High-Temperature Chemical Stability of Cr(III) Oxide Refractories in the Presence of Calcium Aluminate Cement
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Pre-Synthesis of (Al1−xCrx)2O3 Powders
3.2. Cr(VI) Leachability
3.3. Phase Evolution of the Castables
3.4. Reaction Mechanism
4. Conclusions
- (1)
- Compared with Cr2O3, the stability of the (Al1−xCrx)2O3 solid solution in contact with CAC was much higher. Furthermore, the substitution of Cr2O3 with (Al1−xCrx)2O3 in the Al2O3-CaO-Cr2O3 castables can completely inhibit the mid-temperature (300–1100 °C) formation of Cr(VI) compound CaCrO4 and relatively higher temperature Cr(VI) phase Ca4Al6CrO16 (hauyne) drastically reduced at 900 to 1300 °C. Therefore, replacing Cr2O3 with (Al1−xCrx)2O3 can effectively lower the Cr(VI) concentration of the castables after being treated at various temperatures, and a reduction in Cr(VI) amounts up to 98.1% with (Al1−xCrx)2O3 addition could be achieved. Most importantly, Cr(III) present within the in situ (Al1−xCrx)2O3 and Ca(Al,Cr)12O19 solid solution phases showed maximum reoxidation resistance and thus need further investigation.
- (2)
- In comparison with the CA2 phase, CA was more likely to react with Cr2O3/(Al1−xCrx)2O3, resulting in Cr(VI) compound formation. Simultaneously, calcium in CA6 was much more stable than in CA and CA2, which only caused slight oxidation of Cr2O3 and would not undergo a chemical reaction with (Al1−xCrx)2O3 solid solution. Thus, incorporating some Al2O3 powders in the matrix of the Al2O3-CaO-Cr2O3 castables to form CA6 at a temperature above 1300 °C was also essential for inhibiting Cr(VI) formation when using (Al1−xCrx)2O3 solid solution as a substitute for Cr2O3.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weinberg, A.V.; Varona, C.; Chaucherie, X.; Goeuriot, D.; Poirier, J. Extending refractory lifetime in rotary kilns for hazardous waste incineration. Ceram. Int. 2016, 42, 17626–17634. [Google Scholar] [CrossRef]
- Kaneko, T.K.; Zhu, J.; Howell, N.; Rozelle, P.; Sridhar, S. The effects of gasification feedstock chemistries on the infiltration of slag into the porous high chromia refractory and their reaction products. Fuel 2014, 115, 248–263. [Google Scholar] [CrossRef]
- Nath, M.; Song, S.; Liu, H.; Li, Y. CaAl2Cr2O7: Formation, Synthesis, and Characterization of a New Cr(III) Compound under Air Atmosphere in the Al2O3-CaO-Cr2O3 System. Ceram. Int. 2019, 45, 16476–16481. [Google Scholar] [CrossRef]
- Nath, M.; Song, S.; Liao, N.; Xu, T.; Liu, H.; Tripathi, H.S.; Li, Y. Co-existence of a Cr3+ phase (CaAl2Cr2O7) with hydraulic calcium aluminate phases at high-temperature in the Al2O3-CaO-Cr2O3 system. Ceram. Int. 2021, 47, 2624–2630. [Google Scholar] [CrossRef]
- Perez, I.; Moreno-Ventas, I.; Rios, G. Chemical degradation of magnesia-chromite refractory used in the conversion step of the pyrometallurgical copper-making process: A thermochemical approach. Ceram. Int. 2018, 44, 18363–18375. [Google Scholar] [CrossRef]
- Wu, Y.; Song, S.; Xue, Z.; Nath, M. Formation mechanisms and leachability of hexavalent chromium in Cr2O3-containing refractory castables of Electric Arc Furnace cover. Mater. Metallur. Trans. B 2019, 50, 808–815. [Google Scholar] [CrossRef]
- da Luz, A.P.; Braulio, M.A.L.; Pandolfelli, V.C. Refractory Castable Engineering; Goller Verlag GmbH: Baden, Germany, 2015. [Google Scholar]
- Tomšů, F.; Palčo, S. Refractory monolithics versus shaped refractory products. Interceram. Int. Ceram. Rev. 2017, 66, 20–23. [Google Scholar] [CrossRef]
- Nath, M.; Song, S.; Li, Y.; Xu, Y. Effect of Cr2O3 addition on corrosion mechanism of refractory castables for waste melting furnaces and concurrent formation of hexavalent chromium. Ceram. Int. 2018, 44, 2383–2389. [Google Scholar] [CrossRef]
- Heikal, M.; Radwan, M.M.; Al-Duaij, O.K. Physico-mechanical characteristics and durability of calcium aluminate blended cement subject to different aggressive media. Constr. Build. Mater. 2015, 78, 379–385. [Google Scholar] [CrossRef]
- Verbinnen, B.; Billen, P.; Coninckxloo, M.V.; Vandecasteele, C. Heating temperature dependence of Cr(III) oxidation in the presence of alkali and alkaline earth salts and subsequent Cr(VI) leaching behavior. Environ. Sci. Technol. 2013, 47, 5858–5863. [Google Scholar] [CrossRef]
- Garcia-Ramos, E.; Romero-Serrano, A.; Zeifert, B.; Flores-Sanchez, P.; Hallen-Lopez, M.; Palacios, E.G. Immobilization of chromium in slags using MgO and Al2O3. Steel. Res. Int. 2008, 79, 332–339. [Google Scholar] [CrossRef]
- Mao, L.; Gao, B.; Deng, N.; Liu, L.; Cui, H. Oxidation behavior of Cr(III) during thermal treatment of chromium hydroxide in the presence of alkali and alkaline earth metal chlorides. Chemosphere 2016, 145, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Nandi, R.; Saha, B. Sources and toxicity of hexavalent chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Bishop, M.E.; Glasser, P.; Dong, H.; Arey, B.; Kovarik, L. Reduction and immobilization of hexavalent chromium by microbially reduced Fe-bearing clay minerals. Geochim. Et Cosmochim. Acta 2014, 133, 186–203. [Google Scholar] [CrossRef]
- Lee, Y.; Nassaralla, C.L. Minimization of hexavalent chromium in magnesite-chrome refractory. Met. Mater. Trans. A 1997, 28, 855–859. [Google Scholar] [CrossRef]
- Chen, J.; Jiao, F.; Zhang, L.; Yao, H.; Ninomiya, Y. Elucidating the mechanism of Cr(VI) formation upon the interaction with metal oxides during coal oxy-fuel combustion. J. Hazar. Mater. 2013, 261, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Nath, M.; Xu, Y.; Li, Y.; Liao, N.; Sang, S. Thermal evolution of Al2O3–CaO–Cr2O3 castables in different atmospheres. Ceram. Int. 2021, 47, 11043–11051. [Google Scholar] [CrossRef]
- Mao, L.; Deng, N.; Liu, L.; Cui, H.; Zhang, W. Effects of Al2O3, Fe2O3, SiO2 on Cr(VI) formation during heating of solid waste containing Cr(III). Chem. Eng. J. 2016, 304, 216–222. [Google Scholar] [CrossRef]
- Nath, M.; Song, S.; Xu, T.; Wu, Y.; Li, Y. Effective inhibition of Cr(VI) in the Al2O3-CaO-Cr2O3 refractory castables system through silica gel assisted in-situ secondary phase tuning. J. Clean Prod. 2019, 233, 1038–1046. [Google Scholar] [CrossRef]
- Mao, L.; Deng, N.; Liu, L.; Cui, H.; Zhang, W. Inhibition of Cr(III) oxidation during thermal treatment of simulated tannery sludge: The role of phosphate. Chem. Eng. J. 2016, 294, 1–8. [Google Scholar] [CrossRef]
- Lin, S.H.; Chen, C.N.; Juang, R.S. Structure and thermal stability of toxic chromium(VI) species doped onto TiO2 powders through heat treatment. J. Environ. Manag. 2009, 90, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.G.T.; Luz, A.P.; Braulio, M.A.L.; Pandolfelli, V.C. Creep behavior modeling of silica fume containing Al2O3-MgO refractory castables. Ceram. Int. 2012, 38, 327–332. [Google Scholar] [CrossRef]
- Bie, C.; Sang, S.; Li, Y.; Xu, Y.; Qiao, J.; Fang, M. Effects of firing and operating atmospheres on microstructure and properties of phosphate bonded Cr2O3-Al2O3-ZrO2 bricks. China’s Refract. 2015, 49, 168–174. [Google Scholar]
- Wu, Y.; Song, S.; Garbers-Craig, A.M.; Xue, Z. Formation and leachability of hexavalent chromium in the Al2O3-CaO-MgO-Cr2O3 system. J. Eur. Ceram. Soc. 2018, 38, 2649–2661. [Google Scholar] [CrossRef]
- Liu, H.; Song, S.; Garbers-Craig, A.M.; Xue, Z. High-temperature stability of Mg(Al,Cr)2O4 spinel co-existing with calcium aluminates in air. Ceram. Int. 2019, 45, 16166–16172. [Google Scholar] [CrossRef]
- Klyucharov, Y.V.; Eger, V.G. On the reaction between magnesiochromite and calcium oxide. Refractories 1963, 4, 137–144. [Google Scholar] [CrossRef]
- Wu, Y.; Song, S.; Xue, Z.; Nath, M. Effect of temperature on hexavalent chromium formation in (Al,Cr)2O3 with calcium aluminate cement in air. ISIJ Int. 2019, 59, 1178–1183. [Google Scholar] [CrossRef] [Green Version]
- Nath, M.; Song, S.; Garbers-Craig, A.M.; Li, Y. Phase evolution with temperature in chromium-containing refractory castables used for waste melting furnaces and Cr(VI) leachability. Ceram. Int. 2018, 44, 20391–20398. [Google Scholar] [CrossRef]
- Nath, M.; Liao, N.; Xu, T.; Xu, Y.; Wu, Y.; Song, S.; Li, Y. Recent research on Cr-containing refractory castables. Refract. Worldforum 2021, 13, 48–57. [Google Scholar]
- Nath, M.; Ghosh, A.; Tripathi, H.S. Hot corrosion behaviour of Al2O3–Cr2O3 refractory by molten glass at 1200 °C under static condition. Corros. Sci. 2016, 102, 153–160. [Google Scholar] [CrossRef]
- Nath, M.; Kumar, P.; Song, S.; Li, Y.; Tripathi, H.S. Thermo-mechanical stability of bulk (Al1–xCrx)2O3 solid solution. Ceram. Int. 2019, 45, 12411–12416. [Google Scholar] [CrossRef]
- Nath, M.; Dana, K.; Gupta, S.; Tripathi, H.S. Hot corrosion behavior of slip-cast alumina-chrome refractory crucible against molten glass. Mater. Corros. 2014, 65, 742–747. [Google Scholar] [CrossRef]
- Toxicological Review of Hexavalent Chromium. In CAS No. 18540-29-9; EPA: Washington, DC, USA, 1998.
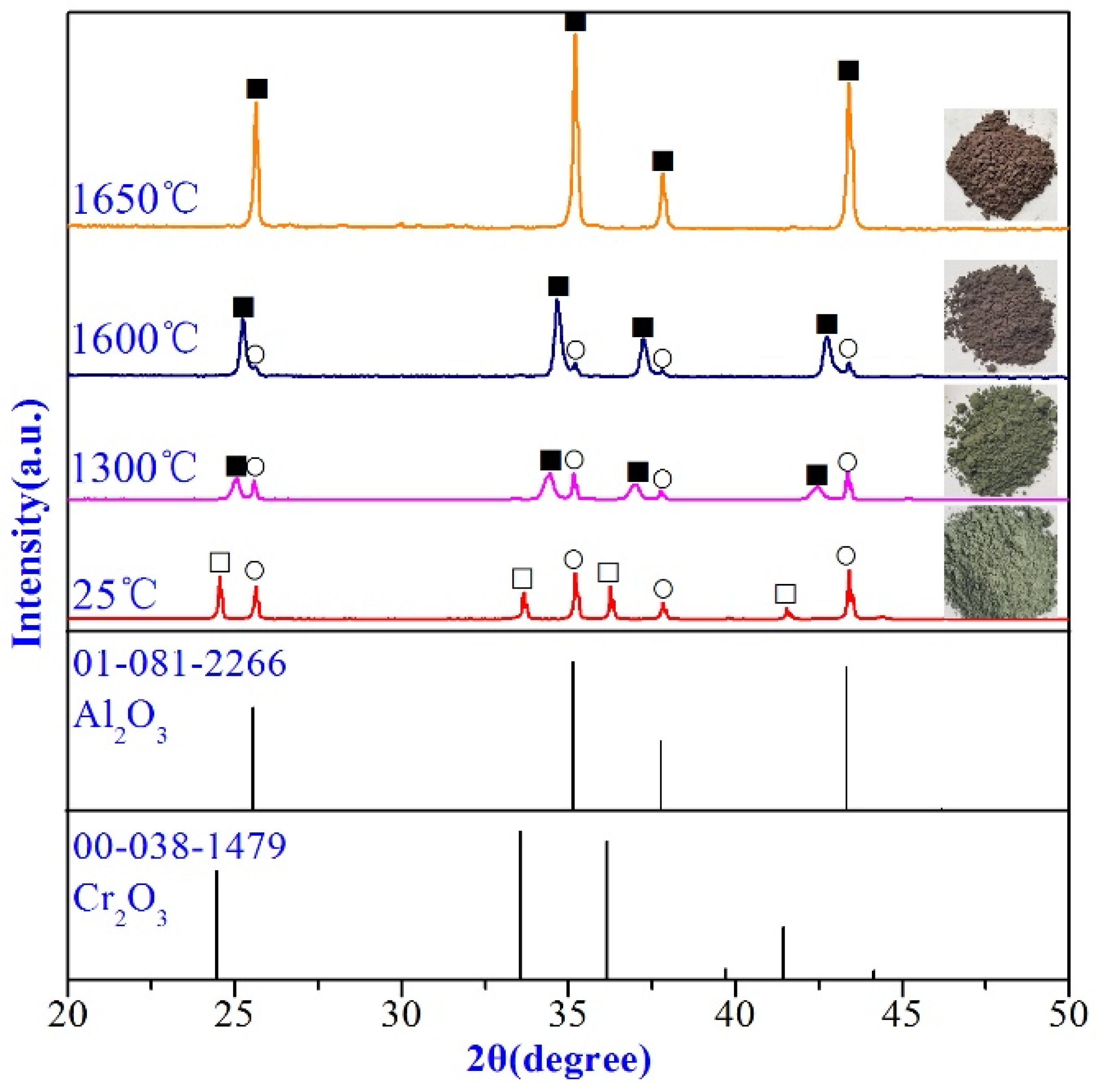

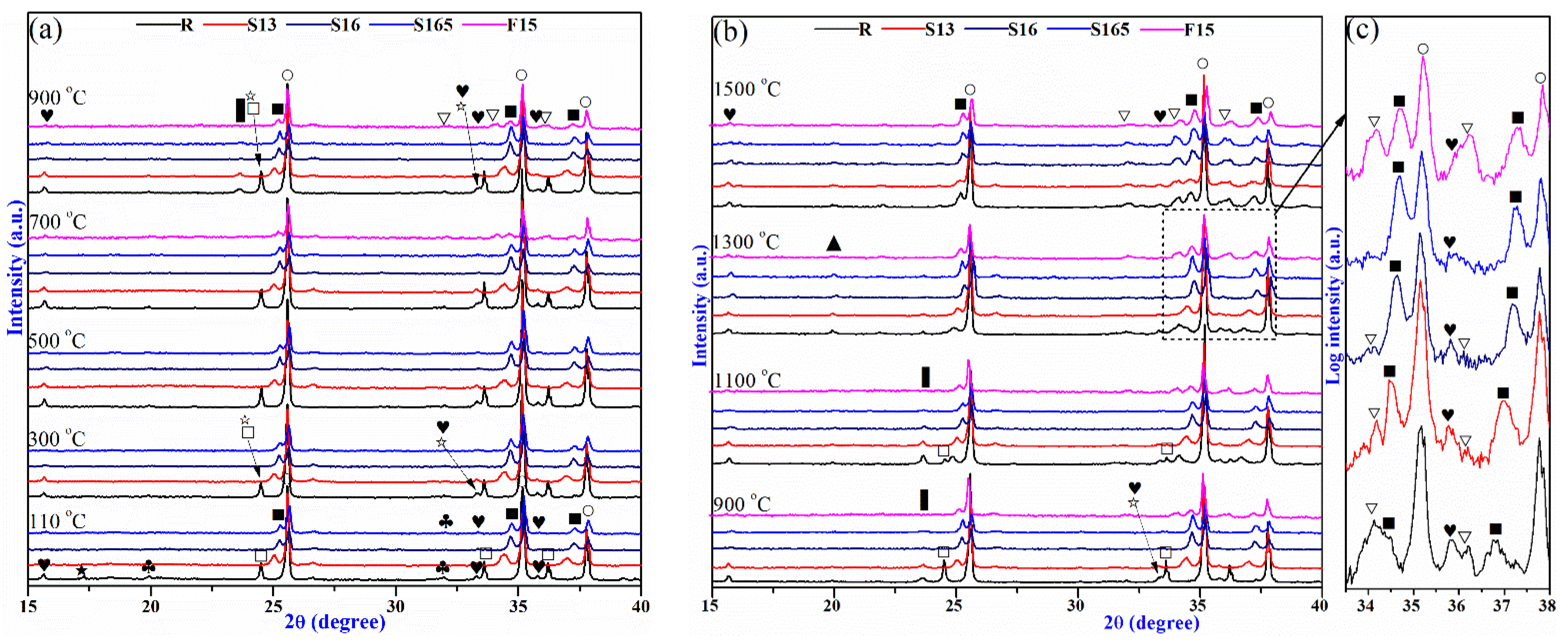
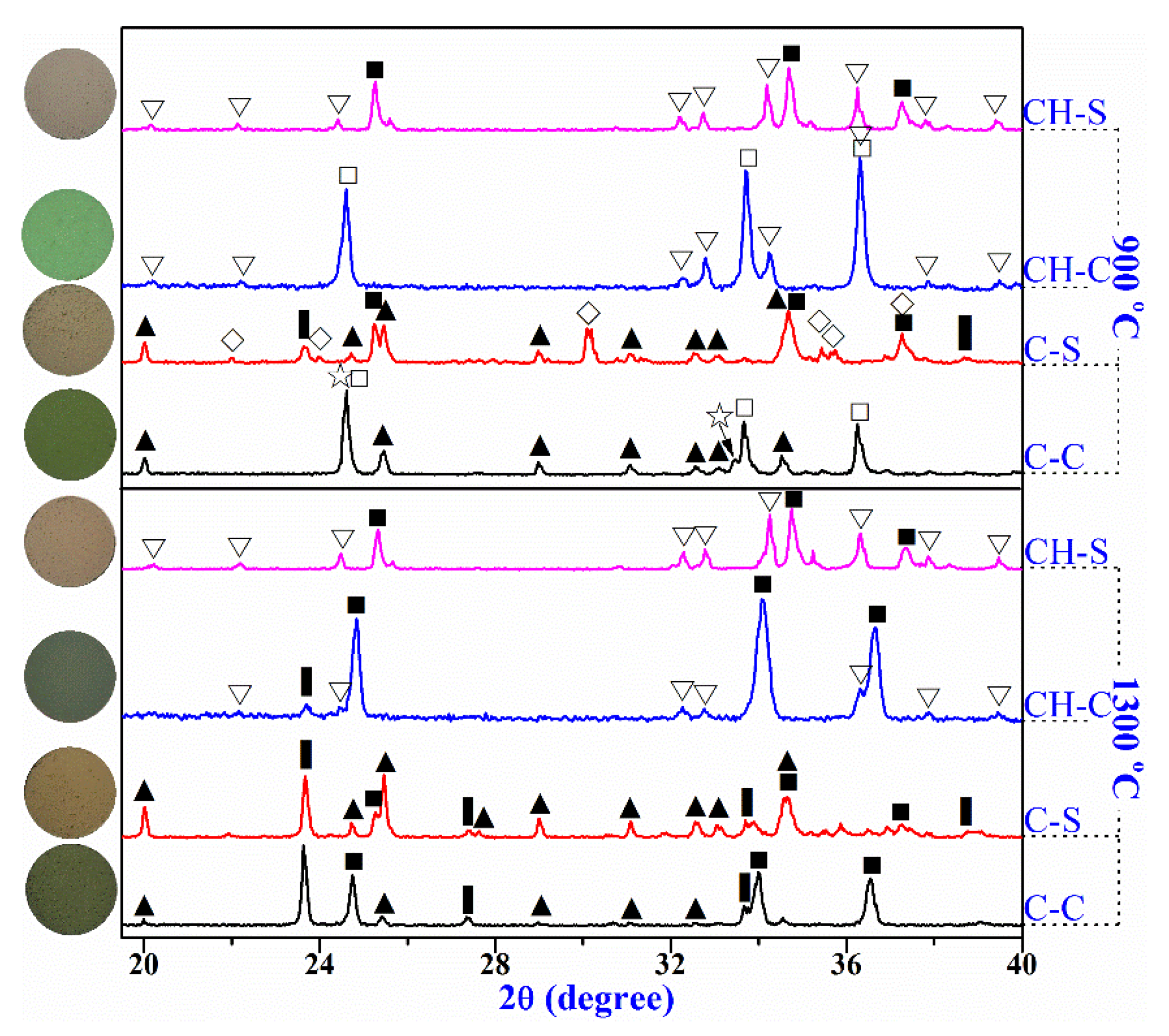

| Raw Materials | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | Na2O | K2O | Cr2O3 |
|---|---|---|---|---|---|---|---|---|
| Tabular alumina | 0.08 | 99.30 | - | 0.02 | - | 0.28 | - | - |
| Reactive α-alumina | 0.28 | 98.87 | 0.07 | 0.13 | 0.12 | 0.10 | 0.005 | - |
| Fused chromium oxide | 0.82 | 0.59 | 0.38 | 0.73 | 0.27 | 0.14 | 0.01 | 94.02 |
| Calcium aluminate cement | 0.40 | 70.6 | 28.4 | 0.20 | - | - | - | - |
| Code | Aggregates Al2O3 (wt%) | Fine Powders (wt%) | Pre-Treatment Temperature (°C) | |||
|---|---|---|---|---|---|---|
| Al2O3 | Cr2O3 | CAC | (Al1−x,Crx)2O3 | |||
| R | 70 | 17 | 8 | 5 | - | - |
| F15 | 70 | 17 | 8 | 5 | - | In situ treated at 1500 |
| S13 | 70 | - | - | 5 | 25 | (Al1−x,Crx)2O3 made at 1300 |
| S16 | 70 | - | - | 5 | 25 | (Al1−x,Crx)2O3 made at 1600 |
| S165 | 70 | - | - | 5 | 25 | (Al1−x,Crx)2O3 made at 1650 |
| Specimens | CAC | CA6 | Cr2O3 | (Al1−x,Crx)2O3 |
|---|---|---|---|---|
| C-C | 50 | 50 | ||
| C-S | 50 | 50 | ||
| CH-C | 50 | 50 | ||
| CH-S | 50 | 50 |
| Specimens | Temperature (°C) | |||||||
|---|---|---|---|---|---|---|---|---|
| 110 | 300 | 500 | 700 | 900 | 1100 | 1300 | 1500 | |
| S13 | 43.7 | −19.5 | 61.7 | 81.9 | 16.1 | 21.2 | 10.5 | 12.6 |
| S16 | 47.4 | 48.6 | 93.4 | 95.0 | 57.2 | 24.0 | 28.0 | 38.7 |
| S165 | 38.5 | 58.0 | 87.4 | 98.1 | 67.6 | 35.8 | −91.4 | −202.4 |
| F15 | - | - | - | 98.9 | 99.1 | 99.0 | 93.5 | −30.8 |
| Specimens | 900 °C | 1300 °C |
|---|---|---|
| C-C | (1) | (3) (4) (5) |
| C-S | (2) | (2) |
| CH-C | - | (5) (6) |
| CH-S | - | - |
| (1) | ||
| (2) | ||
| (3) | ||
| (4) | ||
| (5) | ||
| (6) | ||
| Phase | Al | Ca | Cr | O |
|---|---|---|---|---|
| CaCrO4 | - | 28.36 | 41.27 | 30.37 |
| Ca6Al4CrO16 | 34.27 | 18.58 | 6.34 | 40.82 |
| CaAl2O4 | 40.39 | 16.94 | - | 42.68 |
| CaAl4O7 | 49.87 | 4.13 | - | 46.00 |
| (Al,Cr)2O3 | 48.68 | - | 15.27 | 36.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Xu, Y.; Liao, N.; Li, Y.; Nath, M. High-Temperature Chemical Stability of Cr(III) Oxide Refractories in the Presence of Calcium Aluminate Cement. Materials 2021, 14, 6590. https://doi.org/10.3390/ma14216590
Xu T, Xu Y, Liao N, Li Y, Nath M. High-Temperature Chemical Stability of Cr(III) Oxide Refractories in the Presence of Calcium Aluminate Cement. Materials. 2021; 14(21):6590. https://doi.org/10.3390/ma14216590
Chicago/Turabian StyleXu, Tengteng, Yibiao Xu, Ning Liao, Yawei Li, and Mithun Nath. 2021. "High-Temperature Chemical Stability of Cr(III) Oxide Refractories in the Presence of Calcium Aluminate Cement" Materials 14, no. 21: 6590. https://doi.org/10.3390/ma14216590
APA StyleXu, T., Xu, Y., Liao, N., Li, Y., & Nath, M. (2021). High-Temperature Chemical Stability of Cr(III) Oxide Refractories in the Presence of Calcium Aluminate Cement. Materials, 14(21), 6590. https://doi.org/10.3390/ma14216590






