Resistance Switching Effect of Memory Device Based on All-Inorganic Cspbbri2 Perovskite
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of CsPbBrI2 Perovskite Precursor Solution
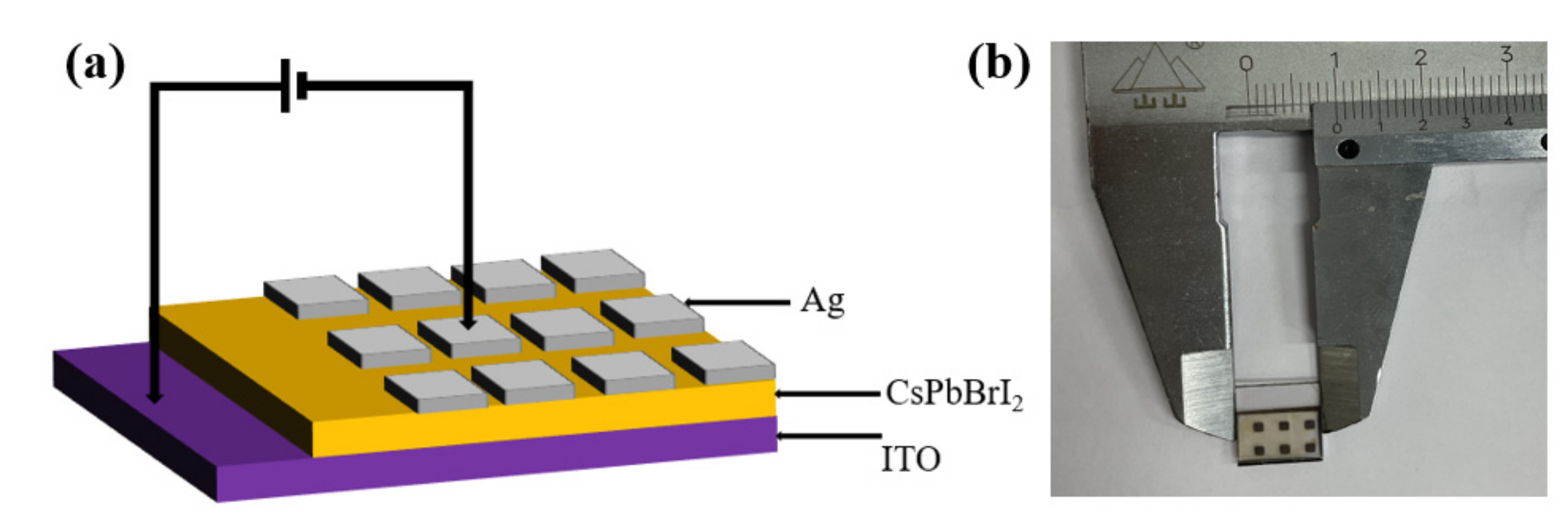
2.2. Ag/CsPbBrI2/ITO Memory Device Fabrication
2.3. Analysis and Characterization of Device
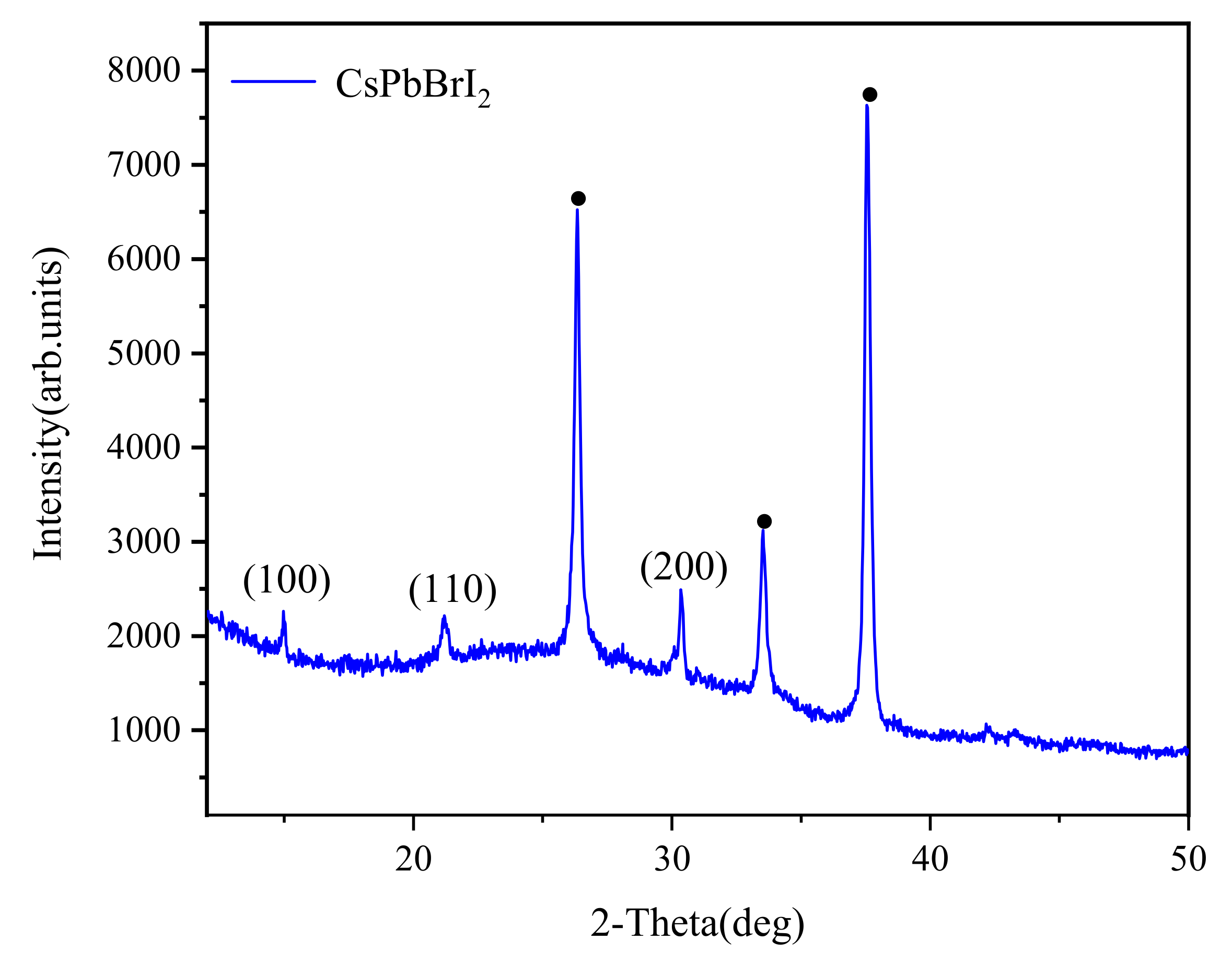
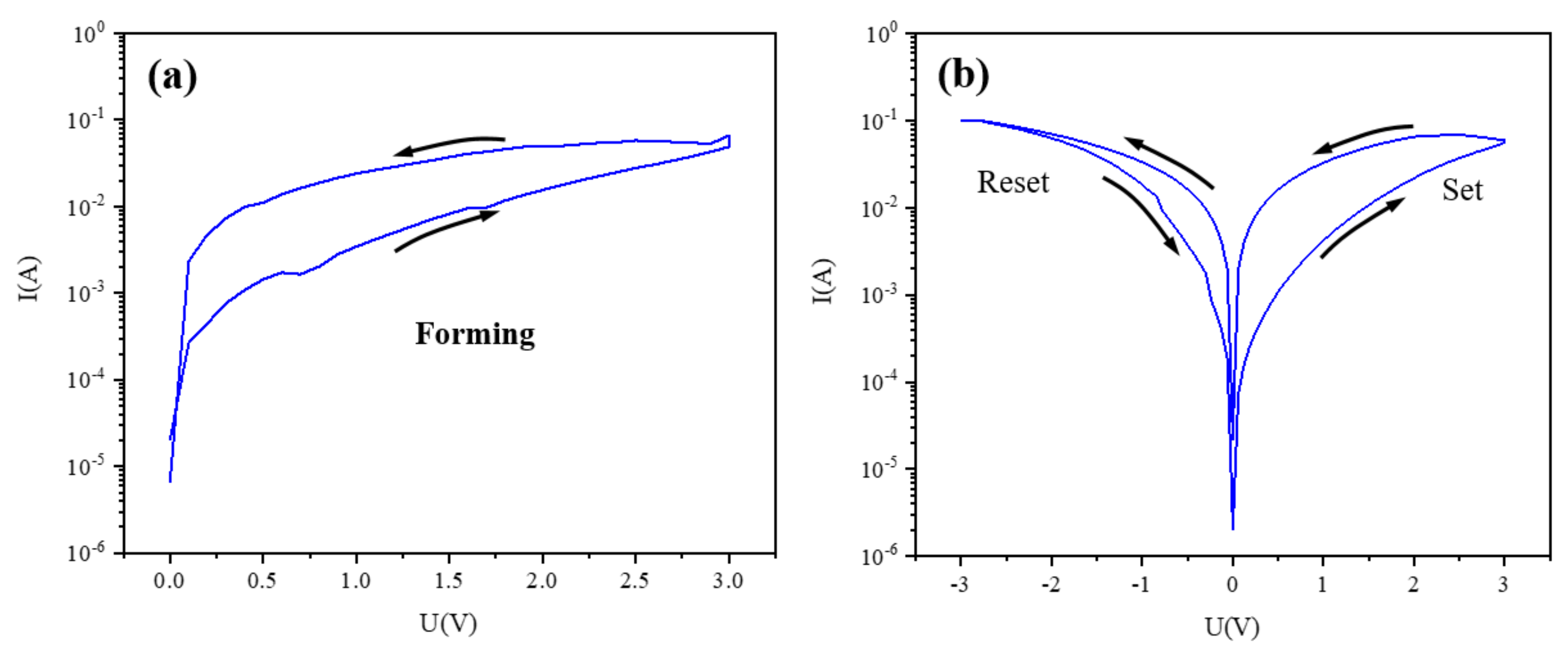
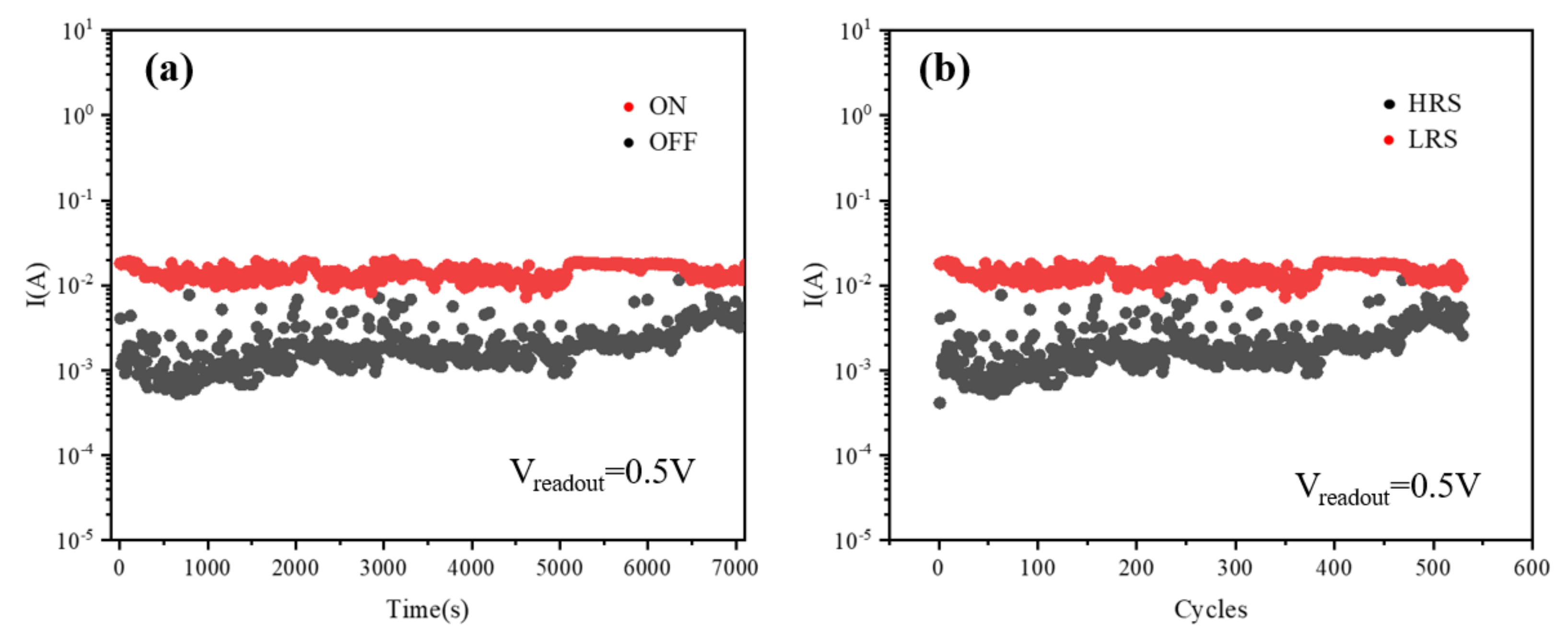
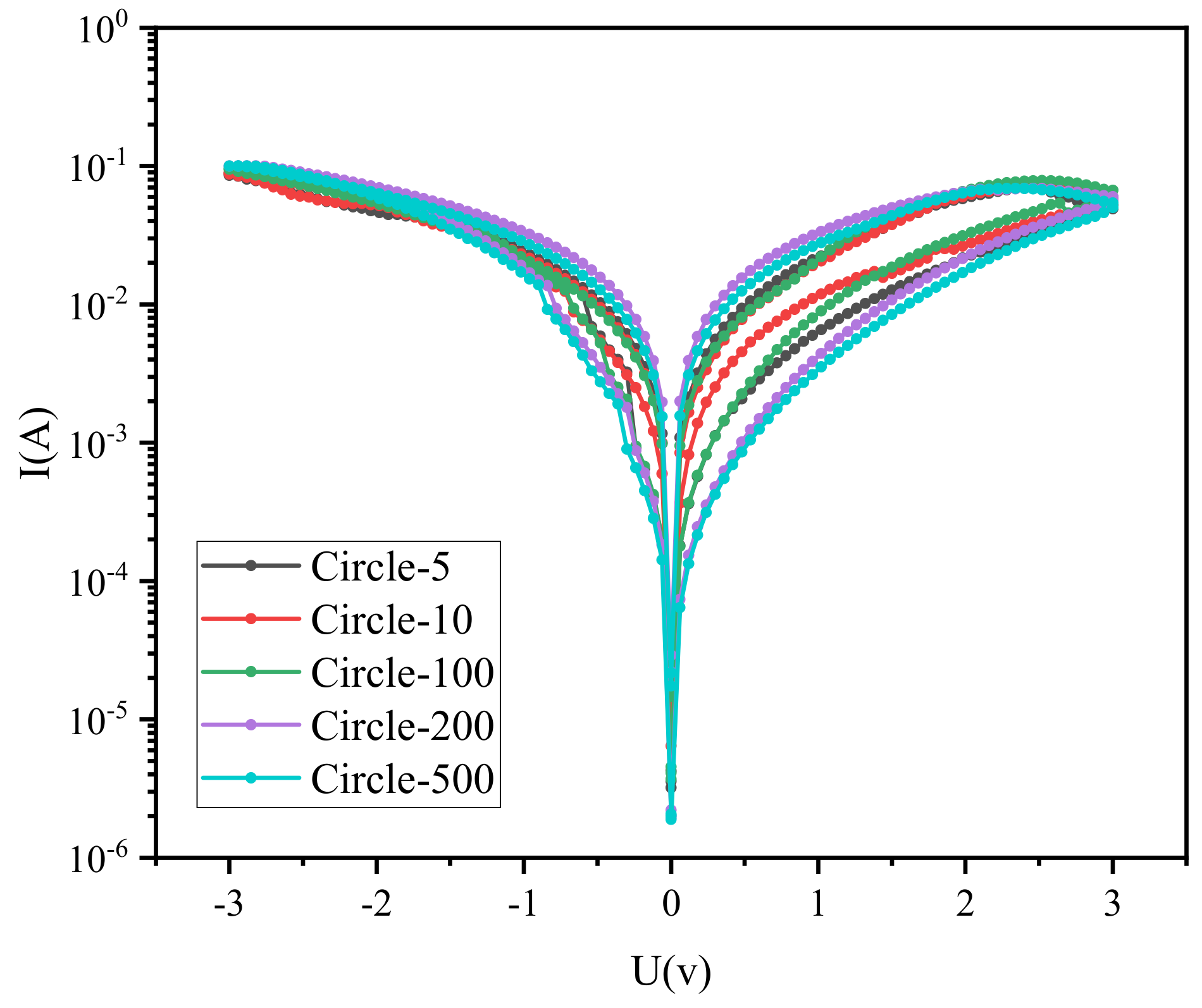
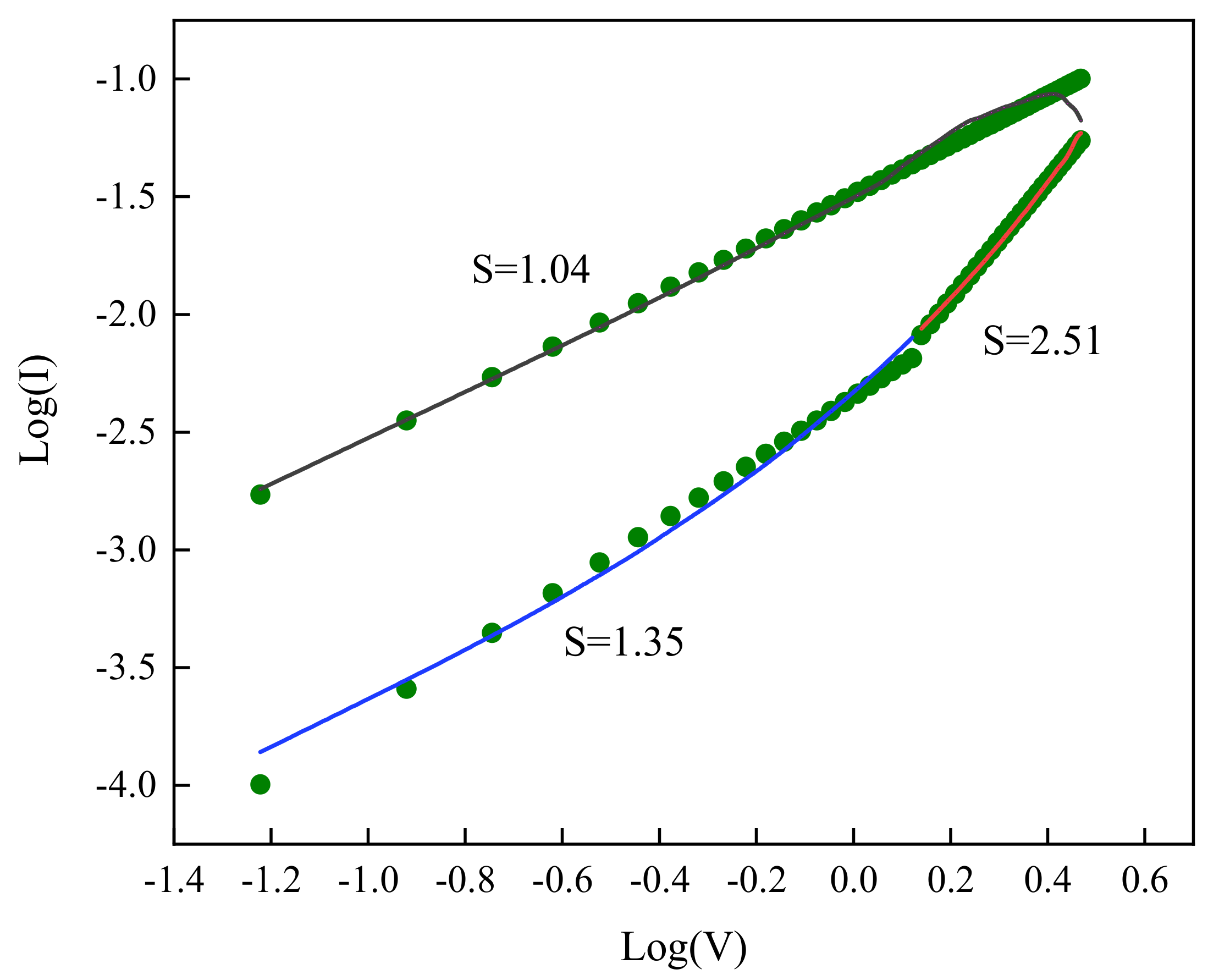
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Correa-Baena, J.P.; Abate, A.; Saliba, M.; Tress, W.; Jacobsson, T.J.; Grätzel, M.; Hagfeldt, A. The rapid evolution of highly efficient perovskite solar cells. Energy Environ. Sci. 2017, 10, 710–727. [Google Scholar] [CrossRef]
- Luo, J.M.; Lin, S.P.; Zheng, Y.; Wang, B. Nonpolar resistive switching in Mn-doped BiFeO3 thin films by chemical solution deposition. Appl. Phys. Lett. 2012, 101, 2632. [Google Scholar] [CrossRef]
- Yang, J.J.; Pickett, M.D.; Li, X.; Ohlberg, D.A.; Stewart, D.R.; Williams, R.S. Memristive Switching Mechanism for Meta. Nat. Nanotechnol. 2008, 3, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Wang, W.; Zhang, C.; Jin, Y.; Zhu, G.; Sun, Q.; Zhang, D.W.; Zhou, P.; Xiu, F. Tunable charge-trap memory based on few-layer MoS2. ACS Nano 2015, 9, 612. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.; Khan, M.F.; Rahmani, M.K.; Kim, H.; Patil, H.; Khan, S.A.; Kang, M.H.; Kim, D.K. Neuro-Transistor Based on UV-Treated Charge Trapping in MoTe 2 for Artificial Synaptic Features. Nanomaterials 2020, 10, 2326. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, Y.; Lee, S.-K.; Ruan, G.; Kim, T.-W.; Fei, H.; Lee, S.-H.; Kim, D.-Y.; Yoon, J.; Tour, J.M. Flexible Nanoporous WO3−x Nonvolatile Memory Device. ACS Nano 2016, 10, 7598–7603. [Google Scholar] [CrossRef]
- Porro, S.; Risplendi, F.; Cicero, G.; Bejtka, K.; Milano, G.; Rivolo, P.; Jasmin, A.; Chiolerio, A.; Pirri, C.F.; Ricciardi, C. Multiple Resistive Switching in Core-Shell ZnO Nanowires Exhibiting Tunable Surface States. J. Mater. Chem. C 2017, 5, 10517–10523. [Google Scholar] [CrossRef]
- Herpers, A.; Lenser, C.; Park, C.; Offi, F.; Borgatti, F.; Panaccione, G.; Menzel, S.; Waser, R.; Dittmann, R. Spectroscopic Proof of the Correlation between Redox-State and Charge-Carrier Transport at the Interface of Resistively Switching Ti/PCMO Devices. Adv. Mater. 2014, 26, 2730–2735. [Google Scholar] [CrossRef]
- Arndt, B.; Herpers, A.; Lenser, C.; Offi, F.; Borgatti, F.; Panaccione, G.; Dittmann, R.; Waser, R. Resistive switching characteristics of Pr0.48Ca0.52ManO3 heterostructures. In Proceedings of the DPG Frühjahrstagung DPG2014, Dresden, Germany, 30 March–4 April 2014. [Google Scholar]
- Hota, M.K.; Hedhili, M.N.; Wehbe, N.; McLachlan, M.A.; Alshareef, H.N. Multistate Resistive Switching Memory for Synaptic Memory Applications. Adv. Mater. Interfaces 2016, 3, 1600192. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, L.; Liu, G.; Liu, L. Non-Destructive Photovoltaic Reading of Interface Type Memristors Using Graphene as Transparent Electrode. J. Alloys Compd. 2018, 740, 273–277. [Google Scholar]
- Guan, X.; Hu, W.; Haque, M.A.; Wei, N.; Liu, Z.; Chen, A.; Wu, T. Light-Responsive Ion-Redistribution-Induced Resistive Switching in Hybrid Perovskite Schottky Junctions. Adv. Funct. Mater. 2018, 28, 1704665. [Google Scholar] [CrossRef]
- Xiao, J.W.; Liu, L.; Zhang, D.; Marco, N.D.; Lee, J.W.; Lin, O.; Chen, Q.; Yang, Y. The Emergence of the Mixed Perovskites and Their Applications as Solar Cells. Adv. Energy Mater. 2017, 7, 1700491. [Google Scholar] [CrossRef]
- Park, Y.H.; Jeong, I.; Bae, S.; Son, H.J.; Lee, P.; Lee, J.; Lee, C.H.; Ko, M.J. Inorganic Rubidium Cation as an Enhancer for Photovoltaic Performance and Moisture Stability of HC(NH2)2PbI3 Perovskite Solar Cells. Adv. Funct. Mater. 2017, 27, 1605988. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, S.; Li, W.; Liu, H.; Zhu, L.; Yang, S. Inorganic Perovskite Solar Cells: A Rapidly Growing Field. Sol. RRL 2018, 2, 1700188. [Google Scholar] [CrossRef]
- Kim, D.J.; Tak, Y.J.; Kim, W.G.; Kim, J.K.; Kim, J.H.; Kim, H.J. Resistive Switching Properties through Iodine Migrations of a Hybrid Perovskite Insulating Layer. Adv. Mater. Interfaces 2017, 4, 1601035. [Google Scholar] [CrossRef]
- Beal, R.E.; Slotcavage, D.J.; Leijtens, T.; Bowring, A.R.; Belisle, R.A.; Nguyen, W.H.; Burkhard, G.F.; Hoke, E.T.; McGehee, M.D. Cesium Lead Halide Perovskites with Improved Stability for Tandem Solar Cells. J. Phys. Chem. Lett. 2016, 7, 746–751. [Google Scholar] [CrossRef]
- Han, Y.; Meyer, S.; Dkhissi, Y.; Weber, K.; Pringle, J.M.; Bach, U.; Spiccia, L.; Cheng, Y.-B. Degradation Observations of Encapsulated Planar CH3NH3PbI3 Perovskite Solar Cells at High Temperatures and Humidity. J. Mater. Chem. A 2015, 3, 8139–8147. [Google Scholar] [CrossRef]
- Salado, M.; Calio, L.; Berger, R.; Kazim, S.; Ahmad, S. Influence of the Mixed Organic Cation Ratio in Lead Iodide Based Perovskite on the Performance of Solar Cells. Phys. Chem. Chem. Phys. 2016, 18, 27148–27157. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Liu, J.; Jin, Z. All-Inorganic Halide Perovskites for Optoelectronics: Progress and Prospects. Sol. RRL 2017, 1, 1700086. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, Y.; He, B.; Tang, Q. High-Purity Inorganic Perovskite Films for Solar Cells with 9.72% Efficiency. Angew. Chem. Int. Ed. 2018, 57, 3787–3791. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.-S.; Wei, S.-H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Eperon, G.E.; Paterno, G.M.; Sutton, R.J.; Zampetti, A.; Haghighirad, A.A.; Cacialli, F.; Snaith, H.J. Inorganic Caesium Lead Iodide Perovskite Solar Cells. J. Mater. Chem. A 2015, 3, 19688–19695. [Google Scholar] [CrossRef]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum Dot-Induced Phase Stabilization of α-CsPbI3 Perovskite for High-Efficiency Photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Li, W.; Zhang, C.; Ma, Y.; Fan, J.; Mai, Y. All-Inorganic CsPbI2Br Perovskite Solar Cells with High Efficiency Exceeding 13%. J. Am. Chem. Soc. 2018, 140, 3825–3828. [Google Scholar] [CrossRef]
- Sutton, R.J.; Eperon, G.E.; Miranda, L.; Parrott, E.S.; Kamino, B.A.; Patel, J.B.; Hörantner, M.T.; Johnston, M.B.; Haghighirad, A.A.; Moore, D.T. Bandgap-Tunable Cesium Lead Halide Perovskites with High Thermal Stability for Efficient Solar Cell. Adv. Energy Mater. 2016, 6, 1502458. [Google Scholar] [CrossRef]
- Lau, C.F.J.; Deng, X.; Ma, Q.; Zheng, J.; Yun, J.S.; Green, M.A.; Huang, S.; Ho-Baillie, A.W. CsPbIBr2 Perovskite Solar Cell by Spray-Assisted Deposition. ACS Energy Lett. 2016, 1, 573–577. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Chen, J.; Fan, J.; Mai, Y.; Schropp, R.E. Ultra- Thin MoOx as Cathode Buffer Layer for the Improvement of All- Inorganic CsPbIBr2 Perovskite Solar Cells. Nano Energy 2017, 41, 75–83. [Google Scholar] [CrossRef]
- Han, J.S.; Le, Q.V.; Choi, J.; Hong, K.; Moon, C.W.; Kim, T.L.; Kim, H.; Kim, S.Y.; Jang, H.W. Air-Stable Cesium Lead Iodide Perovskite for Ultra-Low Operating Voltage Resistive Switching. Adv. Funct. Mater. 2018, 28, 1705783. [Google Scholar] [CrossRef]
- Hu, Y.; Bai, F.; Liu, X.; Ji, Q.; Miao, X.; Qiu, T.; Zhang, S. Bismuth Incorporation Stabilized α-CsPbI3 for Fully Inorganic Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 2219–2227. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, D.; Zhou, L.; Zhang, C.; Chang, J.; Lin, Z.; Zhang, J.; Hao, Y. Intermediate Phase Intermolecular Exchange Triggered Defect Elimination in CH3NH3PbI3 toward Room- Temperature Fabrication of Efficient Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 40378–40385. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Wang, D.-S.; Wang, J.; Zhao, W.-L.; Wang, C.-W. High Transmittance and Superhydrophilicity of Porous TiO2/SiO2 bilayer Films without UV Rrradiation. Surf. Coatings Technol. 2011, 205, 3596–3599. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, L.; Yan, H.; Guo, S.; Wang, S.; Wang, M.; Jin, K. Bandgap Narrowing in Bi-Doped CH3NH3PbCl3 Perovskite Single Crystals and Thin Films. J. Phys. Chem. C 2017, 121, 17436–17441. [Google Scholar] [CrossRef]
- Nam, J.K.; Jung, M.S.; Chai, S.U.; Choi, Y.J.; Kim, D.; Park, J.H. Unveiling the Crystal Formation of Cesium Lead Mixed-Halide Perovskites for Efficient and Stable Solar Cells. J. Phys. Chem. Lett. 2017, 8, 2936–2940. [Google Scholar] [CrossRef]
- Bae, D.; Palmstrom, A.; Roelofs, K.; Mei, B.; Chorkendorff, I.; Bent, S.F.; Vesborg, P.C. Tailoring Mixed-Halide, Wide-Gap Perovskites via Multistep Conversion Process. ACS Appl. Mater. Interfaces 2016, 8, 14301. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Zhang, Q.; Zhang, C.; Zhang, Z.; Chen, D.; Lin, Z.; Chang, J.; Zhang, J.; Hao, Y. Aged Precursor Solution toward Low-Temperature Fabrication of Efficient Carbon-Based All-Inorganic Planar CsPbIBr2 Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 4991–4997. [Google Scholar] [CrossRef]
- Li, W.; Rothmann, M.U.; Liu, A.; Wang, Z.; Zhang, Y.; Pascoe, A.R.; Lu, J.; Jiang, L.; Chen, Y.; Huang, F.; et al. Phase Segregation Enhanced Ion Movement in Efficient Inorganic CsPbIBr 2 Solar Cells. Adv. Energy Mater. 2017, 7, 1700946. [Google Scholar] [CrossRef]
- Li, C.; Song, Z.; Zhao, D.; Xiao, C.; Subedi, B.; Shrestha, N.; Junda, M.M.; Wang, C.; Jiang, C.-S.; Al-Jassim, M.; et al. Reducing Saturation-Current Density to Realize High-Efficiency Low-Bandgap Mixed Tin-Lead Halide Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1803135. [Google Scholar] [CrossRef]
- Du, Y.; Pan, H.; Wang, S.; Wu, T.; Feng, Y.P.; Pan, J.; Wee, A.T.S. Symmetrical negative differential resistance behavior of a resistive switching device. ACS Nano 2012, 6, 2517. [Google Scholar] [CrossRef]
- Fu, Y.J.; Xia, F.J.; Jia, Y.L.; Jia, C.J.; Li, J.Y.; Dai, X.H.; Fu, G.S.; Zhu, B.Y.; Liu, B.T. Bipolar Resistive Switching Behavior of La0.5Sr0.5CoO3−σ Films for Nonvolatile Memory Applications. Appl. Phys. Lett. 2014, 104, 223505. [Google Scholar] [CrossRef]
- Jing, M.; Song, W.; Chen, L.; Ma, S.; Deng, J.; Zheng, H.; Li, Y.; Liu, J.; Zhao, Z. Density Functional Theory Study of the Formaldehyde Catalytic Oxidation Mechanism on a Au-Doped CeO2(111) Surface. J. Phys. Chem. C. Nanomater. Interf. 2018, 122, 438–448. [Google Scholar]
- Kuan-Liang, L.; Tuo-Hung, H.; Yao-Jen, L.; Jhe-Wei, C.; Jun-Hung, L.; Jiann, S.; Cheng-Tung, C.; Tan-Fu, L.; Wen-Hsiung, C.; Wen-Yueh, J.; et al. Switching Mode and Mechanism in Binary Oxide Resistive Random Access Memory Using Ni Electrode. J. Appl. Phys. 2013, 52, 031801. [Google Scholar]
- Lin, Q.; Hu, W.; Zang, Z.; Zhou, M.; Du, J.; Wang, M.; Han, S.; Tang, X. Transient Resistive Switching Memory of CsPbBr3 Thin Films. Adv. Electron. Mater. 2018, 4, 1700596. [Google Scholar] [CrossRef]
- Chen, R.; Xu, J.; Lao, M.; Liang, Z.; Chen, Y.; Zhong, C.; Huang, L.; Hao, A.; Ismail, M. Transient Resistive Switching for Nonvolatile Memory Based on Water–Soluble Cs4PbBr6 Perovskite Films. Phys. Status Solidi RRL Rapid Res. Lett. 2019, 13, 1900397. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, W.; Yang, X.; Liu, T. Resistance Switching Effect of Memory Device Based on All-Inorganic Cspbbri2 Perovskite. Materials 2021, 14, 6629. https://doi.org/10.3390/ma14216629
Ke W, Yang X, Liu T. Resistance Switching Effect of Memory Device Based on All-Inorganic Cspbbri2 Perovskite. Materials. 2021; 14(21):6629. https://doi.org/10.3390/ma14216629
Chicago/Turabian StyleKe, Wang, Xiaoting Yang, and Tongyu Liu. 2021. "Resistance Switching Effect of Memory Device Based on All-Inorganic Cspbbri2 Perovskite" Materials 14, no. 21: 6629. https://doi.org/10.3390/ma14216629
APA StyleKe, W., Yang, X., & Liu, T. (2021). Resistance Switching Effect of Memory Device Based on All-Inorganic Cspbbri2 Perovskite. Materials, 14(21), 6629. https://doi.org/10.3390/ma14216629




