Plasma Electrolytic Polishing of Nitinol: Investigation of Functional Properties
Abstract
:1. Introduction
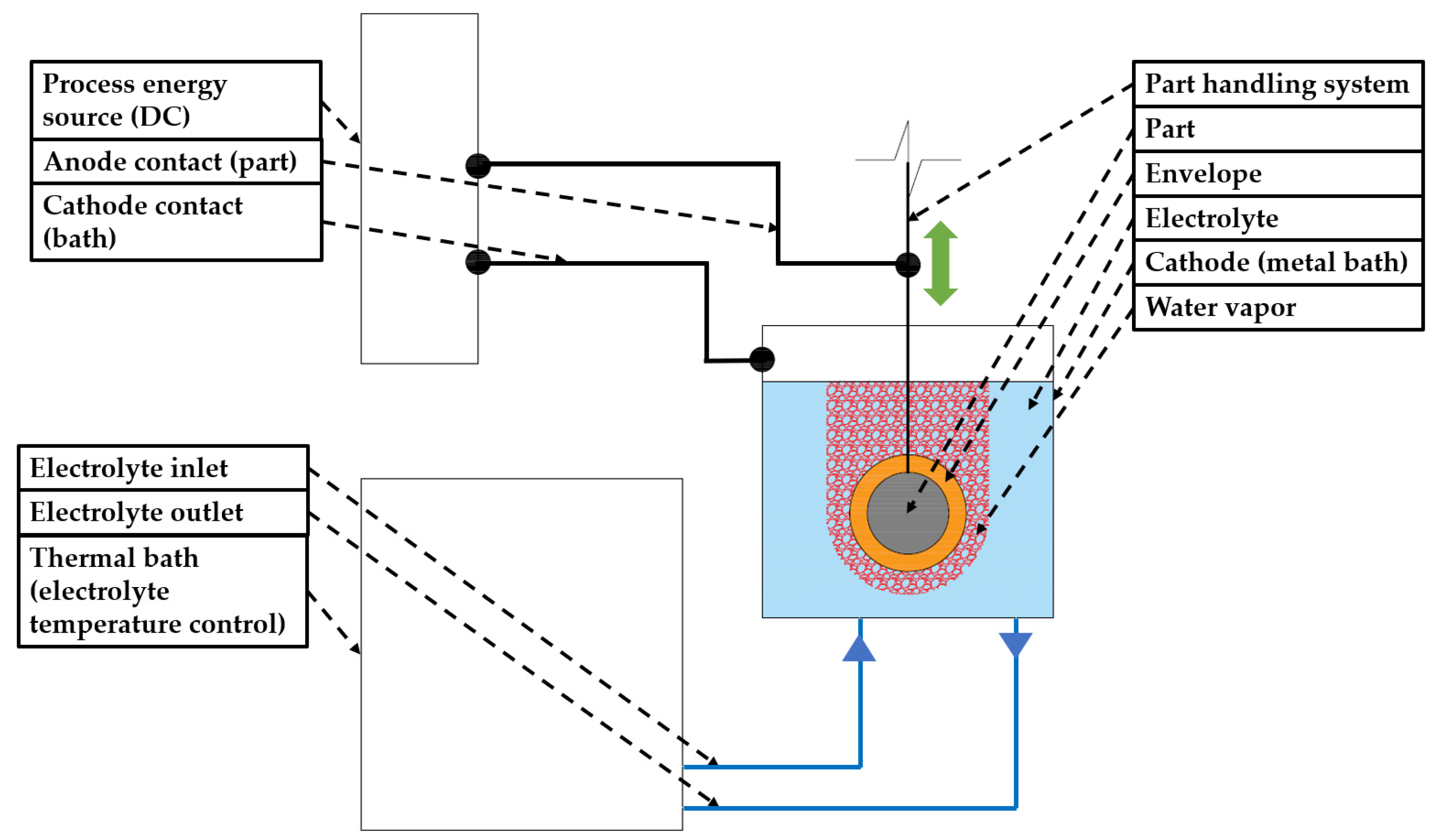
2. Materials and Methods
Nitinol Samples
3. Results and Discussion
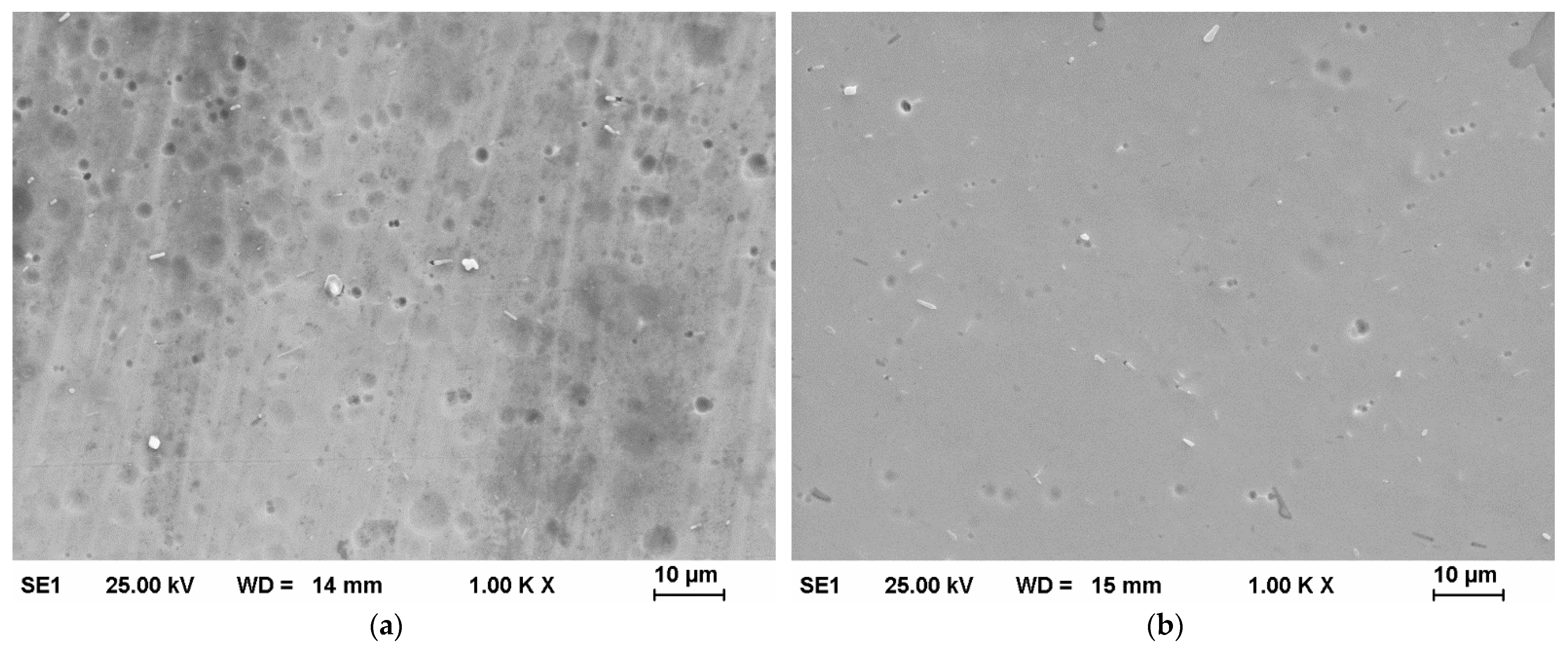
3.1. Plasma Electrolytic Polishing
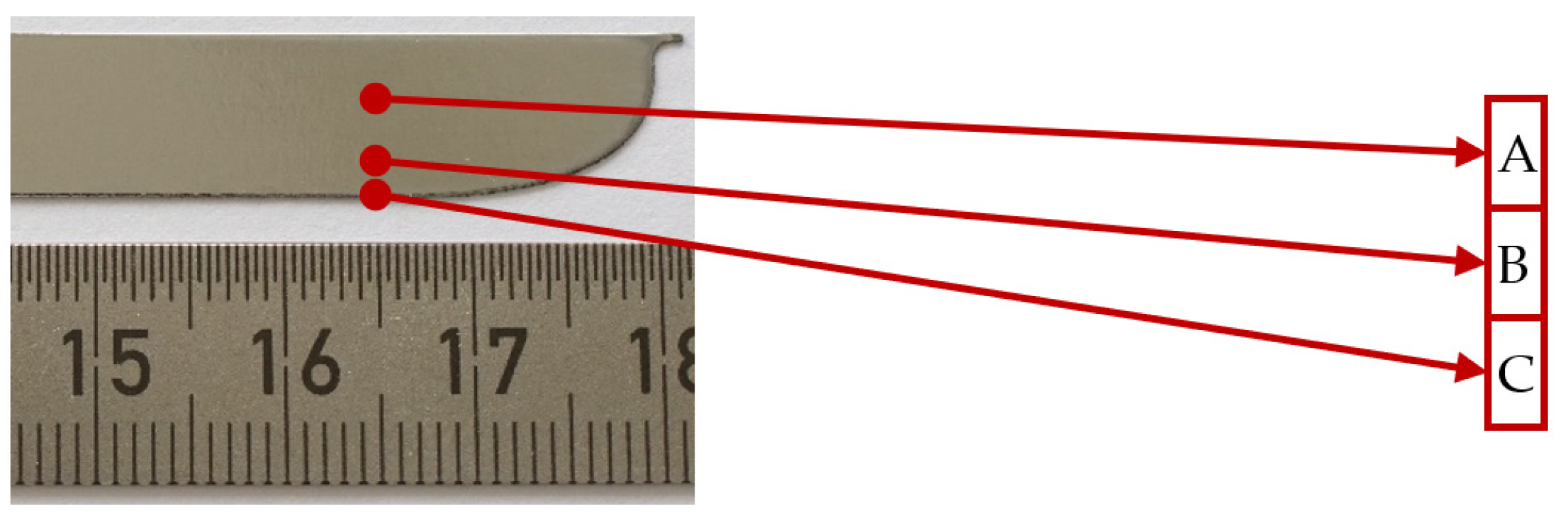
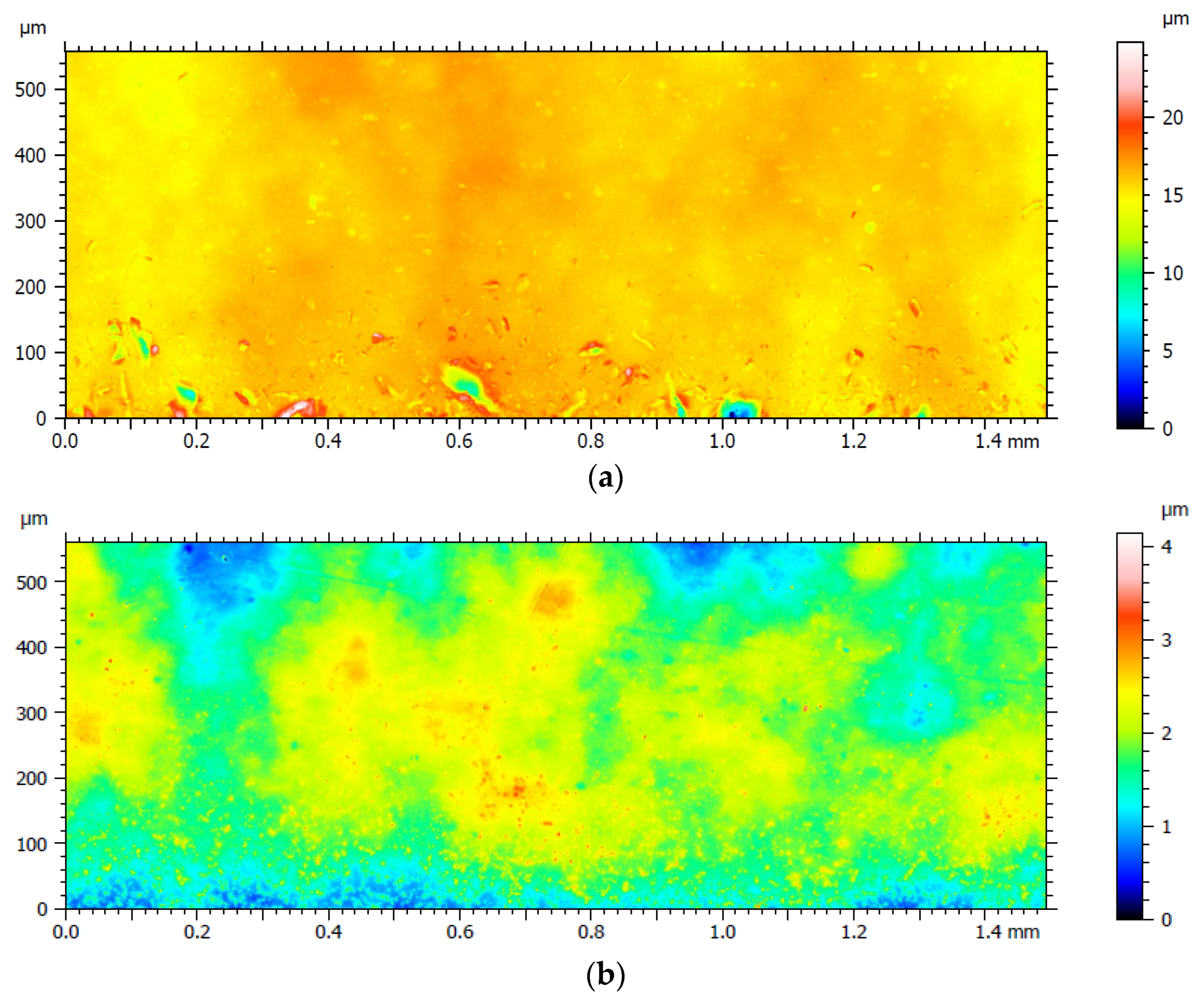
3.2. Surface Roughness Measurements
3.3. EDX Measurements
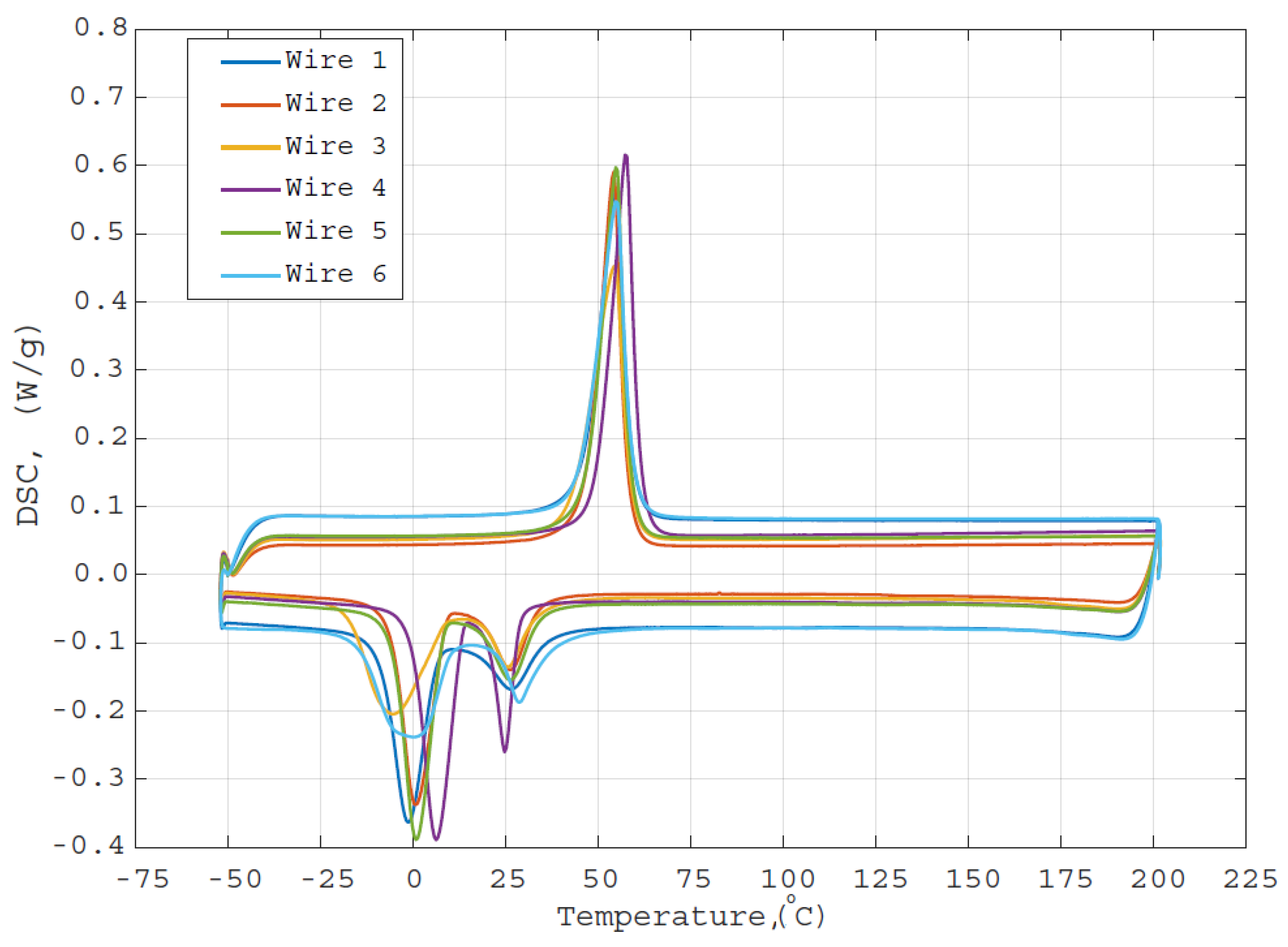
3.4. DSC Measurements
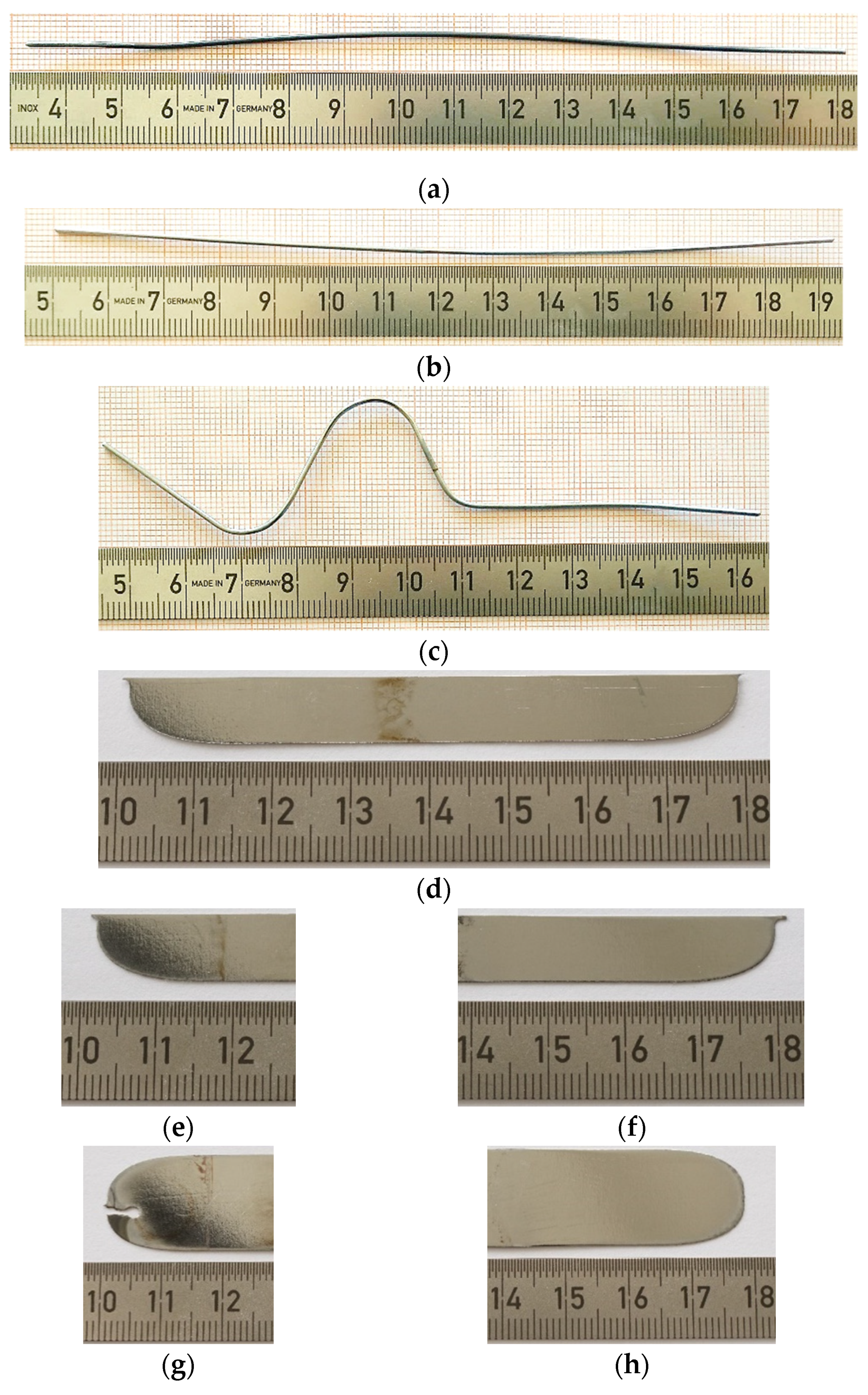
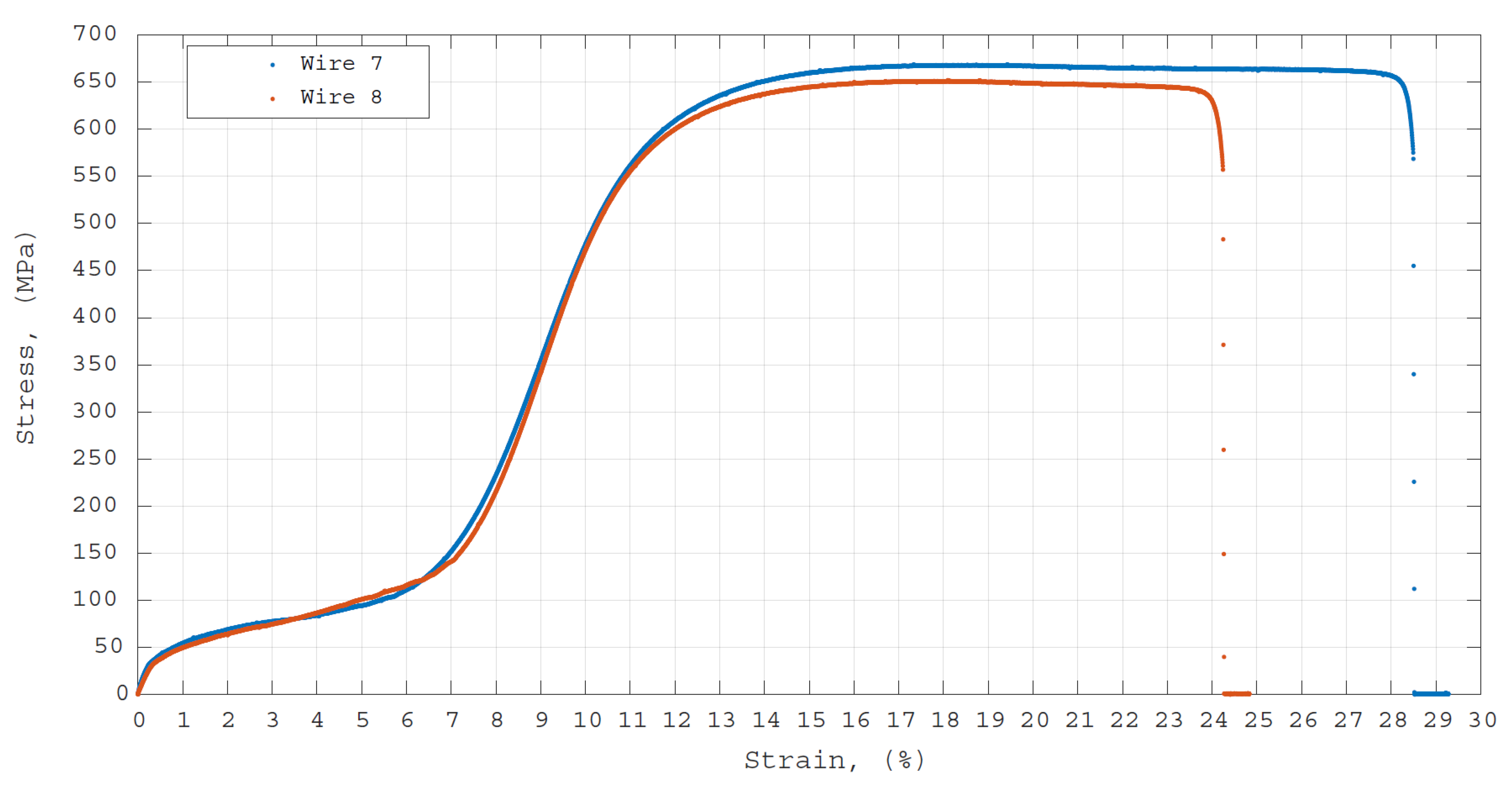
3.5. Mechanical Tensile Strength Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duerig, T.; Pelton, A.; Stöckel, D. An overview of nitinol medical applications. Mater. Sci. Eng. A 1999, 273–275, 149–160. [Google Scholar] [CrossRef]
- Kauffman, G.B.; Mayo, I. The story of nitinol: The serendipitous discovery of the memory metal and its applications. Chem. Educ. 1997, 2, 1–21. [Google Scholar] [CrossRef]
- Mwangi, J.W.; Nguyen, L.T.; Bui, V.D.; Berger, T.; Zeidler, H.; Schubert, A. Nitinol manufacturing and micromachining: A review of processes and their suitability in processing medical-grade nitinol. J. Manuf. Process. 2019, 38, 355–369. [Google Scholar] [CrossRef]
- Pelton, A.R.; Duerig, T.W.; Stöckel, D. A guide to shape memory and superelasticity in Nitinol medical devices. Minim. Invasive Ther. Allied Technol. 2004, 13, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Tušek, J.; Žerovnik, A.; Čebron, M.; Brojan, M.; Žužek, B.; Engelbrecht, K.; Cadelli, A. Elastocaloric effect vs fatigue life: Exploring the durability limits of Ni-Ti plates under pre-strain conditions for elastocaloric cooling. Acta Mater. 2018, 150, 295–307. [Google Scholar] [CrossRef] [Green Version]
- Engelbrecht, K. Future prospects for elastocaloric devices. J. Phys. Energy 2019, 1, 2. [Google Scholar] [CrossRef]
- Bruederlin, F.; Bumke, L.; Chluba, C.; Ossmer, H.; Quandt, E.; Kohl, M. Elastocaloric cooling on the miniature scale: A review on materials and device engineering. Energy Technol. 2018, 6, 8. [Google Scholar] [CrossRef]
- Kirsch, S.-M.; Welsch, F.; Michaelis, N.; Schmidt, M.; Wieczorek, A.; Frenzel, J.; Eggeler, G.; Schütze, A.; Seelecke, S. NiTi-based elastocaloric cooling on the macroscale: From basic concepts to realization. Energy Technol. 2018, 6, 1567–1587. [Google Scholar] [CrossRef]
- Kabirifar, P.; Žerovnik, A.; Ahčin, Ž.; Porenta, L.; Brojan, M.; Tušek, J. Elastocaloric cooling: State-of-the-art and future challenges in designing regenerative elastocaloric devices. Stroj. Vestn. J. Mech. Eng. 2019, 65, 11–12. [Google Scholar] [CrossRef] [Green Version]
- Tušek, J.; Engelbrecht, K.; Mañosa, L.; Vives, E.; Pryds, N. Understanding the thermodynamic properties of the elastocaloric effect through experimentation and modelling. Shape Mem. Superelasticity 2016, 2, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Nikitin, S.A.; Myalikgulyev, G.; Annaorazov, M.P.; Tyurin, A.L.; Myndyev, R.W.; Akopyan, S.A. Giant elastocaloric effect in FeRh alloy. Phys. Lett. A 1992, 171, 234–236. [Google Scholar] [CrossRef]
- Porenta, L.; Kabirifar, P.; Žerovnik, A.; Čebron, M.; Žužek, B.; Dolenec, M.; Brojan, M.; Tušek, J. Thin-walled Ni-Ti tubes under compression: Ideal candidates for efficient and fatigue-resistant elastocaloric cooling. Appl. Mater. Today 2020, 20, 100712. [Google Scholar] [CrossRef]
- Ossmer, H.; Chluba, C.; Krevet, B.; Quandt, E.; Rohde, M.; & Kohl, M. Elastocaloric cooling using shape memory alloy films. J. Phys. Conf. Ser. 2013, 476, 012138. [Google Scholar] [CrossRef]
- Slaughter, J.; Czernuszewicz, A.; Griffith, L.; Pecharsky, V. Compact and efficient elastocaloric heat pumps—Is there a path forward? J. Appl. Phys. 2020, 127, 194501. [Google Scholar] [CrossRef]
- Navickaitė, K.; Penzel, M.; Bahl, C.; Engelbrecht, K.; Tušek, J.; Martin, A.; Zinecker, M.; Schubert, A. CFD-simulation assisted design of elastocaloric regenerator geometry. Sustainability 2020, 12, 9013. [Google Scholar] [CrossRef]
- Jahadakbar, A.; Nematollahi, M.; Safaei, K.; Bayati, P.; Giri, G.; Dabbaghi, H.; Dean, D.; Elahinia, M. Design, modeling, additive manufacturing, and polishing of stiffness-modulated porous nitinol bone fixation plates followed by thermomechanical and composition analysis. Metals 2020, 10, 151. [Google Scholar] [CrossRef] [Green Version]
- Engelbrecht, K.; Tušek, J.; Sanna, S.; Eriksen, D.; Mishin, O.V.; Bahl, C.R.H.; Pryds, N. Effects of surface finish and mechanical training on Ni-Ti sheets for elastocaloric cooling. APL Mater. 2016, 4, 064110. [Google Scholar] [CrossRef] [Green Version]
- Hansen, A.W.; Beltrami, L.V.R.; Antonini, L.M.; Villarinho, D.J.J.; das Neves, C.K.; Marino, C.E.B.; de Fraga Malfatti, C. Oxide formation on NiTi surface: Influence of the heat treatment time to achieve the shape memory. Mater. Res. 2015, 18, 1053–1061. [Google Scholar] [CrossRef] [Green Version]
- Wirth, C.; Comte, V.; Lagneau, C.; Exbrayat, P.; Lissac, M.; Jaffrezic-Renault, N.; Ponsonnet, L. Nitinol surface roughness modulates in vitro cell response: A comparison between fibroblasts and osteoblasts. Mater. Sci. Eng. C 2005, 25, 51–60. [Google Scholar] [CrossRef]
- Vopát, T.; Podhorský, Š.; Sahul, M.; Haršáni, M. Cutting edge preparation of cutting tools using plasma discharges in electrolyte. J. Manuf. Process. 2019, 46, 234–240. [Google Scholar] [CrossRef]
- Belkin, P.N.; Kusmanov, S.A.; Parfenov, E.V. Mechanism and technological opportunity of plasma electrolytic polishing of metals and alloys surfaces. Appl. Surf. Sci. Adv. 2020, 1, 100016. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.K.; Kim, H.K.; Unnithan, A.R.; Kim, C.S.; Park, C.H. Optimization of electropolishing on NiTi alloy stents and its influence on corrosion behavior. J. Nanosci. Nanotechnol. 2017, 17, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Rokicki, R.; Hryniewicz, T. Nitinol surface finishing by magnetoelectropolishing. Trans. Inst. Met. Finish. 2008, 86, 280–285. [Google Scholar] [CrossRef]
- Barbosa, F.O.G.; da, J.A.; Gomes, C.P.; de Araújo, M.C.P. Influence of electrochemical polishing on the mechanical properties of K3 nickel-titanium rotary instruments. J. Endod. 2008, 34, 1533–1536. [Google Scholar] [CrossRef]
- Mwangi, J.W. Micro-Electrical Discharge Machining of Nitinol for Medical Applications. Ph.D. Thesis, Chemnitz Technical University, Chemnitz, Germany.
- Zanotti, C.; Giuliani, P.; Bassani, P.; Zhang, Z.; Chrysanthou, A. Comparison between the thermal properties of fully dense and porous NiTi SMAs. Intermetallics 2010, 18, 14–21. [Google Scholar] [CrossRef]
- Kashapov, L.N.; Kashapov, N.F.; Kashapov, R.N.; Denisov, D.G. Plasma electrolytic treatment of products after selective laser melting. J. Phys. Conf. Ser. 2016, 669, 1. [Google Scholar] [CrossRef]
- Navickaitė, K. Nature-Inspired Double Corrugated Geometry for Enhanced Heat Transfer. Ph.D. Thesis, Technical University of Denmark, Risø, Denmark, 2018. [Google Scholar]
- Nestler, K.; Böttger-Hiller, F.; Adamitzki, W.; Glowa, G.; Zeidler, H.; Schubert, A. Plasma electrolytic polishing—An overview of applied technologies and current challenges to extend the polishable material range. Procedia CIRP 2016, 42, 503–507. [Google Scholar] [CrossRef]
- Nevyantseva, R.R.; Gorbatkov, S.A.; Parfenov, E.V.; Bybin, A.A. The influence of vapor-gaseous envelope behavior on plasma electrolytic coating removal. Surf. Coatings Technol. 2001, 148, 30–37. [Google Scholar] [CrossRef]
- Yerokhin, A.; Mukaeva, V.R.; Parfenov, E.V.; Laugel, N.; Matthews, A. Charge transfer mechanisms underlying contact glow discharge electrolysis. Electrochim. Acta 2019, 312, 441–456. [Google Scholar] [CrossRef]
- Kröning, O.; Schulze, H.-P.; Zeidler, H.; Kranhold, C. Das Plasma-elektrolytische Polieren von Werkstücken für den medizin- technischen Einsatz. Magdebg. Ing. 2019, 9, 2–9. [Google Scholar]
- Cornelsen, M.; Deutsch, C.; Seitz, H. Electrolytic plasma polishing of pipe inner surfaces. Metals 2018, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Parfenov, E.V.; Farrakhov, R.G.; Mukaeva, V.R.; Gusarov, A.V.; Nevyantseva, R.R.; Yerokhin, A. Electric field effect on surface layer removal during electrolytic plasma polishing. Surf. Coat. Technol. 2016, 307, 1329–1340. [Google Scholar] [CrossRef]
- Kusmanov, S.A.; Tambovskiy, I.V.; Kusmanova, I.A.; Belkin, P.N. Some features of anodic plasma electrolytic processes in aqueous solution. J. Phys. Conf. Ser. 2019, 1396, 012025. [Google Scholar] [CrossRef]
- Zeidler, H.; Nestler, K.; Boettger-Hiller, F.; Schubert, A.; Previtali, B.; Demir Gökhan, A. Surface finish machining of medical parts using plasma electrolytic polishing. Procedia CIRP 2016, 49, 83–87. [Google Scholar] [CrossRef]
- Lochyński, P.; Charazińska, S.; Łyczkowska-Widłak, E.; Sikora, A. Electropolishing of stainless steel in laboratory and industrial scale. Metals 2019, 9, 854. [Google Scholar] [CrossRef] [Green Version]
- Vana, D.; Podhorsky, S.; Hurajt, M.; Hanzen, V. Surface properties of the stainless steel X10 CrNi 18/10 after aplication of plasma polishing in electrolyte. Int. J. Mod. Eng. Res. 2013, 3, 788–792. [Google Scholar]
- Stepputat, V. Influence of Plasma-Electrolytic Polishing on the Functional and Mechanical Properties of Additively Manufactured Nitinol Smart Springs. Master’s Thesis, Technische Universität Bergakademie Freiberg, Freiberg, Germany, 2020. [Google Scholar]
- Stepputat, V.N.; Zeidler, H.; Safranchik, D.; Strokin, E.; Böttger-Hiller, F. Investigation of post-processing of additively manufactured nitinol smart springs with plasma-electrolytic polishing. Materials 2021, 14, 4093. [Google Scholar] [CrossRef] [PubMed]
- Andani, M.; Haberland, T.; Walker, C.J.; Elahinia, M. An Investigation of Effective Process Parameters on Phase Transformation Temperature of Nitinol Manufactured by Selective Laser Melting. In Proceedings of the ASME 2014 Conference on Smart Materials, Adaptive Structures and Intelligent Systems, Newport, RI, USA, 8–10 September 2014. [Google Scholar]
- Walker, J.; Andani, M.T.; Haberland, C.; Elahinia, M. Additive manufacturing of Nitinol shape memory alloys to overcome challenges in conventional Nitinol fabrication. In ASME International Mechanical Engineering Congress and Exposition; American Society of Mechanical Engineers: New York, NY, USA, 2014; pp. 1–5. [Google Scholar]
- Haberland, C.; Elahinia, M.; Walker, J.; Meier, H. Visions, concepts and strategies for smart Nitinol actuators and complex Nitinol structures produced by additive manufacturing. In Proceedings of the ASME 2013 Conference on Smart Materials, Adaptive Structures and Intelligent Systems SMASIS 2013, Snowbird, UT, USA, 16–18 September 2013. [Google Scholar]
- Bormann, T.; Schumacher, R.; Müller, B.; Mertmann, M.; De Wild, M. Tailoring selective laser melting process parameters for niti implants. J. Mater. Eng. Perform. 2012, 21, 2519–2524. [Google Scholar] [CrossRef] [Green Version]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, W.J.; Milewski, J.O.; Beese, M.A.; Wilson-Heid, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Gilbert, H.B.; Webster, R.J. Rapid, reliable shape setting of superelastic nitinol for prototyping robots. IEEE Robot. Autom. Lett. 2016, 1, 98–105. [Google Scholar] [CrossRef] [Green Version]
| Sample Name | Length, l, (mm) | Diameter/Width x Thickness, d, w x σ, (mm) | Austenite Finish, Af, (°C) | Shape Setting, (Y/N) | PeP Time, t, (s) | Voltage, U, (V) | Current, I, (A) | Electrolyte Temp., Te, (°C) |
|---|---|---|---|---|---|---|---|---|
| Wire 1 | 150 | 1 | 70 ± 5 1 | N | 0 | 0 | 0 | 0 |
| Wire 2 | 150 | 1 | 70 ± 5 1 | N | 120 | 330 | 1 | 80 |
| Wire 3 | 150 | 1 | 70 ± 5 1 | Y | 120 | 330 | 1 | 80 |
| Wire 4 | 150 | 1 | 70 ± 5 1 | Y | 0 | 0 | 0 | 0 |
| Wire 5 | 150 | 1 | 70 ± 5 1 | N | 60 | 330 | 1 | 80 |
| Wire 6 | 150 | 1 | 70 ± 5 1 | Y | 120 | 330 | 1 | 80 |
| Wire 7 | 40 | 1 | 70 ± 5 1 | N | 0 | 0 | 0 | 0 |
| Wire 8 | 40 | 1 | 70 ± 5 1 | N | 120 | 331 | 3 | 80 |
| Plate 1 | 78 | 90 × 0.25 | 0 ± 7 1 | N | 10 | 335 | 3 | 80 |
| Plate 2_1 | 39 | 90 × 0.25 | 0 ± 7 1 | N | 30 | 334 | 3 | 80 |
| Plate 2_2 | 39 | 90 × 0.25 | 0 ± 7 1 | N | 60 | 334 | 3 | 80 |
| Plate 3_1 | 39 | 180 × 0.25 | 0 ± 7 1 | N | 120 | 334 | 4 | 80 |
| Plate 3_2 | 39 | 180 × 0.25 | 0 ± 7 1 | N | 180 | 333 | 4 | 80 |
| Plate Name | PeP Time, t, (s) | Before PeP | After PeP | ||||
|---|---|---|---|---|---|---|---|
| Ra_A, (μm) | Ra_B, (μm) | Ra_C, (μm) | Ra_A, (μm) | Ra_B, (μm) | Ra_C, (μm) | ||
| Plate 1 | 10 | 0.12 | 0.12 | 0.37 | 0.11 | 0.11 | 0.54 |
| Plate 2_1 | 30 | 0.11 | 0.13 | 0.17 | 0.11 | 0.13 | 0.30 |
| Plate 2_2 | 60 | 0.10 | 0.11 | 0.40 | |||
| Plate 3_1 | 120 | 0.15 | 0.13 | 0.85 | 0.08 | 0.08 | 0.17 |
| Plate 3_2 | 180 | 0.09 | 0.13 | 0.16 | |||
| Sample Name | Element in at% | Before PeP | After PeP |
|---|---|---|---|
| Plate 1 | Ni | 51 | 51 |
| Ti | 49 | 49 | |
| Plate 2_1 | Ni | 52 | 51 |
| Ti | 48 | 49 | |
| Plate 2_2 | Ni | 52 | 51 |
| Ti | 48 | 49 | |
| Plate 3_1 | Ni | 52 | 51 |
| Ti | 48 | 49 | |
| Plate 3_2 | Ni | 52 | 51 |
| Ti | 48 | 49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navickaitė, K.; Ianniciello, L.; Tušek, J.; Engelbrecht, K.; Bahl, C.R.H.; Penzel, M.; Nestler, K.; Böttger-Hiller, F.; Zeidler, H. Plasma Electrolytic Polishing of Nitinol: Investigation of Functional Properties. Materials 2021, 14, 6450. https://doi.org/10.3390/ma14216450
Navickaitė K, Ianniciello L, Tušek J, Engelbrecht K, Bahl CRH, Penzel M, Nestler K, Böttger-Hiller F, Zeidler H. Plasma Electrolytic Polishing of Nitinol: Investigation of Functional Properties. Materials. 2021; 14(21):6450. https://doi.org/10.3390/ma14216450
Chicago/Turabian StyleNavickaitė, Kristina, Lucia Ianniciello, Jaka Tušek, Kurt Engelbrecht, Christian R. H. Bahl, Michael Penzel, Klaus Nestler, Falko Böttger-Hiller, and Henning Zeidler. 2021. "Plasma Electrolytic Polishing of Nitinol: Investigation of Functional Properties" Materials 14, no. 21: 6450. https://doi.org/10.3390/ma14216450
APA StyleNavickaitė, K., Ianniciello, L., Tušek, J., Engelbrecht, K., Bahl, C. R. H., Penzel, M., Nestler, K., Böttger-Hiller, F., & Zeidler, H. (2021). Plasma Electrolytic Polishing of Nitinol: Investigation of Functional Properties. Materials, 14(21), 6450. https://doi.org/10.3390/ma14216450







