Collagen/Chitosan Gels Cross-Linked with Genipin for Wound Healing in Mice with Induced Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chitosan
2.3. Characterization of Chitosan
Antibacterial Tests
2.4. Preparation of Silver Nanoparticles
2.5. Preparation of Gels
2.6. Swelling Test
2.7. IR Spectroscopy and SEM Characterization
2.8. Rheological Measurements
2.9. Cytotoxicity of Gels
2.10. In Vivo Healing Assays
2.10.1. Modeling Diabetes in Mice Using Alloxan
2.10.2. Modeling Wound Healing in Mice
2.11. Analysis of Gene Expression
2.11.1. Gene Expression
2.11.2. Analysis of Gene Expression by Real-Time Polymerase Chain Reaction (qPCR)
2.12. Histological Analysis of Wound Tissue
Statistical Analysis
3. Results and Discussion
3.1. Chitosan Properties
3.2. Preparation of NPs
3.3. Preparation of Gels
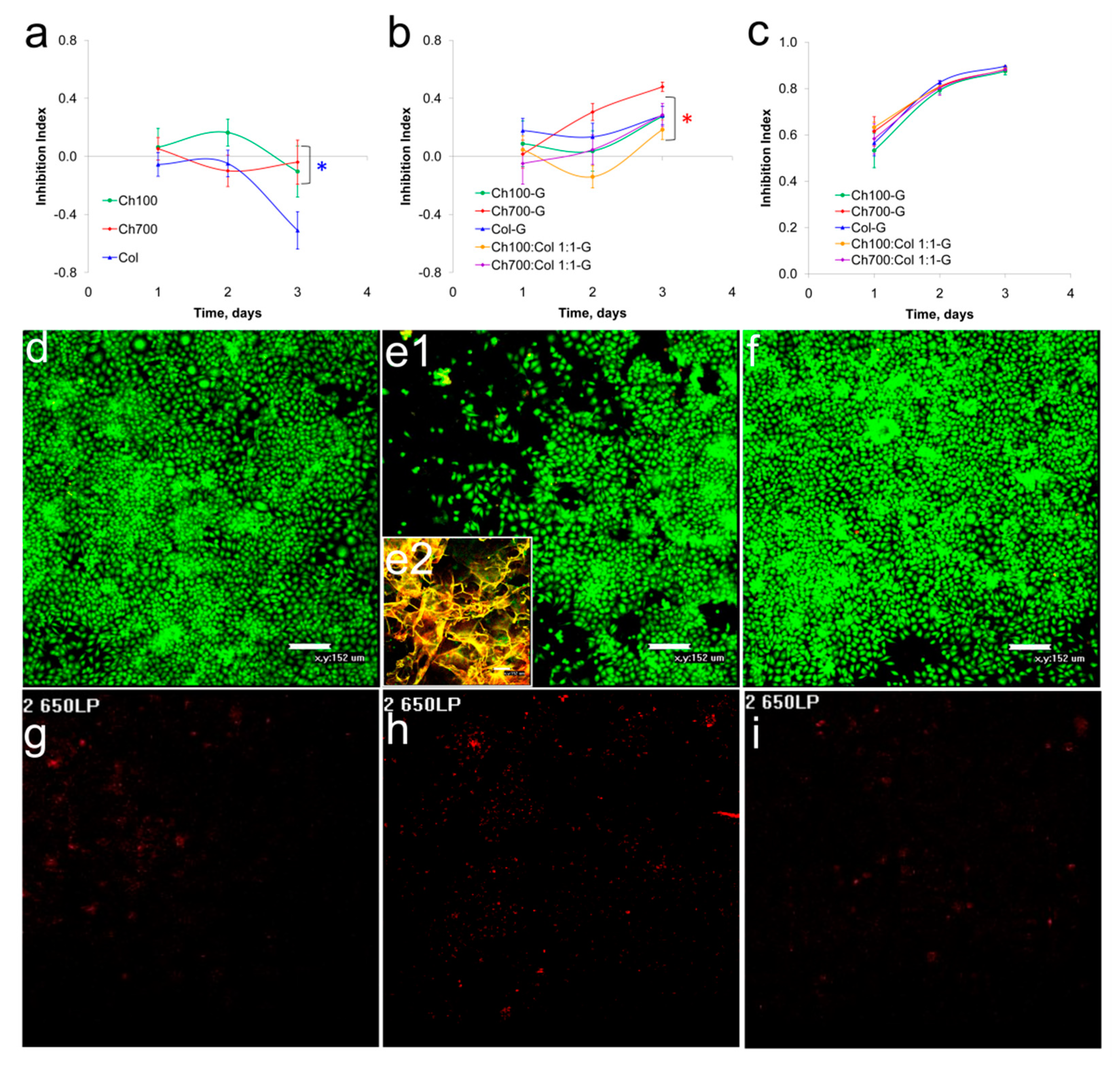
3.4. Cytotoxicity of Gels
3.5. Swelling Properties of Gels
3.6. Fourier Transform Infrared Spectroscopy (FTIR)
3.7. Scanning Electron Microscopy
3.8. Rheological Measurements
3.9. Wound Healing
3.10. Genes Expression
3.11. Histological Analysis of Wound Tissue
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Standl, E.; Khunti, K.; Hansen, T.B.; Schnell, O. The global epidemics of diabetes in the 21st century: Current situation and perspectives. Eur. J. Prev. Cardiol. 2019, 26, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.L.; Veves, A. A review of the mechanisms implicated in the pathogenesis of the diabetic foot. Int. J. Low. Extrem. Wounds 2005, 4, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, L.; Wicks, J.; Ling, C.; Zhao, X.; Yan, Y.; Qi, J.; Cui, W.; Deng, L. Quickly promoting angiogenesis by using a DFO-loaded photo-crosslinked gelatin hydrogel for diabetic skin regeneration. J. Mater. Chem. B 2016, 4, 3770–3781. [Google Scholar] [CrossRef]
- Abdelhady, S.; Honsy, K.M.; Kurakula, M. Electro Spun- Nanofibrous Mats: A Modern Wound Dressing Matrix with a Potential of Drug Delivery and Therapeutics. J. Eng. Fiber. Fabr. 2015, 10, 155892501501000. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.K.; Kiran, V.; Hasnain, M.S.; Nayak, A.K. Alginate-Based Hydrogel Systems for Drug Releasing in Wound Healing; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128176405. [Google Scholar]
- Kurakula, M.; Raghavendra Naveen, N. Electrospraying: A facile technology unfolding the chitosan based drug delivery and biomedical applications. Eur. Polym. J. 2021, 147, 110326. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Wang, J.; Kong, M.; Zhou, Z.; Yan, D.; Yu, X.; Cheng, X.; Feng, C.; Liu, Y.; Chen, X. Mechanism of surface charge triggered intestinal epithelial tight junction opening upon chitosan nanoparticles for insulin oral delivery. Carbohydr. Polym. 2017, 157, 596–602. [Google Scholar] [CrossRef]
- Vllasaliu, D.; Exposito-Harris, R.; Heras, A.; Casettari, L.; Garnett, M.; Illum, L.; Stolnik, S. Tight junction modulation by chitosan nanoparticles: Comparison with chitosan solution. Int. J. Pharm. 2010, 400, 183–193. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.-Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti Infect. Ther. 2011, 9, 857. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Irastorza, A.; Zarandona, I.; Andonegi, M.; Guerrero, P.; de la Caba, K. The versatility of collagen and chitosan: From food to biomedical applications. Food Hydrocoll. 2021, 116, 106633. [Google Scholar] [CrossRef]
- Mienaltowski, M.J.; Birk, D.E. Structure, physiology, and biochemistry of collagens. Adv. Exp. Med. Biol. 2014, 802, 5–29. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, P.; Vulesevic, B.; Kuraitis, D.; Li, F.; Yang, A.F.; Griffith, M.; Ruel, M.; Suuronen, E.J. A collagen-chitosan hydrogel for endothelial differentiation and angiogenesis. Tissue Eng.-Part A 2010, 16, 3099–3109. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Best, S.M.; Cameron, R.E. Crosslinking collagen constructs: Achieving cellular selectivity through modifications of physical and chemical properties. Appl. Sci. 2020, 10, 6911. [Google Scholar] [CrossRef]
- Torkaman, S.; Rahmani, H.; Ashori, A.; Najafi, S.H.M. Modification of chitosan using amino acids for wound healing purposes: A review. Carbohydr. Polym. 2021, 258, 117675. [Google Scholar] [CrossRef]
- Flores, E.E.E.; Cardoso, F.D.; Siqueira, L.B.; Ricardi, N.C.; Costa, T.H.; Rodrigues, R.C.; Klein, M.P.; Hertz, P.F. Influence of reaction parameters in the polymerization between genipin and chitosan for enzyme immobilization. Process. Biochem. 2019, 84, 73–80. [Google Scholar] [CrossRef]
- Ramos-de-la-Peña, A.M.; Renard, C.M.G.C.; Montañez, J.; de la Luz Reyes-Vega, M.; Contreras-Esquivel, J.C. A review through recovery, purification and identification of genipin. Phytochem. Rev. 2016, 15, 37–49. [Google Scholar] [CrossRef]
- Yao, C.H.; Chen, K.Y.; Cheng, M.H.; Chen, Y.S.; Huang, C.H. Effect of genipin crossliked chitosan scaffolds containing SDF-1 on wound healing in a rat model. Mater. Sci. Eng. C 2020, 109, 110368. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, W.; Deng, W.; Xu, C.; Cai, Y.; Wang, X. Antibacterial and Hemostatic Thiol-Modified Chitosan-Immobilized AgNPs Composite Sponges. ACS Appl. Mater. Interfaces 2020, 12, 20307–20320. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zheng, J. Antibacterial activity of silver nanoparticles: Structural effects. Adv. Healthc. Mater. 2018, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Tang, S.; Deng, W.; Luo, J.; Wang, X. Antibacterial chitosan composite films with food-inspired carbon spheres immobilized AgNPs. Food Chem. 2021, 363, 130342. [Google Scholar] [CrossRef]
- Das, B.; Tripathy, S.; Adhikary, J.; Chattopadhyay, S.; Mandal, D.; Dash, S.K.; Das, S.; Dey, A.; Dey, S.K.; Das, D.; et al. Surface modification minimizes the toxicity of silver nanoparticles: An in vitro and in vivo study. J. Biol. Inorg. Chem. 2017, 22, 893–918. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Kumar, S.; Majhi, R.K.; Goswami, L.; Goswami, C.; Mohapatra, H. Antibacterial efficacy of polysaccharide capped silver nanoparticles is not compromised by AcrAB-TolC efflux pump. Front. Microbiol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lee, P.Y.; Ho, C.M.; Lui, V.C.H.; Chen, Y.; Che, C.M.; Tam, P.K.H.; Wong, K.K.Y. Silver nanoparticles mediate differential responses in keratinocytes and fibroblasts during skin wound healing. ChemMedChem 2010, 5, 468–475. [Google Scholar] [CrossRef]
- Das, S.; Baker, A.B. Biomaterials and nanotherapeutics for enhancing skin wound healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.T.; Chang, C.H.; Lin, Y.Y.; Wu, M.F.; Wang, J.L.; Young, T.H.; Han, J.L.; Hsieh, K.H. Evaluation of chitosan/γ-poly(glutamic acid) polyelectrolyte complex for wound dressing materials. Carbohydr. Polym. 2011, 84, 812–819. [Google Scholar] [CrossRef]
- Shagdarova, B.T.; Ilyina, A.V.; Lopatin, S.A.; Kartashov, M.I.; Arslanova, L.R.; Dzhavakhiya, V.G.; Varlamov, V.P. Study of the Protective Activity of Chitosan Hydrolyzate Against Septoria Leaf Blotch of Wheat and Brown Spot of Tobacco. Appl. Biochem. Microbiol. 2018, 54. [Google Scholar] [CrossRef]
- Shagdarova, B.; Lunkov, A.; Il’ina, A.; Varlamov, V. Investigation of the properties of N-[(2-hydroxy-3-trimethylammonium) propyl] chloride chitosan derivatives. Int. J. Biol. Macromol. 2019, 124, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, S.N.; Lisovskaya, S.A.; Zelenikhin, P.V.; Bezrodnykh, E.A.; Shakirova, D.R.; Blagodatskikh, I.V.; Tikhonov, V.E. Antifungal activity of oligochitosans (short chain chitosans) against some Candida species and clinical isolates of Candida albicans: Molecular weight-activity relationship. Eur. J. Med. Chem. 2014, 74, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Lunkov, A.; Shagdarova, B.; Konovalova, M.; Zhuikova, Y.; Drozd, N.; Il’ina, A.; Varlamov, V. Synthesis of silver nanoparticles using gallic acid-conjugated chitosan derivatives. Carbohydr. Polym. 2020, 234, 115916. [Google Scholar] [CrossRef]
- Xiao, B.; Wan, Y.; Wang, X.; Zha, Q.; Liu, H.; Qiu, Z.; Zhang, S. Synthesis and characterization of N-(2-hydroxy)propyl-3-trimethyl ammonium chitosan chloride for potential application in gene delivery. Colloids Surf. B. Biointerfaces 2012, 91, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Anderson, J.D.; Bozell, J.J.; Zivanovic, S. The effect of solvent composition on grafting gallic acid onto chitosan via carbodiimide. Carbohydr. Polym. 2016, 140, 171–180. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Rubio-Valle, J.F.; Jiménez-Rosado, M.; Guerrero, A.; Romero, A. Chitosan as a potential alternative to collagen for the development of genipin-crosslinked scaffolds. React. Funct. Polym. 2020, 146, 104414. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bialik-Wąs, K.; Kijkowska, R.; Sobczak-Kupiec, A. Preparation and cytotoxicity of chitosan-based hydrogels modified with silver nanoparticles. Colloids Surf. B Biointerfaces 2017, 160, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Karpushkin, E.; Dušková-Smrčková, M.; Remmler, T.; Lapčíková, M.; Dušek, K. Rheological properties of homogeneous and heterogeneous poly(2-hydroxyethyl methacrylate) hydrogels. Polym. Int. 2012, 61, 328–336. [Google Scholar] [CrossRef]
- Zhang, Z.; Ni, J.; Chen, L.; Yu, L.; Xu, J.; Ding, J. Biodegradable and thermoreversible PCLA-PEG-PCLA hydrogel as a barrier for prevention of post-operative adhesion. Biomaterials 2011, 32, 4725–4736. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Maji, U.; Khan, G.A.; Das, R.; Sinha, A.K.; Ghosh, C.; Maiti, S. Antidiabetic role of a novel protein from garlic via NO in expression of Glut-4/insulin in liver of alloxan induced diabetic mice. Biomed. Pharmacother. 2019, 111, 1302–1314. [Google Scholar] [CrossRef]
- Dunn, L.; Prosser, H.C.G.; Tan, J.T.M.; Vanags, L.Z.; Ng, M.K.C.; Bursill, C.A. Murine model of wound healing. J. Vis. Exp. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, S.; Nezhad, M.N.; Zarrintaj, P.; Jafari, S.H.; Gholizadeh, S.S.; Saeb, M.R.; Mozafari, M. Chitosan in biomedical engineering: A critical review. Curr. Stem Cell Res. Ther. 2018, 14, 93–116. [Google Scholar] [CrossRef]
- Venkatesh, N. Metallic nanoparticle: A review. Biomed. J. Sci. Tech. Res. 2018, 4, 3765–3775. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Marques, A.P.; Silva, T.H.; Reis, R.L. Evaluation of the potential of collagen from codfish skin as a biomaterial for biomedical applications. Mar. Drugs 2018, 16, 495. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.P.; Wang, Y.J.; Ren, L.; Wu, G.; Caridade, S.G.; Fan, J.B.; Wang, L.Y.; Ji, P.H.; Oliveira, J.M.; Oliveira, J.T.; et al. Genipin-cross-linked collagen/chitosan biomimetic scaffolds for articular cartilage tissue engineering applications. J. Biomed. Mater. Res.-Part A 2010, 95A, 465–475. [Google Scholar] [CrossRef]
- Sundararaghavan, H.G.; Monteiro, G.A.; Lapin, N.A.; Chabal, Y.J.; Miksan, J.R.; Shreiber, D.I. Genipin-induced changes in collagen gels: Correlation of mechanical properties to fluorescence. J. Biomed. Mater. Res.-Part A 2008, 87, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.; Prabakaran, P.; Prasad, E. Augmenting Photoinduced Charge Transport in a Single-Component Gel System: Controlled In Situ Gel–Crystal Transformation at Room Temperature. Chem.-A Eur. J. 2018, 24, 6217–6230. [Google Scholar] [CrossRef]
- Satapathy, S.; Prasad, E. Charge Transfer Modulated Self-Assembly in Poly(aryl ether) Dendron Derivatives with Improved Stability and Transport Characteristics. ACS Appl. Mater. Interfaces 2016, 8, 26176–26189. [Google Scholar] [CrossRef]
- Amorim, P.A.; d’Ávila, M.A.; Anand, R.; Moldenaers, P.; Van Puyvelde, P.; Bloemen, V. Insights on shear rheology of inks for extrusion-based 3D bioprinting. Bioprinting 2021, 22, e00129. [Google Scholar] [CrossRef]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical properties of hydrogels and their experimental determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef]
- Schramm, G. A Practical Approach to Rheology and Rheometry, 2nd ed.; Gebrueder HAAKE GmbH: Karlsruhe, Germany, 1994; ISBN 0.0.010.2-2004. [Google Scholar]
- Wani, T.A.; Masoodi, F.A.; Akhter, R. Preparation and characterization of chitosan flake and chitosan nanopowder gels: A comparative study of rheological, thermal and morphological perspectives. LWT 2021, 148, 111771. [Google Scholar] [CrossRef]
- Porter, G.C.; Schwass, D.R.; Tompkins, G.R.; Bobbala, S.K.R.; Medlicott, N.J.; Meledandri, C.J. AgNP/Alginate Nanocomposite hydrogel for antimicrobial and antibiofilm applications. Carbohydr. Polym. 2021, 251, 117017. [Google Scholar] [CrossRef]
- Lipatova, I.M.; Yusova, A.A.; Losev, N.V.; Indeikin, E.A. Gelation in solutions of low deacetylated chitosan initiated by high shear stresses. Int. J. Biol. Macromol. 2019, 139, 550–557. [Google Scholar] [CrossRef]
- Valluru, M.; Staton, C.A.; Reed, M.W.R.; Brown, N.J. Transforming growth factor-β and endoglin signaling orchestrate wound healing. Front. Physiol. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H. Topical formulations and wound healing applications of chitosan 2. Topical findings of healing with chitosan at early phase of experimental open skin wound. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Heimbuck, A.M.; Priddy-Arrington, T.R.; Padgett, M.L.; Llamas, C.B.; Barnett, H.H.; Bunnell, B.A.; Caldorera-Moore, M.E. Development of Responsive Chitosan-Genipin Hydrogels for the Treatment of Wounds. ACS Appl. Bio Mater. 2019, 2, 2879–2888. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in wound healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, J.; Wu, X.; Ren, Y.; Li, Z.; Ren, J. Controlled release of silver ions from AgNPs using a hydrogel based on konjac glucomannan and chitosan for infected wounds. Int. J. Biol. Macromol. 2020, 149, 148–157. [Google Scholar] [CrossRef]
- Ferreira, M.O.G.; Sá Lima, I.; Ribeiro, A.B.; Lobo, A.O.; Rizzo, M.S.; Osajima, J.A.; Estevinho, L.M.; Silva-Filho, E.C. Biocompatible gels of chitosan-buriti oil for potential wound healing applications. Materials 2020, 13, 1977. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Yamano, S.; Kuo, W.P.; Sukotjo, C. Downregulated gene expression of TGF-βs in diabetic oral wound healing. J. Cranio-Maxillofac. Surg. 2013, 41, e42–e48. [Google Scholar] [CrossRef]
- Lichtman, M.K.; Otero-Vinas, M.; Falanga, V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016, 24, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Ahmad, J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev. Endocr. Metab. Disord. 2019, 20, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Artlett, C.M. Inflammasomes in wound healing and fibrosis. J. Pathol. 2013, 229, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and wound healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Dissemond, J.; Augustin, M.; Dietlein, M.; Faust, U.; Keuthage, W.; Lobmann, R.; Münter, K.C.; Strohal, R.; Stücker, M.; Traber, J.; et al. Efficacy of MMP-inhibiting wound dressings in the treatment of hard-to-heal wounds: A systematic review. J. Wound Care 2020, 29, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Suvik, A.; Effendy, A.W. The use of modified Masson’s trichrome staining in collagen evaluation in wound healing study. Malays. J. Vet. Res. 2012, 3, 39–47. [Google Scholar]
- Mahajan, M.A.; Das, S.; Zhu, H.; Tomic-Canic, M.; Samuels, H.H. The nuclear hormone receptor coactivator NRC is a pleiotropic modulator affecting growth, development, apoptosis, reproduction, and wound repair. Mol. Cell. Biol. 2004, 24, 4994–5004. [Google Scholar] [CrossRef] [PubMed][Green Version]








| N | Gene | Forward Primer | Reverse Primer |
|---|---|---|---|
| 1 | GAPDH | TGCACCACCAACTGCTTAG | GGATGCAGGGATGATGTTC |
| 2 | VEGF | AGGATGTCCTCACTCGGATG | CTGTCCTCTTGACTCAGGGC |
| 3 | TGF-b1 | ACTGGAGTTGTACGGCAGTG | GGGGCTGATCCCGTTGATTT |
| 4 | IL-1b | TGCCACCTTTTGACAGTGATG | GGAGCCTGTAGTGCAGTTGT |
| 5 | MMP-1 | GTGAATGGCAAGGAGATGATGG | ACGAGGATTGTTGTGAGTAATGG |
| 6 | MMP-9 | GGGTCTAGGCCCAGAGGTAA | AGACACGCCCCTTGCTGA |
| 5 | MMP-12 | TGCACTCTGCTGAAAGGAGTC | TGAGTTGTCCAGTTGCCCAG |
| 6 | TIMP-1 | GGACCTGGTCATAAGGGCTA | GGCATATCCACAGAGGCTTT |
| HNO3, % | Mw, kDa | Ip | DD,% | |
|---|---|---|---|---|
| 1 | 6.5 | 28 | 2.00 | 93 |
| 2 | 3.25 | 54 | 2.25 | 93 |
| 3 | 1.95 | 100 | 2.22 | 93 |
| 4 | 0.65 | 700 | 1.20 | 92 |
| Strain | S. epidermidis | S. aureus | E. coli | C. albicans | |
|---|---|---|---|---|---|
| Sample | |||||
| 100 kDa | 125 | >500 | ≥500 | 125 | |
| 700 kDa | 250 | >500 | 500 | 31.25 | |
| Nomenclature | Gel Composition |
|---|---|
| Col | Collagen, 1% solution in acetic acid |
| Ch100 | Chitosan (100 kDa), 1% solution in acetic acid |
| Ch700 | Chitosan (700 kDa), 1% solution in acetic acid |
| Ch100:Col 1:1; Ch100:Col 1:3; Ch100:Col 1:5 | Chitosan (100 kDa) and collagen in ratios 1:1, 1:3, 1:5 (mass) |
| Ch700:Col 1:1; Ch700:Col 1:3; Ch700:Col 1:5 | Chitosan (700 kDa) and collagen in ratios 1:1, 1:3, 1:5 (mass) |
| Col-G | Collagen crosslinked with genipin |
| Ch100-G | Chitosan (100 kDa) crosslinked with genipin |
| Ch700-G | Chitosan (700 kDa) crosslinked with genipin |
| Ch100:Col 1:1-G; Ch100:Col 1:3-G; Ch100:Col 1:5-G | Chitosan (100 kDa) and collagen in ratios 1:1, 1:3, 1:5 (mass) crosslinked with genipin |
| Ch700:Col 1:1-G; Ch700:Col 1:3-G; Ch700:Col 1:5-G | Chitosan (700 kDa) and collagen in ratios 1:1, 1:3, 1:5 (mass) crosslinked with genipin |
| Ch700:Col 1:1NPs-G | Chitosan (700 kDa) and collagen in 1:1 ratios with silver nanoparticles synthesized in a medium of quaternized chitosan with gallic acid, crosslinked with genipin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shagdarova, B.; Konovalova, M.; Zhuikova, Y.; Lunkov, A.; Zhuikov, V.; Khaydapova, D.; Il’ina, A.; Svirshchevskaya, E.; Varlamov, V. Collagen/Chitosan Gels Cross-Linked with Genipin for Wound Healing in Mice with Induced Diabetes. Materials 2022, 15, 15. https://doi.org/10.3390/ma15010015
Shagdarova B, Konovalova M, Zhuikova Y, Lunkov A, Zhuikov V, Khaydapova D, Il’ina A, Svirshchevskaya E, Varlamov V. Collagen/Chitosan Gels Cross-Linked with Genipin for Wound Healing in Mice with Induced Diabetes. Materials. 2022; 15(1):15. https://doi.org/10.3390/ma15010015
Chicago/Turabian StyleShagdarova, Balzhima, Mariya Konovalova, Yuliya Zhuikova, Alexey Lunkov, Vsevolod Zhuikov, Dolgor Khaydapova, Alla Il’ina, Elena Svirshchevskaya, and Valery Varlamov. 2022. "Collagen/Chitosan Gels Cross-Linked with Genipin for Wound Healing in Mice with Induced Diabetes" Materials 15, no. 1: 15. https://doi.org/10.3390/ma15010015
APA StyleShagdarova, B., Konovalova, M., Zhuikova, Y., Lunkov, A., Zhuikov, V., Khaydapova, D., Il’ina, A., Svirshchevskaya, E., & Varlamov, V. (2022). Collagen/Chitosan Gels Cross-Linked with Genipin for Wound Healing in Mice with Induced Diabetes. Materials, 15(1), 15. https://doi.org/10.3390/ma15010015






