Augmentation Stability of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with L-shaped Porcine-Derived Block Bone Substitute
Abstract
:1. Introduction
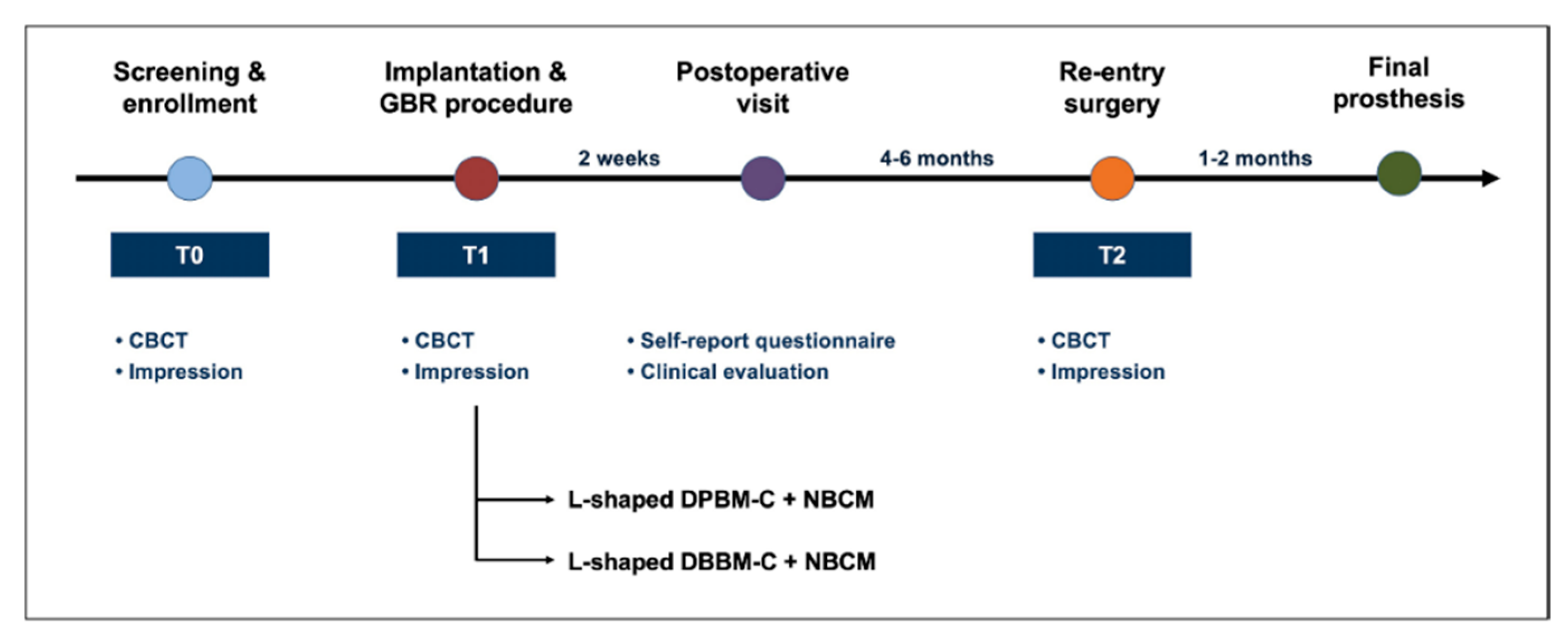
2. Materials and Methods
2.1. Ethical Statements
2.2. Participants
- Presence of peri-implant buccal dehiscence defects (≥1 mm);
- Re-entry surgery within 4–6 months;
- Healthy or mild systemic diseases(American Society of Anesthesiologists physical status classification I/II);
- Good or acceptable oral hygiene(full-mouth bleeding score on probing and full-mouth plaque score < 25%).
- Implant surgery with GBR within 1 month after tooth extraction;
- Heavy smokers (≥10 cigarettes/day);
- Uncontrolled diabetes mellitus or bone metabolic diseases.
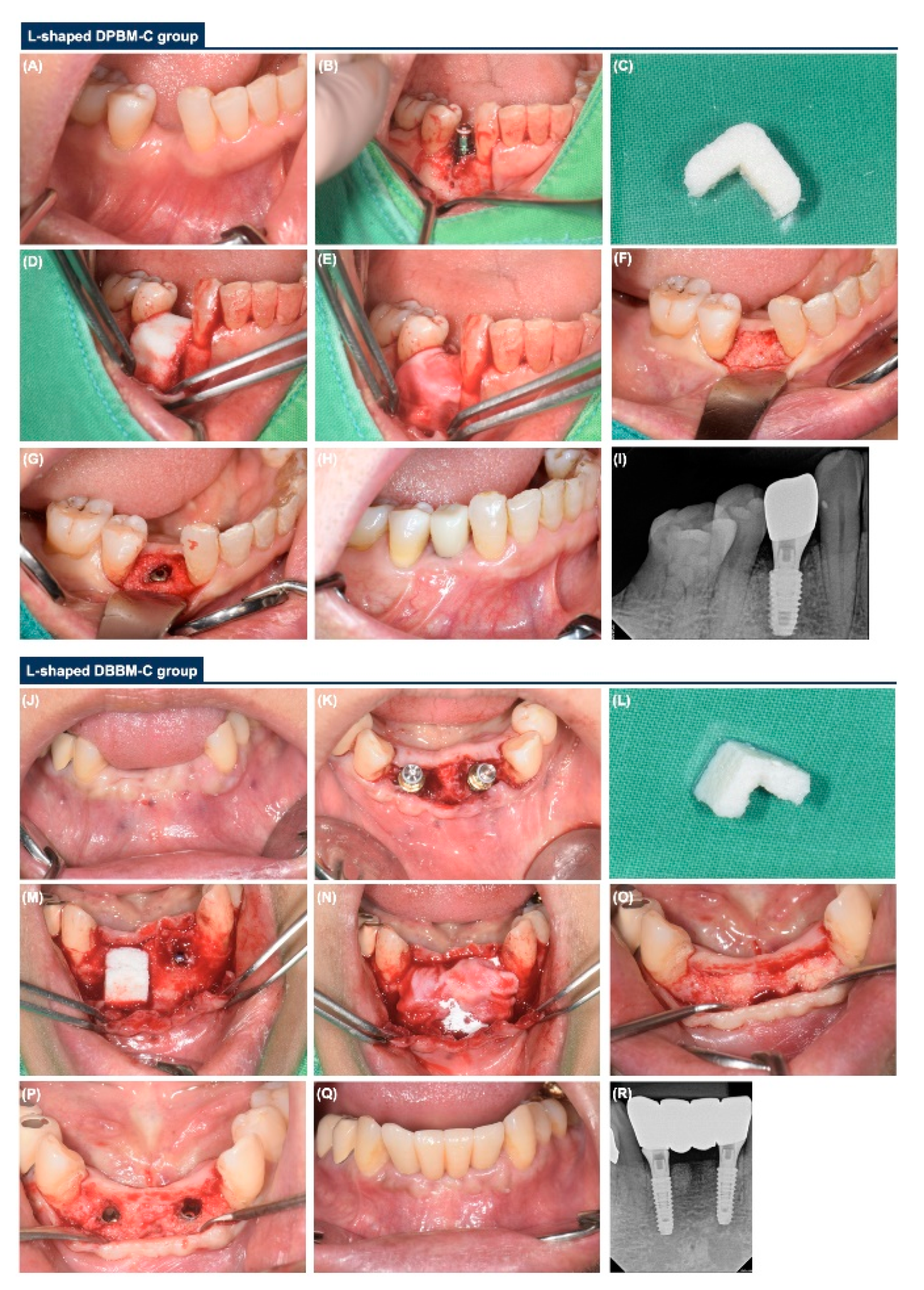
2.3. Surgical Procedure
2.4. Re-Entry Sugery
- L-shaped DPBM-C group: DPBM-C (Legograft®, Purgo Biologics, Seongnam, Korea), which was composed of a porcine-derived bone mineral matrix from cancellous bone and 10% atelocollagen from porcine tendon, was directly trimmed to an L-shape using a #15 blade. DPBM-C was manually adapted to the peri-implant dehiscence defect without using additional fixation devices (e.g., bone screws, pins, bone tack, or titanium mesh), and the defect was augmented to ≥1 mm of the buccal and occlusal aspects. The peri-implant dehiscence defect was augmented to ≥1 mm of the buccal and occlusal aspects. The L-shaped DPBM-C was covered with an absorbable native bilayer collagen membrane (NBCM, Geistlich Bio-Gide®, Geistlich Pharma AG, Wolhusen, Switzerland).
- L-shaped DBBM-C group: DBBM-C (Geistlich Bio-Oss® Collagen, Geistlich Pharma AG, Wolhusen, Switzerland) was appropriately trimmed to an L-shape and applied to the peri-implant dehiscence defect. DBBM-C was manually adapted to the peri-implant dehiscence defect without using additional fixation devices, and the defect was augmented to ≥1 mm of the buccal and occlusal aspects. The defects were augmented to achieve a ≥1 mm over-contour for both the buccal and occlusal aspects. Subsequently, the L-shaped DBBM-C was covered with the NBCM.
2.5. Radiograhic Analysis
2.6. Volumetric Analysis
2.7. Self-Reported Questionnaire and Clinical Analysis
2.8. Outcome Variables
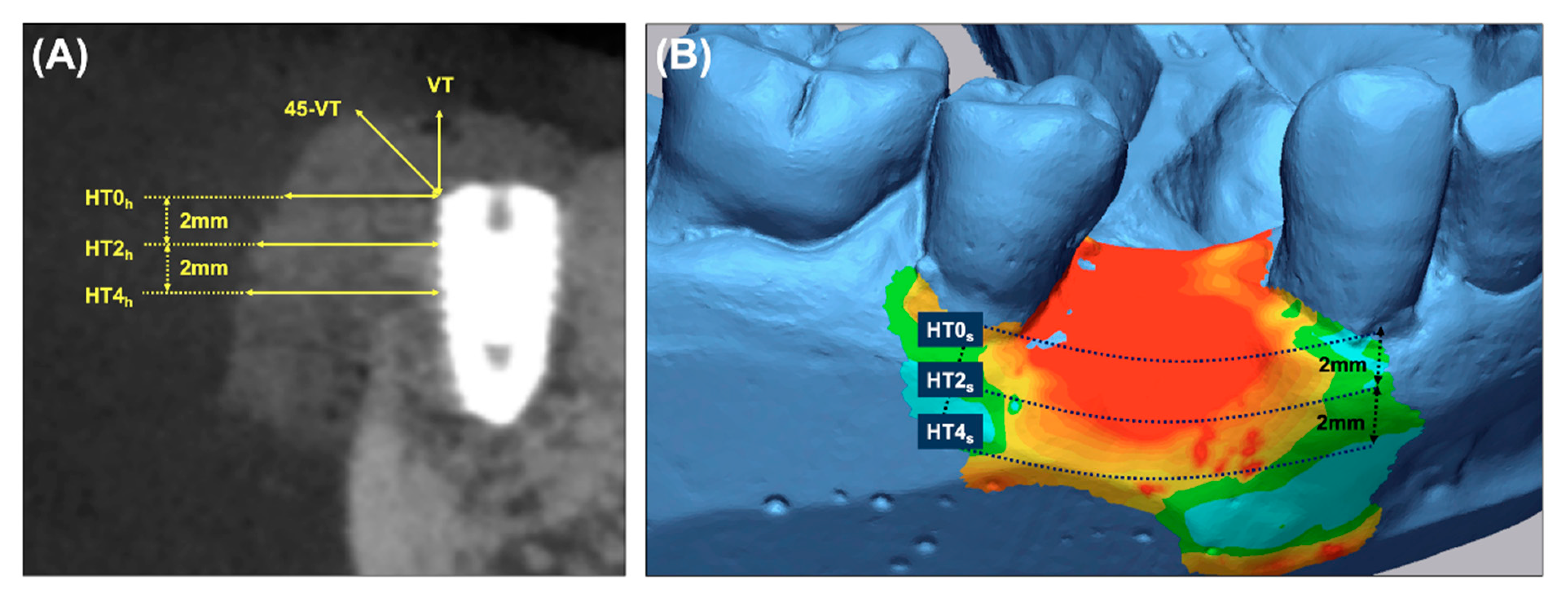
- Primary outcome: To measure the horizontal thickness of the augmented hard tissue, lines perpendicular to the long axis of the fixture at its shoulder (HT0h) and 2 mm (HT2h) and 4 mm (HT4h) below it were drawn on the sagittal CBCT images.
- Secondary outcomes: (a) The vertical thickness (VT) of the augmented hard tissue following the long axis of the implant and the 45° vertical thickness (45-VT) at a 45° positive angle relative to the long axis of the fixture were measured on the sagittal CBCT images. (b) The horizontal thickness of the augmented volume was measured on the cross-sectional images of the STL products. The ROI was limited by the mid-point of the facial cementoenamel junction of the mesial and distal teeth and extended 4 mm apically. At the cross-section of the baseline, the lines parallel to the occlusal plane were drawn at the buccal crest (HT0s) and 2 mm (HT2s) and 4 mm (HT4s) below it. (c) Subjective postoperative discomfort and the early wound healing outcomes were assessed using a self-report questionnaire and based on the clinical evaluation during the suture removal. (d) The stability of the implants (PTVs) were measured during the re-entry surgery.
2.9. Statistical Analysis
3. Results
3.1. Baseline Characteristics
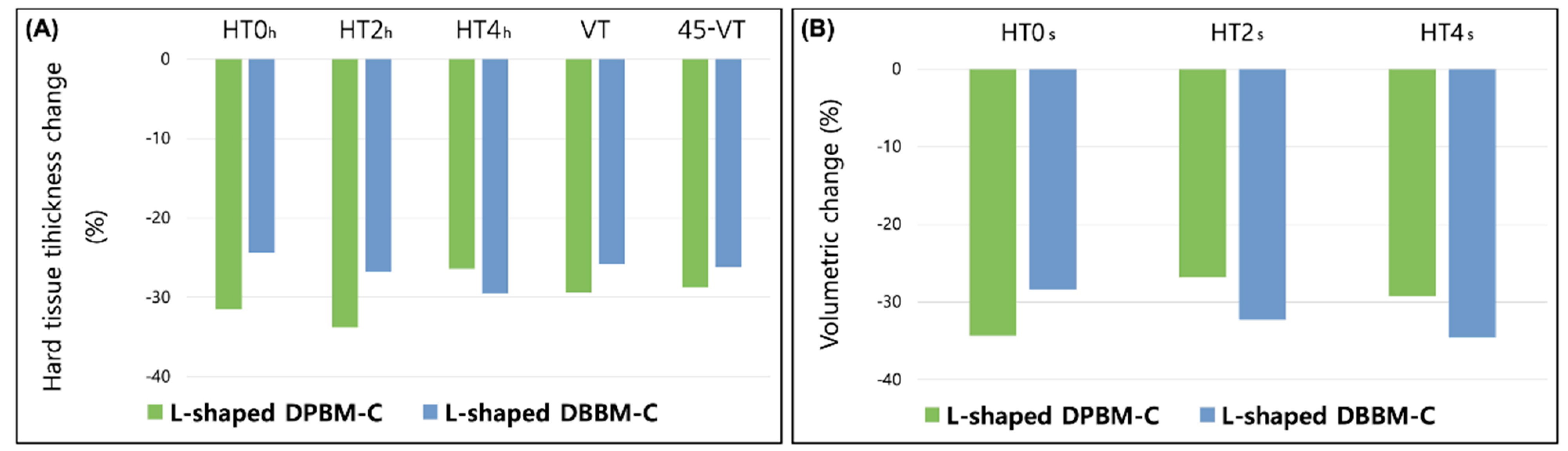
3.2. Radiographic and Volumetric Outcomes
3.3. Postoperative Discomfort and Wound Healing Outcomes
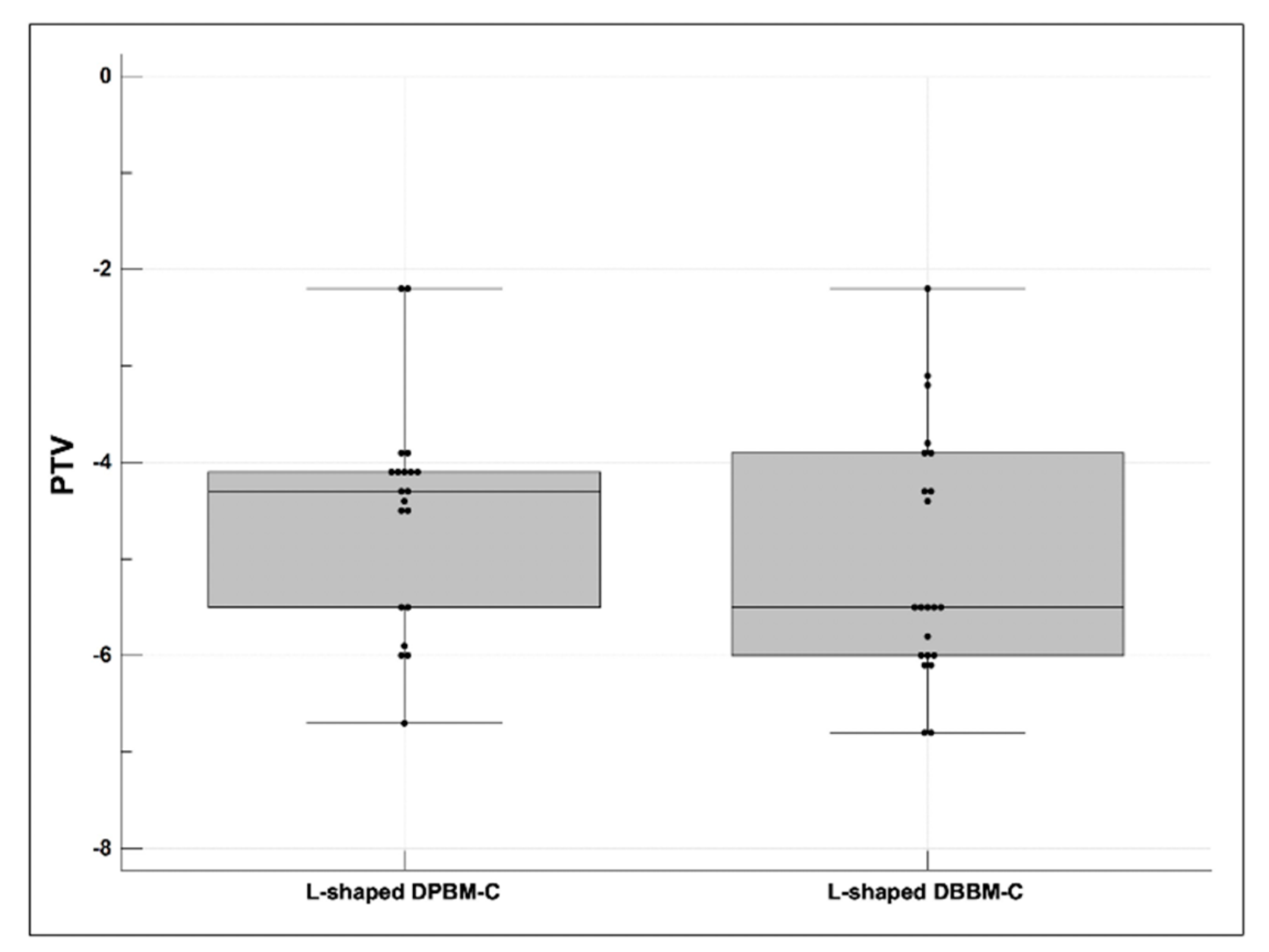
3.4. Stability of the Dental Implants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bazrafshan, N.; Darby, I. Retrospective success and survival rates of dental implants placed with simultaneous bone augmentation in partially edentulous patients. Clin. Oral Implant. Res. 2014, 25, 768–773. [Google Scholar] [CrossRef]
- Jensen, S.S.; Terheyden, H. Bone augmentation procedures in localized defects in the alveolar ridge: Clinical results with different bone grafts and bone-substitute materials. Int. J. Oral Maxillofac. Implant. 2009, 24, 218–236. [Google Scholar]
- Chiapasco, M.; Zaniboni, M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: A systematic review. Clin. Oral Implant. Res. 2009, 20 (Suppl. 4), 113–123. [Google Scholar] [CrossRef]
- Yamada, M.; Egusa, H. Current bone substitutes for implant dentistry. J. Prosthodont. Res. 2018, 62, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Chavda, S.; Levin, L. Human Studies of Vertical and Horizontal Alveolar Ridge Augmentation Comparing Different Types of Bone Graft Materials: A Systematic Review. J. Oral Implant. 2018, 44, 74–84. [Google Scholar] [CrossRef]
- Mir-Mari, J.; Wui, H.; Jung, R.E.; Hammerle, C.H.; Benic, G.I. Influence of blinded wound closure on the volume stability of different gbr materials: An in vitro cone-beam computed tomographic examination. Clin. Oral Implant. Res. 2016, 27, 258–265. [Google Scholar] [CrossRef]
- Benic, G.I.; Eisner, B.M.; Jung, R.E.; Basler, T.; Schneider, D.; Hämmerle, C.H.F. Hard tissue changes after guided bone regeneration of peri-implant defects comparing block versus particulate bone substitutes: 6-month results of a randomized controlled clinical trial. Clin. Oral Implant. Res. 2019, 30, 1016–1026. [Google Scholar] [CrossRef]
- Troeltzsch, M.; Troeltzsch, M.; Kauffmann, P.; Gruber, R.; Brockmeyer, P.; Moser, N.; Rau, A.; Schliephake, H. Clinical efficacy of grafting materials in alveolar ridge augmentation: A systematic review. J. Cranio-Maxillofac. Surg. 2016, 44, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Rabie, A. Effect of Bio-Oss® Collagen and Collagen Matrix on Bone Formation. Open Biomed. Eng. J. 2010, 4, 71–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Kim, D.-H.; Jeong, S. Comparative assessment of anterior maxillary alveolar ridge preservation with and without adjunctive use of enamel matrix derivative: A randomized clinical trial. Clin. Oral Implant. Res. 2020, 31, 1–9. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, S.N. Effect of enamel matrix derivative on alveolar ridge preservation in the posterior maxilla: A randomized controlled clinical trial. Clin. Implant. Dent. Relat. Res. 2020, 22, 622–630. [Google Scholar] [CrossRef]
- Mir-Mari, J.; Benic, G.I.; Valmaseda-Castellón, E.; Hämmerle, C.H.; Jung, R.E. Influence of wound closure on the volume stability of particulate and non-particulate GBR materials: An in vitro cone-beam computed tomographic examination. Part II. Clin. Oral Implant. Res. 2017, 28, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Jung, E.; Jeong, S.; Lee, J. Augmentation stability and early wound healing outcomes of guided bone regeneration in peri-implant dehiscence defects with L- and I-shaped soft block bone substitutes: A clinical and radiographic study. Clin. Oral Implant. Res. 2021. Early View. [Google Scholar] [CrossRef]
- Morris, K. Revising the Declaration of Helsinki. Lancet 2013, 381, 1889–1890. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Park, Y.-S.; Kim, Y.-T.; Kim, D.-H.; Jeong, S.-N. Assessment of early discomfort and wound healing outcomes after periodontal surgery with and without enamel matrix derivative: An observational retrospective case-control study. Clin. Oral Investig. 2020, 24, 229–237. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Kim, Y.; Nowzari, H.; Rich, S.K. Risk of Prion Disease Transmission through Bovine-Derived Bone Substitutes: A Systematic Review. Clin. Implant. Dent. Relat. Res. 2013, 15, 645–653. [Google Scholar] [CrossRef]
- Bae, E.-B.; Kim, H.-J.; Ahn, J.-J.; Bae, H.-Y.; Kim, H.-J.; Huh, J.-B. Comparison of Bone Regeneration between Porcine-Derived and Bovine-Derived Xenografts in Rat Calvarial Defects: A Non-Inferiority Study. Materials 2019, 12, 3412. [Google Scholar] [CrossRef] [Green Version]
- Koo, T.; Song, Y.W.; Cha, J.; Jung, U.; Kim, C.; Lee, J. Histologic analysis following grafting of damaged extraction sockets using deproteinized bovine or porcine bone mineral: A randomized clinical trial. Clin. Oral Implant. Res. 2020, 31, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Cha, J.-K.; Kim, C.-S. Alveolar ridge regeneration of damaged extraction sockets using deproteinized porcine versus bovine bone minerals: A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2018, 20, 729–737. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.; Jeong, S. Adjunctive use of enamel matrix derivatives to porcine-derived xenograft for the treatment of one-wall intrabony defects: Two-year longitudinal results of a randomized controlled clinical trial. J. Periodontol. 2020, 91, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, S. Long-term stability of adjunctive use of enamel matrix protein derivative on porcine-derived xenograft for the treatment of one-wall intrabony defects: A 4-year extended follow-up of a randomized controlled trial. J. Periodontol. 2021. Early View. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Lozada, J.L.; Wessing, B.; Del Amo, F.S.-L.; Wang, H.-L. Vertical Bone Grafting and Periosteal Vertical Mattress Suture for the Fixation of Resorbable Membranes and Stabilization of Particulate Grafts in Horizontal Guided Bone Regeneration to Achieve More Predictable Results: A Technical Report. Int. J. Periodontics Restor. Dent. 2016, 36, 153–159. [Google Scholar] [CrossRef]
| Variables | L-shaped DPBM-C (n = 20) | L-shaped DBBM-C (n = 22) | Total (n = 42) | p Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 11 (55.0%) | 8 (36.4%) | 19 (45.2%) | 0.231 |
| Female | 9 (45.0%) | 14 (63.6%) | 23 (54.8%) | |
| Age | ||||
| Mean ± SD | 58.9 ± 7.7 | 58.0 ± 13.6 | 58.4 ± 11.1 | 0.797 |
| Smoking habits | ||||
| Non-smoker | 12 (60.0%) | 13 (59.1%) | 25 (59.5%) | 0.756 |
| Former smoker | 3 (15.0%) | 5 (22.7%) | 8 (19.0%) | |
| Smoker (<10 cigarettes/day) | 5 (25.0%) | 4 (18.2%) | 9 (21.4%) | |
| Diabetes mellitus | ||||
| Yes | 6 (30.0%) | 5 (22.7%) | 11 (26.2%) | 0.596 |
| Location | ||||
| Maxillary anterior | 6 (30.0%) | 8 (36.4%) | 14 (33.3%) | 0.923 |
| Maxillary posterior | 3 (15.0%) | 4 (18.2%) | 7 (16.7%) | |
| Mandibular anterior | 7 (35.0%) | 7 (31.8%) | 14 (33.3%) | |
| Mandibular posterior | 4 (20.0%) | 3 (13.6%) | 7 (16.7%) |
| L-shaped DPBM-C (n = 20) | L-shaped DBBM-C (n = 22) | |||||
|---|---|---|---|---|---|---|
| T0–T1 | T0–T2 | p Value | T0–T1 | T0–T2 | p Value | |
| Changes in hard tissue thickness (mm) | ||||||
| HT0h | 2.46 ± 1.06 (2.60, (1.60, 3.25)) | 1.63 ± 0.87 (1.42, (0.92, 2.20)) | <0.001 | 2.50 ± 0.85 (2.33, (2.09, 2.98)) | 1.88 ± 0.85 (1.99, (1.21, 2.12)) | <0.001 |
| HT2h | 2.19 ± 0.90 (2.20, (1.28, 2.85)) | 1.37 ± 0.75 (1.25, (0.70, 2.09)) | <0.001 | 2.35 ± 0.85 (2.38, (1.85, 2.90)) | 1.71 ± 0.86 (2.01, (0.98, 2.34)) | <0.001 |
| HT4h | 2.21 ± 0.87 (2.20, (1.29, 2.85)) | 1.60 ± 0.74 (1.54, (1.04, 2.12)) | <0.001 | 2.58 ± 1.03 (2.61, (1.85, 3.01)) | 1.84 ± 0.94 (1.73, (1.12, 2.23)) | <0.001 |
| VT | 2.28 ± 0.89 (2.38, (1.60, 2.95)) | 1.62 ± 0.91 (1.88, (0.69, 2.30)) | <0.001 | 2.57 ± 0.89 (2.20, (2.01, 3.01)) | 1.79 ± 0.70 (1.77, (1.47, 2.04)) | <0.001 |
| 45-VT | 2.34 ± 1.01 (2.30, (1.41, 2.95)) | 1.64 ± 0.97 (1.48, (0.72, 2.64)) | <0.001 | 2.57 ± 0.75 (2.53, (2.09, 2.98)) | 1.80 ± 0.76 (1.77, (1.19, 2.04)) | <0.001 |
| Changes in volume (mm) | ||||||
| HT0s | 3.97 ± 0.94 (4.12, (3.31, 4.50)) | 2.55 ± 0.98 (2.33, (2.03, 3.28)) | <0.001 | 4.22 ± 0.86 (4.21, (3.44, 4.63)) | 2.98 ± 0.91 (3.05, (2.30, 3.47)) | <0.001 |
| HT2s | 4.03 ± 0.99 (4.12, (3.34, 4.50)) | 2.49 ± 1.11 (2.33, (1.83, 3.35)) | <0.001 | 4.32 ± 1.01 (4.28, (3.85, 5.02)) | 2.86 ± 0.97 (2.93, (2.33, 3.52)) | <0.001 |
| HT4s | 4.17 ± 1.10 (4.17, (3.23, 4.90)) | 2.90 ± 1.11 (2.89, (2.15, 3.41)) | <0.001 | 4.28 ± 0.86 (4.17, (3.75, 4.83)) | 2.78 ± 0.97 (2.93, (2.20, 3.14)) | <0.001 |
| Variables | L-shaped DPBM-C (n = 20) | L-shaped DBBM-C (n = 22) | p Value |
|---|---|---|---|
| Subjective pain | |||
| Severity (VAS) a | 4.8 ± 1.5 | 4.5 ± 1.6 | 0.501 |
| Duration (days) | 4.3 ± 2.5 | 4.9 ± 2.3 | 0.569 |
| Subjective swelling | |||
| Severity (VAS) a | 4.7 ± 2.0 | 4.2 ± 1.5 | 0.385 |
| Duration (days) | 6.9 ± 2.5 | 5.5 ± 2.5 | 0.086 |
| Wound dehiscence and membrane exposure | |||
| No | 17 (85.0%) | 20 (90.9%) | 0.348 |
| Yes | 3 (15.0%) | 2 (9.1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Jung, E.-H.; Jeong, S.-N. Augmentation Stability of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with L-shaped Porcine-Derived Block Bone Substitute. Materials 2021, 14, 6580. https://doi.org/10.3390/ma14216580
Lee J-H, Jung E-H, Jeong S-N. Augmentation Stability of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with L-shaped Porcine-Derived Block Bone Substitute. Materials. 2021; 14(21):6580. https://doi.org/10.3390/ma14216580
Chicago/Turabian StyleLee, Jae-Hong, Eun-Hee Jung, and Seong-Nyum Jeong. 2021. "Augmentation Stability of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with L-shaped Porcine-Derived Block Bone Substitute" Materials 14, no. 21: 6580. https://doi.org/10.3390/ma14216580
APA StyleLee, J.-H., Jung, E.-H., & Jeong, S.-N. (2021). Augmentation Stability of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with L-shaped Porcine-Derived Block Bone Substitute. Materials, 14(21), 6580. https://doi.org/10.3390/ma14216580






