The Effect of AlI3 Nanoadditive on the Thermal Behavior of PMMA Subjected to Thermoanalytical Py-GC-MS Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Poly (Methyl Methacrylate)
2.3. Synthesis of PMMA Composite with AlI3
2.4. Preparation of Strip for HBT
2.5. Horizontal Burning Test (HBT)
2.6. Pyrolytic Study of PMMA Composite
2.7. Instrumentation
3. Results
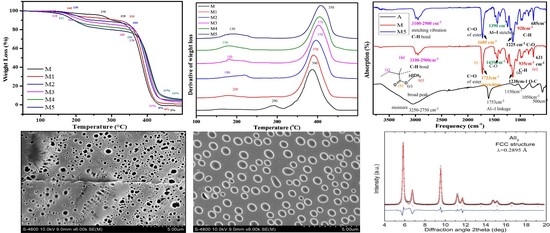
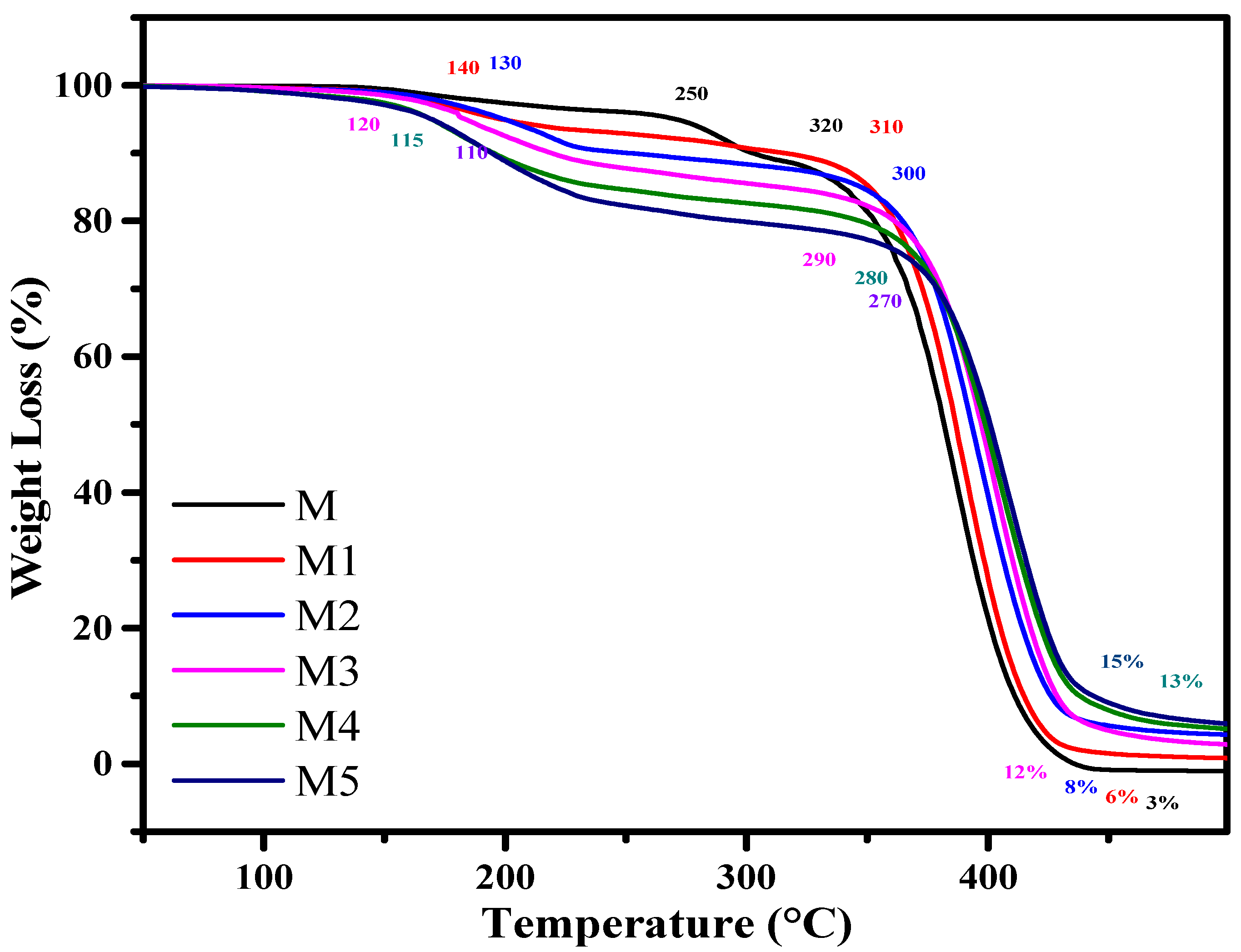
3.1. Thermoanalytical Study
3.2. FTIR Analysis
3.3. Py-GC-MS Analysis
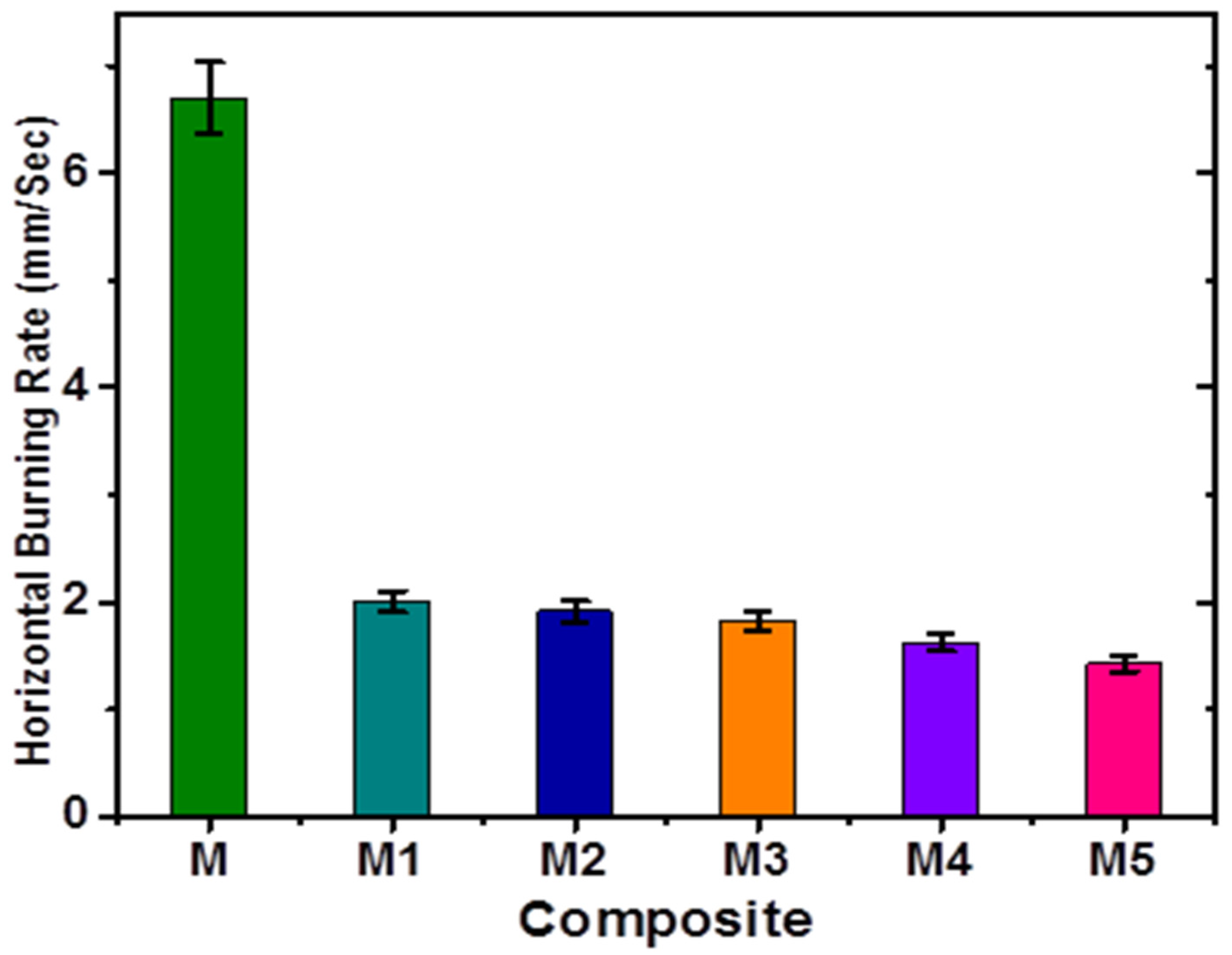
3.4. Horizontal Burning Test
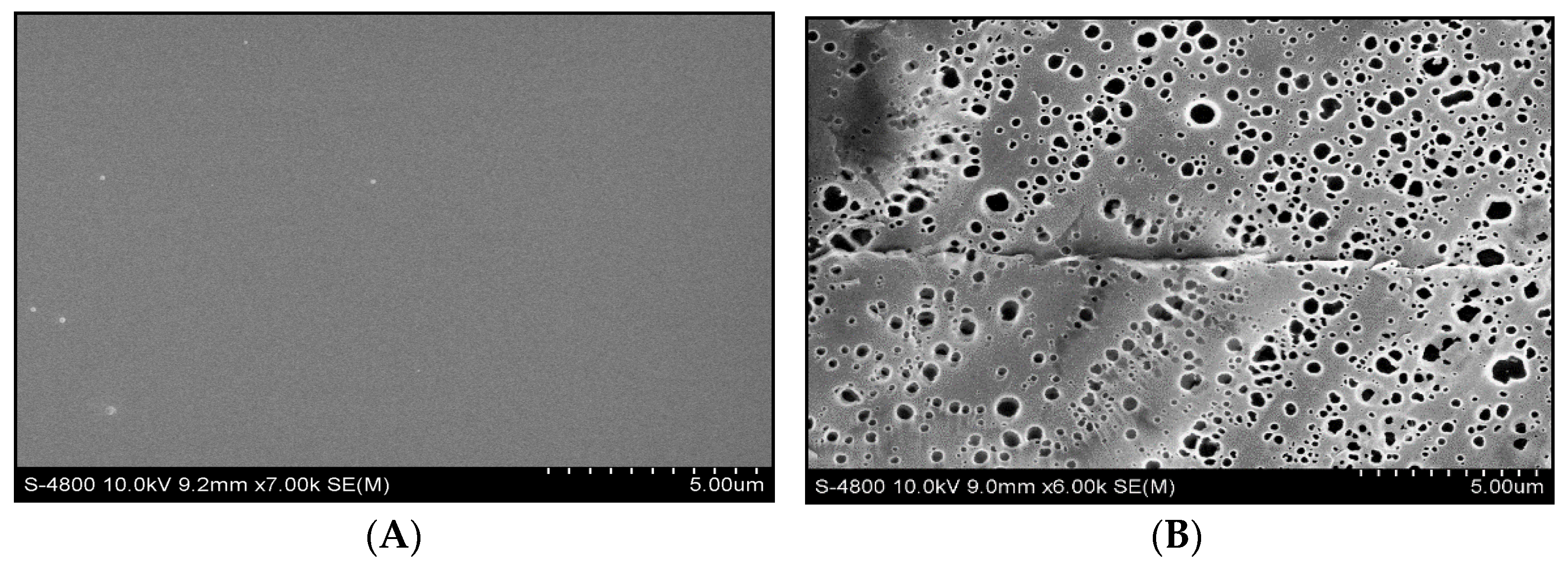
3.5. Morphology Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Attar, T. Current Review on Synthesis, Composites and Multifunctional Properties of Graphene. Top. Curr. Chem. 2019, 377, 1–45. [Google Scholar]
- Jones, M.; Bhat, T.; Kandare, E.; Thomas, A.; Joseph, P.; Dekiwadia, C.; Wang, C.H. Thermal degradation and fire properties of fungal mycelium and mycelium-biomass composite materials. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Villetti, M.; Crespo, J.; Soldi, M.; Pires, A.; Borsali, R.; Soldi, V. Thermal degradation of natural polymers. J. Therm. Anal. Calorim. 2002, 67, 295–303. [Google Scholar] [CrossRef]
- Zhou, X.X.; Hao, L.T.; Wang, H.Y.Z.; Li, Y.J.; Liu, J.F. Cloud-point extraction combined with thermal degradation for nanoplastic analysis using pyrolysis gas chromatography–mass spectrometry. Anal. Chem. 2018, 91, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Prado, A.R.; Leal-Junior, A.G.; Marques, C.; Leite, S.; De Sena, G.L.; Machado, L.C.; Pontes, M.J. Polymethyl methacrylate (PMMA) recycling for the production of optical fiber sensor systems. Opt. Express 2017, 25, 30051–30060. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Masud, K.; Ameer, Q. Thermal degradation behavior ofblends of phenyl methacrylate-styrene copolymers with aluminum isopropoxide. J. Therm. Anal. Calorim. 2003, 73, 877–886. [Google Scholar] [CrossRef]
- Nikolaidis, A.K.; Achilias, D.S. Thermal degradation kinetics and viscoelastic behavior of poly (methyl methacrylate)/organomodified montmorillonite nanocomposites prepared via in situ bulk radical polymerization. Polymers 2018, 10, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nampoothiri, P.K.; Gandhi, M.N.; Kulkarni, A.R. Elucidating the stabilizing effect of oleic acid coated LaF3: Nd3+ nanoparticle surface in the thermal degradation of PMMA nanocomposites. Mater. Chem. Phys. 2017, 190, 45–52. [Google Scholar] [CrossRef]
- Godiya, C.B.; Gabrielli, S.; Materazzi, S.; Pianesi, M.S.; Stefanini, N.; Marcantoni, E. Depolymerization of waste poly (methyl methacrylate) scraps and purification of depolymerized products. J. Environ. Manag. 2019, 231, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- AL-Harbi, M.; Alshaiban, A.; Yassin, M.F.; Elmi, A. Kinetic analysis and modelling of thermal degradation of perspex (PMMA) and perspex blend plastic waste. Can. J. Chem. Eng. 2012, 91, 1281–1288. [Google Scholar] [CrossRef]
- García-Garrido, C.; Pérez-Maqueda, L.A.; Criado, J.M.; Sánchez-Jiménez, P.E. Combined kinetic analysis of multistep processes of thermal decomposition of polydimethylsiloxane silicone. Polymer 2018, 153, 558–564. [Google Scholar] [CrossRef]
- Rosli, N.A.; Ahmad, I.; Anuar, F.H.; Abdullah, I. Application of polymethylmethacrylate-grafted cellulose as reinforcement for compatibilised polylactic acid/natural rubber blends. Carbohydr. Polym. 2019, 213, 50–58. [Google Scholar] [CrossRef]
- Ozdemir, E.; Hacaloglu, J. Poly (methyl methacrylate) organoclay composites; interactions of organic modifier with the polymer effecting thermal degradation behavior. Eur. Polym. J. 2017, 95, 474–481. [Google Scholar] [CrossRef]
- Nenonen, V.; Sammaljärvi, J.; Johanson, B.; Voutilainen, M.; Dick, P.; Siitari-Kauppi, M. Porosity distribution in a heterogeneous clay-rich fault core by image processing of 14 C-PMMA Autoradiographs and Scanning Electron Microscopy. MRS Adv. 2018, 3, 1167–1173. [Google Scholar] [CrossRef] [Green Version]
- Ozkutlu, M.; Dilek, C.; Bayram, G. Effects of hollow glass microsphere density and surface modification on the mechanical and thermal properties of poly (methyl methacrylate) syntactic foams. Compos. Struct. 2018, 202, 545–550. [Google Scholar] [CrossRef]
- Durkin, D.P.; Gallagher, M.J.; Frank, B.P.; Knowlton, E.D.; Trulove, P.C.; Fairbrother, D.H.; Fox, D.M. Phosphorus-functionalized multi-wall carbon nanotubes as flame-retardant additives for polystyrene and poly (methyl methacrylate). J. Therm. Anal. Calorim. 2017, 130, 735–753. [Google Scholar] [CrossRef]
- Aouachria, K.; Massardier, V.; Benaniba, M.T.; Belhaneche-Bensemra, N. Evaluation of the effects of acetyl tributyl citrate as bio-based plasticizer on the physical, thermal and dynamical mechanical properties of poly (vinyl chloride)/polymethyl methacrylate blends. J. Vinyl Addit. Technol. 2019, 25, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Wijesinghe, W.L.; Mantilaka, M.M.M.G.P.G.; Karunarathne, T.S.E.F.; Rajapakse, R.M.G. Synthesis of a hydroxyapatite/poly (methyl methacrylate) nanocomposite using dolomite. Nanoscale Adv. 2019, 1, 86–88. [Google Scholar] [CrossRef] [Green Version]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A review of the properties and applications of poly (methyl methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Alkelaby, A.S.; Abass, K.H.; Mubarak, T.H.; Habubi, N.F.; Chiad, S.S.; Al-Baidhany, I. Effect of MnCl2 additive on optical and dispersion parameters of poly methyl methacrylate films. J. Glob. Pharma Technol. 2019, 11, 347–352. [Google Scholar]
- Bahadur, A.; Shoaib, M.; Iqbal, S.; Saeed, A.; ur Rahman, M.S.; Channar, P.A. Regulating the anticancer drug release rate by controlling the composition of waterborne polyurethane. React. Funct. Polym. 2018, 131, 134–141. [Google Scholar] [CrossRef]
- Arshad, M.; Masud, K.; Arif, M.; Rehman, S.U.; Saeed, A.; Zaidi, J.H. Characterization of Poly (methyl methacrylate)-tin (IV) Chloride Blend by TG-DTG-DTA, IR and Pyrolysis-GC-MS Techniques. Bull. Korean Chem. Soc. 2011, 32, 3295–3305. [Google Scholar] [CrossRef] [Green Version]
- Bahadur, A.; Saeed, A.; Shoaib, M.; Iqbal, S.; Anwer, S. Modulating the burst drug release effect of waterborne polyurethane matrix by modifying with polymethylmethacrylate. J. Appl. Polym. Sci. 2019, 136, 47253. [Google Scholar] [CrossRef]
- Bahadur, A.; Shoaib, M.; Saeed, A.; Iqbal, S. FT-IR spectroscopic and thermal study of waterborne polyurethane-acrylate leather coatings using tartaric acid as an ionomer. e-Polymer 2016, 16, 463–474. [Google Scholar] [CrossRef]



| Ingredients | Composite Mix Number | |||||
|---|---|---|---|---|---|---|
| M | M1 | M2 | M3 | M4 | M5 | |
| PMMA (%) | 100 | 98 | 96 | 94 | 92 | 90 |
| AlI3 (%) | - | 2 | 4 | 6 | 8 | 10 |
| Sample | All3% | Stage | Temperature Range (°C) | Weight Loss (%) | T0 | T25 | T50 | Tmax |
|---|---|---|---|---|---|---|---|---|
| M | 0 | I | 250–320 | 10 | ||||
| II | 320–450 | 90 | 250 | 390 | 400 | 450 | ||
| M1 | 2 | I | 140–250 | 10 | 140 | 380 | 390 | 460 |
| II | 250–460 | 87 | ||||||
| Resi. | >460 | 3 | ||||||
| M2 | 4 | I | 130–240 | 12 | 130 | 370 | 380 | 460 |
| II | 240–460 | 82 | ||||||
| Resi. | >470 | 6 | ||||||
| M3 | 6 | I | 120–250 | 14 | 120 | 360 | 370 | 470 |
| II | 250–470 | 78 | ||||||
| Resi. | >470 | 8 | ||||||
| M4 | 8 | I | 115–260 | 16 | 115 | 340 | 350 | 470 |
| II | 260–470 | 72 | ||||||
| Resi. | >470 | 12 | ||||||
| M5 | 10 | I | 110–270 | 18 | 110 | 320 | 390 | 475 |
| II | 270–475 | 67 | ||||||
| Resi. | >475 | 15 | ||||||
| AlI3 | I | 60–160 | 20 | 60 | 150 | 200 | 490 | |
| II | 165–490 | 72 | ||||||
| Resi. | >490 | 8 |
| Peak | R.T (mint.) | MS (m/e) | Fragments Identified |
|---|---|---|---|
| 1 | 3.9 | 210, 183, 167, 143, 140, 127 | C3H4OIAl |
| 2 | 4.8 | 254, 239, 225, 210, 194, 179, 52 | C8H15OI |
| 3 | 5.2 | 253, 252, 221, 193, 180, 167, 40 | C7H11O2I |
| 4 | 11.2 | 347, 314, 298, 267, 235, 209, 194, 179, 162 | C6H9O7IAl |
| 5 | 18.1 | 480, 449, 322, 308, 294, 277, 206, 249, 221 | C10H15O4I2Al |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adnan, M.; Rahman, T.U.; Bahadur, A.; Zeb, M.A.; Liaqat, W.; Akitsu, T.; Abdel-Hafez, S.H.; El-Sayed, W.A. The Effect of AlI3 Nanoadditive on the Thermal Behavior of PMMA Subjected to Thermoanalytical Py-GC-MS Technique. Materials 2021, 14, 7036. https://doi.org/10.3390/ma14227036
Adnan M, Rahman TU, Bahadur A, Zeb MA, Liaqat W, Akitsu T, Abdel-Hafez SH, El-Sayed WA. The Effect of AlI3 Nanoadditive on the Thermal Behavior of PMMA Subjected to Thermoanalytical Py-GC-MS Technique. Materials. 2021; 14(22):7036. https://doi.org/10.3390/ma14227036
Chicago/Turabian StyleAdnan, Muhammad, Taj Ur Rahman, Ali Bahadur, Muhammad Aurang Zeb, Wajiha Liaqat, Takashiro Akitsu, Shams H. Abdel-Hafez, and Wael A. El-Sayed. 2021. "The Effect of AlI3 Nanoadditive on the Thermal Behavior of PMMA Subjected to Thermoanalytical Py-GC-MS Technique" Materials 14, no. 22: 7036. https://doi.org/10.3390/ma14227036
APA StyleAdnan, M., Rahman, T. U., Bahadur, A., Zeb, M. A., Liaqat, W., Akitsu, T., Abdel-Hafez, S. H., & El-Sayed, W. A. (2021). The Effect of AlI3 Nanoadditive on the Thermal Behavior of PMMA Subjected to Thermoanalytical Py-GC-MS Technique. Materials, 14(22), 7036. https://doi.org/10.3390/ma14227036