Physico-Chemical Properties of NaV3O8 Prepared by Solid-State Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Samples Characterization
2.3. Measurement of Conductivity
3. Results and Discussion
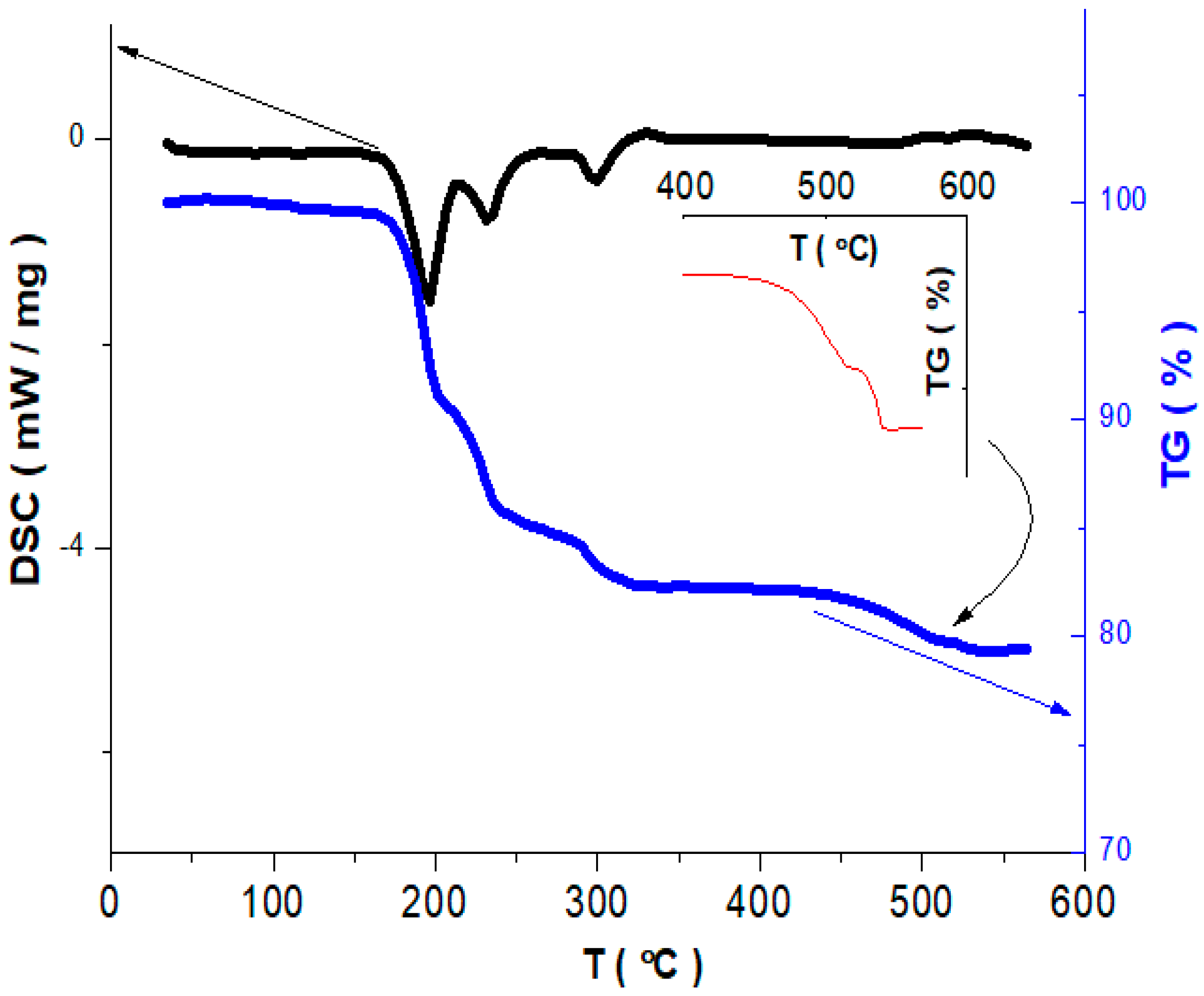
3.1. Synthesis of Sodium–Vanadium Oxide NaV3O8
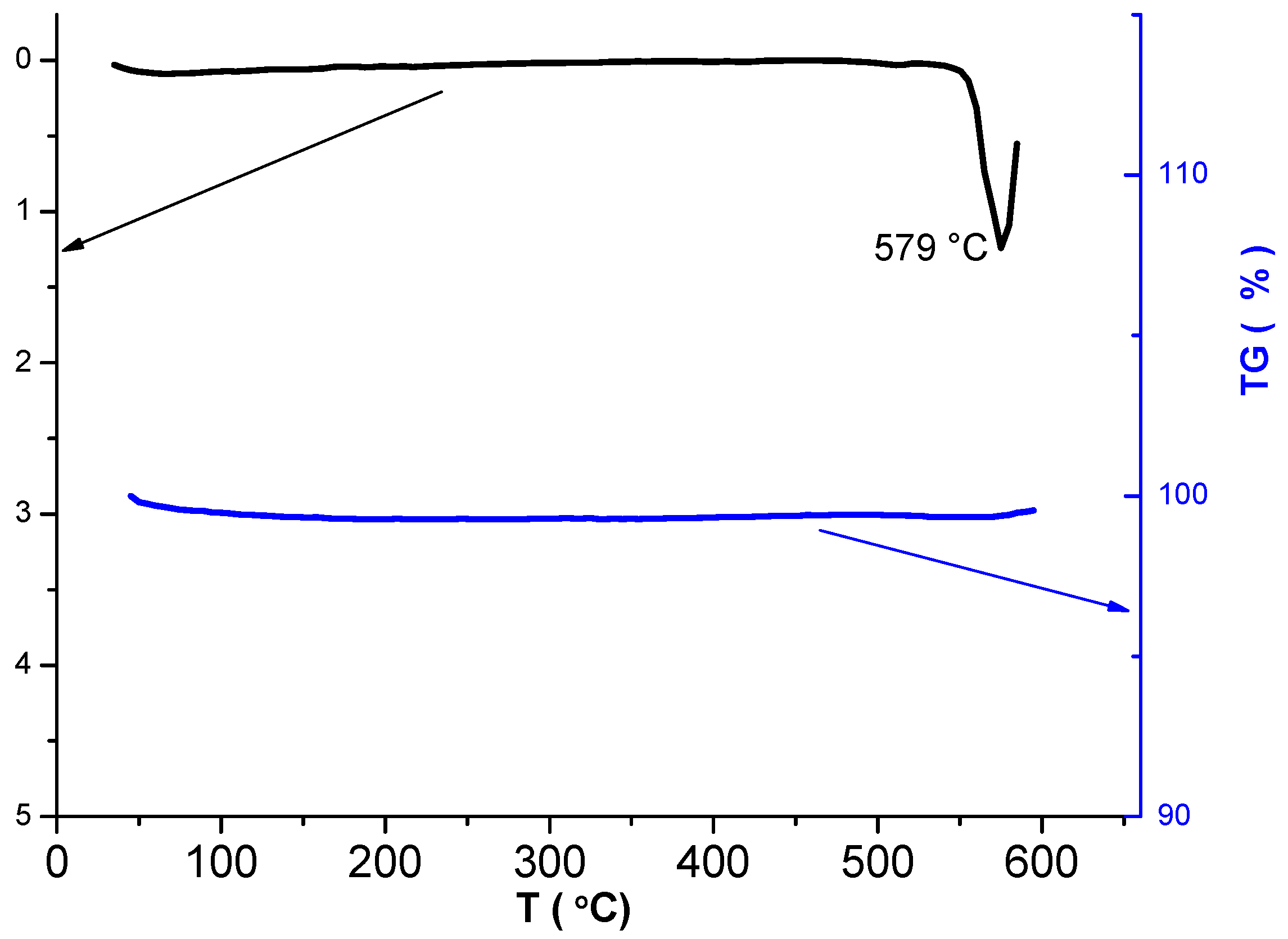
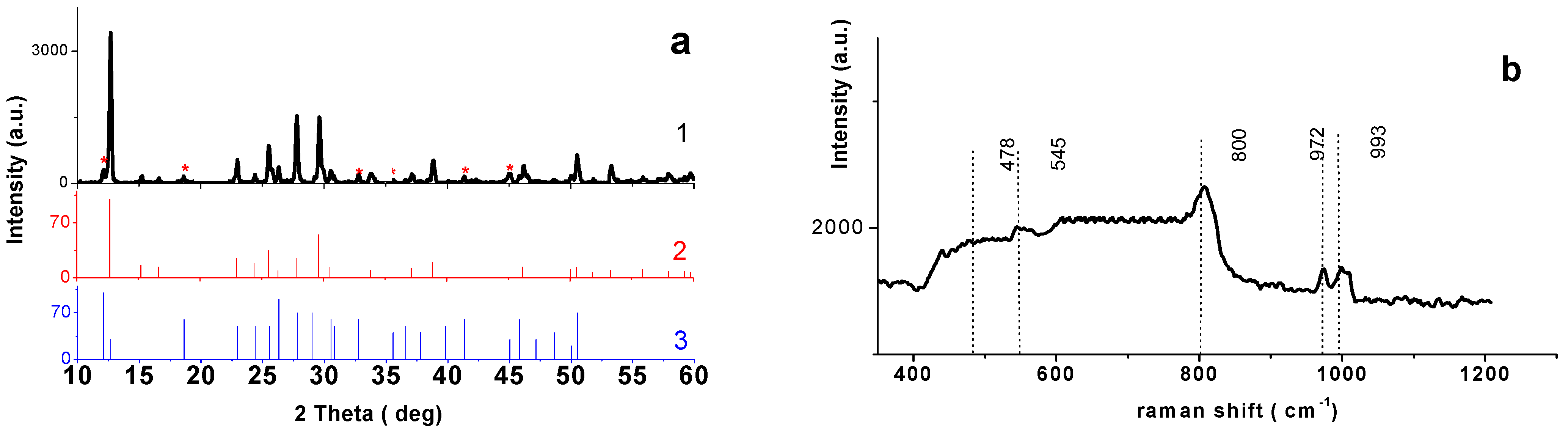
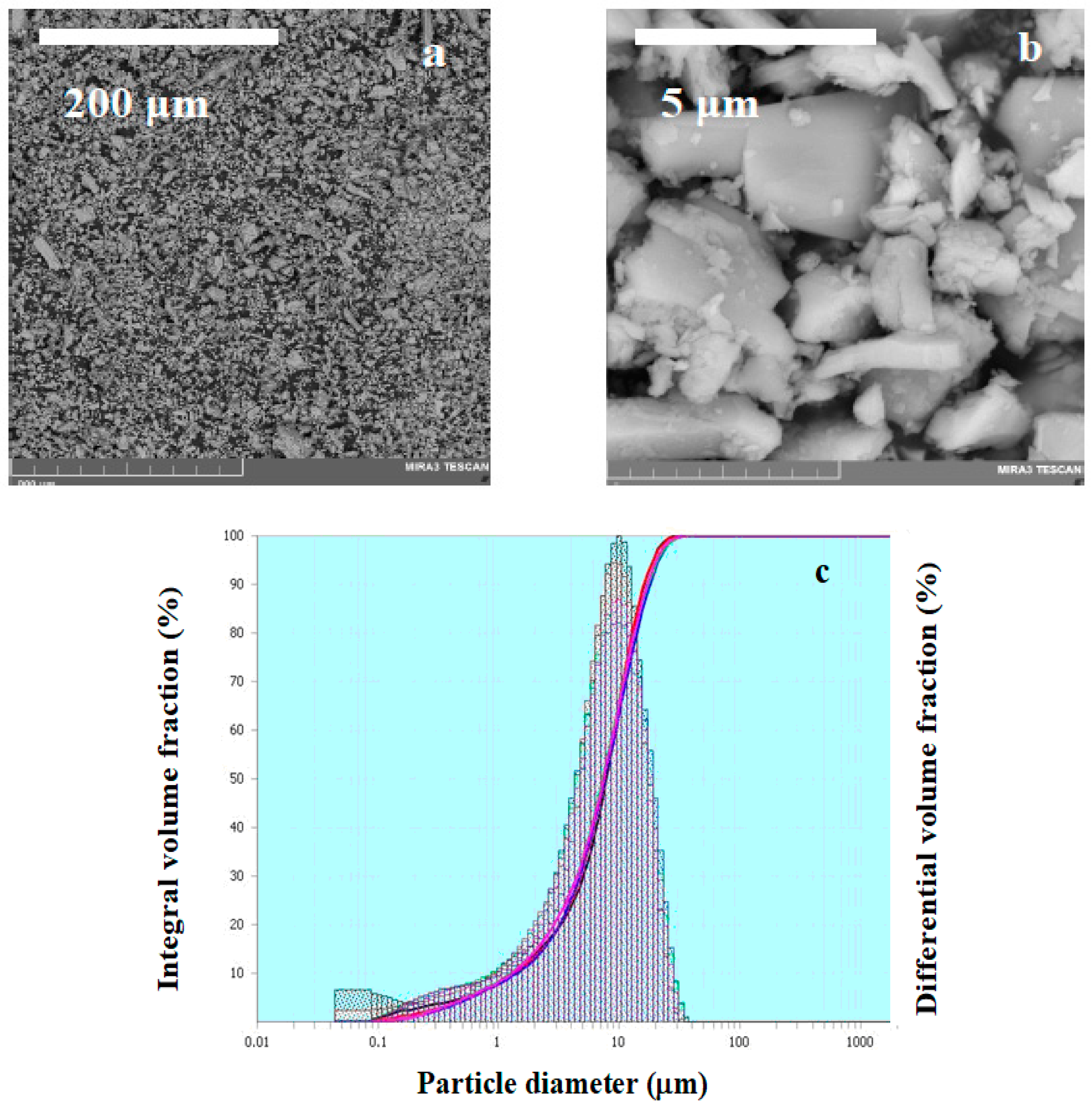
3.2. Phase Composition and Microstructure of NaV3O8
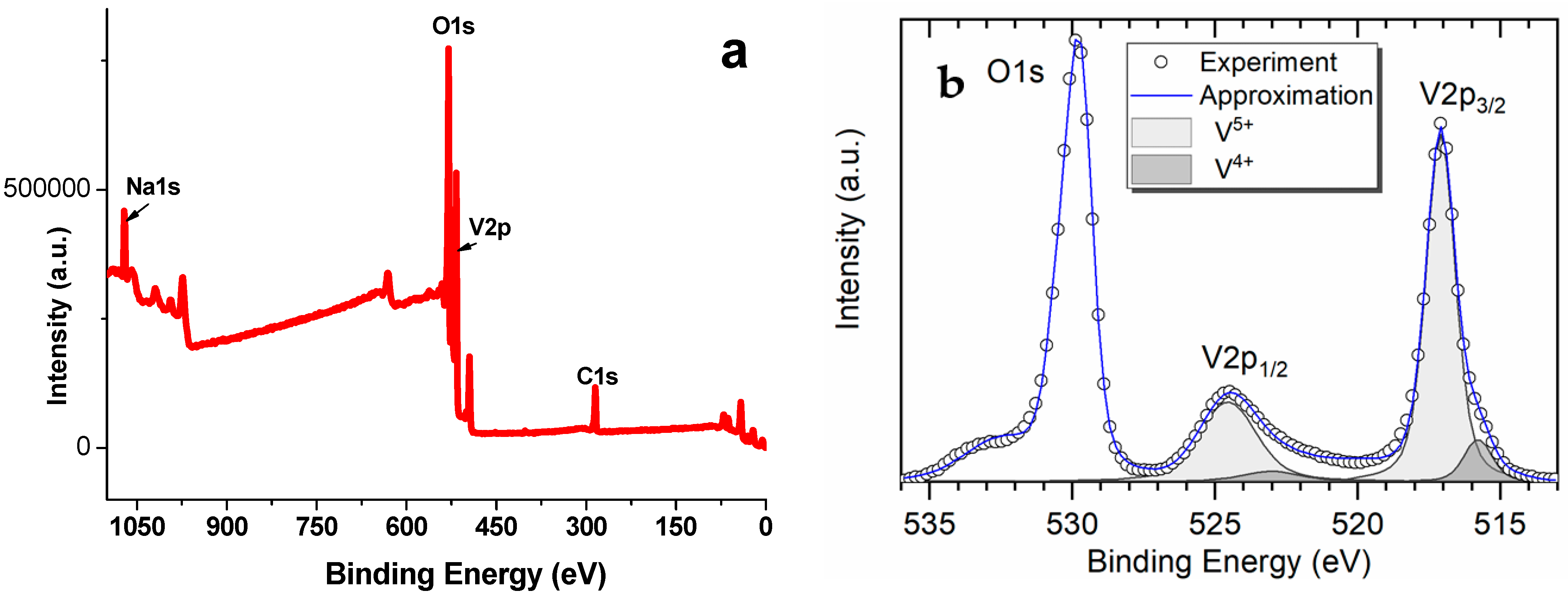
3.3. Determining the Content of V4+ Ions in the Synthesized NaV3O8; Conductivity Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium ion batteries: Present and future. Chem. Soc. Rew. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [Green Version]
- Skundin, A.M.; Kulova, T.L.; Yaroslavtsev, A.B. Sodium-Ion Batteries (A Review). Russ. J. Electrochem. 2018, 54, 113–152. [Google Scholar] [CrossRef]
- Zhou, C.; Bag, S.; Thangadurai, V. Engineering Materials for Progressive All-Solid-State Na Batteries. ACS Energy Lett. 2018, 3, 2181–2198. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Qi, X.; Lu, Y.; Wu, F.; Zhao, J.; Yu, Y.; Hu, Y.-S.; Chen, L. Solid State Sodium Batteries. Adv. Energy Mater. 2018, 8, 1703012. [Google Scholar] [CrossRef]
- Mutta, G.R.; Popuri, S.R.; Ruterana, P.; Buckman, J. Single step hydrothermal synthesis of mixed valent V6O13 nano-architectures: A case study of the possible applications in electrochemical energy conversion. J. Alloy Comp. 2017, 706, 562–567. [Google Scholar] [CrossRef]
- Chernova, N.A.; Roppolo, M.; Dillon, A.C.; Wittingham, M.S. Layered vanadium and molybdenum oxides: Batteries and electrochromics. J. Mater. Chem. 2009, 19, 2526–2552. [Google Scholar] [CrossRef]
- Wang, H.L.; Bi, X.X.; Bai, J.; Wu, C.; Gu, S.-C.; Chen, S.; Wu, F.; Amine, K.; Lu, J. Open-structured V2O5·nH2O nanoflakes as highly reversible cathode material for monovalent and multivalent intercalation batteries. Adv. Energy Mater. 2017, 7, 1602720. [Google Scholar] [CrossRef]
- Wittingham, M.S. Lithium Batter and Cathode Materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef] [PubMed]
- Bensalah, N.; Dawood, H. Revieaw on Synthesis, Characterization and Electrochemical Properties of Cathode Materials for Lithium Ion Batteries. J. Mater. Sci. 2016, 5, 258. [Google Scholar] [CrossRef] [Green Version]
- McNulty, D.; Bucley, D.N.; O’Dwyer, C. Synthesis and electrochemical properties of vanadium oxide materials and structures as Li-ion battery positive electrodes. J. Power Sources 2014, 267, 831–873. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, J.; Zhang, W.; Mao, M.; Wei, Z.; Wang, L.; Cui, C.; Zhu, Y.; Ma, J. Research progress on vanadium-based cathode materials for sodium ion batteries. J. Mater. Chem. A 2018, 19, 8815–8838. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, H.; Chen, L.; Tang, Z.; Yang, W. Electrochemical insertion/deinsertion of sodium on NaV6O15 nanorods as cathode material of rechargeable sodium-based batteries. J. Power Sources 2011, 196, 814–819. [Google Scholar] [CrossRef]
- Nagaraju, G.; Sarkar, S.; Dupont, J.; Sampath, S. Na0,33V2O5·1,5H2O nanorings/nanorods and Na0,33V2O5·1,5H2O/RGO composite fabricated by facile one pot synthesis and its lithium storage behavior. Solid State Ionics 2012, 227, 30–38. [Google Scholar] [CrossRef]
- Venkatesh, G.; Pralong, V.; Lebedev, O.; Caignaert, V.; Bazin, P.; Raveau, B. Amorphous sodium vanadate Na1,5+yVO3, a promising matrix for reversible sodium intercalation. Electrochem. Commun. 2014, 40, 100–102. [Google Scholar] [CrossRef]
- Nguyen, D.; Gim, J.; Mathew, V.; Song, J.; Kim, S.; Ahn, D.; Kim, J. Plate-Type NaV3O8 Cathode by Solid State Reaction for Sodium-Ion Batteries. ECS Electrochem. Lett. 2014, 3, A69–A71. [Google Scholar] [CrossRef]
- Zhu, L.; Li, W.; Xie, L.; Yang, Q.; Cao, X. Rod-like NaV3O8 as cathode materials with high capacity and stability for sodium storage. Chem. Eng. J. 2019, 372, 1056–1065. [Google Scholar] [CrossRef]
- He, H.; Jin, G.; Wang, H.; Huang, X.; Chen, Z.; Sunaand, D.; Tanga, Y. Annealed NaV3O8 nanowires with good cycling stability as a novel cathode for Na-ion batteries. J. Mater. Chem. A 2014, 2, 3563–3570. [Google Scholar] [CrossRef]
- Hu, F.; Xie, D.; Cui, F.; Zhang, D.; Song, G. Synthesis and electrochemical performance of NaV3O8 nanobelts for Li/Na-ion batteries and aqueous zinc-ion batteries. RCS Adv. 2019, 9, 20549. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Liu, Y.; Shang, M.; Lu, T.; Wang, Y.; Jiao, L. NaVO3 Nanosheets@Polypirrole Core-Shell Composites with Good Electrochemical Performance as Cathodes for Na-Ion Batteries. Nanoscale 2015, 7, 9261. [Google Scholar] [CrossRef]
- Shchelkanova, M.S.; Shekhtman, G.S.; Pershina, S.V.; Pankratov, A.A.; Khodimchuk, A.V.; Pryakhina, V.I. The study of sodium-vanadium oxide NaV3O8 as an electrode material for all-solid-state sodium-ion batteries. J. Alloys Compd. 2021, 864, 158516. [Google Scholar] [CrossRef]
- Range, K.-J.; Zintl, R.; Heyns, A.M. The Thermal Decomposition of Ammonium Metavanadate in Open and Closed Systems. Z. Naturforsch. 1988, 43, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Fotiev, A.A.; Slobodin, B.V.; Hodos, M.; Vanadaty, Y. Sostav, Sintez, Struktura, Svoistva (Vanadates. Composition, Synthesis, Structure, Properties); Nauka: Moscow, Russia, 1988. [Google Scholar]
- Zavaliy, P.Y.; Wittingham, M.S. Structural chemistry of vanadium oxides with open frameworks. Acta Crystallogr. 1999, 55, 627–663. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Yu, J.C. Large-scale in situ synthesis and characterization of ternary single-crystal NaV6O15 nanoneedles. J. Mater. Chem. Phys. 2007, 104, 362–366. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Liang, C.; Cai, Y.; Pan, A.; Tan, X.; Tang, Y.; Liang, S. General synthesis of three-dimensional alkali metal vanadate aerogels with superior lithium storage properties. J. Mater. Chem. A 2016, 4, 14408. [Google Scholar] [CrossRef]
- Zhang, X.; Frech, R. Spectroscopic investigation of Li1+xV3O8. Electrochim. Acta 1998, 43, 861–868. [Google Scholar] [CrossRef]
- West, K.; Zachau-Christiansen, B.; Ostergard, M.J.L.; Jacobsen, T. Vanadium Oxides as Electrode Materials for Rechargeable Lithium Cells. J. Power Sources 1987, 20, 165–172. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. J. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Sawatzki, G.A.; Post, D. X-ray fotoelectron and Auger spectroscopy study of some vanadium oxides. Phys. Rev. B 1979, 20, 1546–1555. [Google Scholar] [CrossRef]
- Shchelkanova, M.S.; Shekhtman, G.S.; Druzhinin, K.V.; Pankratov, A.A.; Pryakhina, V.I. The study of lithium vanadium oxide LiV3O8 as an electrode material for all-solid-state lithium-ion batteries with solid electrolyte Li3,4Si0,4P0,6O4. Electrochim. Acta 2019, 320, 134570. [Google Scholar] [CrossRef]
- Kawakita, J.; Miura, T.; Kishi, T. Comparison of Na1+xV3O8 with Li1+xV3O8 as lithium insertion host. Solid State Ion. 1999, 124, 21–28. [Google Scholar] [CrossRef]
- Kawakita, J.; Miura, T.; Kishi, T. Effect of crystallinity on lithium insertion behavior of Na1+xV3O8. Solid State Ion. 1999, 124, 29–35. [Google Scholar] [CrossRef]

| Physico-Chemical Properties | Composition | |||
|---|---|---|---|---|
| NaV3O8 Solid-State Synthesis | NaV3O8 Aqueous Solution Technique [21] | LiV3O8 Solid-State Synthesis [31] | LiV3O8 Aqueous Solution Technique [31] | |
| particle size | 1–10 μm | 100 nm | 1–10 μm | 200 nm |
| conductivity, S × cm−1 | 6.3 × 10−2 | 3.2 × 10−2 | 7.9 × 10−2 | 2.5 × 10−2 |
| V4+ content, % | 8 | 7 | 5 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shchelkanova, M.; Shekhtman, G.; Pershina, S.; Vovkotrub, E. Physico-Chemical Properties of NaV3O8 Prepared by Solid-State Reaction. Materials 2021, 14, 6976. https://doi.org/10.3390/ma14226976
Shchelkanova M, Shekhtman G, Pershina S, Vovkotrub E. Physico-Chemical Properties of NaV3O8 Prepared by Solid-State Reaction. Materials. 2021; 14(22):6976. https://doi.org/10.3390/ma14226976
Chicago/Turabian StyleShchelkanova, Mariya, Georgiy Shekhtman, Svetlana Pershina, and Emma Vovkotrub. 2021. "Physico-Chemical Properties of NaV3O8 Prepared by Solid-State Reaction" Materials 14, no. 22: 6976. https://doi.org/10.3390/ma14226976
APA StyleShchelkanova, M., Shekhtman, G., Pershina, S., & Vovkotrub, E. (2021). Physico-Chemical Properties of NaV3O8 Prepared by Solid-State Reaction. Materials, 14(22), 6976. https://doi.org/10.3390/ma14226976





