1. Introduction
Lithium zirconates are a subject of wide-ranging studies during the last years due to the wide range of their practical applications. In particular, their feasibility of using as tritium breeder materials in thermonuclear fusion reactors [
1,
2,
3], and carbon dioxide sorbents [
4,
5], has been demonstrated. Besides, lithium zirconates are thermodynamically stable against Li [
6], so may be used in lithium power sources. So, attempts have been made to use lithium zirconates as component of electrode mass [
7,
8,
9,
10,
11] and solid lithium conducting electrolytes [
12,
13] for various types of lithium batteries. For effective application of lithium zirconates data on their conductivity is of great importance. However, data on lithium zirconates conduction and influence of doping are rather scarce and literature data are contradictory.
According to [
14,
15], in Li
2O–ZrO
2 system form following compounds: Li
2ZrO
3, Li
6Zr
2O
7, and Li
8ZrO
6. Initial study of Li
2ZrO
3 and Li
8ZrO
6 conductivity revealed that their Li
+-ion conductivity is 10
−3–10
−4 S·cm
−1 at 400 °C and due to their high activation energy they show quick lowering with drop of temperature [
12,
16]. In more recent publications it was reported that Li
2ZrO
3 near 430 °C transforms into superionic state with sharp rise in ionic conductivity and a heavy drop in activation energy from 98.4 to 13.8 kJ·mol
−1 [
17]. A jump-like growth in Li ion conductivity at 400–470 °C was also recorded for Li
8ZrO
6 [
18]. For that reason, the authors supposed that both compounds transform into superionic state with disordering of Li
+ sublattice. However, efforts to stabilize high-temperature phases at room temperature by doping, were not successful [
19,
20]. Besides that, the heavy rise in Li
2ZrO
3 conductivity at 430 °C was not reproduced later [
13].
Li
+ ion conductivity of Li
6Zr
2O
7 was first measured in [
12]. The authors aimed to synthesize Li
4ZrO
4, but later investigations show that lithium orthozirconate does not exist, and received X-ray patterns earlier ascribed to Li
4ZrO
4 [
21] belong to Li
6Zr
2O
7 [
15]. The conductivity of Li
6Zr
2O
7 measured in [
12] is rather low: 3.0 × 10
−6 S·cm
−1 at 300 °C. However, subsequently it was found that the conductivity of Li
6Zr
2O
7 can be considerably increased by heterovalent doping. Thus, when lithium is substituted for divalent magnesium or zinc cations, the conductivity of the resulting solid electrolytes is 10
−4 at 300 °C [
22]. A more considerable conductivity growth is observed when Zr
4+ ions are substituted for pentavalent cations (Nb
5+ or Ta
5+) with the positive charge excess compensation by lithium vacancies. Lithium-cation conductivity of Li
5.85Zr
1.85Ta
0.15O
7 solid solution is above 10
−3 S·cm
−1 at 300 °C [
23], (
Table 1).
On substitution of Li
+ by double charged ions and on substitution of Zr
4+ by pentavalent ions Nb
5+ or Ta
5+, charge compensation is accomplished by the creation of lithium vacancies. Electromigration in that kind of solid solutions is realized via the vacancy mechanism, i.e., lithium vacancies are the charge carriers. The amount of vacancies increases with the concentration of the dopant and depends on the range of the single phase region. However, the work [
23], investigates only two compositions of solid electrolytes: Li
5.85Zr
1.85A
0.15O
7 (A = Nb, Ta). Such high conductivity values obtained in the initial studies give reason to extend study of the conductivity of Li
6Zr
2O
7-based solid solutions in Li
6-xZr
2-xNb
xO
7 and Li
6-xZr
2-xTa
xO
7 systems in the wide concentration region.
The present paper investigates the effect the concentration of pentavalent doping cations in Li
6-xZr
2-xA
xO
7 (A = Nb, Ta) systems has on the lithium-cation conductivity of Li
6Zr
2O
7. It is assumed that when A
5+ ions replace Zr
4+, the compensation of the charge imbalance by lithium vacancies can be described as
2. Materials and Methods
Li2CO3 (reagent grade), Zr(OH)2CO3·5.5H2O (analytical grade), and Nb2O5 or Ta2O5 (analytical grade) all VECTON RF were used as starting reagents to synthesize the substances under study. Lithium carbonate was previously dried at 300 °C, niobium and tantalum oxides were annealed at 1000 °C.
Several methods of Li
6Zr
2O
7 synthesis are described in literature: solid state reaction [
15,
24,
25,
26], co-precipitation of hydroxides [
5], a combined method [
27]. In [
24], solid solutions based on Li
6Zr
2O
7 were produced via urea combustion followed by heat treatment. A similar method is used in the present work, except for the fact that instead of urea we use glycine.
The required quantities of the starting reagents were weighed (FX40-CJ analytical balance, Tokyo, Japan BMI Surplus) within the accuracy of ±10−4 g and ground together in a jasper mortar. To compensate for Li2O loss at heat treatment, a slight (5 wt%) excess of Li2CO3 was added to the starting mixtures. The resulting mixtures were dissolved in a water solution of HNO3 (reagent grade), evaporated under stirring, then glycine (C2H5NO2) of analytical purity was added. After portion of water evaporates, the reaction mixtures turn into a syrup-like liquid, which ignites spontaneously at further heating. The powder obtained was calcinated in air at 650 °C for 3 h to remove the organic compounds, then the mixtures were ground and sintered at 850–950 °C for 8–16 h. The obtained materials were ground into powder with particles less than 50 µm and pressed in stainless steel die into pellets 10 mm in diameter and 1–1.5 mm thick at 100–300 MPa. The pressed pellets were sintered at 900 °C for 5 h in the powder of the same composition. The density of the sintered pellets was 90–95% of the theoretical.
The phase composition of the samples was studied by XRD on a Rigaku D/MAX-2200VL/PC instrument (RIGAKU, Tokyo, Japan) in filtered Cu Kα-radiation generated at 40 kW, 30 mA (
λ = 1.54178 Å) stepwise with 0.3 s counting time and the step of 0.02° at room temperature in air. MDI Jade 6.5 software and PDF-2 ICDD database were used to analyze phase composition and to calculate unit cell parameters. The error of the cell parameters calculation did not exceed 0.02%. The data for full-profile Rietveld analysis in the range of 15° < 2Θ < 90° were recorded with 4 s holding time per step. Crystal structure refinement was performed using the FullProf program [
28].
The morphology and microstructure of the material synthesized were examined using MIRA 3 LMU scanning electron microscope (TESCAN, Brno, Czech Republic).
The electrical resistance of the samples was measured in air across 200–600 °C temperature range by P-40xpotentiostat-galvanostat (Elins, Zelenograd, Russia) with FRA-24M module for electrochemical impedance measurements over the frequency range of 50 Hz–500 kHz. Silver applied by a thermochemical method was used as electrodes. The measurements were performed during stepwise cooling with the step of 20 °C. The samples were kept for 15–20 min at every measurement temperature to allow the electric resistance to reach a stable value. The resistance of the samples was determined by analyzing the impedance frequency dispersion. The resultant equivalent circuit is given in
Figure 1, where C
g is the geometrical capacity, R
total is the resistance of the sample, CPE is the constant phase element reflecting the polarization at the electrode–electrolyte interface. The resistance of the sample in this case is the sum of the volume resistance (R
v) and grain boundary resistance (R
gb), i.e., R
total = R
v + R
gb.
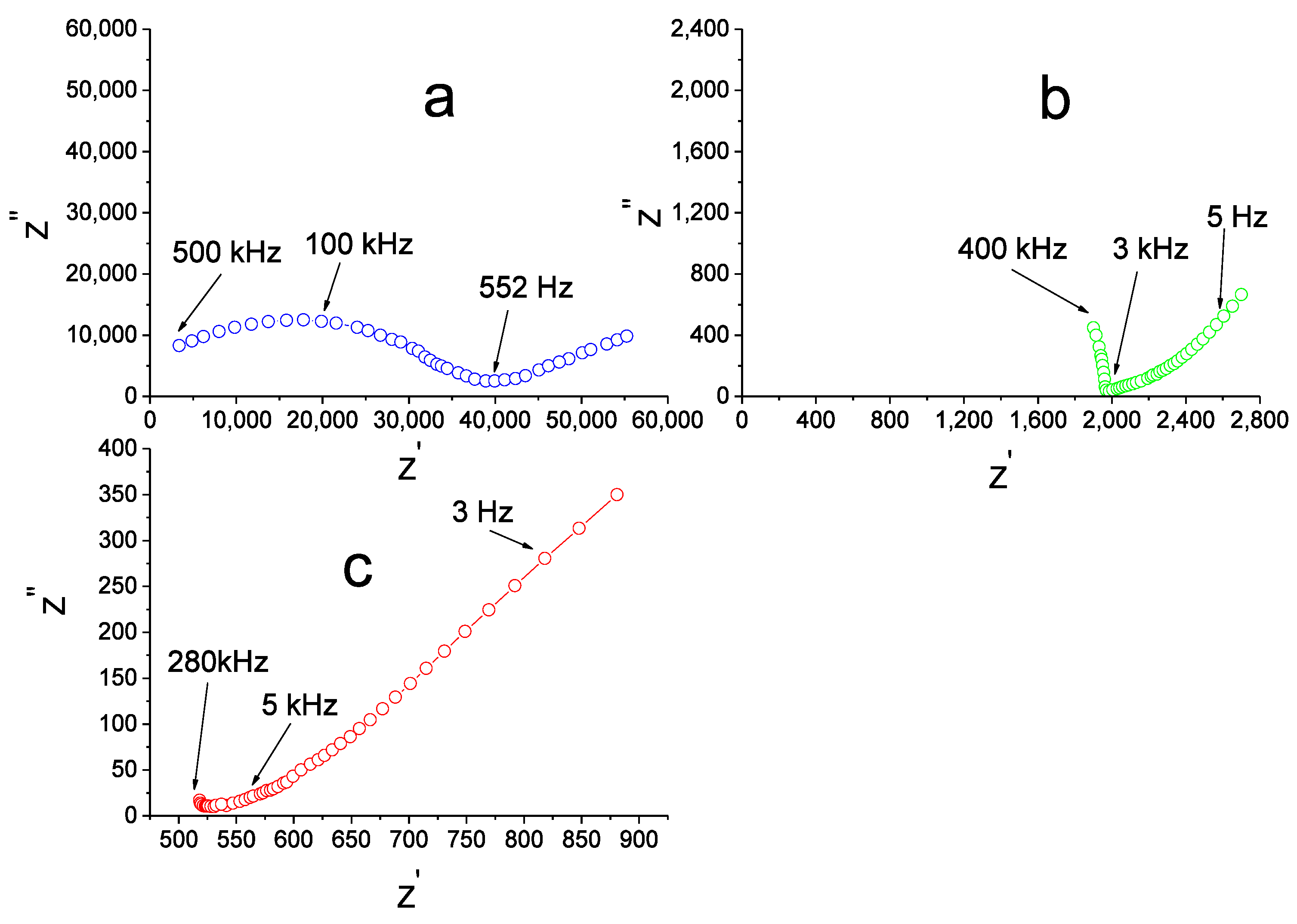
The impedance spectra for the undoped Li
6Zr
2O
7 and for the solid solutions are identical. The spectra for the Ag|Li
5.95Zr
1.95Nb
0.05O
7|Ag cell at different temperatures are given in
Figure 2 as examples. At low temperatures the spectra consist of a semicircular arc in the high-frequency region and a tail in the low-frequency region (
Figure 2a).
The resistance R
total of the sample was determined as the value corresponding to the point of intersection between the high-frequency arc and the Z
’ axis. As the temperature rises, the semicircle becomes smaller (
Figure 2b), and above 500–550 °C only a tail is observed across the whole accessible frequency range (
Figure 2c). In this case R
total was found by the extrapolation of the low-frequency linear portion onto the Z
’ axis. The corresponding value of specific conductivity was calculated according to the formula:
where l is the length of the sample, S is the area of its cross-section.
The electron conductivity was determined using DC method with gold electrodes at 40–50 mV and in all the cases did not exceed 1% of total conductivity.
3. Results and Discussion
The crystal structure of Li
6Zr
2O
7 was studied by X-ray analysis of single crystals [
27,
29], in [
26], the X-ray method was enlarged with the neutron diffraction in order to refine the positions of Li
+ ions. These data show a good agreement. Li
6Zr
2O
7 has a monoclinic structure, space group C2/c, a = 10.4428(1) Ǻ, b = 5.9877(1) Ǻ, c = 10.2014(1) Ǻ, β = 100.266(1)° [
25]. The ions of oxygen form a distorted cubic close-packed lattice, in which 1/8 of the sites are vacant. The distribution of lithium and zirconium ions is similar to the distribution of cations in NaCl; besides, zirconium is octahedrally coordinated by oxygen, and Li coordination number is five (a square pyramid).
According to [
21], at low temperatures there exists a metastable α-form of Li
6Zr
2O
7, which is produced by annealing the mixture of Li
2CO
3 and ZrO
2 at 850–900 °C and which passes into β-form at high temperatures. The work [
15] confirms the existence of α-modification of Li
6Zr
2O
7, which has a triclinic structure with a = 6.0153(4) Å, b = 9.1941(12) Å, c = 5.3112(6) Å, α = 96.30(1)°, β = 107.16(1)°, γ = 89.74(1)°, however, the authors point out that this phase can only be formed if Li
2CO
3 is used as lithium source. Synthesis at 750 °C produces single-phase Li
6Zr
2O
7 samples with triclinic structure. If the annealing temperature is 800 °C, the triclinic and monoclinic modifications co-exist in the sample, and at higher temperatures the triclinic one disappears. Using ZrO
2 and Li
2O oxides as the starting components yielded a monoclinic phase irrespective of the synthesis temperature. Thus, the formation of the metastable triclinic modification of Li
6Zr
2O
7 via synthesis from Li
2CO
3 and ZrO
2 is confirmed by literature data, but the temperature and the time of heat treatment required to produce single-phase samples with a stable monoclinic structure are quite different in [
15,
22].
In the present work, the glycine-nitrate stage of Li
6Zr
2O
7 synthesis was followed by keeping the reaction mixture at 850, 900, and 950 °C. The results indicate that heat treatment at 850 and 900 °C produces a mixture of triclinic and monoclinic modifications. Notably, if the heat treatment time is increased from 8 to 16 h, the proportion of the triclinic modification decreases.
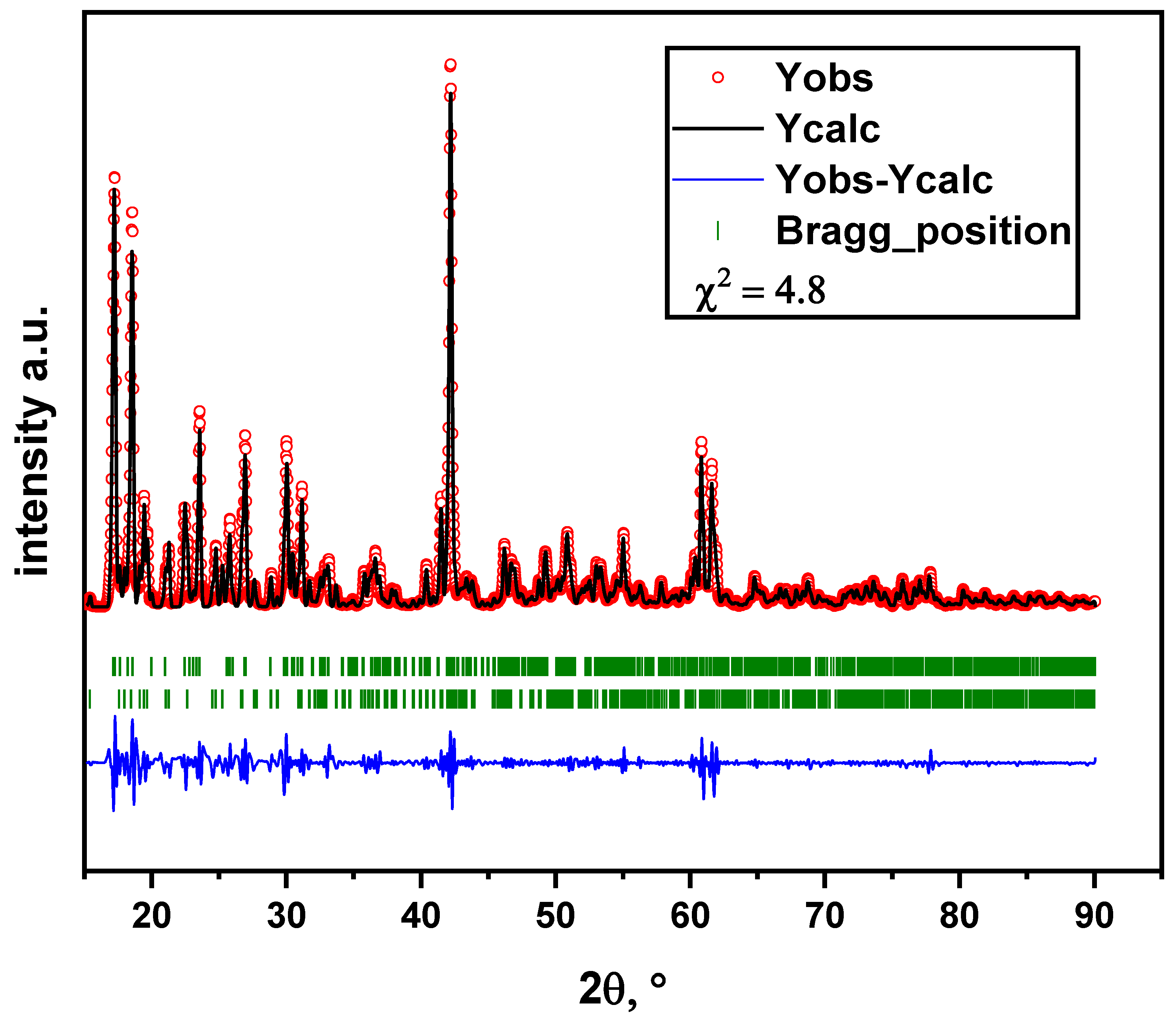
Figure 3 contains Rietveld refinement of the powder XRD pattern for the sample sintered at 900 °C for 8 h. The data indicate that Li
6Zr
2O
7 powder is non-single-phase. The main phase is characterized by a monoclinic syngony with space group C2/c (PDF No 88-2213). The impurity phase of the same chemical composition has a triclinic syngony with space group P1 (PDF No 36-0122). The content of the triclinic phase is estimated to be 8.9%.
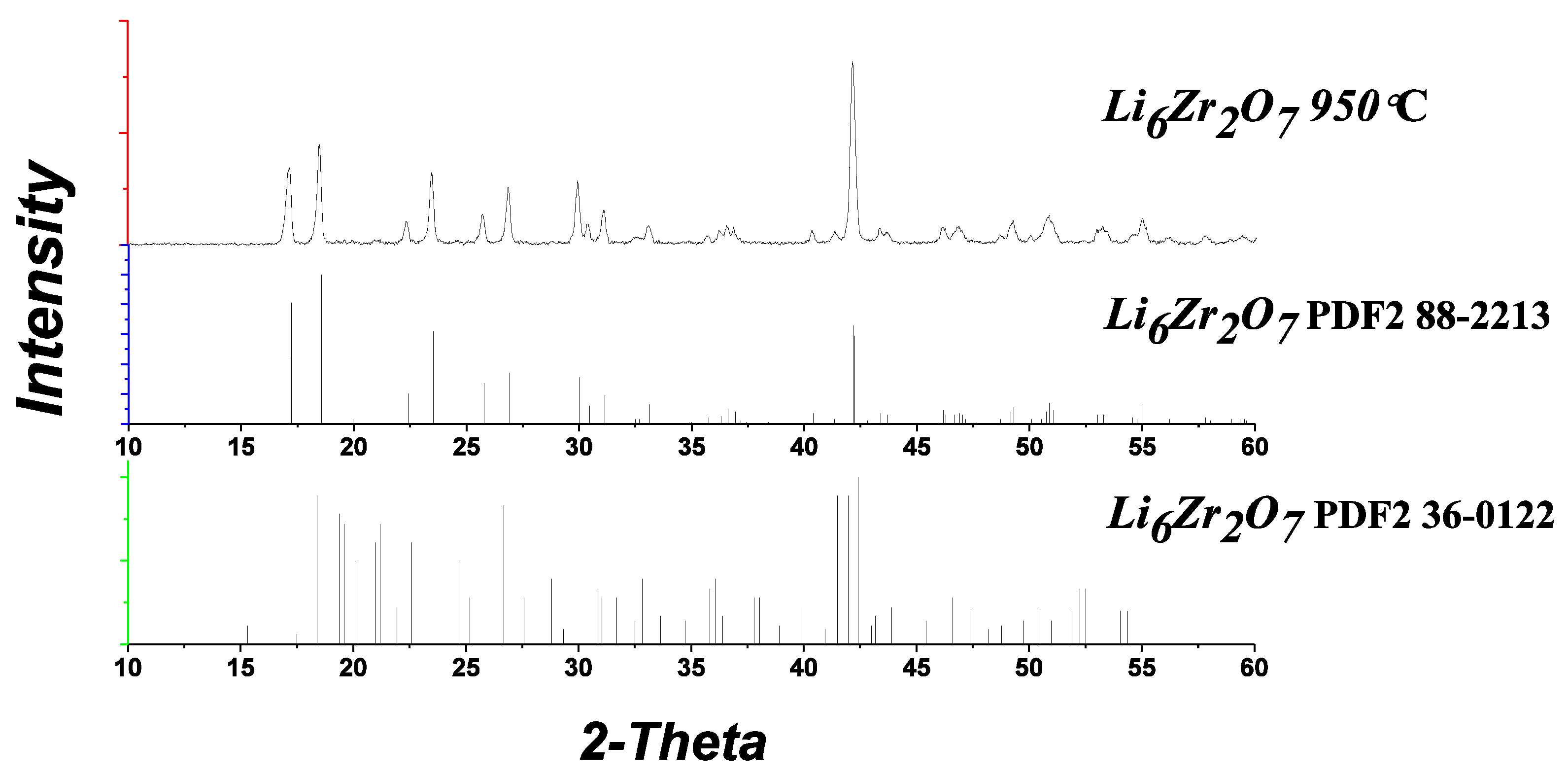
The XRD patterns of the samples annealed at 950 °C contain only the reflections of monoclinic Li
6Zr
2O
7 (
Figure 4), the same applies to the doped samples.
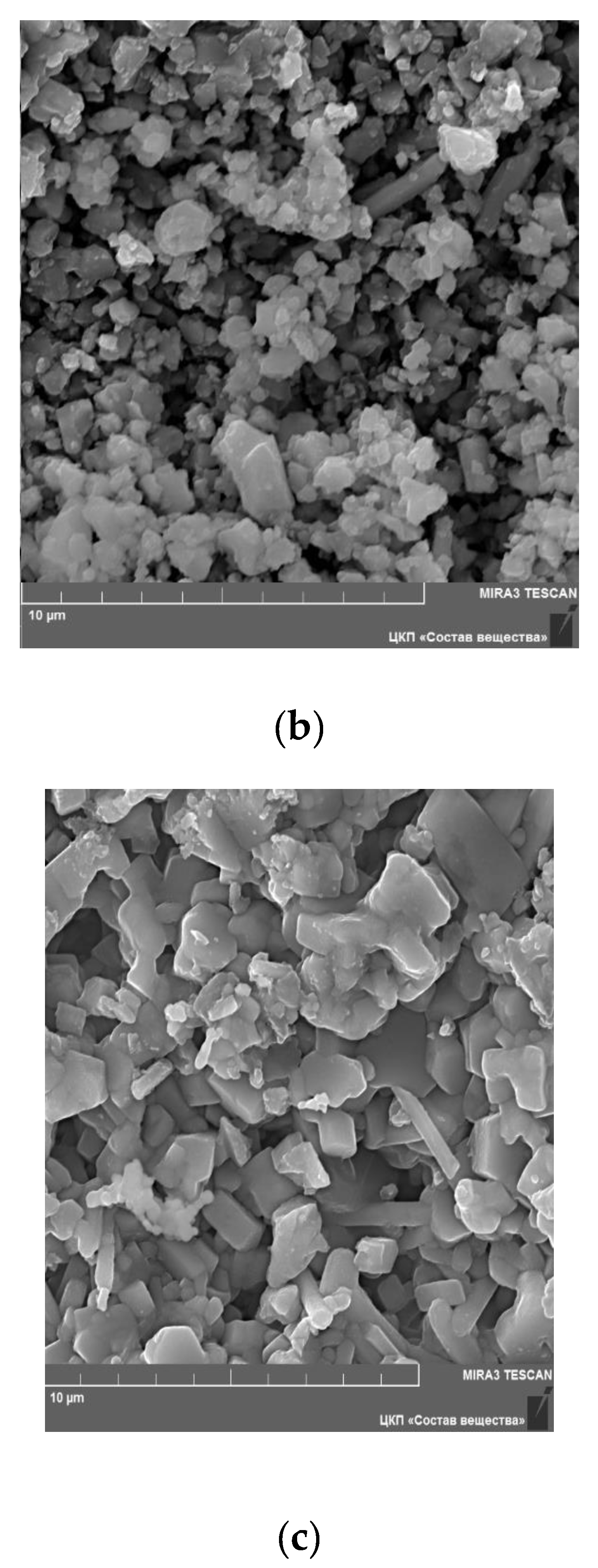
Figure 5 shows SEM images for the surface of Li
6Zr
2O
7 sample annealed at 950 °C, as well as for Li
5.8Zr
1.8Ta
0.2O
7 and Li
5.8Zr
1.8Nb
0.2O
7 solid solutions. One can see that the microstructure of the samples is non-homogeneous, and in the case of Li
6Zr
2O
7 it consists mainly of particles 0.5–2.0 µm in size (
Figure 5a), though some grains are as large as 3 µm. In Li
5.8Zr
1.8Ta
0.2O
7 and Li
5.8Zr
1.8Nb
0.2O
7 the structure is more homogeneous and consists of grains 1–2 µm in size (
Figure 5b,c).
It should be pointed out that at temperatures of 900 °C and above lithium oxide is quite volatile, therefore, increasing the temperature and time of heat treatment may cause a considerable loss of Li2O. On the other hand, decreasing the temperature results in the formation of triclinic phase in the samples. Thus, a question arises about how the presence of the triclinic modification of Li6Zr2O7 affects the conductivity, i.e., what is the relation between the conductivity of the samples having triclinic and monoclinic structure.
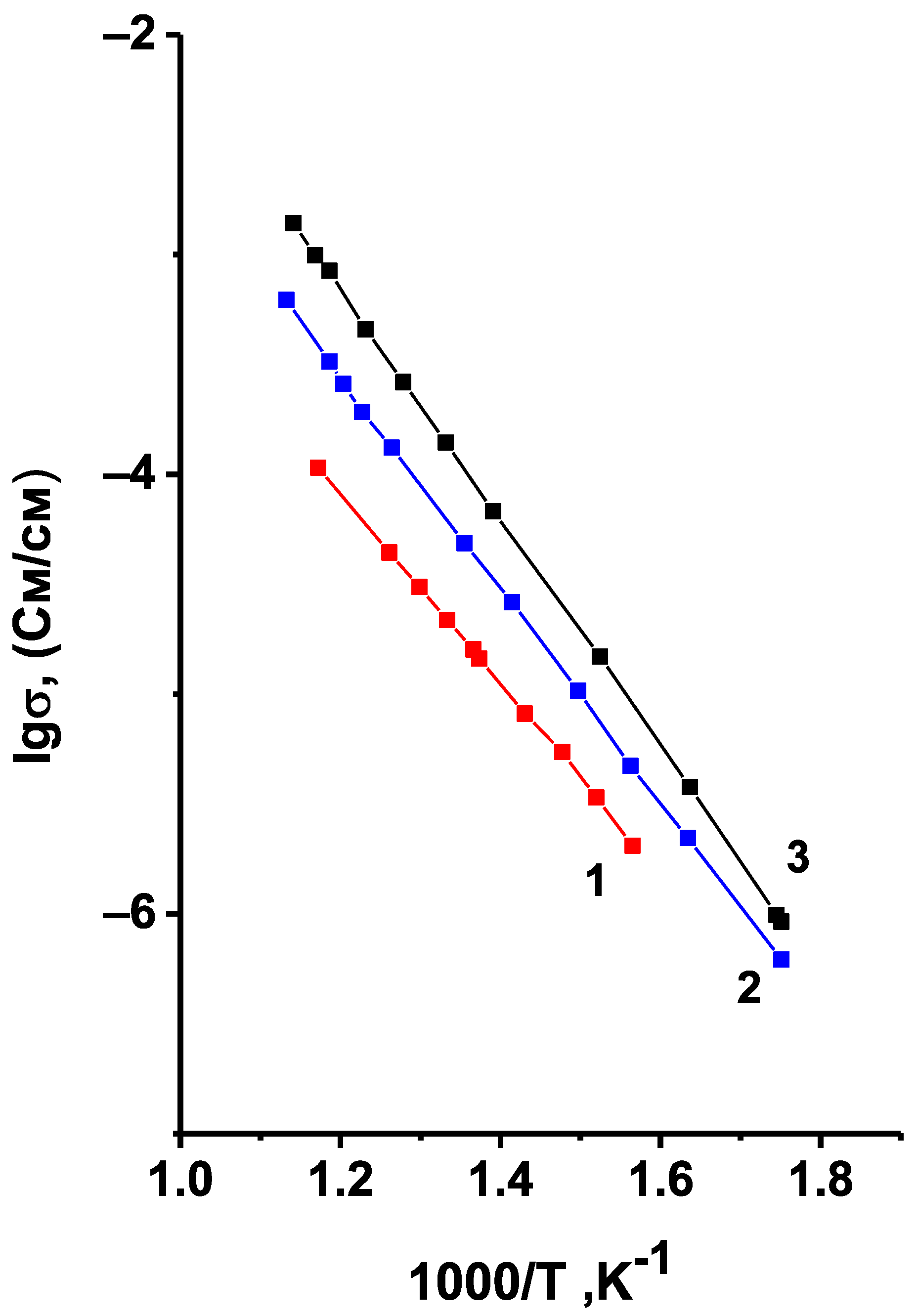
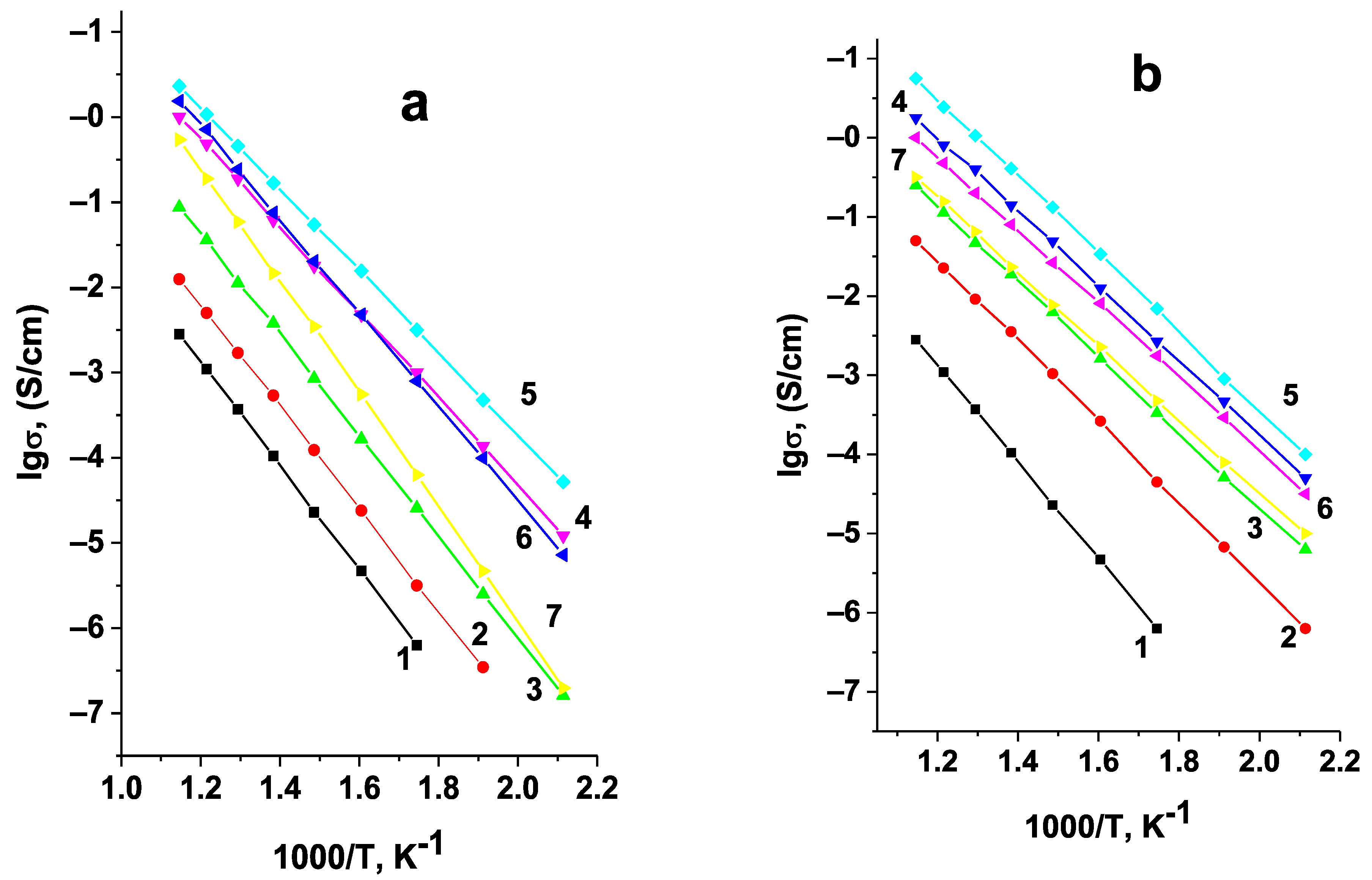
Figure 6 shows the temperature dependences of conductivity for Li
6Zr
2O
7 samples synthesized at different temperatures. Lines 1 and 2 correspond to the two-phase samples that contain triclinic phase alongside the monoclinic one. It can be assumed from the intensity of the reflections on the XRD pattern, that the content of triclinic phase in sample 1, synthesized at 850 °C, is higher than in sample 2, synthesized at 900 °C. Line 3 corresponds to the single-phase sample with monoclinic structure. Its electric characteristics are close to those given in [
12] (
Table 1).
As can be seen from
Figure 6, the conductivity of Li
6Zr
2O
7 decreases with the appearance of triclinic phase and with the further growth of its content. However, one should take into account that the samples sintered at lower temperatures have a higher porosity, e.g., the porosity of the samples sintered at 850, 900, and 950 °C assessed from saturating the samples with an organic liquid was 24, 19, and 13%, respectively. An increase in the porosity of the samples always leads to a decrease in their conductivity, while the triclinic modification of Li
6Zr
2O
7 may have both higher and lower conduction as compared to the monoclinic one. To get reliable data on the relation between the conductivity of triclinic and monoclinic forms of Li
6Zr
2O
7, it is necessary to eliminate the effect of porosity. In order to do that we corrected the calculation of conductivity for samples 1, 2, and 3 to the conductivity of non-porous ceramics using the formula for heterogeneous materials [
30]:
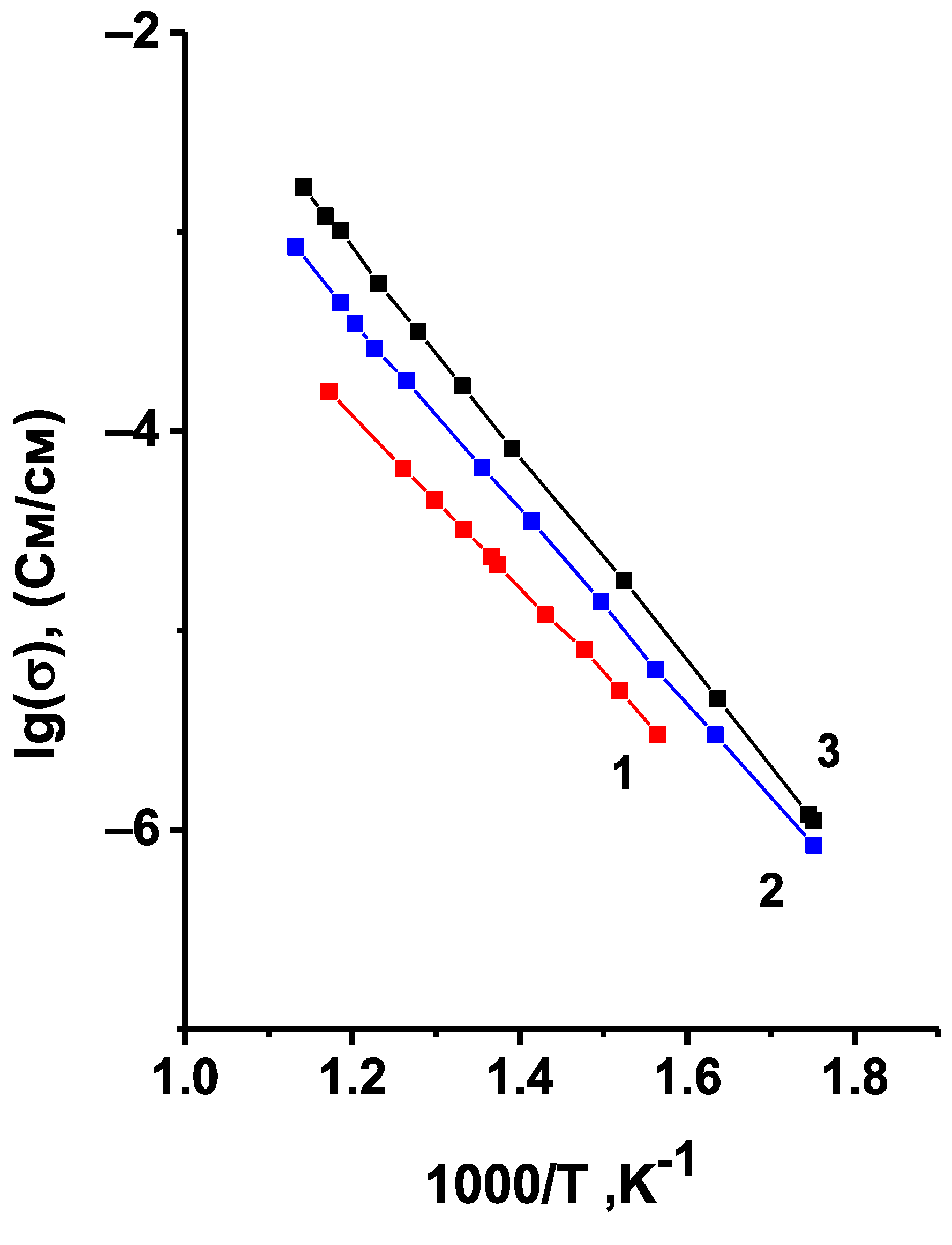
where
σ* is the measured value of conductivity,
σ1 is the conductivity of the non-porous sample,
m2 is the volume fraction of pores,
σ2 in this case is the conductivity of air, i.e.,
σ2 = 0. The temperature dependences of the corrected conductivity values are given in
Figure 7. One can observe here the same tendency for the conductivity to decrease with decreasing synthesis temperature, and, consequently, with the increasing content of the triclinic modification. Thus, across the investigated temperature range the triclinic modification of Li
6Zr
2O
7 has a much lower conductivity compared to the monoclinic one, therefore, the final temperature of synthesis for the doped samples was 950 °C, and the absence of the triclinic phase in the synthesized samples was monitored by XRD.
Phase diagrams for Li
2O-ZrO
2-Nb
2O
5 (Ta
2O
5) systems were investigated in [
31], but the authors focused mainly on the study of perovskite-like phases near LiNbO
3 and LiTaO
3, and, as a result, the domain of Li
6-xZr
2-xNb(Ta)
xO
7 was not considered. According to our results, in both systems under investigation, i.e., Li
6-xZr
2-xA
xO
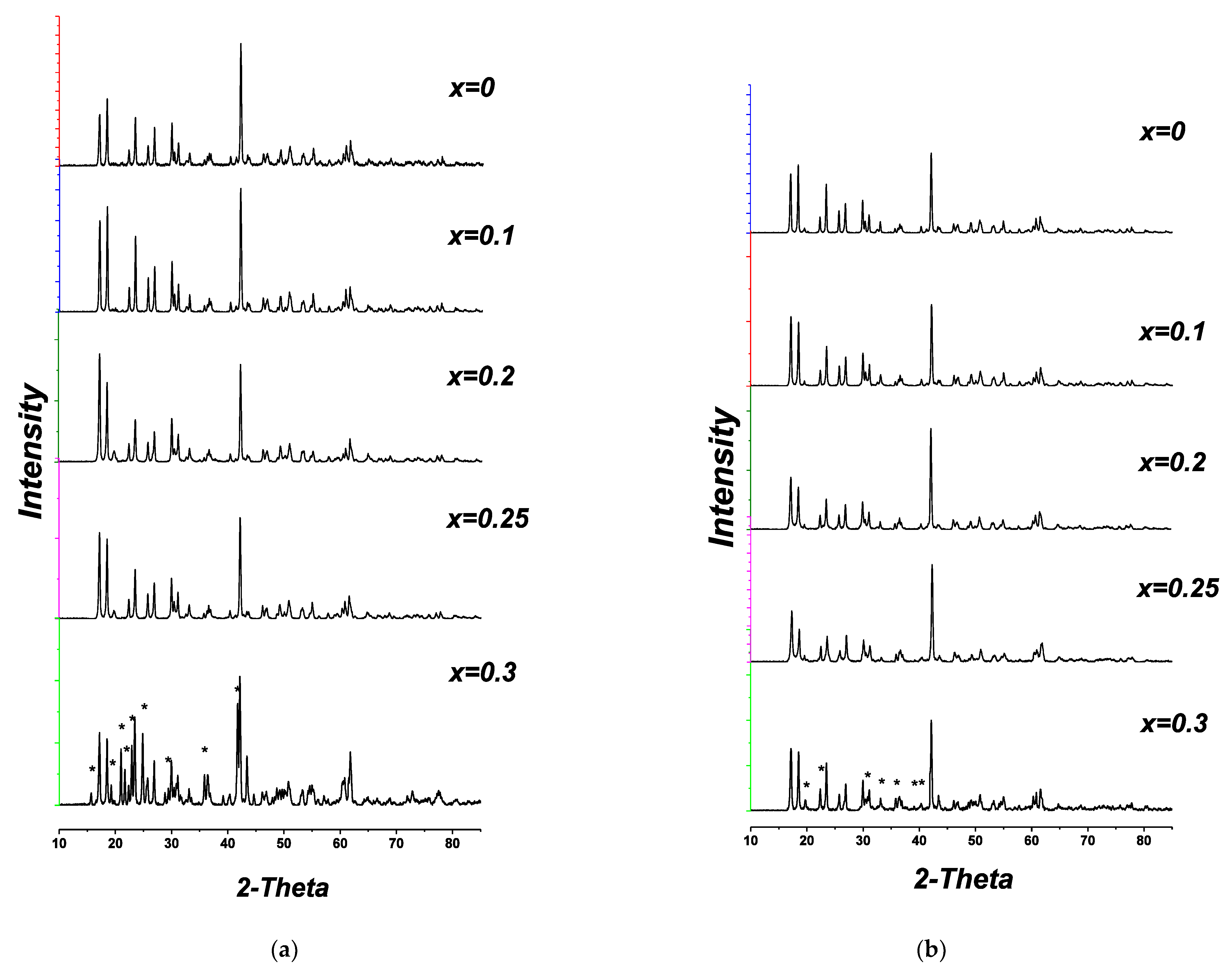
7 (A = Nb, Ta), the samples with 0 <x< 0.25 retain the monoclinic structure of the initial Li
6Zr
2O
7 (
Figure 8a,b, XRD patterns for x = 0–0.20).
When x ≥ 0.25, reflections of impurity phases appear on the XRD patterns of the samples, their intensity grows with increasing content of the dopants (
Figure 8a,b, XRD patterns for x = 0.25, 0.30). In Li
6-xZr
2-xNb
xO
7 system these reflections correspond to the recently investigated Li
29Zr
9Nb
3O
40 compound [
32], (PDF 02-082-2342). The phase appearing in the samples of Li
6-xZr
2-xTa
xO
7 with x ≥ 0.25 could not be identified using PDF 2, but all the lines that cannot be assigned to Li
6Zr
2O
7 practically coincide with the reflections of Li
29Zr
9Nb
3O
40. Taking into account the closeness of chemical properties that have niobium and tantalum and close values of ionic radii for Nb
5+ and Ta
5+, we can assume that there exists a tantalum analog isostructural with Li
29Zr
9Nb
3O
40. The results of XRD analysis for the samples of the systems studied are listed in
Table 2.
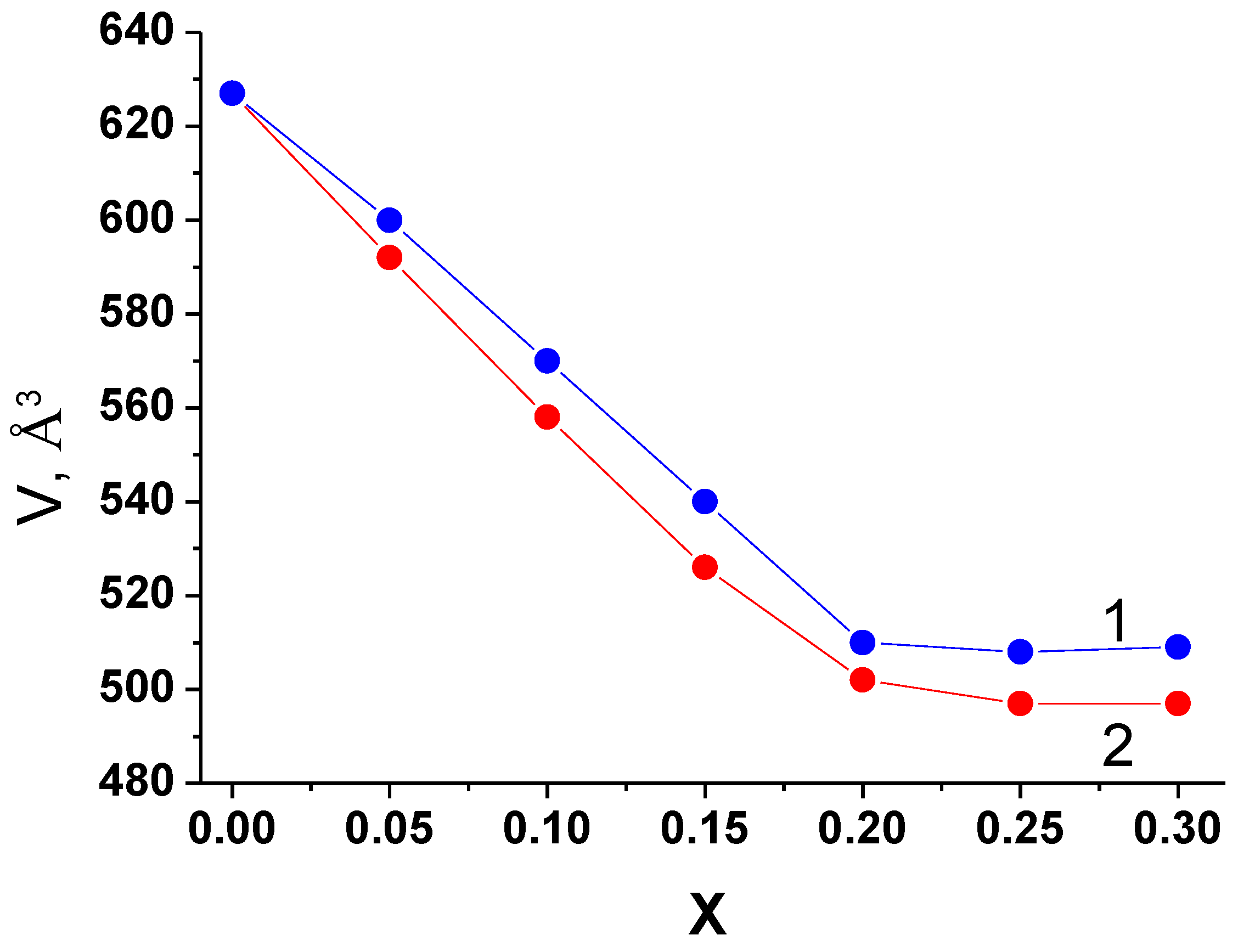
The plots of concentration vs. the volumes of elementary cells for the phases with monoclinic C2/c structure in the systems investigated are given in
Figure 9. The radii of Nb
5+ and Ta
5+ ions are practically the same (0.78 Å), but they are slightly smaller than Zr
4+ (0.86 Å) [
33], therefore, the volumes of the elementary cells decrease with the growth of A
5+ content, and for the samples with the same value of
x in the systems with niobium and tantalum their values are close. Overall, the plots of
V vs.
x correlate with the results of the phase composition studies (
Table 2).
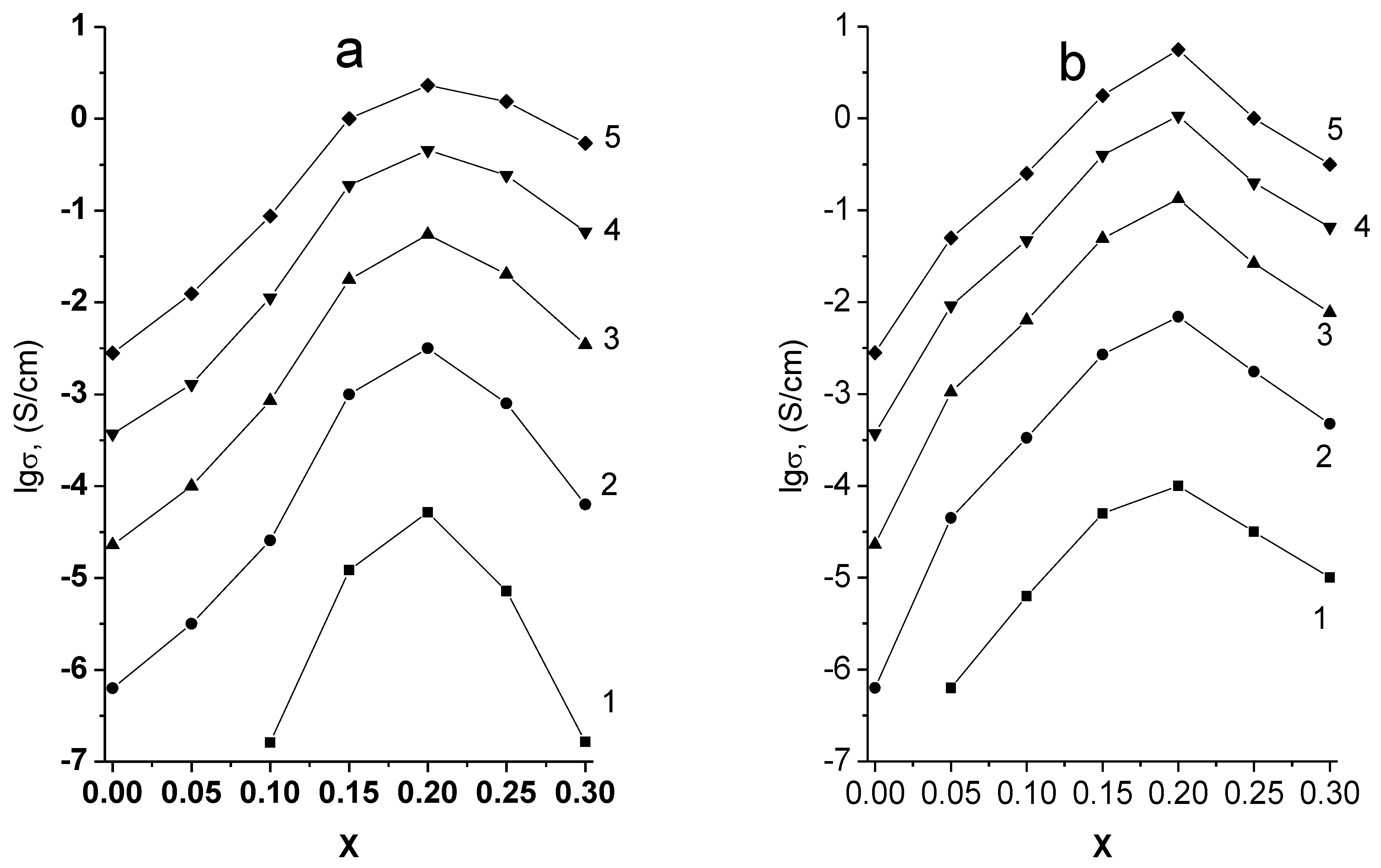
The Arrhenius plots of specific conductivity in both systems studied are linear (
Figure 10). The maximum conductivity values in both cases correspond to the samples on the boundaries of single-phase regions (
Figure 11,
Table 2), i.e., to Li
5.8Zr
1.8Nb(Ta)
0.2O
7.
The appearance of impurity phases when x ≥ 0.25 is accompanied by a drop of conductivity in both systems. The conductivity of Li
5.8Zr
1.8Ta
0.2O
7 is 6.92 × 10
−3 S × cm
−1 at 300 °C, which is approximately four times higher than the conductivity of Li
5.85Zr
1.85Ta
0.15O
7 reported in [
23] (1.7 × 10
−3 S × cm
−1 at the same temperature), which is explained by a higher content of Ta
5+ and, consequently, a higher concentration of charge carriers, i.e., lithium vacancies. The conductivity of Li
5.85Zr
1.85Nb
0.15O
7 given in [
23], is by an order of magnitude lower than the one of the tantalum-containing sample with the same dopant concentration, which is not quite understandable considering the similarity of the ionic radii, electronegativities, and other characteristics of niobium and tantalum that may affect the conductivity of solid solutions. In the present work, the ionic conductivity of Li
5.8Zr
1.8Nb
0.2O
7 sample was found to be 3.16 × 10
−3 S × cm
−1 at 300 °C, which is close to the conductivity of tantalum-containing sample of the same composition.
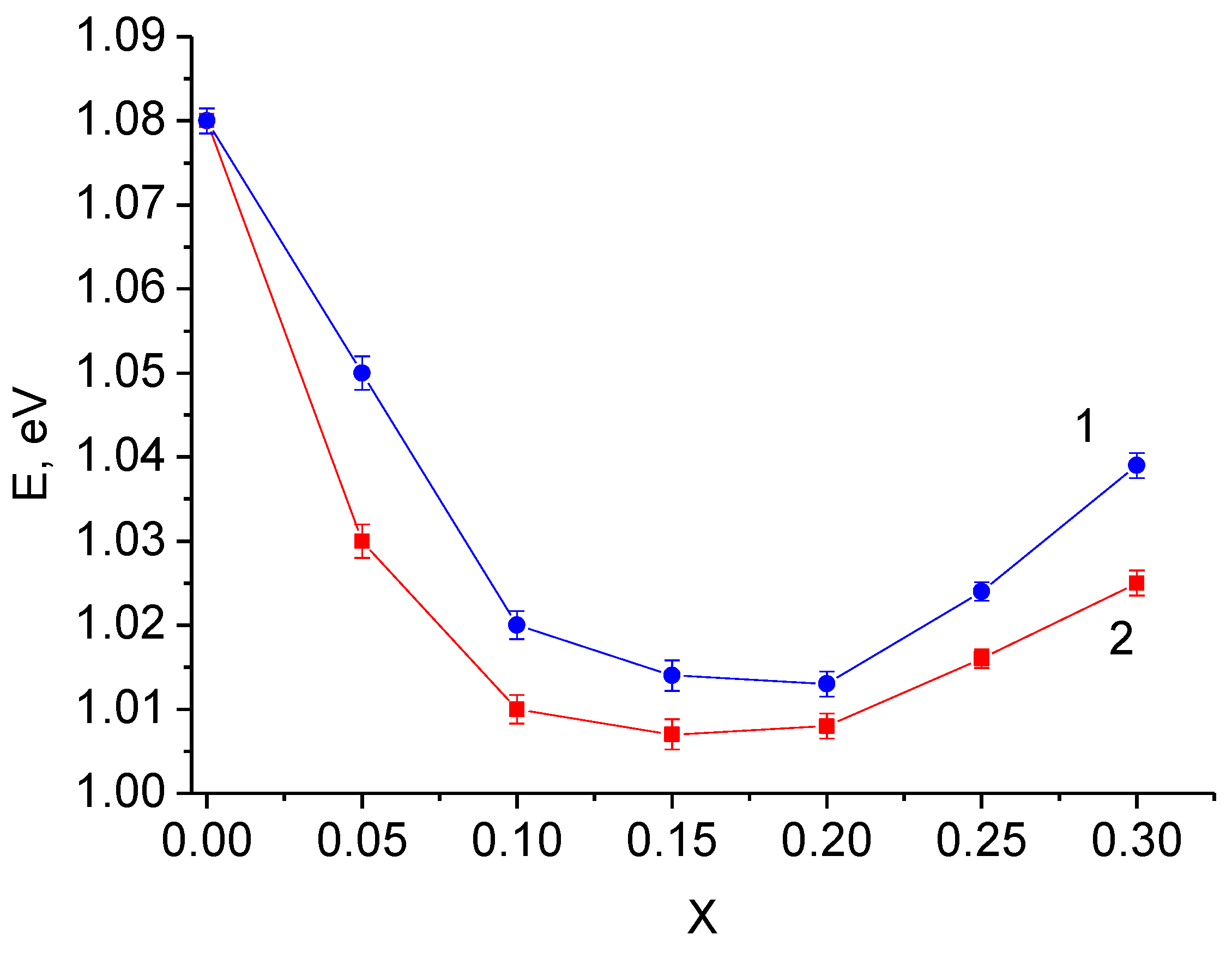
Solid electrolytes with maximum conductivity in the investigated systems have the lowest activation energy (
Figure 12). In the systems with niobium and tantalum these values are similar and equal approximately 1.01 eV, which is close to the values given in literature for vacancy solid solutions based on Li
6Zr
2O
7 [
23,
25].
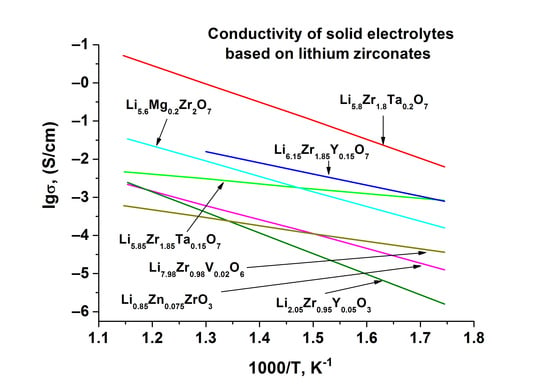
Table 3 contains transport properties of the materials investigated in the present paper compared to the properties of other solid electrolytes based on lithium zirconates. One can see that Li
5.8Zr
1.8Nb(Ta)
0.2O
7 solid solutions have the highest lithium-cation conductivity at 300 °C compared to electrolytes based on Li
2ZrO
3 [
34], Li
8ZrO
6 [
20,
34], and Li
6Zr
2O
7-based solid solutions that form when lithium is substituted for double-charged cations [
22], or when zirconium is substituted for triple-charged cations [
24]. It should be noted, however, that the activation energy of Li
6.15Zr
1.85Y(In)
0.15O
4 interstitial solid solution is much lower compared to vacancy solid solution based on Li
6Zr
2O
7 (
Table 3). The authors of [
24] assign this to the fact that the coulombic interaction between Li
+ ions that occupy interstitial positions and the neighbor Li
+ on normal sites displaces the neighboring normal-site of Li
+ from the centers of the sites, thus reducing the potential barrier for Li
+ ions migration. As a result, at 300 °C solid solutions of Li
6.15Zr
1.85Y(In)
0.15O
4 have a lower conductivity compared to the solid electrolytes studied in the present work, however, at lower temperatures they will apparently have an advantage.
Vacancy solid solutions in
Table 3 display the following pattern: solid electrolytes with substitutions in lithium sublattice (Li
1.85Ca(Zn)
0.075ZrO
3, Li
5.6Mg
0.2Zr
2O
7, Li
7.85Mg
0.075ZrO
6) have a rather lower activation energy compared to the solid electrolytes with substitution of Zr
+ by Nb
5+ or Ta
5+. This can be explained by the influence of the size factor. Activation energy depends on the mobility of the carriers, which, in its turn, depends on the size of crystallographic channels for Li
+ ions migration. The change of channel dimensions that accompanies heterovalent substitutions depends on the substituted ion and dopant radii. Li
+ and double charged ions (Mg
2+, Zn
2+) have rather close values of ionic radii (0.90, 0.86 и 0.88 Å, respectively [
33]). Therefore, the substitution of Li
+ by Mg
2+or Zn
2+ has no significant effect on the mobility of lithium ions. In contrast, Nb
5+ and Ta
5+ ions (r = 0.78 Å) are smaller than Zr
4+ (r = 0.86 Å), so substitution of zirconium by niobium or tantalum leads to a significant decrease in cell volume (
Figure 8) and, therefore, in the average radii of channels, which hinders Li
+ migration and increases the activation energy. However, the maximum dopant solubility in the case of doping Li
6Zr
2O
7 by double charged cations is far less compared to the solubility of the pentavalent ions, so the ionic conductivity of the former is smaller (
Table 3).
High lithium-cation conductivity of solid solutions in Li
6-xZr
2-xNb(Ta)
xO
7 systems can be assigned to a relatively open framework structure of the monoclinic Li
6Zr
2O
7 [
23] and, compared to other lithium zirconates, a rather high solubility of the dopants, and, consequently, a higher concentration of charge carriers. Nevertheless, compared to the best solid lithium-cation conductors [
36], the conductivity of the materials under study is relatively low, therefore, they can be used as solid electrolytes in LIBs only together with additional means of reducing the internal resistance of the battery, e.g., by using solid electrolytes in the form of thin films.