The Preparation and Characterization of Quantum Dots in Polysaccharide Carriers (Starch/Chitosan) as Elements of Smart Packaging and Their Impact on the Growth of Microorganisms in Food
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Polymer Matrix Preparation
2.2.2. Control Sample K Preparation
2.2.3. QD Sample Preparation—ZnS
2.2.4. QD Sample Preparation—CdS
2.3. SEM/TEM Microscopy
2.4. FTIR Spectroscopy
2.5. UV-VIS Spectroscopy
2.6. Surface Color Measurements
2.7. Mechanical Properties of the Films
2.8. Water Content, Solubility, and Degree of Swelling Determination
2.9. Contact Angle Determination
2.10. Particle/Aggregate Sizes (DLS) and Zeta Potential
2.11. Microbiological Testing
2.12. Photoluminescence Spectroscopy
3. Results and Discussion
3.1. Surface Color of Foils
3.2. Mechanical Properties of the Foils
3.3. Determination of Water Content, Solubility, and Degree of Swelling
3.4. Optical Properties
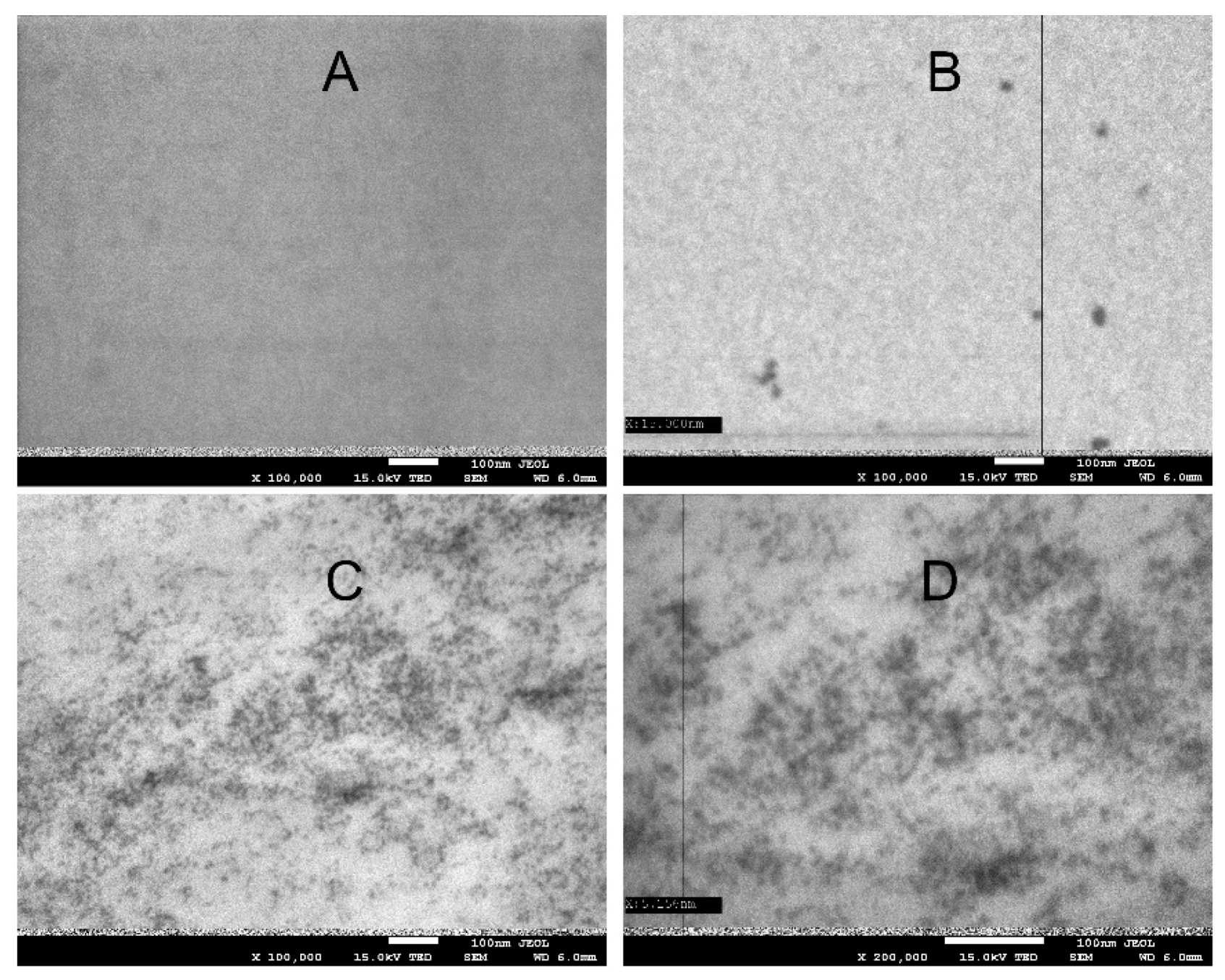
3.5. SEM/TEM Microscopy
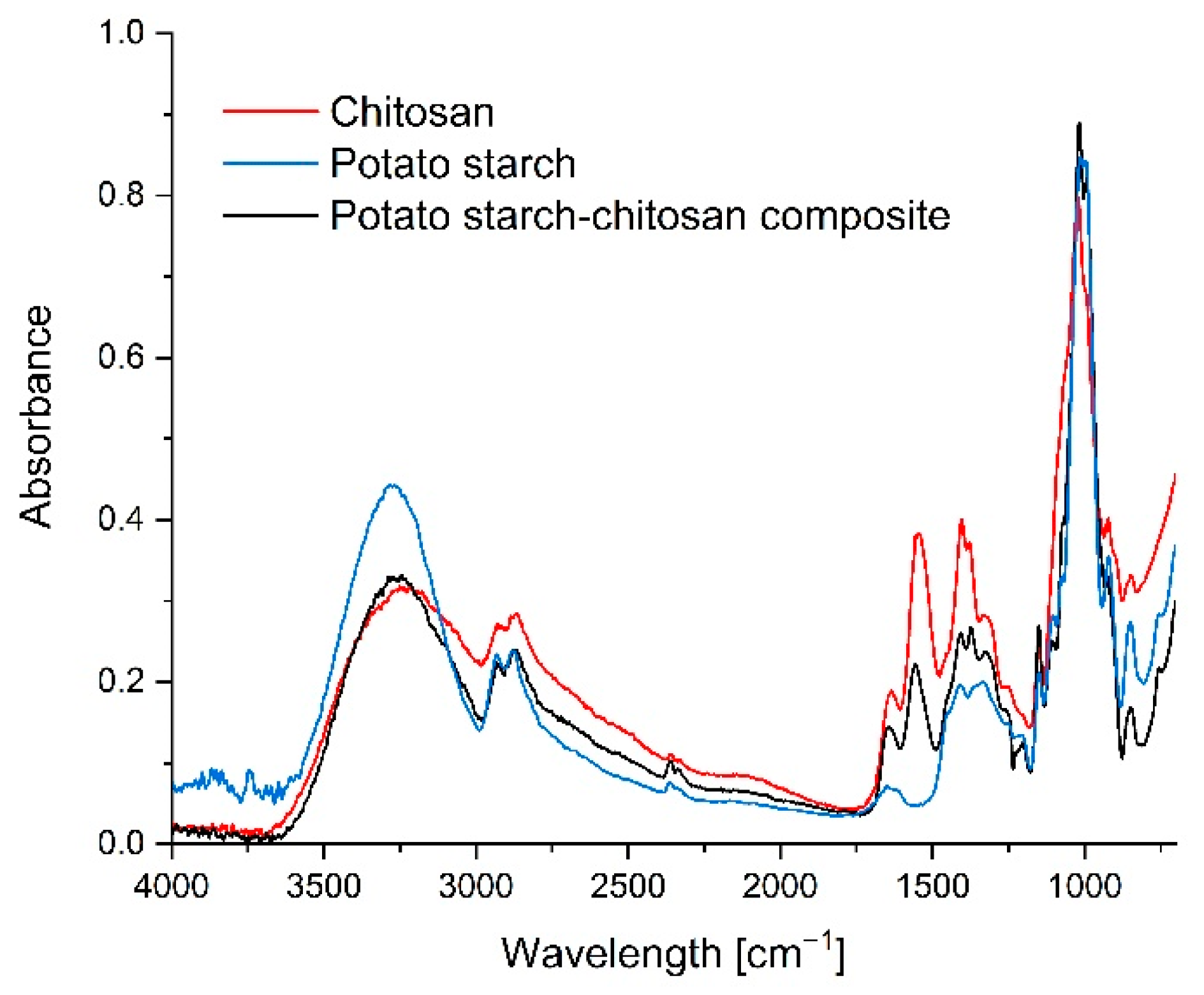
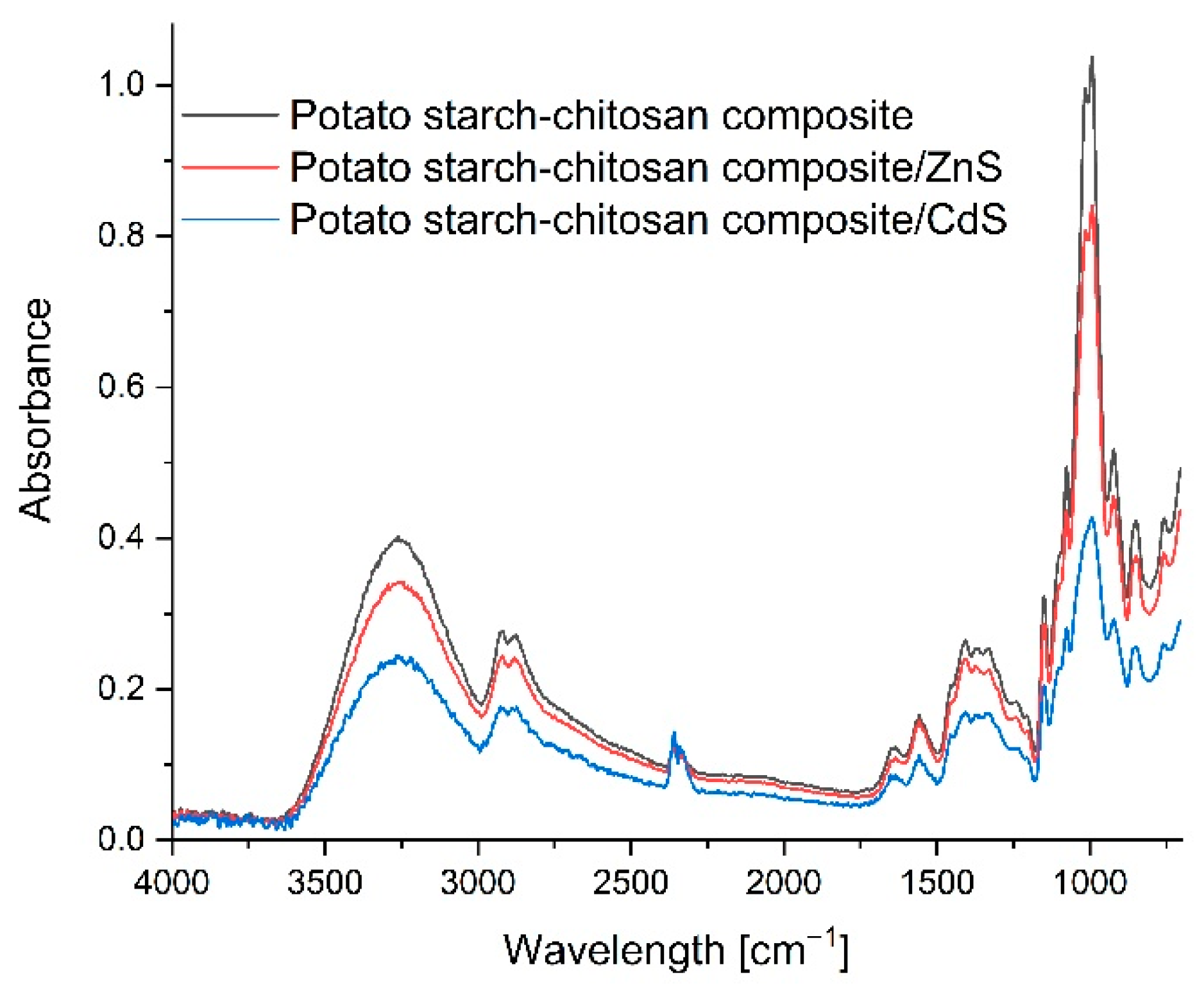
3.6. FTIR Infra-Red Spectra
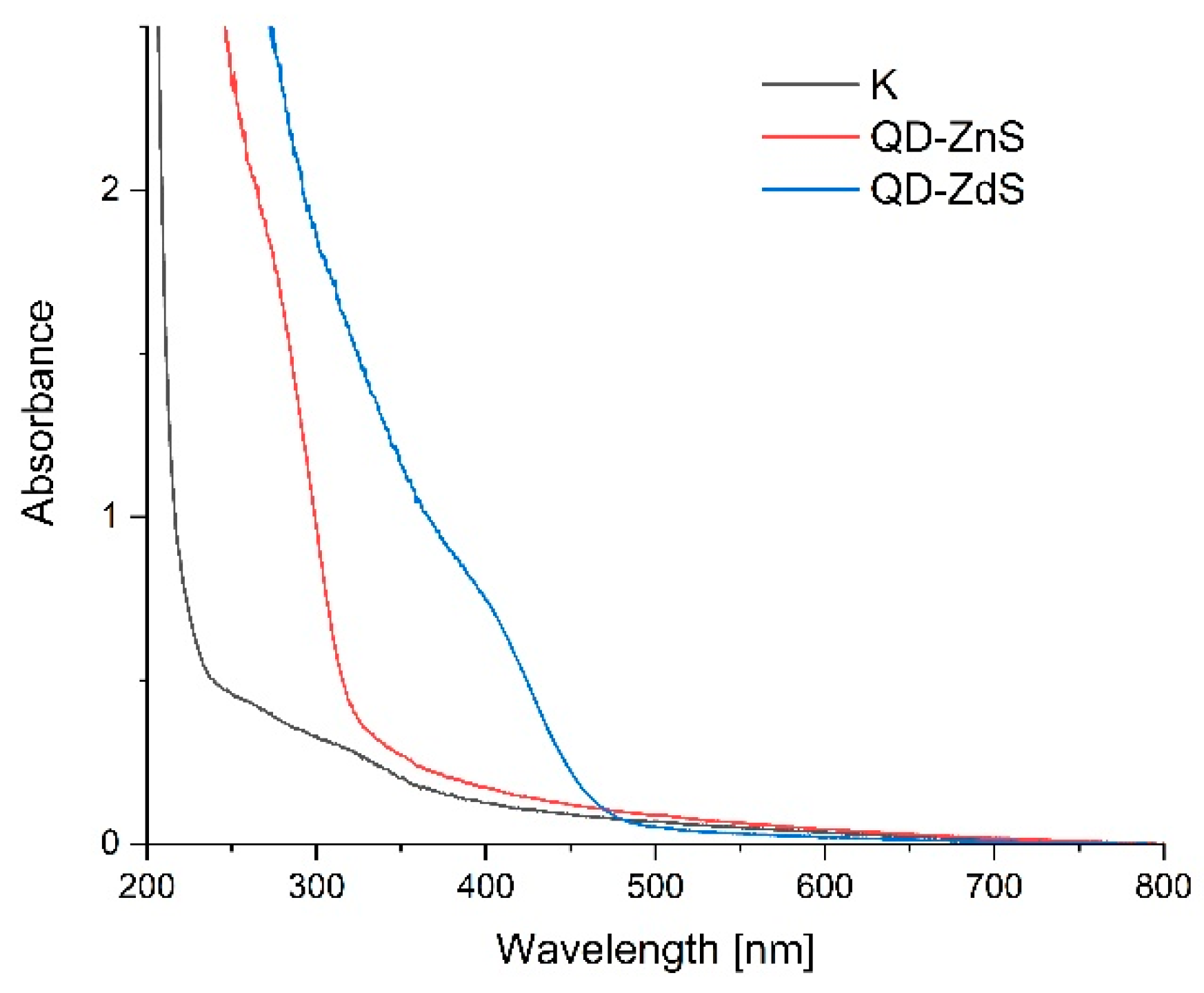
3.7. UV-VIS Spectroscopy
3.8. Wetting Angles
3.9. DLS
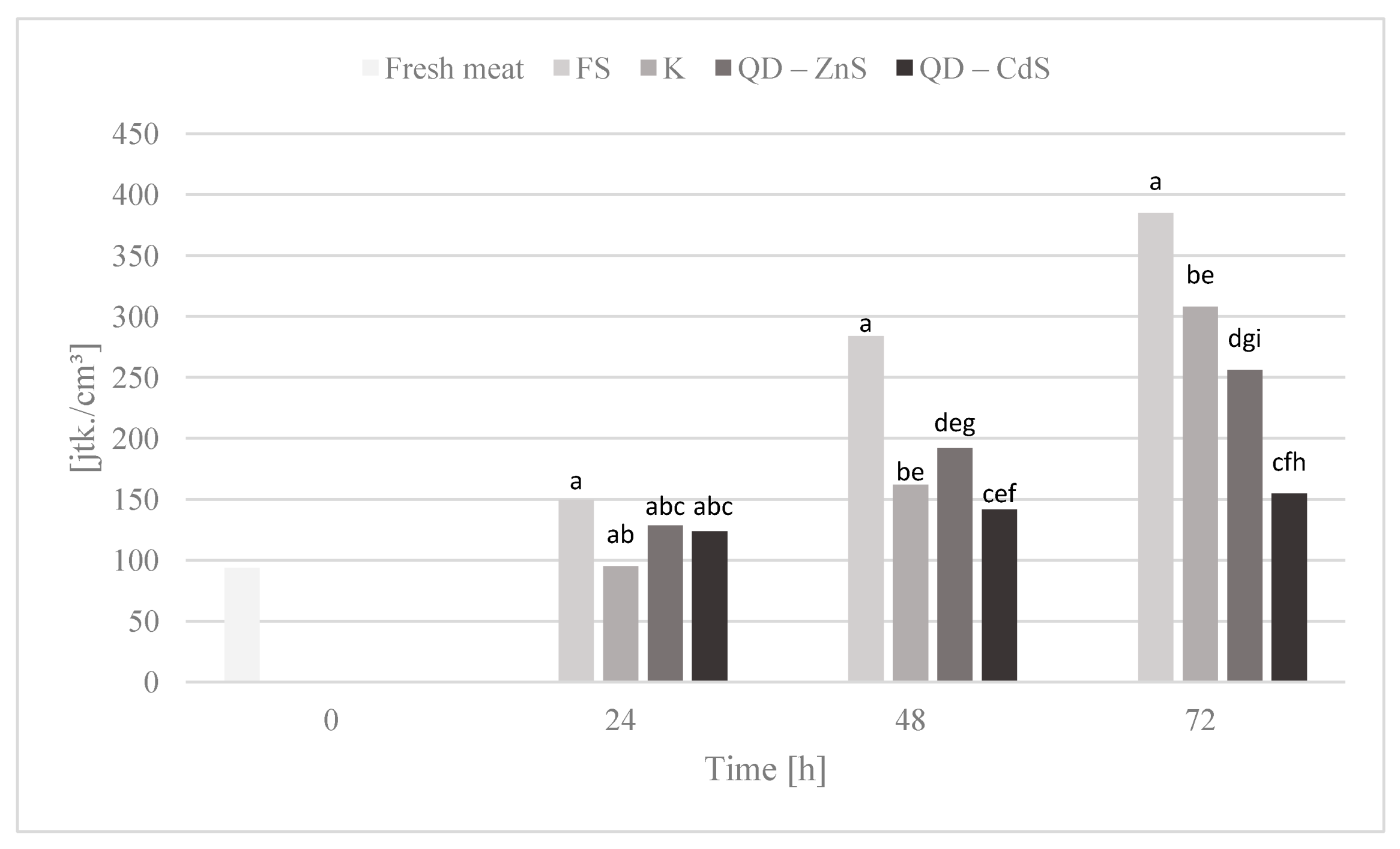
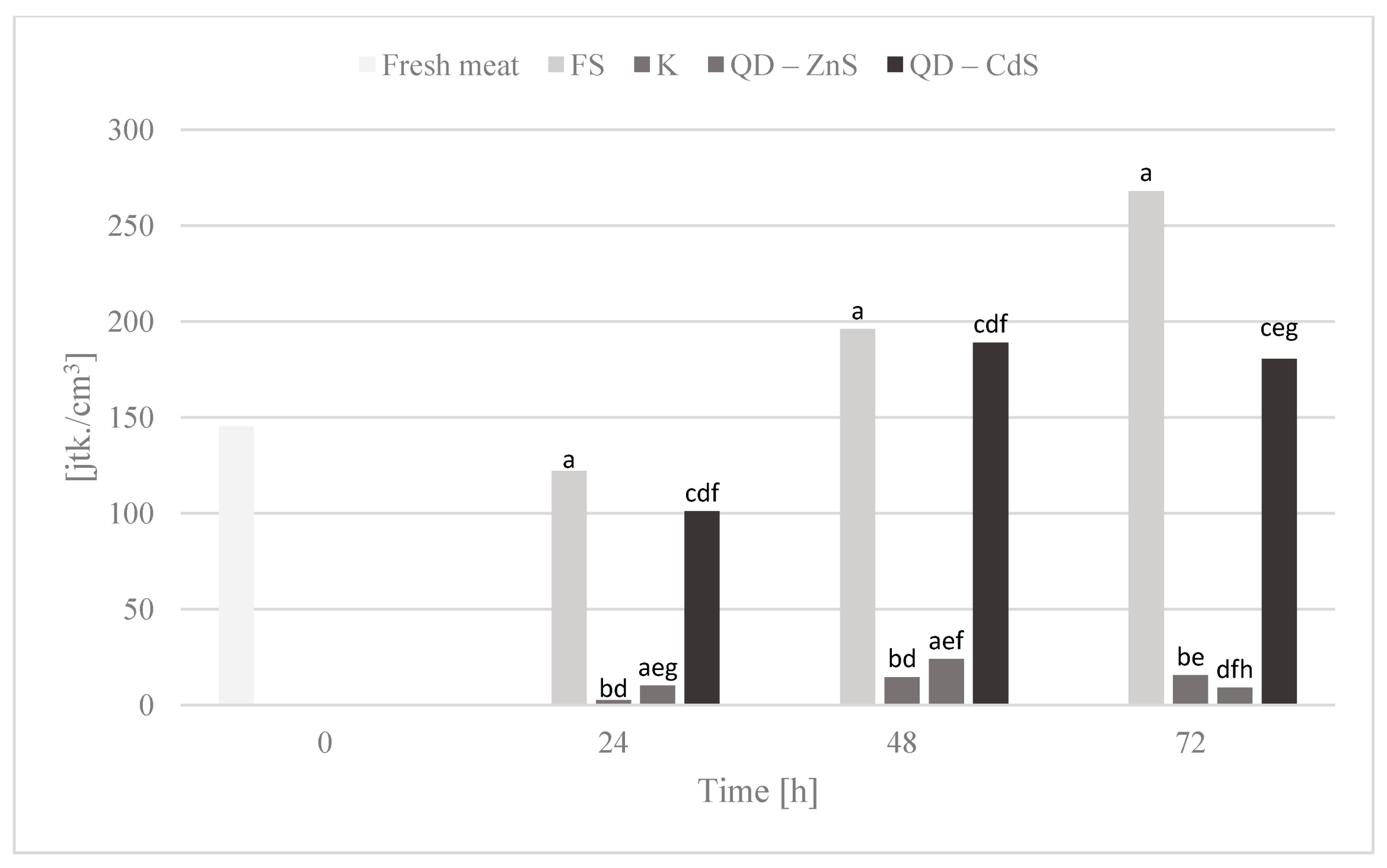
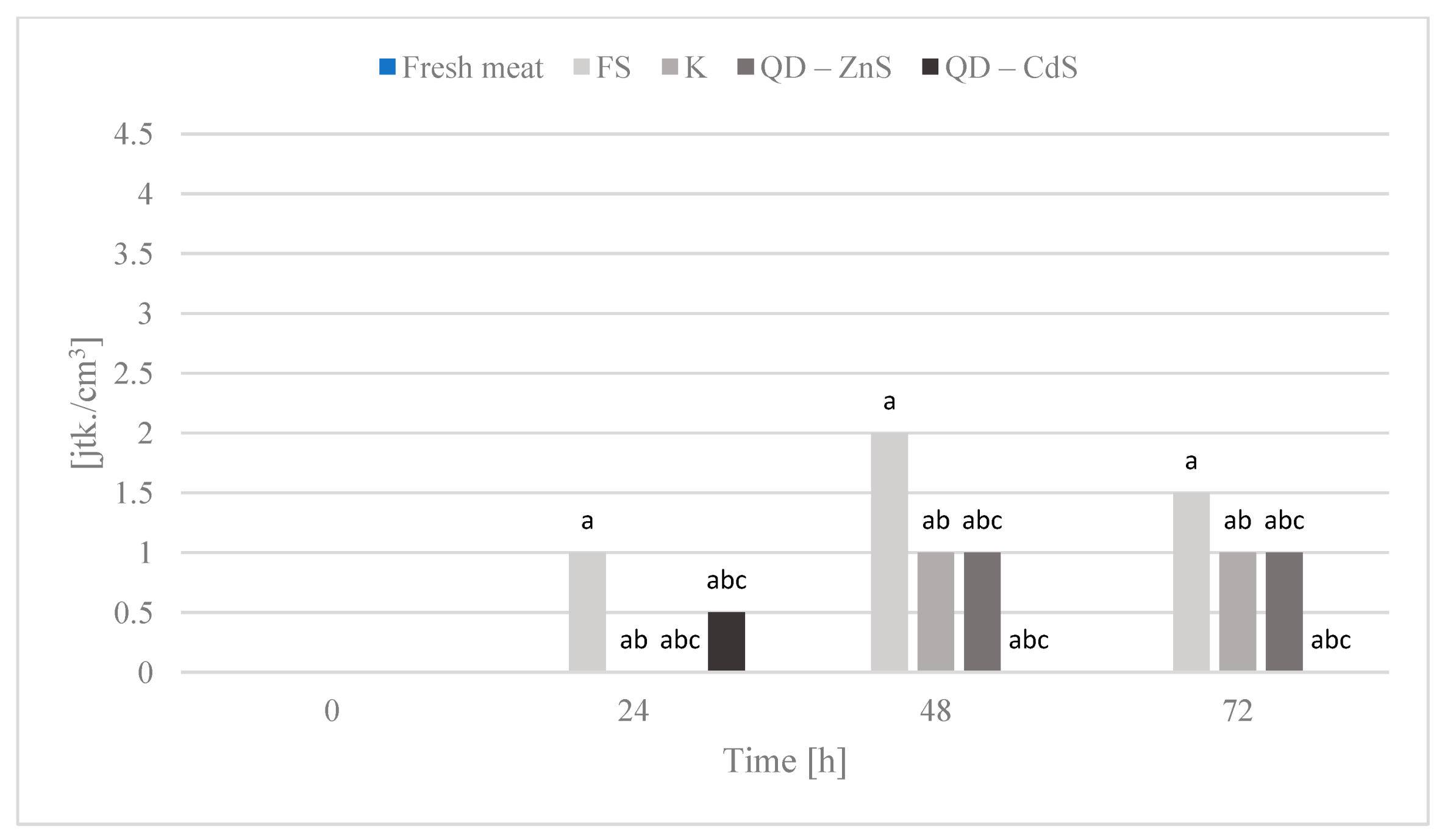
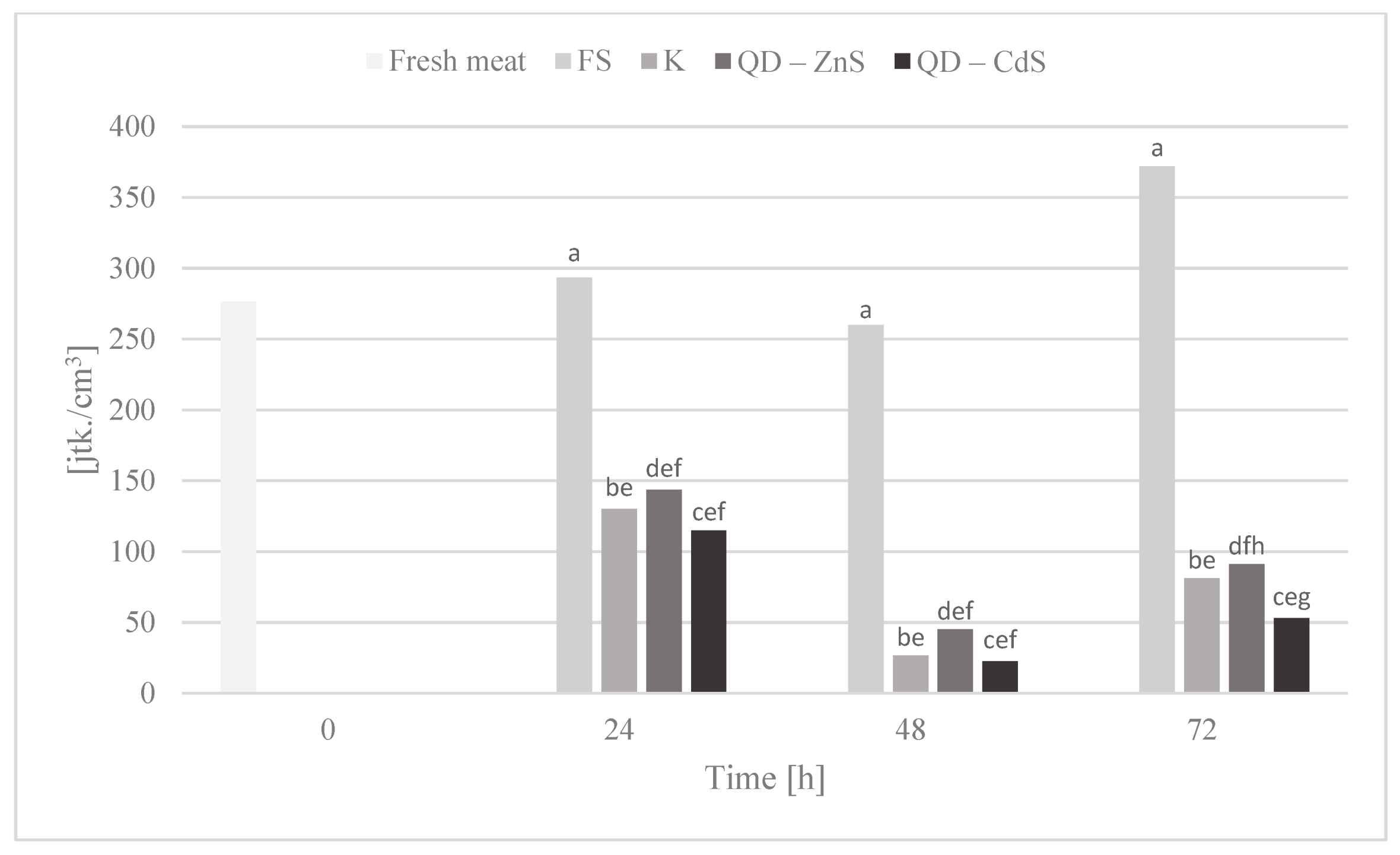
3.10. Storage Test and Microbiological Testing
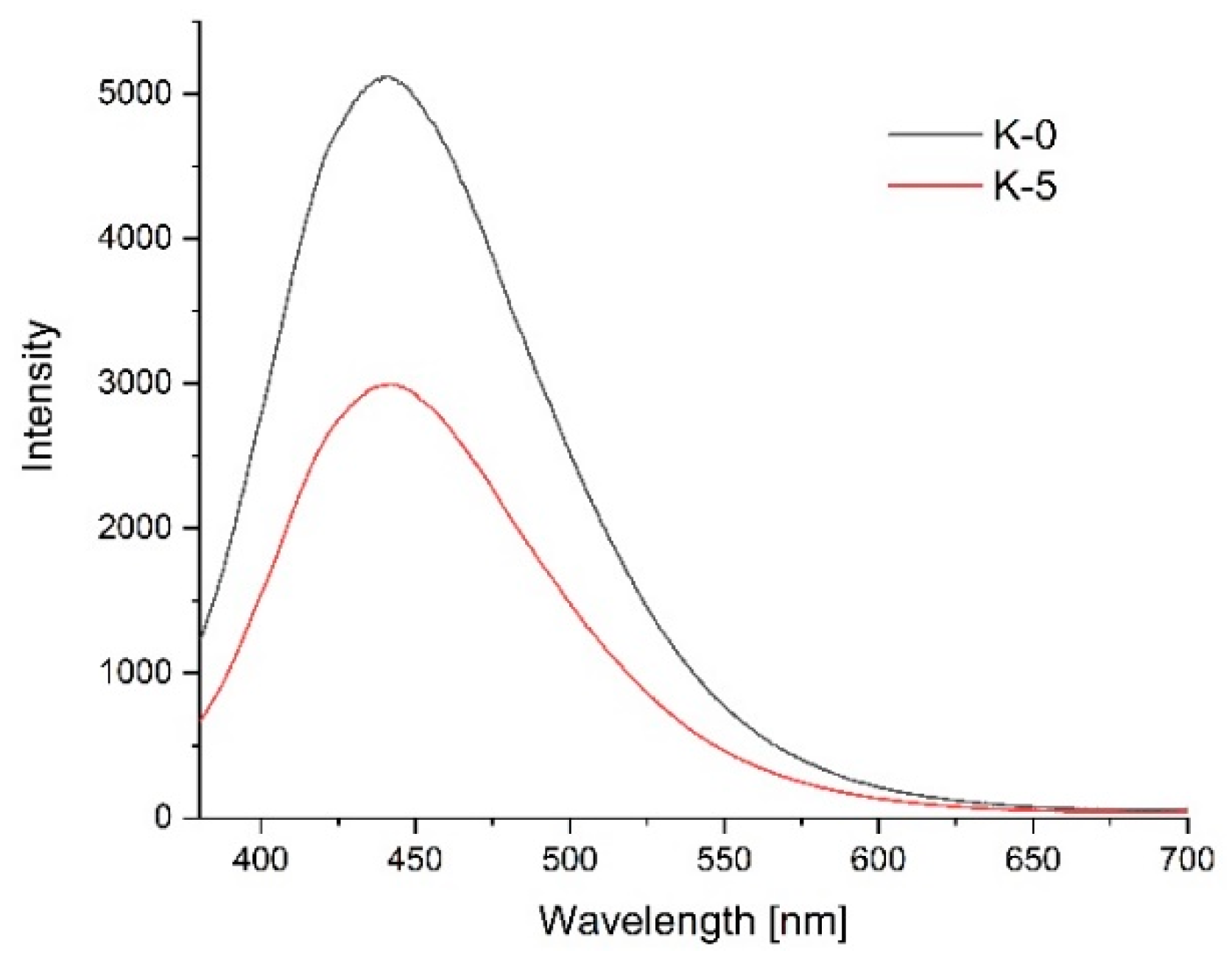
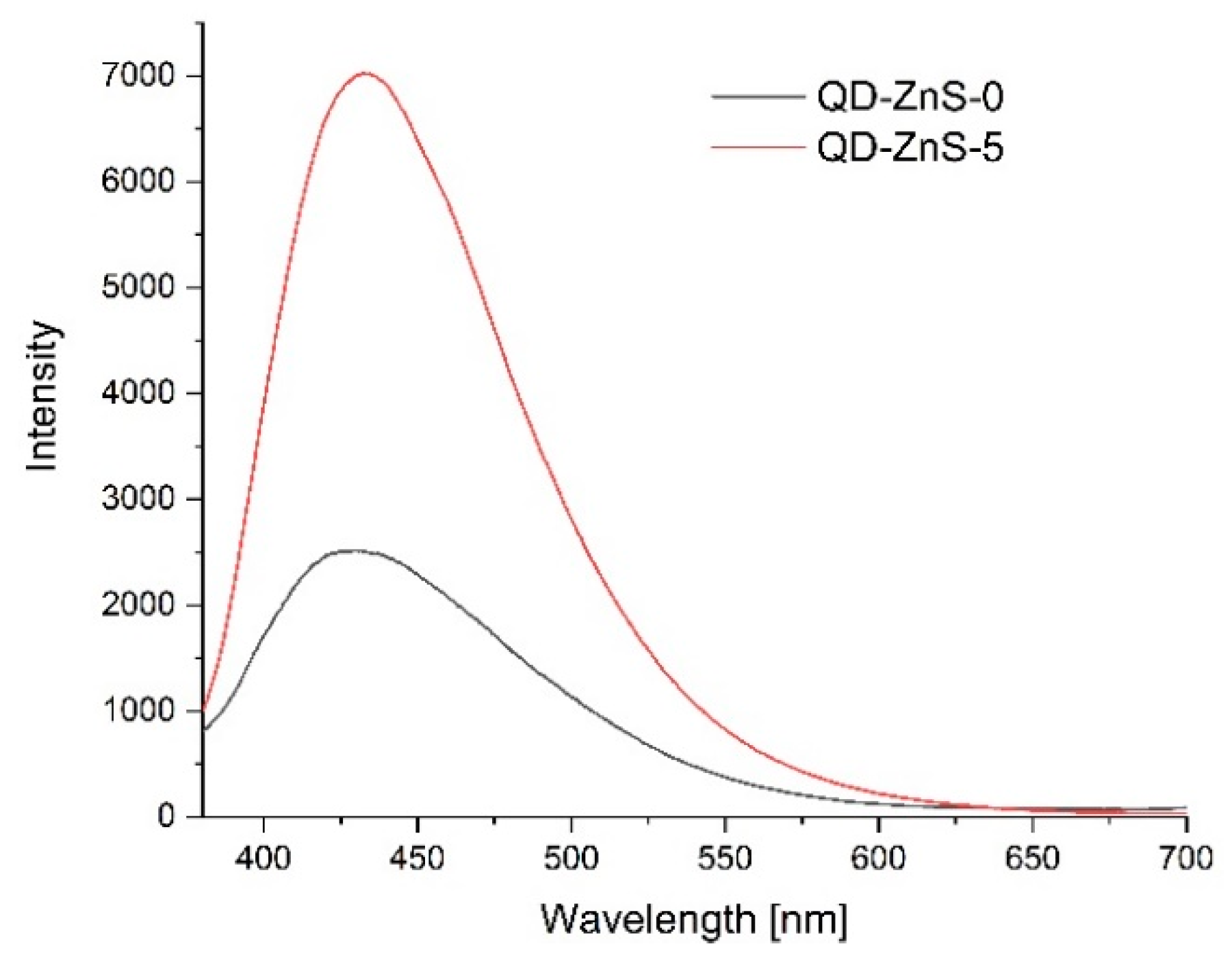
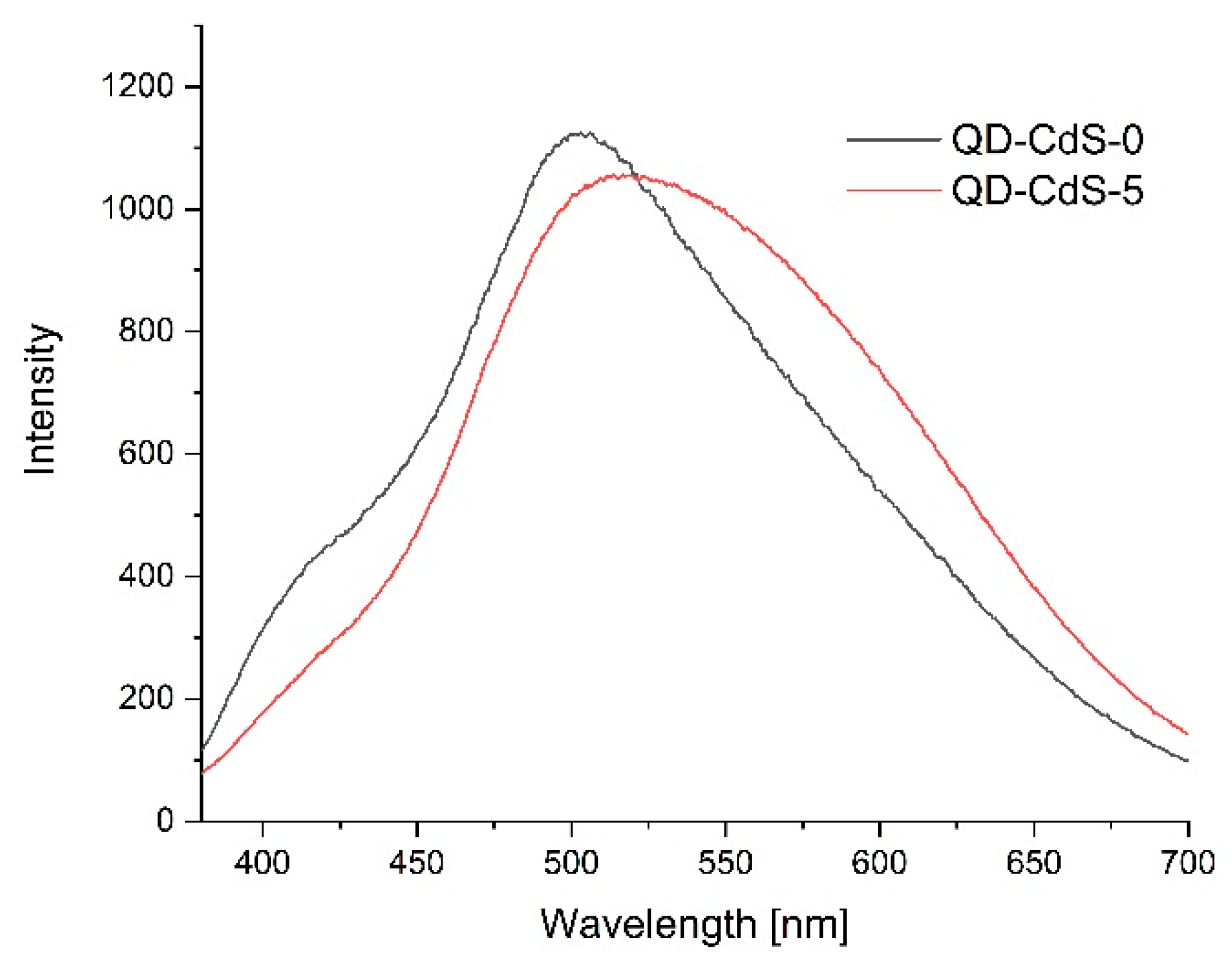
3.11. Photoluminescence Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khachatryan, G.; Khachatryan, K. Starch Based Nanocomposites as Sensors for Heavy Metals–Detection of Cu2+ and Pb2+ Ions. Int. Agrophys. 2019, 33, 121–126. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Al-Asmar, A.; D’Angelo, A.; Roviello, V.; Esposito, M.; Mariniello, L. Preparation and Characterization of Bioplastics from Grass Pea Flour Cast in the Presence of Microbial Transglutaminase. Coatings 2018, 8, 435. [Google Scholar] [CrossRef]
- Mir, I.A.; Alam, H.; Priyadarshini, E.; Meena, R.; Rawat, K.; Rajamani, P.; Rizvi, M.S.; Bohidar, H.B. Antimicrobial and Biocompatibility of Highly Fluorescent ZnSe Core and ZnSe@ZnS Core-Shell Quantum Dots. J. Nanopart. Res. 2018, 20, 1–11. [Google Scholar] [CrossRef]
- Gamal-Eldeen, A.M.; Ahmed, E.F.; Abo-Zeid, M.A. In vitro Cancer Chemopreventive Properties of Polysaccharide Extract from the Brown Alga, Sargassum Latifolium. Food Chem. Toxicol. 2009, 47, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zeng, H.; Xu, Z.; Zheng, B.; Lin, Y.; Gan, C.; Lo, Y.M. Ultrasonic-Assisted Extraction and Antioxidant Activity of Polysaccharides Recovered from White Button Mushroom (Agaricus Bisporus). Carbohydr. Polym. 2012, 88, 522–529. [Google Scholar] [CrossRef]
- Li, S.; Shah, N.P. Antioxidant and Antibacterial Activities of Sulphated Polysaccharides from Pleurotus Eryngii and Streptococcus Thermophilus ASCC 1275. Food Chem. 2014, 165, 262–270. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Grabacka, M.; Krzan, M.; Witczak, M.; Grzyb, J.; Woszczak, L. Physicochemical, Bacteriostatic, and Biological Properties of Starch/Chitosan Polymer Composites Modified by Graphene Oxide, Designed as New Bionanomaterials. Polymers 2021, 13, 2327. [Google Scholar] [CrossRef]
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Potential Perspectives of Bio-Nanocomposites for Food Packaging Applications. Trends Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- Mandal, A.; Ray Banerjee, E. Introduction to Nanoscience, Nanotechnology and Nanoparticles. In Nanomaterials and Biomedicine; Ray Banerjee, E., Ed.; Springer: Singapore, 2020; pp. 1–39. [Google Scholar] [CrossRef]
- Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Bionanocomposites: A New Concept of Ecological, Bioinspired, and Functional Hybrid Materials. Adv. Mater. 2007, 19, 1309–1319. [Google Scholar] [CrossRef]
- Dias, A.M.G.C.; Hussain, A.; Marcos, A.S.; Roque, A.C.A. A Biotechnological Perspective on the Application of Iron Oxide Magnetic Colloids Modified with Polysaccharides. Biotechnol. Adv. 2011, 29, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Hanemann, T.; Szabó, D.V. Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Jabeen, U.; Adhikari, T.; Shah, S.M.; Pathak, D.; Kumar, V.; Nunzi, J.M.; Aamir, M.; Mushtaq, A. Synthesis, Characterization and Photovoltaic Applications of Noble Metal—Doped ZnS Quantum Dots. Chin. J. Phys. 2019, 58, 348–362. [Google Scholar] [CrossRef]
- Ebrahim, S.; Reda, M.; Hussien, A.; Zayed, D. CdTe Quantum Dots as a Novel Biosensor for Serratia Marcescens and Lipopolysaccharide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 150, 212–219. [Google Scholar] [CrossRef]
- Ali, M.; El Nady, J.; Ebrahim, S.; Soliman, M. Structural and Optical Properties of Upconversion CuInS/ZnS Quantum Dots. Opt. Mater. 2018, 86, 545–549. [Google Scholar] [CrossRef]
- Tungittiplakorn, W.; Cohen, C.; Lion, L.W. Engineered Polymeric Nanoparticles for Bioremediation of Hydrophobic Contaminants. Environ. Sci. Technol. 2005, 39, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Tungittiplakorn, W.; Lion, L.W.; Cohen, C.; Kim, J.Y. Engineered Polymeric Nanoparticles for Soil Remediation. Environ. Sci. Technol. 2004, 38, 1605–1610. [Google Scholar] [CrossRef]
- Shan, G.; Xing, J.; Zhang, H.; Liu, H. Biodesulfurization of Dibenzothiophene by Microbial Cells Coated with Magnetite Nanoparticles. Appl. Environ. Microbiol. 2005, 71, 4497–4502. [Google Scholar] [CrossRef] [PubMed]
- Derfus, A.M.; Chan, W.C.; Bhatia, S.N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Creely, K.S.; Tran, C.L. HSE Health & Safety Executive Nanoparticles: An Occupational Hygiene Review. In Nanoparticles: An Occupational Hygiene Review; HSE Books: Edinburgh, UK, 2004; pp. 41–44. [Google Scholar]
- Halonen, N.; Pálvölgyi, P.S.; Bassani, A.; Fiorentini, C.; Nair, R.; Spigno, G.; Kordas, K. Bio-Based Smart Materials for Food Packaging and Sensors—A Review. Front. Mater. 2020, 7, 82. [Google Scholar] [CrossRef]
- Mohamed Fahmy, H.; Eldin, R.E.S.; Serea, E.S.A.; Mamdouh Gomaa, N.; AboElmagd, G.M.; Salem, S.A.; Elsayed, Z.A.; Edrees, A.; Shams-Eldin, E.; Esmail Shalan, A. Advances in Nanotechnology and Antibacterial Properties of Biodegradable Food Packaging Materials. RSC Adv. 2020, 10, 20467–20484. [Google Scholar] [CrossRef]
- Kalpana, S.; Priyadarshini, S.R.; Maria Leena, M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent Packaging: Trends and Applications in Food Systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Hamad, A.F.; Han, J.H.; Kim, B.C.; Rather, I.A. The Intertwine of Nanotechnology with the Food Industry. Saudi J. Biol. Sci. 2018, 25, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.; Andreescu, S. Chemical and Biological Sensors for Food-Quality Monitoring and Smart Packaging. Foods 2018, 7, 168. [Google Scholar] [CrossRef]
- Khachatryan, K.; Khachatryan, G.; Fiedorowicz, M. Synthesis of ZnS, CdS and Core-Shell Mixed CdS/ZnS, ZnS/CdS Nanocrystals in Tapioca Starch Matrix. J. Mater. Sci. Chem. Eng. 2015, 3, 30. [Google Scholar] [CrossRef][Green Version]
- Khachatryan, K.; Khachatryan, G.; Fiedorowicz, M.; Tomasik, P. Formation and Properties of Selected Quantum Dots in Maize Amylopectin Matrix. J. Alloys Compd. 2014, 607, 39–43. [Google Scholar] [CrossRef]
- Khachatryan, K.; Khachatryan, G.; Fiedorowicz, M. Distarch Phosphate as a Matrix for the Generation of Quantum Dots. Polym. Polym. Compos. 2016, 24, 403–410. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical Properties of Chitosan Films Incorporated with Natural Antioxidants. Ind. Crops Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Khachtryan, K.; Fiedorowicz, M.; Khachatryan, G.; Krzeminska-Fiedorowicz, L. Formation and Properties of ZnS and Cds Nanocrystals in Potato Starch Gel. In Proceedings of the ICCE-18 Eighteenth Annual International Conference on Composites Engineering, Anchorage, AK, USA, 4–10 July 2010. [Google Scholar]
- Lee, H.M.; Kim, M.H.; Yoon, Y.I.; Park, W.H. Fluorescent Property of Chitosan Oligomer and Its Application as a Metal Ion Sensor. Mar. Drugs 2017, 15, 105. [Google Scholar] [CrossRef]
- Rajabi, H.R.; Shamsipur, M.; Khosravi, A.A.; Khani, O.; Yousefi, M.H. Selective Spectrofluorimetric Determination of Sulfide ion Using Manganese Doped ZnS Quantum Dots as Luminescent probe. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 107, 256–262. [Google Scholar] [CrossRef]
- Ibrahim, M.; Mahmoud, A.A.; Osman, O.; Refaat, A.; El-Sayed, E.S.M. Molecular Spectroscopic Analysis of Nano-Chitosan Blend as Biosensor. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 77, 802–806. [Google Scholar] [CrossRef]
- Nowak, N.; Grzebieniarz, W.; Khachatryan, G.; Khachatryan, K.; Konieczna-Molenda, A.; Krzan, M.; Grzyb, J. Synthesis of Silver and Gold Nanoparticles in Sodium Alginate Matrix Enriched with Graphene Oxide and Investigation of Properties of the Obtained Thin Films. Appl. Sci. 2021, 11, 3857. [Google Scholar] [CrossRef]
- Mutavdžić, D.; Xu, J.; Thakur, G.; Triulzi, R.; Kasas, S.; Jeremić, M.; Leblanc, R.; Radotić, K. Determination of the Size of Quantum Dots by Fluorescence Spectroscopy. Analyst 2011, 136, 2391–2396. [Google Scholar] [CrossRef] [PubMed]
- Walaszczyk, E.; Gąsiorek, E.; Podgórski, W. Effect of sucrose concentration on oxalic acid biosynthesis by Aspergillus niger. Zesz. Probl. Post. Nauk Rol. 2017, 588, 129–138. [Google Scholar] [CrossRef]
- Desai, M.L.; Deshmukh, B.; Lenka, N.; Haran, V.; Jha, S.; Basu, H.; Singhal, R.K.; Sharma, P.K.; Kailasa, S.K.; Kim, K.H. Influence of Doping Ion, Capping Agent and pH on the Fluorescence Properties of Zinc Sulfide Quantum Dots: Sensing of Cu2+ and Hg2+ Ions and Their Biocompatibility with Cancer and Fungal Cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Rajendiran, K.; Zhao, Z.; Pei, D.-S.; Fu, A. Antimicrobial Activity and Mechanism of Functionalized Quantum Dots. Polymers 2019, 11, 1670. [Google Scholar] [CrossRef] [PubMed]
- Salunke, B.K.; Sawant, S.S.; Lee, S.-I.; Kim, B.S. Microorganisms as Efficient Biosystem for the Synthesis of Metal Nanoparticles: Current Scenario and Future Possibilities. World J. Microbiol. Biotechnol. 2016, 32, 1–16. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Devi Rajeswari, V. A Review on Green Synthesis, Biomedical Applications, and Toxicity Studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, 3569758. [Google Scholar] [CrossRef]
- Galdiero, E.; Siciliano, A.; Maselli, V.; Gesuele, R.; Guida, M.; Fulgione, D.; Galdiero, S.; Lombardi, L.; Falanga, A. An Integrated Study on Antimicrobial Activity and Ecotoxicity of Quantum Dots and Quantum Dots Coated with the Antimicrobial Peptide Indolicidin. Int. J. Nanomed. 2016, 11, 4199. [Google Scholar] [CrossRef]
- Dwivedi, S.; Wahab, R.; Khan, F.; Mishra, Y.K.; Musarrat, J.; Al-Khedhairy, A.A. Reactive Oxygen Species Mediated Bacterial Biofilm Inhibition Via Zinc Oxide Nanoparticles and Their Statistical Determination. PLoS ONE 2014, 9, e111289. [Google Scholar] [CrossRef]
- Nanda, A.; Raghavan, M. Bactericidal Activity of Zinc Sulphate Bio-Nanoparticles against Enterobacteriaceae Pathogens. Proc. Int. Conf. Nanosci. Eng. Technol. ICONSET 2011, 28, 667–670. [Google Scholar] [CrossRef]
- Jacob, J.M.; Rajan, R.; Tom, T.C.; Kumar, V.S.; Kurup, G.G.; Shanmuganathan, R.; Pugazhendhi, A. Biogenic Design of ZnS Quantum Dots-Insights into Their in-vitro Cytotoxicity, Photocatalysis and Biosensing Properties. Ceram. Int. 2019, 45, 24193–24201. [Google Scholar] [CrossRef]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W.J. Surface Modification, Functionalization and Bioconjugation of Colloidal Inorganic Nanoparticles. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1333–1383. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.B.; Huang, N.; Zhang, Y. Ultrafine Biocompatible Chitosan Nanoparticles Encapsulating Multi-Coloured Quantum Dots for Bioapplications. J. Colloid Interface Sci. 2007, 310, 464–470. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, S.M.; Mansur, A.A.P.; Mansur, H.S.; Guedes, M.I.M.C.; Lobato, Z.I.P.; Leite, M.F. In vitro and in vivo Assessment of Nanotoxicity of CdS Quantum Dot/Aminopolysaccharide Bionanoconjugates. Mater. Sci. Eng. C 2017, 71, 412–424. [Google Scholar] [CrossRef]
- Benn, T.M.; Westerhoff, P. Nanoparticle Silver Released into Water from Commercially Available Sock Fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.P.; Bugge, D.; Ranville, J.F.; Chen, C.Y. Bioavailability, Toxicity, and Bioaccumulation of Quantum Dot Nanoparticles to the Amphipod Leptocheirus Plumulosus. Environ. Sci. Technol. 2012, 46, 5550–5556. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.; Nelson, K. Trophic Level Transfer of Microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ. Pollut. 2013, 177, 1–3. [Google Scholar] [CrossRef]
- Ma, S.; Lin, D. The Biophysicochemical Interactions at the Interfaces between Nanoparticles and Aquatic Organisms: Adsorption and Internalization. Environ. Sci. Process. Impacts 2012, 15, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Krug, H.F. Nanosafety Research—Are We on the Right Track? Angew. Chem. Int. Ed. 2014, 53, 12304–12319. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-Analysis of Cellular Toxicity for Cadmium-containing Quantum Dots. Nat. Nanotechnol. 2016, 11, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Paesano, L.; Marmiroli, M.; Bianchi, M.G.; White, J.C.; Bussolati, O.; Zappettini, A.; Villani, M.; Marmiroli, N. Data on miRNome Changes in Human Cells Exposed to Nano-or Ionic-Forms of Cadmium. Data Br. 2020, 30, 105636. [Google Scholar] [CrossRef] [PubMed]
- Matos, B.; Martins, M.; Samamed, A.C.; Sousa, D.; Ferreira, I.; Diniz, M.S. Toxicity Evaluation of Quantum Dots (ZnS and CdS) Singly and Combined in Zebrafish (Danio Rerio). Int. J. Environ. Res. Public Health 2019, 17, 232. [Google Scholar] [CrossRef]
- Ye, L.; Yong, K.-T.T.; Liu, L.; Roy, I.; Hu, R.; Zhu, J.; Cai, H.; Law, W.-C.C.; Liu, J.J.; Wang, K.; et al. A Pilot Study in Non-human Primates Shows no Adverse Response to Intravenous Injection of Quantum Dots. Nat. Nanotechnol. 2012, 7, 453–458. [Google Scholar] [CrossRef]
- Alexandrakis, D.; Brunton, N.P.; Downey, G.; Scannell, A.G.M. Identification of Spoilage Marker Metabolites in Irish Chicken Breast Muscle Using HPLC, GC–MS Coupled with SPME and Traditional Chemical Techniques. Food Bioprocess Technol. 2012, 5, 1917–1923. [Google Scholar] [CrossRef]
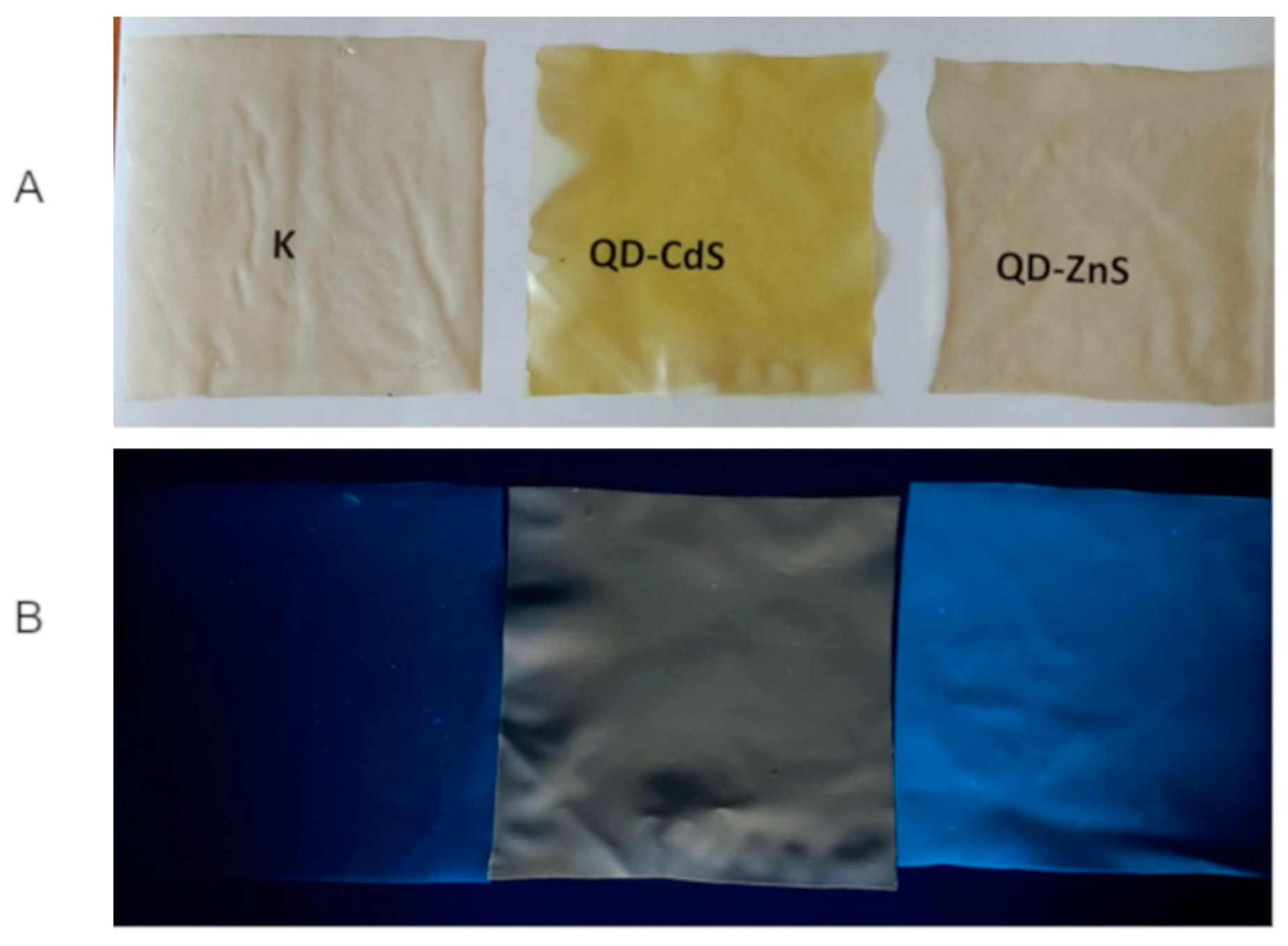

| Sample | L* (D65) | a* (D65) | b* (D65) |
|---|---|---|---|
| K | 94.6 ± 0.4 b | −0.7 ± 0.1 b | 7.2 ± 0.8 b |
| QD-ZnS | 95.6 ± 0.1 a | −0.8 ± 0.04 b | 7.6 ± 0.3 b |
| QD-CdS | 94.3 ± 0.04 b | −11.4 ± 0.07 a | 45.0 ± 0.6 a |
| Sample | Thickness (mm) | TS (MPa) | E (%) |
|---|---|---|---|
| K | 0.12 ± 0.005 b | 7.3 ± 0.6 a | 59± 5.0 a |
| QD-ZnS | 0.13 ± 0.005 a | 5.7 ± 0.5 b | 47.1 ± 6.2 b |
| QD-CdS | 0.13 ± 0.001 a | 4.6 ± 0.2 b | 43.7 ± 8.1 b |
| Sample | Water Content [%] | Solubility [%] | Degree of Swelling [%] |
|---|---|---|---|
| K | 6.0 ± 1.1 a | 33.4 ± 0.5 a | 67.0± 3.7 a |
| QD-ZnS | 5.4 ± 1.5 a | 36.8 ± 0.7 b | 69.3 ± 0.8 a |
| QD-CdS | 5.2 ± 1.0 a | 34.1 ± 1.0 a | 66.2 ± 1.7 a |
| Sample | Contact Angle | Surface Free Energy | |||
|---|---|---|---|---|---|
| Water | DIM | Dispersive | Polar | Total | |
| K | 69.7 | 37.8 | 39.32 | 7.65 | 46.97 |
| QD-ZnS | 76.2 | 34.5 | 43.24 | 4.10 | 47.32 |
| QD-CdS | 88.0 | 40.3 | 43.39 | 0.95 | 44.34 |
| Sample | Size [nm] | Zeta Potential [mV] |
|---|---|---|
| K | 350 | 51.4 |
| QD-ZnS | 3600 | −7.4 |
| QD-CdS | 510 | 35.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzebieniarz, W.; Nowak, N.; Khachatryan, G.; Krzan, M.; Krystyjan, M.; Kosiński, J.; Khachatryan, K. The Preparation and Characterization of Quantum Dots in Polysaccharide Carriers (Starch/Chitosan) as Elements of Smart Packaging and Their Impact on the Growth of Microorganisms in Food. Materials 2021, 14, 7732. https://doi.org/10.3390/ma14247732
Grzebieniarz W, Nowak N, Khachatryan G, Krzan M, Krystyjan M, Kosiński J, Khachatryan K. The Preparation and Characterization of Quantum Dots in Polysaccharide Carriers (Starch/Chitosan) as Elements of Smart Packaging and Their Impact on the Growth of Microorganisms in Food. Materials. 2021; 14(24):7732. https://doi.org/10.3390/ma14247732
Chicago/Turabian StyleGrzebieniarz, Wiktoria, Nikola Nowak, Gohar Khachatryan, Marcel Krzan, Magdalena Krystyjan, Jarosław Kosiński, and Karen Khachatryan. 2021. "The Preparation and Characterization of Quantum Dots in Polysaccharide Carriers (Starch/Chitosan) as Elements of Smart Packaging and Their Impact on the Growth of Microorganisms in Food" Materials 14, no. 24: 7732. https://doi.org/10.3390/ma14247732
APA StyleGrzebieniarz, W., Nowak, N., Khachatryan, G., Krzan, M., Krystyjan, M., Kosiński, J., & Khachatryan, K. (2021). The Preparation and Characterization of Quantum Dots in Polysaccharide Carriers (Starch/Chitosan) as Elements of Smart Packaging and Their Impact on the Growth of Microorganisms in Food. Materials, 14(24), 7732. https://doi.org/10.3390/ma14247732









