Influence of Firing Temperature on Phase Composition and Color Properties of Ceramic Tile Bodies
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Raw Materials Analysis
3.1.1. Chemical Composition Analysis (XRF) of Raw Materials
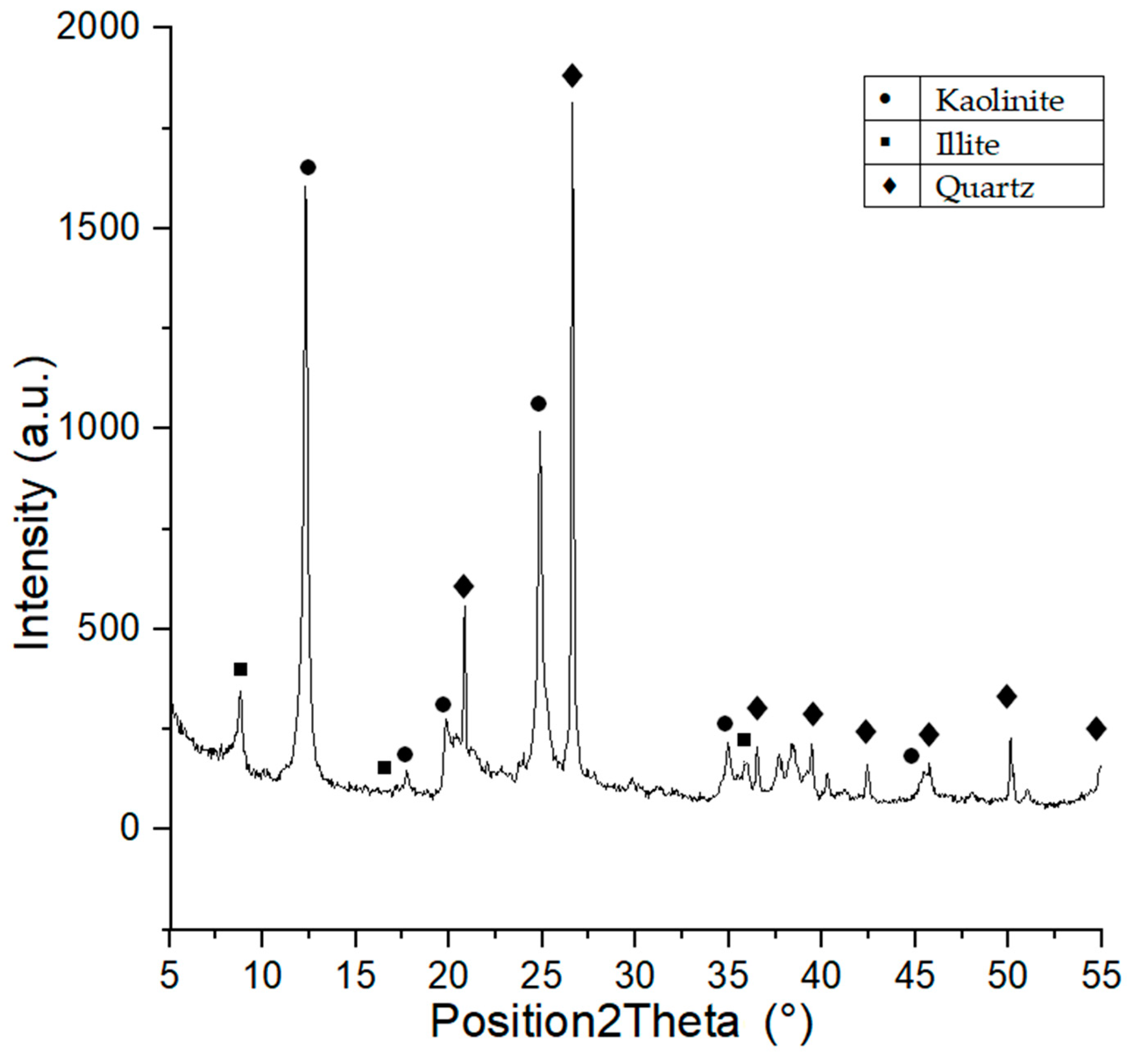
3.1.2. Phase Composition Analysis (XRD) of Raw Materials
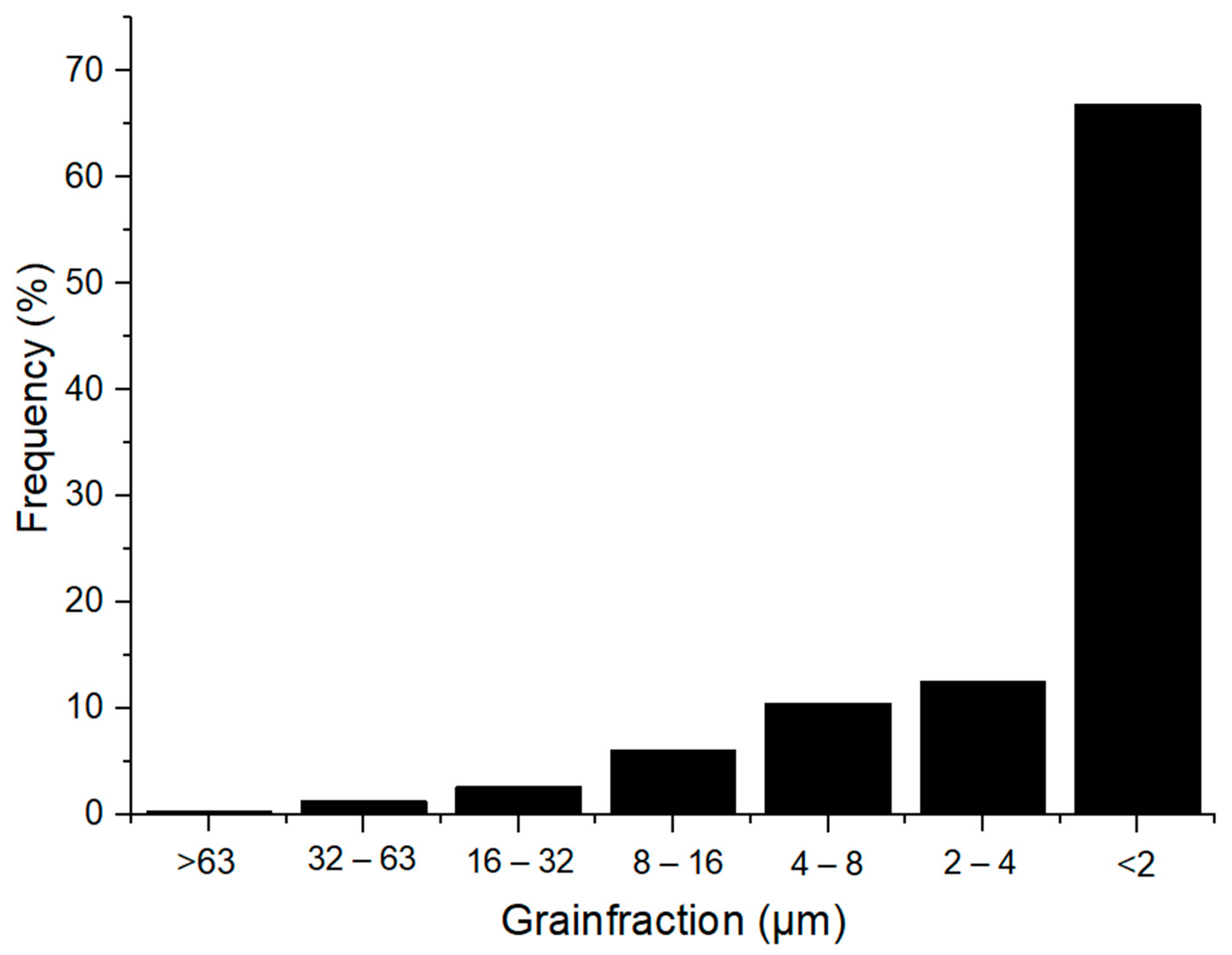
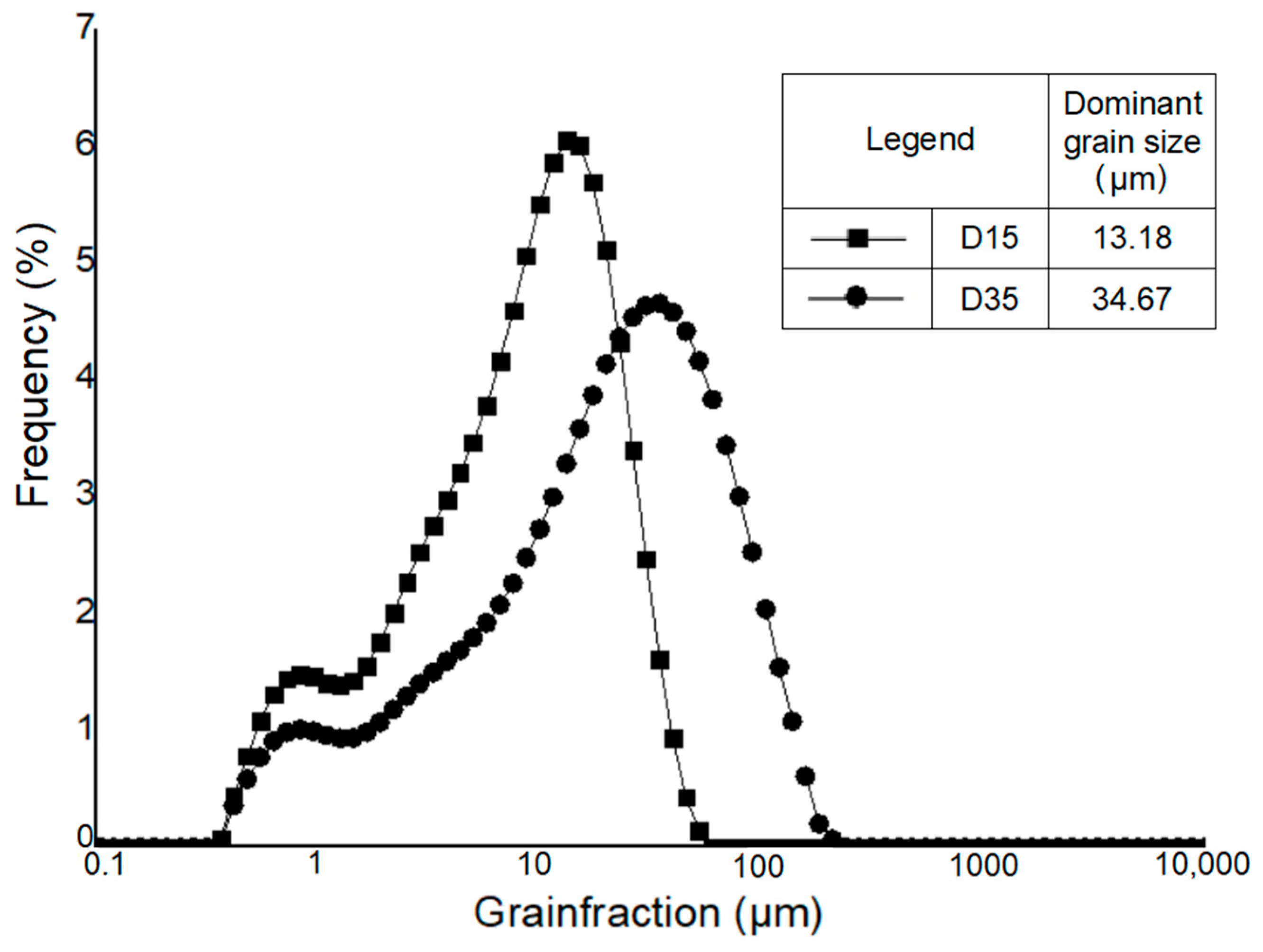
3.1.3. Particle Size Distribution of Raw Materials
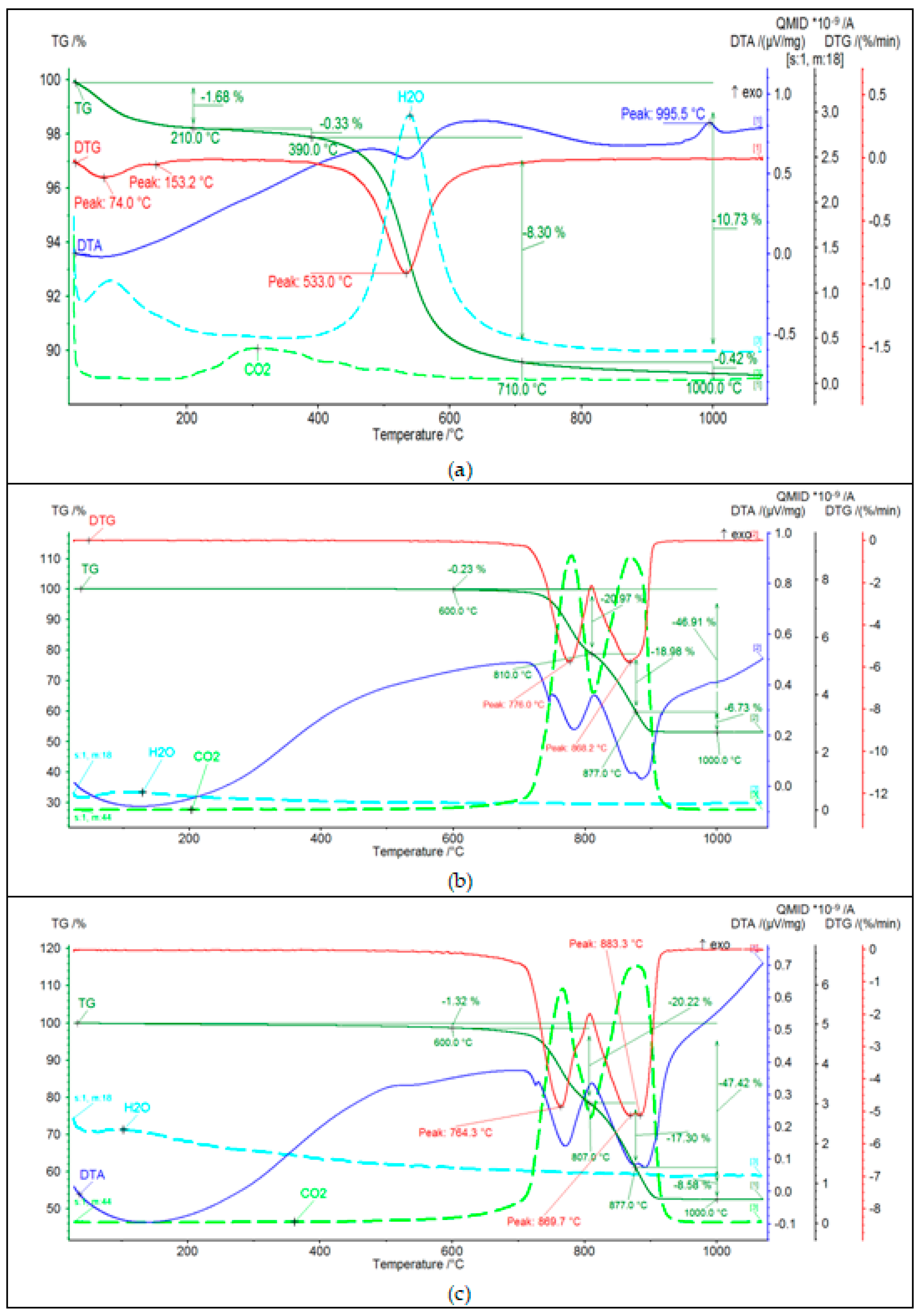
3.1.4. Thermal Analysis of Raw Materials
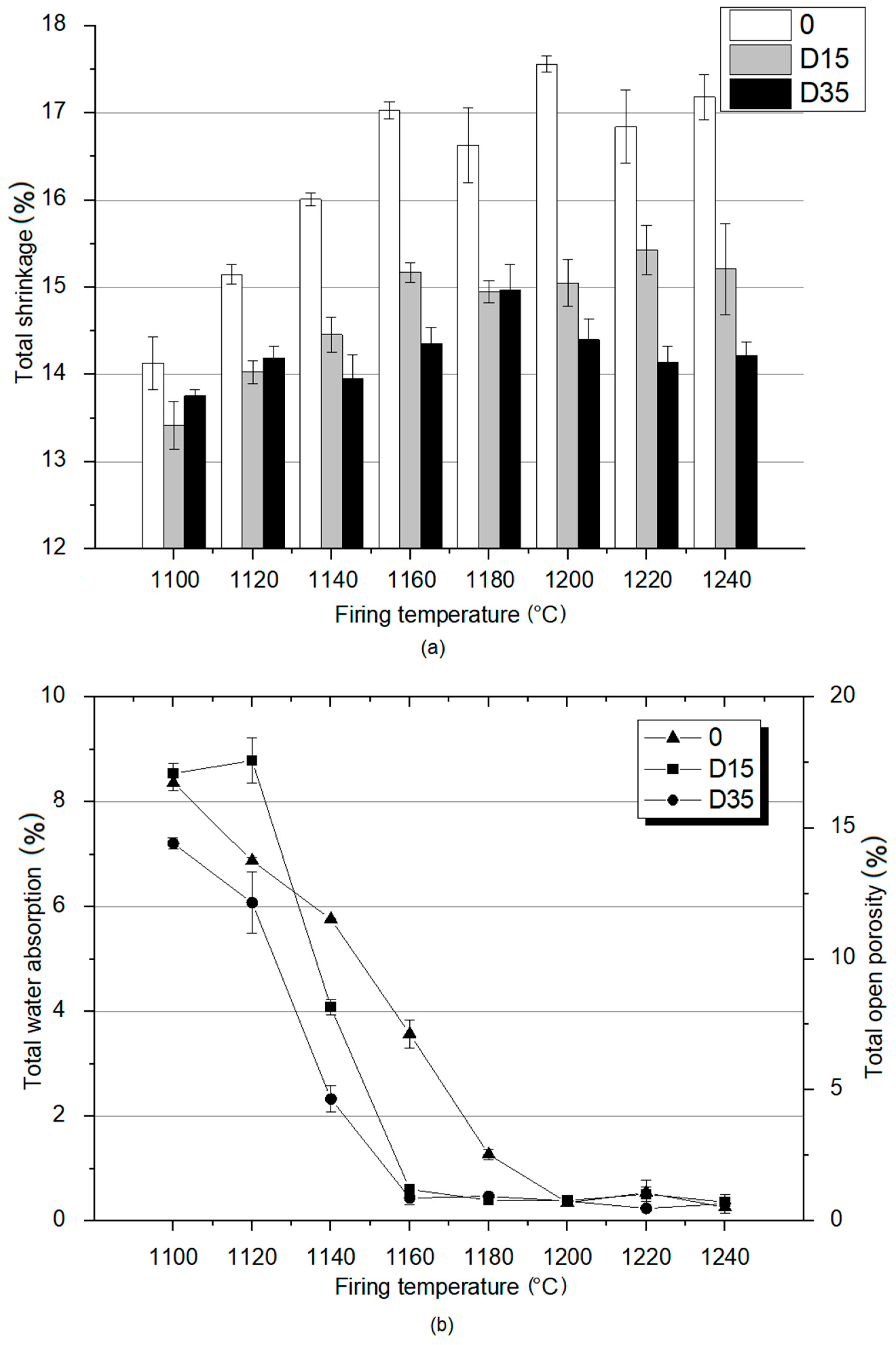
3.2. Material Properties Analysis
3.3. Material Properties Analysis
3.4. Phase Composition Analysis
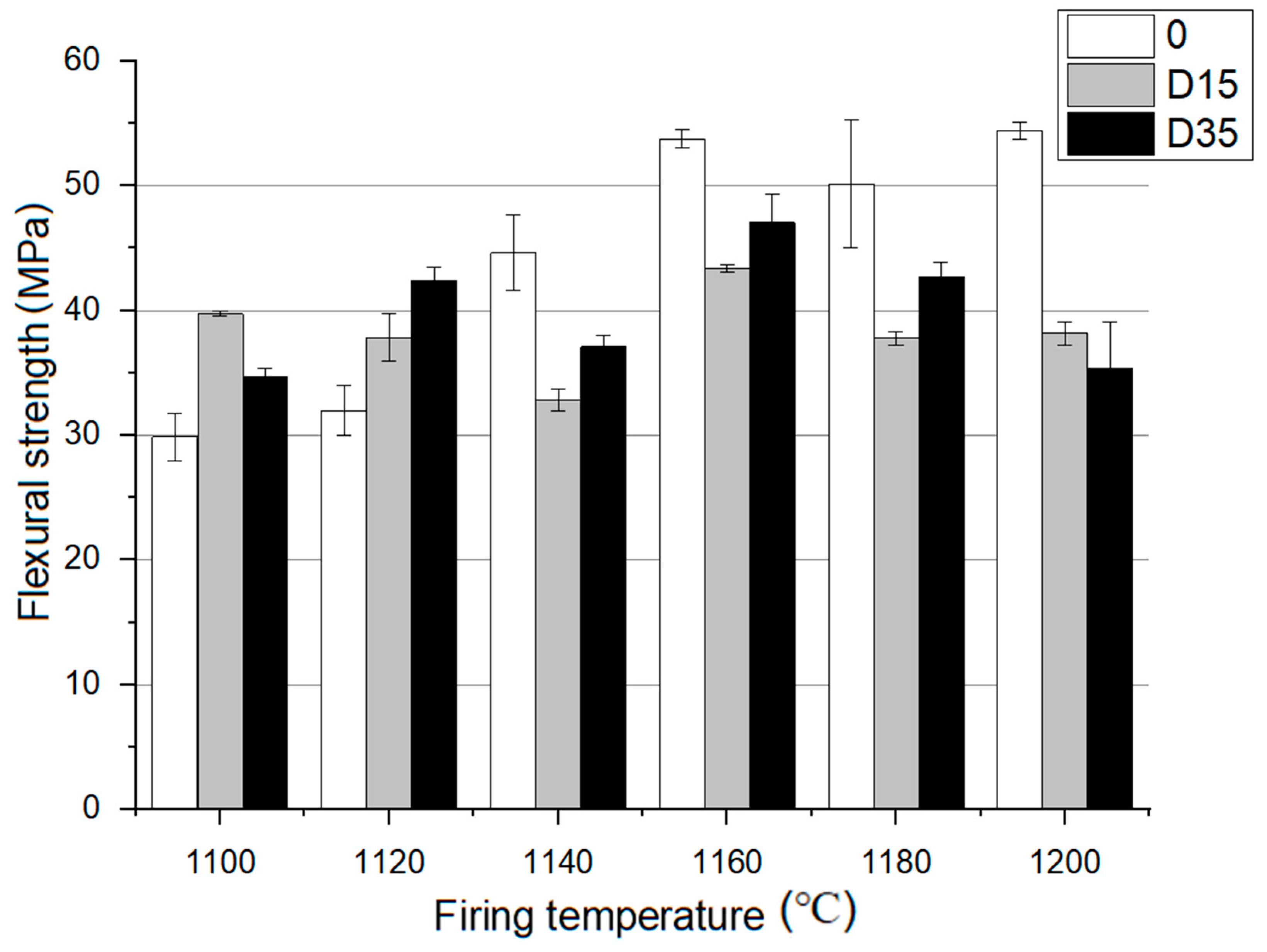
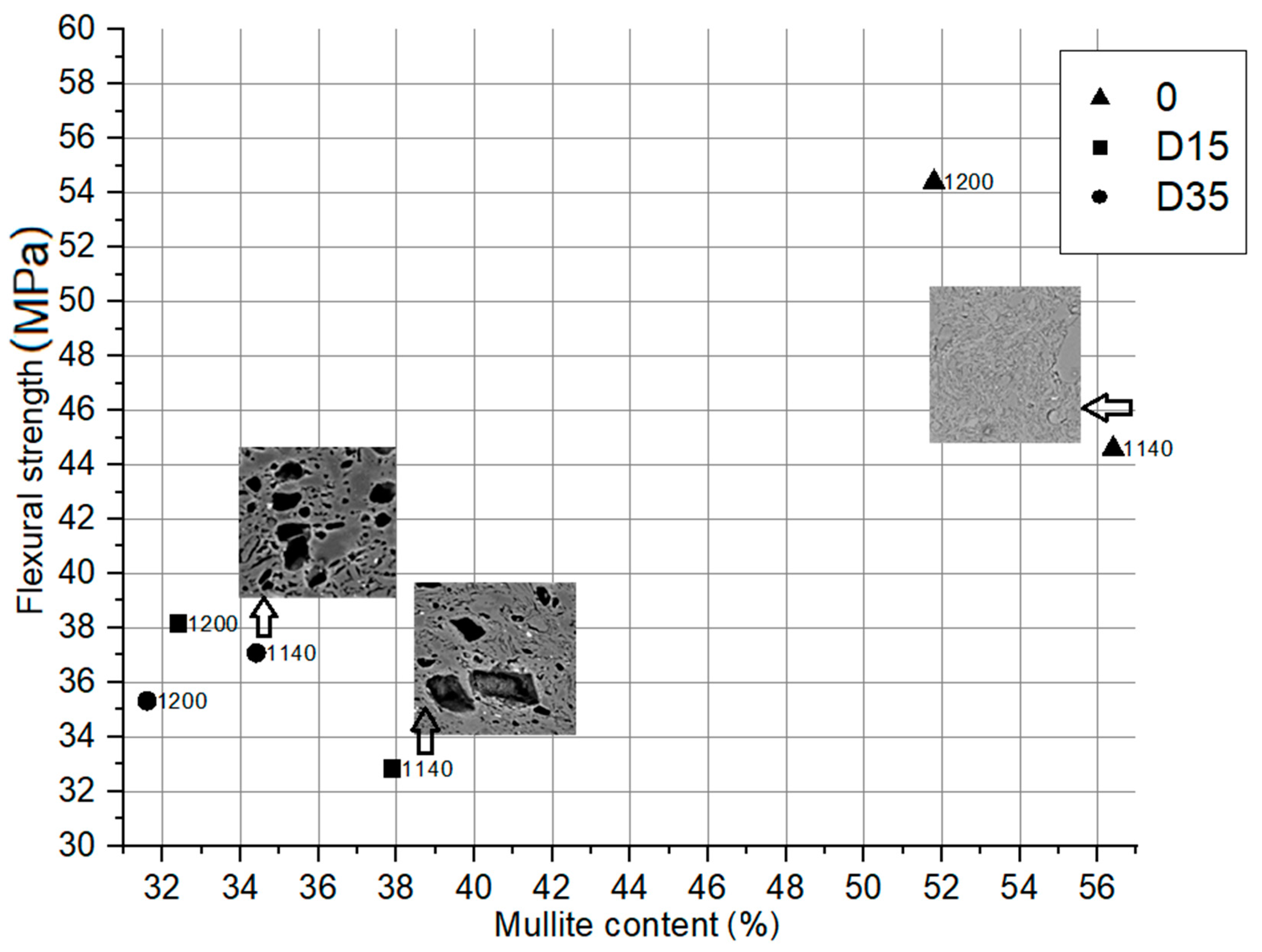
3.4.1. Dependence between Flexural Strength of Obtained Ceramic Materials and Mullite Content in Their Phase Composition
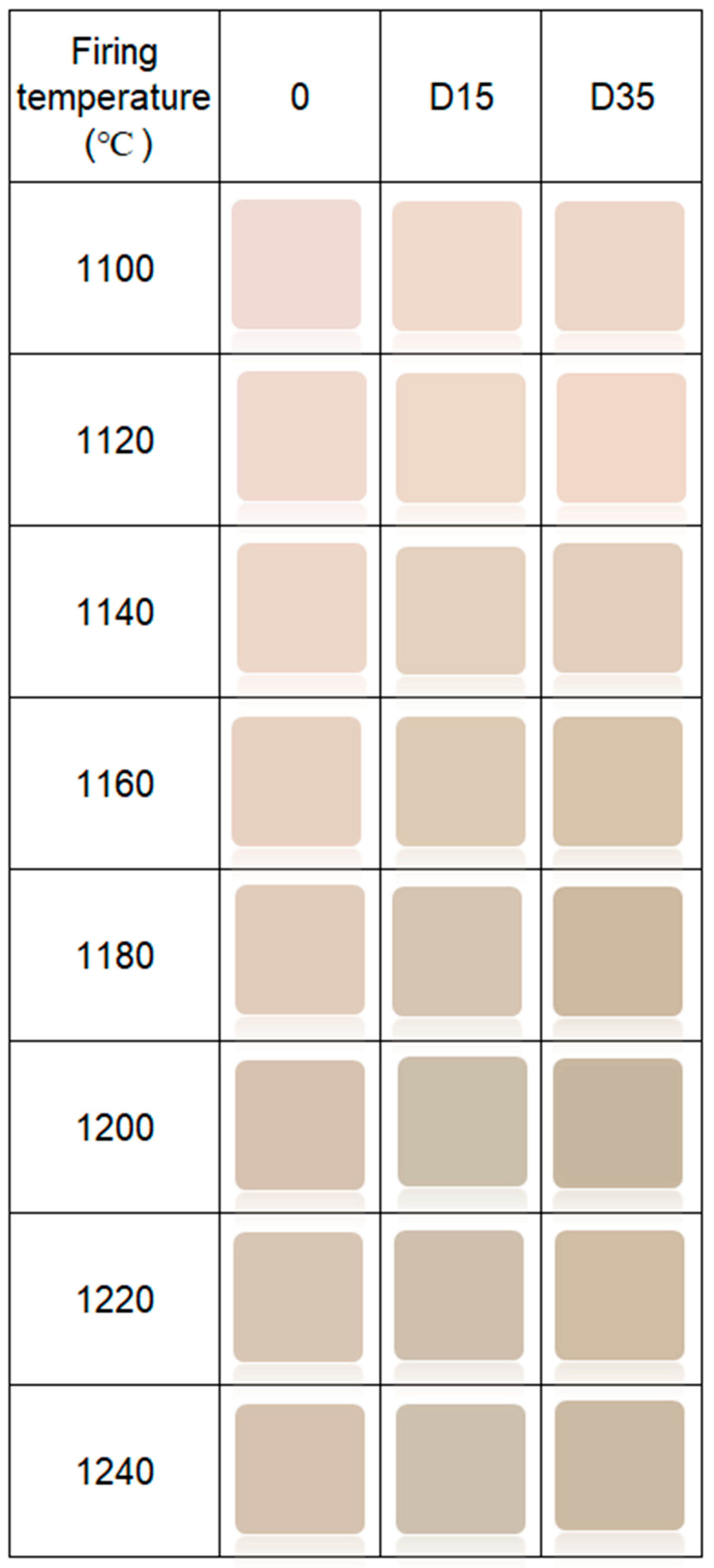
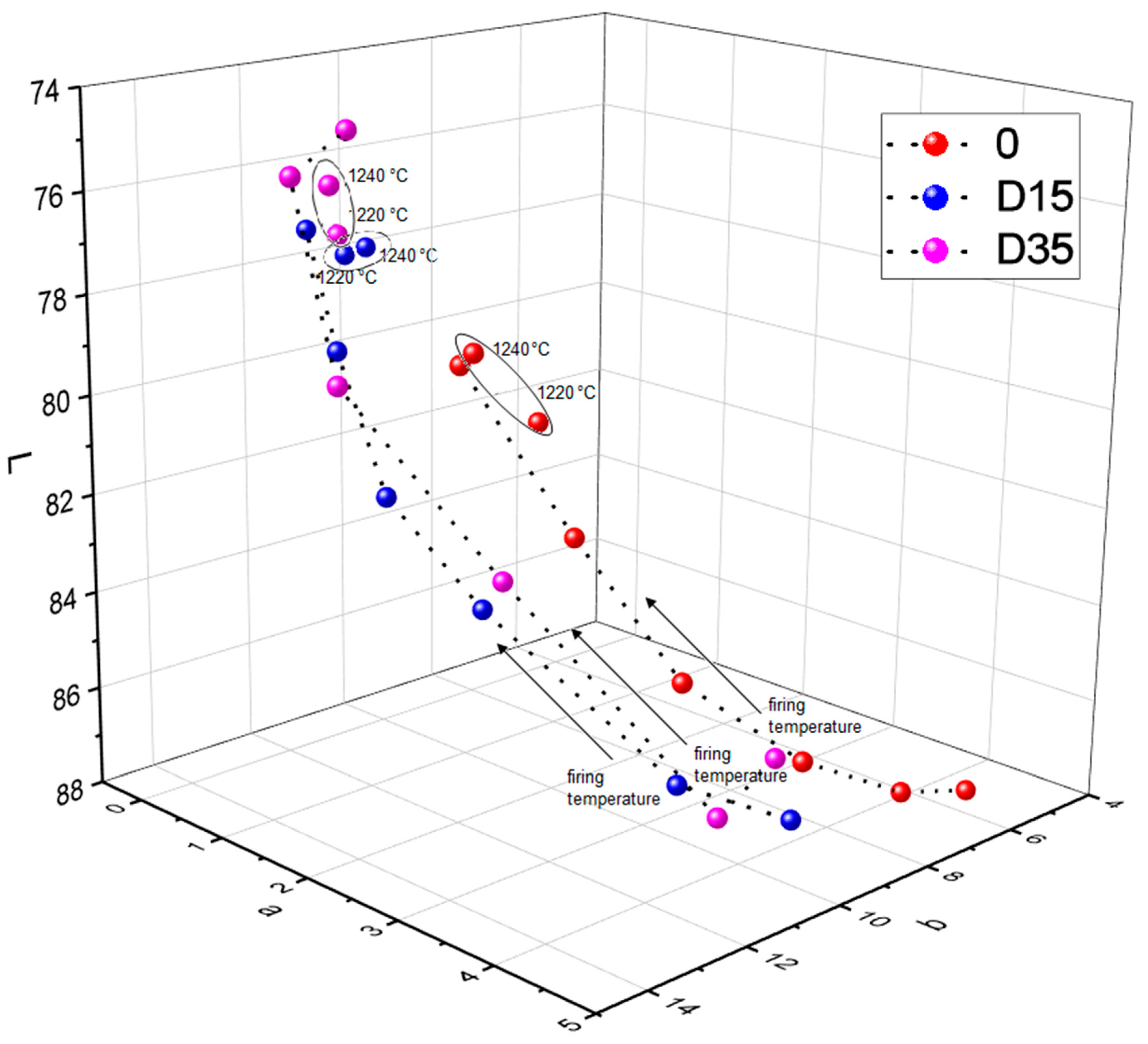
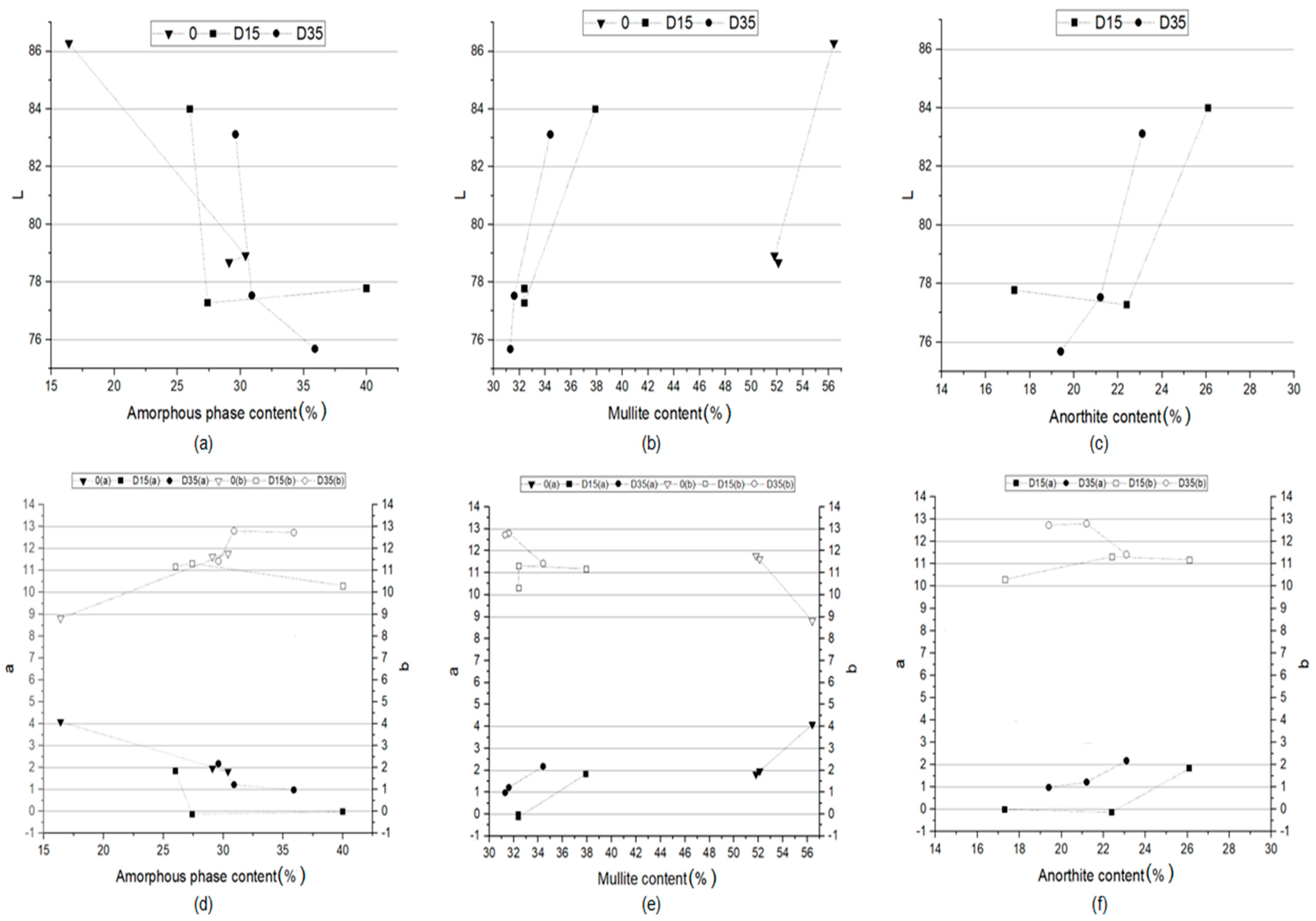
3.4.2. Dependence between Color and Phase Composition of Obtained Ceramic Materials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jordán, M.M.; Boix, T. Firing transformations of cretaceous clays used in the manufacturing of ceramic tiles. Appl. Clay Sci. 1999, 14, 225–234. [Google Scholar] [CrossRef]
- Maggetti, M. Phaseanalysis and its significance for technology and origin. In Archaeological Ceramics; Olin, J.S., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1982; pp. 121–133. [Google Scholar]
- Moropoulou, A.; Bakolas, A.; Bisbikou, K. Thermal analysis as a method of characterizing ancient ceramic technologies. Thermochim. Acta 1995, 269, 743–753. [Google Scholar] [CrossRef]
- Dondi, M.; Ercolani, G.; Melandri, C.; Mingazzini, C.; Marsigli, M. The chemical composition of porcelain stoneware tiles and its influence on microstructural and mechanical properties. Intercam 1999, 48, 75–83. [Google Scholar]
- Lecmote-Nana, G.L.; Bonnet, J.P.; Blanchart, P. Investigation of the sintering mechanisms of kaolin-muscovite. Appl. Clay Sci. 2011, 57, 445–451. [Google Scholar] [CrossRef]
- González-García, F.; Romero-Acosta, V.; García Ramos, G.; González Rodríguez, M. Firing transformations of mixtures of clays containing illite, kaolinite and calcium carbonate used by ornamental tile industries. Appl. Clay Sci. 1990, 5, 361–375. [Google Scholar] [CrossRef]
- Jordán, M.M.; Montero, M.A.; Maseguer, S.; Sanfeliu, T. Influence of firing temperature and mineralogical composition on bending strength and porosity of ceramic tile bodies. Appl. Clay Sci. 2008, 42, 266–271. [Google Scholar] [CrossRef]
- Baccour, H.; Medhioub, M.; Jamoussi, F.; Mhiri, T. Influence of firing temperature on the ceramic properties of Triassic clays from Tunisia. J. Mater. Process. Technol. 2008, 209, 2812–2817. [Google Scholar] [CrossRef]
- Ducman, V.; Škapin, A.S.; Radeka, M.; Ranogajec, J. Frost resistance of clay roofing tiles: Case study. Ceram. Int. 2011, 37, 85–91. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Pichór, W.; Kłosek-Wawrzyn, E. Impact of the addition of dolomite to cream-firing clays on the technological and color properties of sintered ceramics. Int. J. Appl. Ceram. Technol. 2021, 18, 1063–1073. [Google Scholar] [CrossRef]
- Kłosek-Wawrzyn, E.; Bugaj, A. Influence of dolomite addition to masses made from triassic clay on application properties, phase composition and microstructure of ceramic materials produced. Ceram. Mater. 2016, 68, 236–241. [Google Scholar]
- Wyszomirski, P. Ił z Borkowic (rejon opoczyński) jako wartościowy surowiec wielu dziedzin przemysłu ceramicznego (Borkowice clay (Opoczno region) as a valuable raw material in many areas of the ceramics industry). Zesz. Nauk. Inst. Gospod. Surowcami Miner. Energią Pol. Akad. Nauk 2015, 91, 245–260. [Google Scholar]
- Weisser, P.; Lech, R.; Grabski, J. Badanie właściwości dolomitów z trzech złóż, przeznaczonych do przemysłowego stosowania (The examination of the proprieties of dolomites from three deposits, used in the different industries). Cem. Wapno Beton 2014, 3, 194–202. [Google Scholar]
- Zarubica, A.; Miljkovic, M.; Purenovic, M.; Tomic, V. Colour parameters, whiteness indices and physical features of marking paints for horizontal signalization. Facta Univ.-Ser. Phys. Chem. Technol. 2005, 3, 205–216. [Google Scholar] [CrossRef]
- Hiller, S. Accurate quantitative analysis of clay and other minerals in sandstones by XRD: Comparison of a Rietveld and a reference intensity ratio (RIR) method and the importance of sample preparation. Clay Miner. 2000, 35, 291–302. [Google Scholar] [CrossRef]
- Stoch, L. Metody Badań Minerałów i Skał: Metody Termiczne; Wydawnictwo Geologiczne: Warszawa, Poland, 1988. [Google Scholar]
- Lech, R. Termiczny Rozkład Wapieni: Transport Masy i Ciepła; Polskie Towarzystwo Ceramiczne: Kraków, Poland, 2008. [Google Scholar]
- Salvator, A.R.; Calvo, E.G.; Aparicio, C.B. Effects of sample weight, particle size, purge gas and crystalline structure on the observed kinetic parameters of calcium carbonate decomposition. Thermochim. Acta 1989, 143, 339–345. [Google Scholar] [CrossRef]
- Abubakar, M.; Muthuraja, A.; Ahmad, N. Experimental investigation of the effect of temperature on the density of kaolin clay. Meter. Today Proc. 2021, 41, 791–794. [Google Scholar] [CrossRef]
- Niesyt, M.; Psiuk, B. Fused dolomite-magnesia co-clinker for fired dolomite refractories. Ceram. Int. 2017, 43, 51–59. [Google Scholar] [CrossRef]
- Wang, W.; Shin, Z.; Wang, X.; Fan, W. The phase transformation and thermal expansion properties of cordierite ceramics prepared using drift sands to replace pure quartz. Ceram. Int. 2016, 42, 4477–4485. [Google Scholar] [CrossRef]
- Redaoui, D.; Sahnoune, F.; Heraiz, M.; Saheb, N. Phase formation and crystallization kinetics in cordierite ceramics prepared from kaolinite and magnesia. Ceram. Int. 2018, 44, 3649–3657. [Google Scholar] [CrossRef]
- Njoya, D.; Elimbi, A.; Fouejio, D.; Hajjaji, M. Effects of two mixtures of kaolin-talc-bauxite and firing temperatures on the characteristics of cordierite- based ceramics. J. Build. Eng. 2016, 8, 99–106. [Google Scholar] [CrossRef]
- Sembiring, S.; Simanjuntak, W.; Situmeang, R.; Riyanto, A.; Sebayang, K. Preparation of refractory cordierite using amorphous rice husk silica for thermal insulation purposes. Ceram. Int. 2016, 42, 8431–8437. [Google Scholar] [CrossRef]

| Name of the Material | Amount of Raw Material (% by Mass) | |
|---|---|---|
| BorkowiceClay | OłdrzychowiceDolomite | |
| 0 | 100 | 0 |
| D15 * | 90 | 10 |
| D35 * | 90 | 10 |
| Method and Laboratory Equipment | Formation | Drying Process | Firing Process | ||
| Vented laboratory screw press Plastic method | Dried in the room temperature (at about 20 °C) for 24 h–samples positioned at proper distance to allow air circulation Laboratory dryer | Laboratory electric kiln | |||
| Samples characteristics: | |||||
| Beams with dimensions: 150 × 30 × 20 mm | Bricks with dimension: 75 × 30 × 20 mm | ||||
| Conditions | 24 h storage of the masses in humid conditions | Dried to the constant mass | Oxidizing atmosphere | ||
| Process characteristics | - | (I.) 35 °C for the first 6 h | Firing rate: 100 °C/h | ||
| (II.) Increase in the temperature to: 50 °C, 75 °C and 105 °C every 2 h | Dwelling time: − 1 h at selected temperatures: 100 °C, 600 °C, 900 °C − 2 h at maximum temperature | ||||
| (III.) 105 °C for 7 h | Maximum temperature (°C): | ||||
| T1 | 1100 | ||||
| T2 | 1120 | ||||
| T3 | 1140 | ||||
| T4 | 1160 | ||||
| T5 | 1180 | ||||
| T6 | 1200 | ||||
| T7 | 1220 | ||||
| T8 | 1240 | ||||
| Chemical Composition (wt/%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw Material | SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | K2O | Na2O | Cr2O3 | P2O5 | MnO | SO3 | Loss of Ignition |
| Borkowice clay | 53.22 | 30.92 | 0.99 | 1.34 | 0.23 | 0.41 | 1.60 | 0.16 | 0.02 | 0.15 | 0.00 | 0.02 | 10.73 |
| Ołdrzychowice dolomite | 1.39 | 0.36 | 0.19 | 0.02 | 36.06 | 16.79 | 0.06 | 0.06 | 0.00 | 0.01 | 0.06 | 0.01 | 44.95 |
| Temperature
Range (°C) | Name of the Ceramic Materials | ||
|---|---|---|---|
| 0 | D15 | D35 | |
| DeltaE between Ceramic Materials
Fired at Different Temperatures | |||
| 1100–1120 | 1.4802 | 1.4995 | 1.4342 |
| 1120–1140 | 2.2342 | 3.4520 | 4.4325 |
| 1140–1160 | 2.2138 | 2.4182 | 4.6030 |
| 1160–1180 | 2.4945 | 2.6202 | 3.9185 |
| 1180–1200 | 3.8175 | 2.2117 | 1.3143 |
| 1200–1220 | 2.0315 | 0.6427 | 1.9094 |
| 1220–1240 | 2.1461 | 0.6733 | 0.9135 |
| Type of Material | 0 | D15 | D35 | 0 | D15 | D35 | 0 | D15 | D35 |
|---|---|---|---|---|---|---|---|---|---|
| Firing Temperature (°C) | 1140 | 1200 | 1240 | ||||||
| Mullite (Al4+2xSi2−2xO10−x) | 56.4 | 37.9 | 34.4 | 51.8 | 32.4 | 31.6 | 52.1 | 32.4 | 31.3 |
| Quartz (SiO2) | 27.0 | 10.1 | 12.9 | 17.8 | 4.9 | 4.8 | 18.7 | 5.0 | 7.1 |
| Cordierite (Mg2Al3[AlSi5O18]) | 0.0 | 0.0 | 0.0 | 0.0 | 12.8 | 11.6 | 0.0 | 5.2 | 6.2 |
| Anorthite (Ca[Al2Si2O8]) | 0.0 | 26.1 | 23.1 | 0.0 | 22.4 | 21.2 | 0.0 | 17.3 | 19.4 |
| Amorphous phase | 16.4 | 26.0 | 29.6 | 30.4 | 27.4 | 30.9 | 29.1 | 40.0 | 35.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśniewska, K.; Pichór, W.; Kłosek-Wawrzyn, E. Influence of Firing Temperature on Phase Composition and Color Properties of Ceramic Tile Bodies. Materials 2021, 14, 6380. https://doi.org/10.3390/ma14216380
Wiśniewska K, Pichór W, Kłosek-Wawrzyn E. Influence of Firing Temperature on Phase Composition and Color Properties of Ceramic Tile Bodies. Materials. 2021; 14(21):6380. https://doi.org/10.3390/ma14216380
Chicago/Turabian StyleWiśniewska, Kornelia, Waldemar Pichór, and Ewelina Kłosek-Wawrzyn. 2021. "Influence of Firing Temperature on Phase Composition and Color Properties of Ceramic Tile Bodies" Materials 14, no. 21: 6380. https://doi.org/10.3390/ma14216380
APA StyleWiśniewska, K., Pichór, W., & Kłosek-Wawrzyn, E. (2021). Influence of Firing Temperature on Phase Composition and Color Properties of Ceramic Tile Bodies. Materials, 14(21), 6380. https://doi.org/10.3390/ma14216380






