Energy Transfer Studies in Tb3+-Yb3+ Co-Doped Phosphate Glasses
Abstract
:1. Introduction
2. Experimental
3. Theoretical Introduction for the Cooperative Up-Conversion
4. Results and Discussion
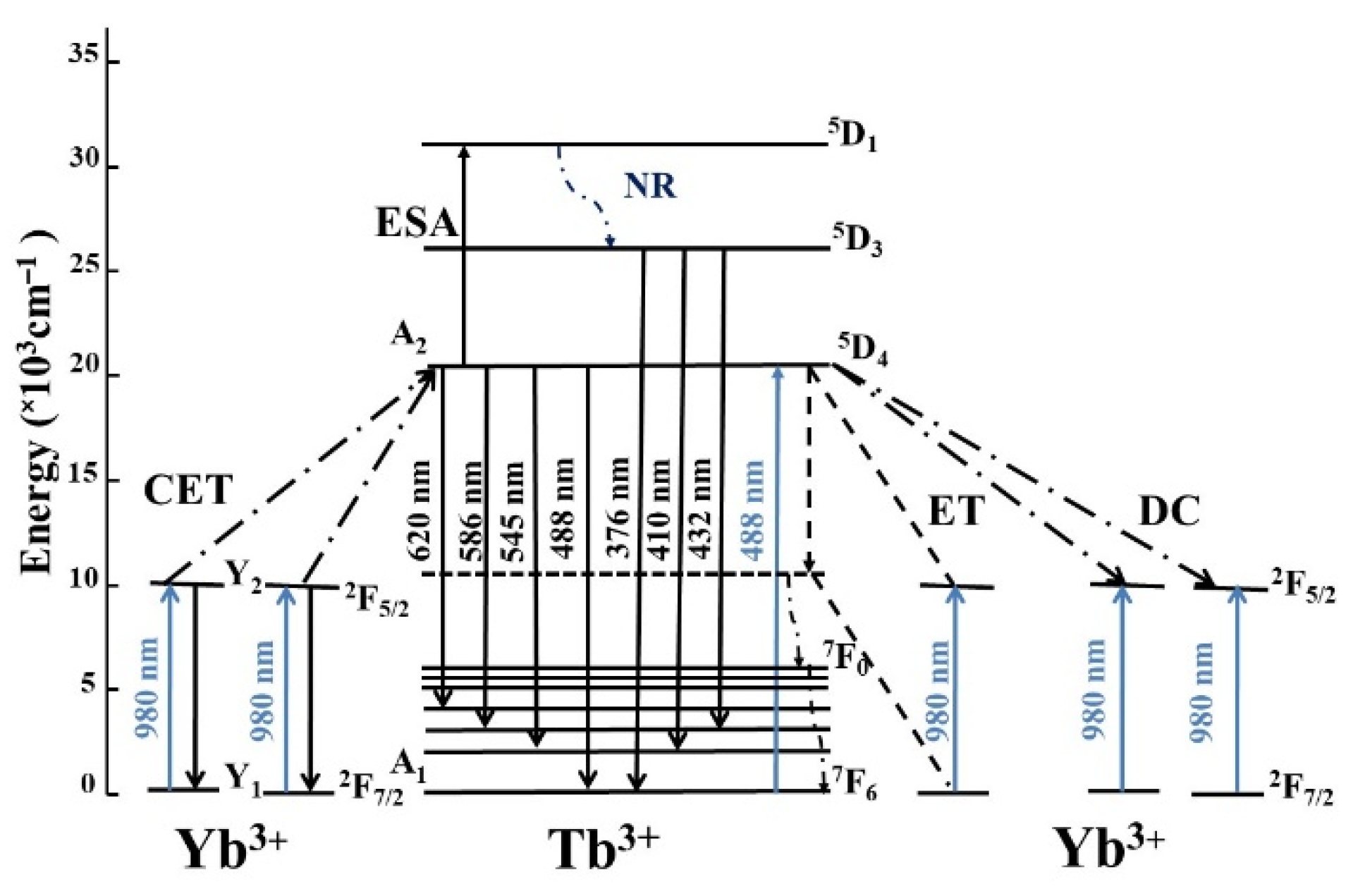
4.1. Energy Transfer from Tb3+ to Yb3+
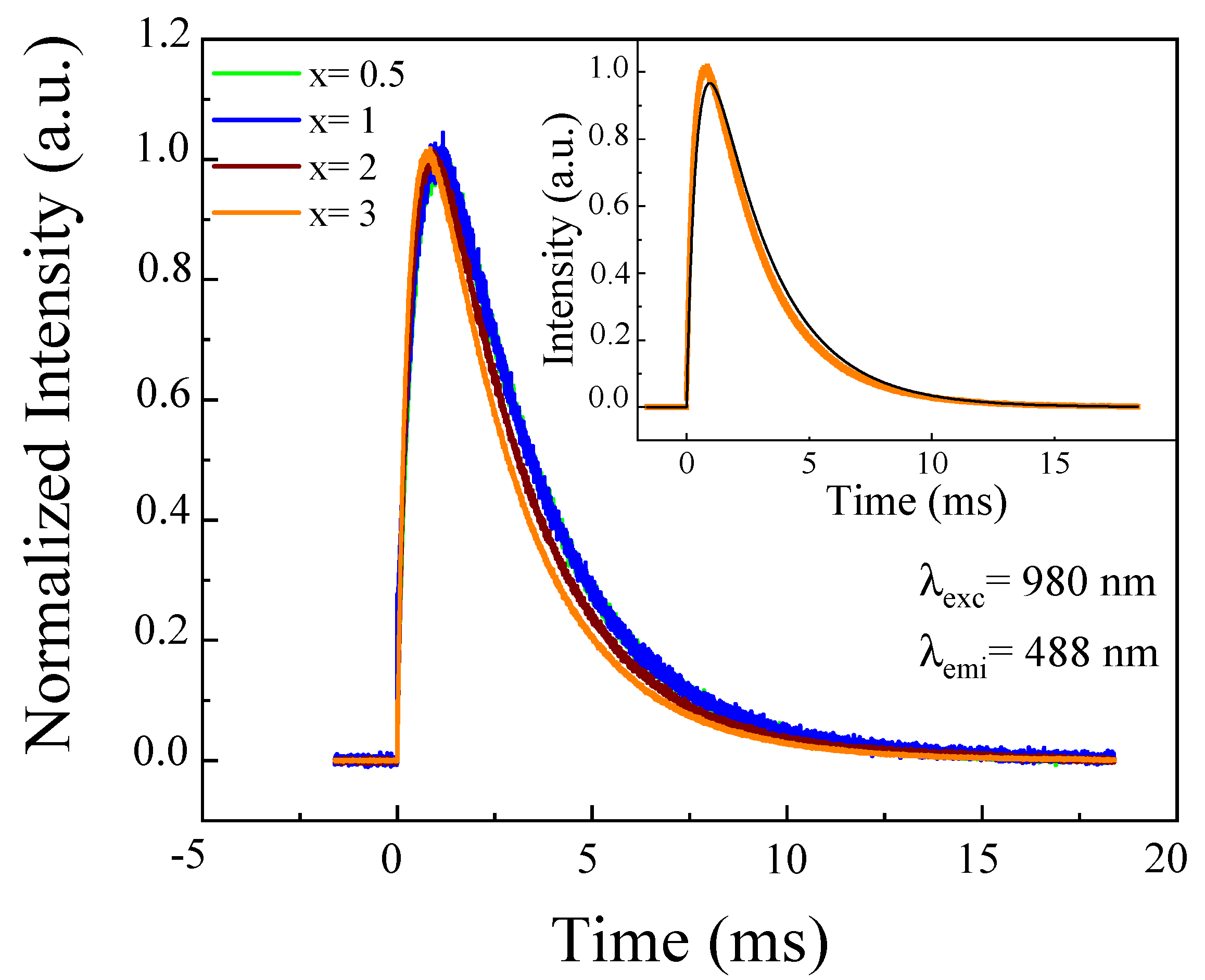
4.1.1. Luminescence Spectra
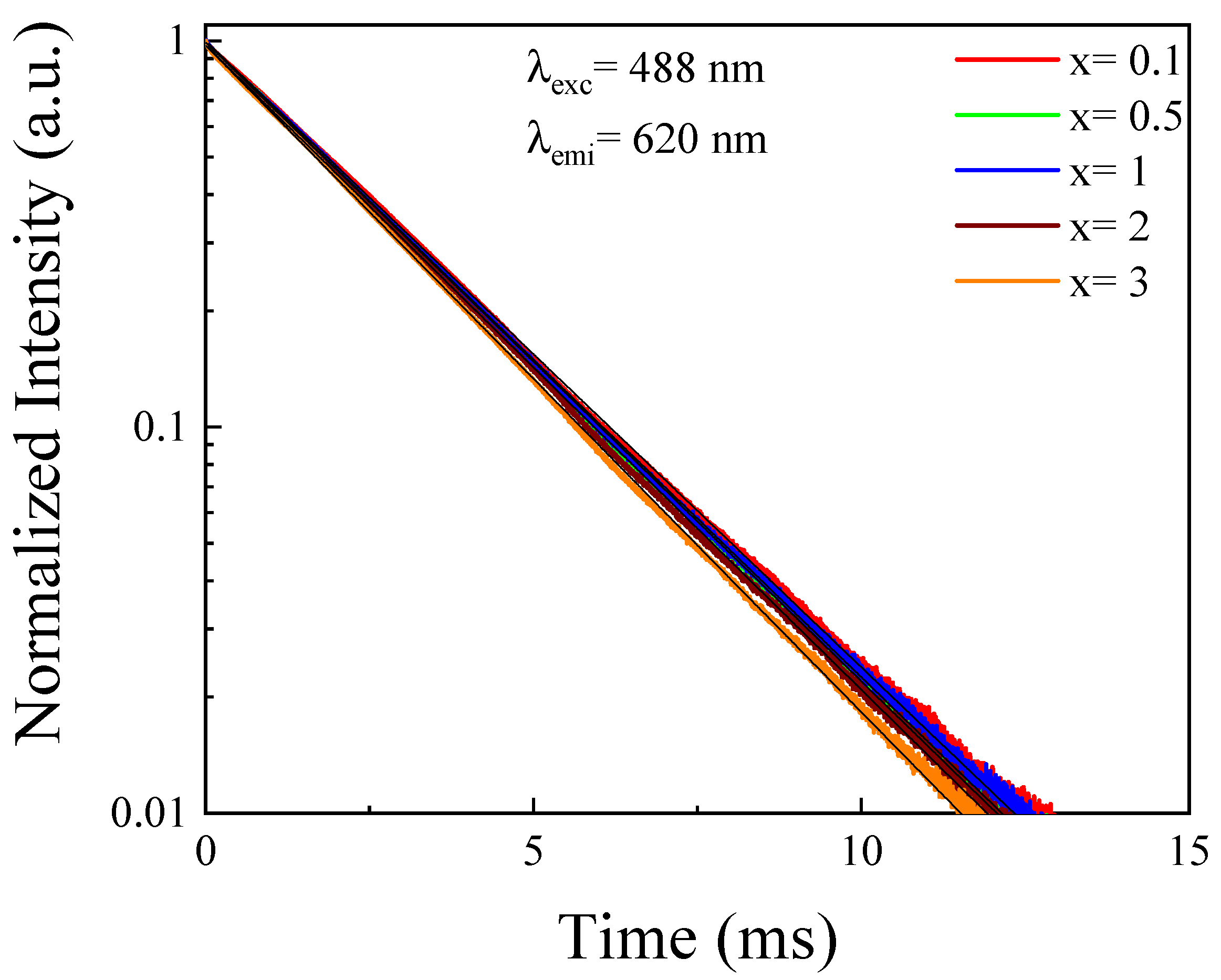
4.1.2. Luminescence Decays
4.2. Cooperative Energy Transfer from Yb3+ to Tb3+
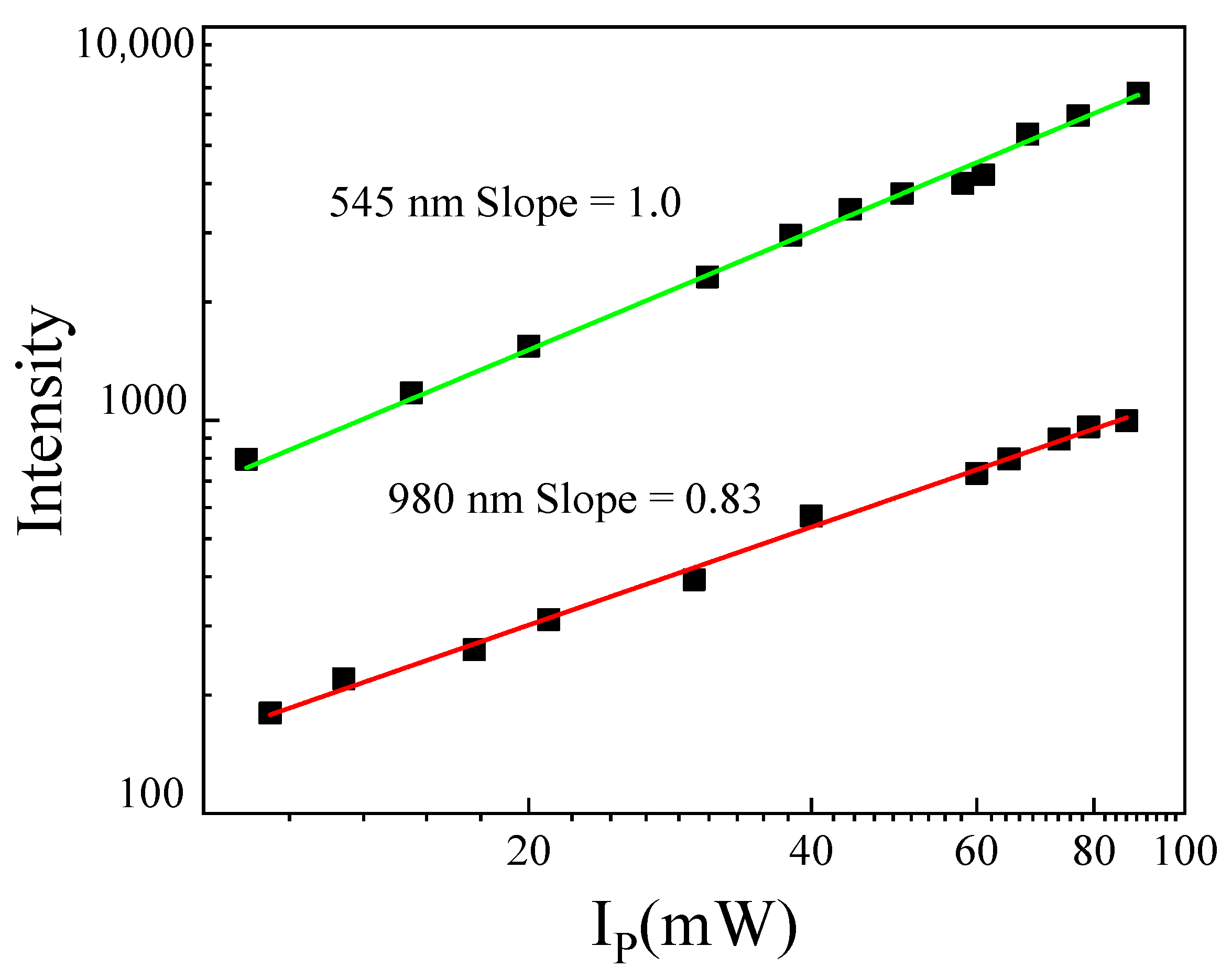
4.2.1. Up-Conversion Luminescence Spectra
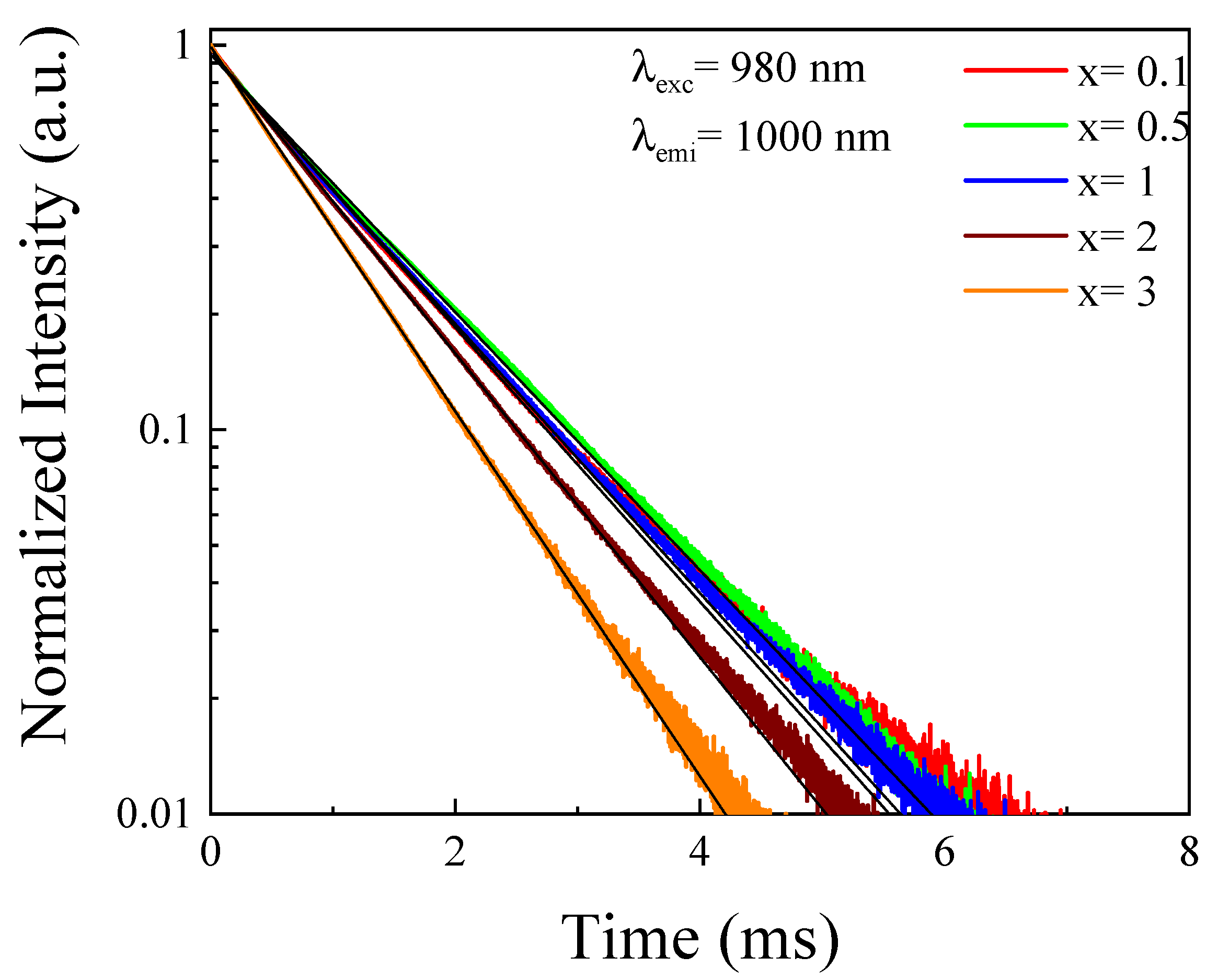
4.2.2. Temporal Evolution of the Up-Conversion Emission
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, X. Broadband dye-sensitized upconversion: A promising new platform for future solar upconverter design. J. Alloy. Compd. 2017, 690, 356–359. [Google Scholar] [CrossRef]
- Dieke, G.H.; Satten, R.A. Spectra and Energy Levels of Rare Earth Ions in Crystals. Am. J. Phys. 1970, 38, 399–400. [Google Scholar] [CrossRef]
- Soltani, I.; Hraiech, S.; Horchani-Naifer, K.; Férid, M. Effects of silver nanoparticles on the enhancement of up conversion and infrared emission in Er3+/Yb3+ co-doped phosphate glasses. Opt. Mater. 2018, 77, 161–169. [Google Scholar] [CrossRef]
- Benrejeb, H.; Soler-Carracedo, K.; Hraiech, S.; Martin, I. Photoluminescence and energy transfer studies in Ce3+-Sm3+ co-doped phosphate glasses. J. Lumin. 2022, 241, 118471. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Dai, S.; Fan, B.; Ma, H.; Adam, J.-L.; Ren, J.; Chen, G. Efficient Near-Infrared Down-Conversion in Pr3+–Yb3+ Codoped Glasses and Glass Ceramics Containing LaF3 Nanocrystals. J. Phys. Chem. C 2011, 115, 13056–13062. [Google Scholar] [CrossRef]
- Jha, A.; Richards, B.D.O.; Jose, G.; Fernandez, T.T.; Hill, C.J.; Lousteau, J.; Joshi, P. Review on structural, thermal, optical and spectroscopic properties of tellurium oxide based glasses for fibre optic and waveguide applications. Int. Mater. Rev. 2012, 57, 357–382. [Google Scholar] [CrossRef]
- Li, J.; Zhang, T.; Zhu, G.; Hairong, Z. Up-conversion photoluminescence emissions of CaMoO4: Pr3+/Yb3+ powder. J. Rare Earths 2017, 35, 645–651. [Google Scholar] [CrossRef]
- Strümpel, C.; McCann, M.; Beaucarne, G.; Arkhipov, V.; Slaoui, A.; Švrček, V.; del Cañizo, C.; Tobias, I. Modifying the solar spectrum to enhance silicon solar cell efficiency—An overview of available materials. Sol. Energy Mater. Sol. Cells 2007, 91, 238–249. [Google Scholar] [CrossRef]
- Duan, Q.; Qin, F.; Zhang, Z.; Cao, W. Quantum cutting mechanism in NaYF4:Tb3+, Yb3+. Opt. Lett. 2012, 37, 521–523. [Google Scholar] [CrossRef]
- Duan, Q.; Qin, F.; Wang, D.; Xu, W.; Cheng, J.; Zhang, Z.; Cao, W. Quantum cutting mechanism in Tb3+-Yb3+ co-doped oxyfluoride glass. J. Appl. Phys. 2011, 110, 113503. [Google Scholar] [CrossRef] [Green Version]
- Meijer, J.-M.; Aarts, L.; van der Ende, B.; Vlugt, T.J.H.; Meijerink, A. Downconversion for solar cells inYF3:Nd3+, Yb3+. Phys. Rev. B 2010, 81. [Google Scholar] [CrossRef]
- Deng, K.; Wei, X.; Wang, X.; Chen, Y.; Yin, M. Near-infrared quantum cutting via resonant energy transfer from Pr3+ to Yb3+ in LaF3. Appl. Phys. A 2011, 102, 555–558. [Google Scholar] [CrossRef]
- Liu, X.; Ye, S.; Qiao, Y.; Dong, G.; Zhu, B.; Chen, D.; Lakshminarayana, G.; Qiu, J. Cooperative downconversion and near-infrared luminescence of Tb3+–Yb3+ codoped lanthanum borogermanate glasses. Appl. Phys. A 2009, 96, 51–55. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C.; Song, P.; Zhu, P.; Zhu, K.; He, J. Luminescence properties in Tb3+/Yb3+ codoped phosphate glasses for solar cells. J. Alloy. Compd. 2016, 662, 89–93. [Google Scholar] [CrossRef]
- Martin, I.R.; Yanes, A.C.; Méndez-Ramos, J.; Torres, M.E.; Rodríguez, V.D. Cooperative energy transfer in Yb3+–Tb3+ codoped silica sol-gel glasses. J. Appl. Phys. 2001, 89, 2520–2524. [Google Scholar] [CrossRef]
- Dos Santos, J.; Zanuto, V.; Soares, A.; Savi, E.; Nunes, L.; Baesso, M.; Catunda, T. Evaluating the link between blue-green luminescence and cross-relaxation processes in Tb3+-doped glasses. J. Lumin. 2021, 240, 118430. [Google Scholar] [CrossRef]
- Puchalska, M.; Zych, E.; Watras, A. Cooperative up-conversion processes in SrAl4O7:Yb and SrAl4O7:Yb,Tb and their dependence on charge compensation by Na. J. Lumin. 2017, 183, 185–192. [Google Scholar] [CrossRef]
- Grzyb, T.; Gruszeczka, A.; Wiglusz, R.J.; Lis, S. The effects of down- and up-conversion on dual-mode green luminescence from Yb3+- and Tb3+-doped LaPO4 nanocrystals. J. Mater. Chem. C 2013, 1, 5410–5418. [Google Scholar] [CrossRef]
- Huang, X.; Yu, D.C.; Zhang, Q.Y. Enhanced near-infrared quantum cutting in GdBO3:Tb3+,Yb3+ phosphors by Ce3+ codoping. J. Appl. Phys. 2009, 106, 113521. [Google Scholar] [CrossRef]
- Terra, I.; Borrero-González, L.; Figueredo, T.; Almeida, J.; Hernandes, A.; Nunes, L.; Malta, O. Down-conversion process in Tb3+–Yb3+ co-doped Calibo glasses. J. Lumin. 2012, 132, 1678–1682. [Google Scholar] [CrossRef]
- Parent, C.; Lurin, C.; Le Flem, G.; Hagenmuller, P. Nd3+ → Yb3+ energy transfer in glasses with composition close to LiLnP4O12 metaphosphate (Ln = La, Nd, Yb). J. Lumin. 1986, 36, 49–55. [Google Scholar] [CrossRef]
- Kochanowicz, M.; Dorosz, D.; Zmojda, J.; Dorosz, J.; Pisarska, J.; Pisarski, W. Up-conversion luminescence of Tb3+ ions in germanate glasses under diode-laser excitation of Yb3+. Opt. Mater. Express 2014, 4, 1050–1056. [Google Scholar] [CrossRef]
- Puchalska, M.; Zych, E.; Sobczyk, M.; Watras, A.; Dereń, P. Cooperative energy transfer in Yb3+–Tb3+ co-doped CaAl4O7 upconverting phosphor. Mater. Chem. Phys. 2015, 156, 220–226. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benrejeb, H.; Soler-Carracedo, K.; Lozano-Gorrín, A.D.; Hraiech, S.; Martin, I.R. Energy Transfer Studies in Tb3+-Yb3+ Co-Doped Phosphate Glasses. Materials 2021, 14, 6782. https://doi.org/10.3390/ma14226782
Benrejeb H, Soler-Carracedo K, Lozano-Gorrín AD, Hraiech S, Martin IR. Energy Transfer Studies in Tb3+-Yb3+ Co-Doped Phosphate Glasses. Materials. 2021; 14(22):6782. https://doi.org/10.3390/ma14226782
Chicago/Turabian StyleBenrejeb, Hadil, Kevin Soler-Carracedo, Antonio Diego Lozano-Gorrín, Sana Hraiech, and Inocencio Rafael Martin. 2021. "Energy Transfer Studies in Tb3+-Yb3+ Co-Doped Phosphate Glasses" Materials 14, no. 22: 6782. https://doi.org/10.3390/ma14226782
APA StyleBenrejeb, H., Soler-Carracedo, K., Lozano-Gorrín, A. D., Hraiech, S., & Martin, I. R. (2021). Energy Transfer Studies in Tb3+-Yb3+ Co-Doped Phosphate Glasses. Materials, 14(22), 6782. https://doi.org/10.3390/ma14226782





