots covalently functionalized PVDF membrane with significantly-enhanced bactericidal and antibiofouling performances. Sci. Rep. 2016, 6, 20142. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Liu, X.; Veldhuis, S.; Zhitomirsky, I. Versatile Strategy for Electrophoretic Deposition of Polyvinylidene Fluoride-Metal Oxide Nanocomposites. Materials 2021, 14, 7902. https://doi.org/10.3390/ma14247902
Zhao Q, Liu X, Veldhuis S, Zhitomirsky I. Versatile Strategy for Electrophoretic Deposition of Polyvinylidene Fluoride-Metal Oxide Nanocomposites. Materials. 2021; 14(24):7902. https://doi.org/10.3390/ma14247902
Chicago/Turabian StyleZhao, Qinfu, Xinqian Liu, Stephen Veldhuis, and Igor Zhitomirsky. 2021. "Versatile Strategy for Electrophoretic Deposition of Polyvinylidene Fluoride-Metal Oxide Nanocomposites" Materials 14, no. 24: 7902. https://doi.org/10.3390/ma14247902
APA StyleZhao, Q., Liu, X., Veldhuis, S., & Zhitomirsky, I. (2021). Versatile Strategy for Electrophoretic Deposition of Polyvinylidene Fluoride-Metal Oxide Nanocomposites. Materials, 14(24), 7902. https://doi.org/10.3390/ma14247902





