Advances Brought by Hydrophilic Ionic Liquids in Fields Involving Pharmaceuticals
Abstract
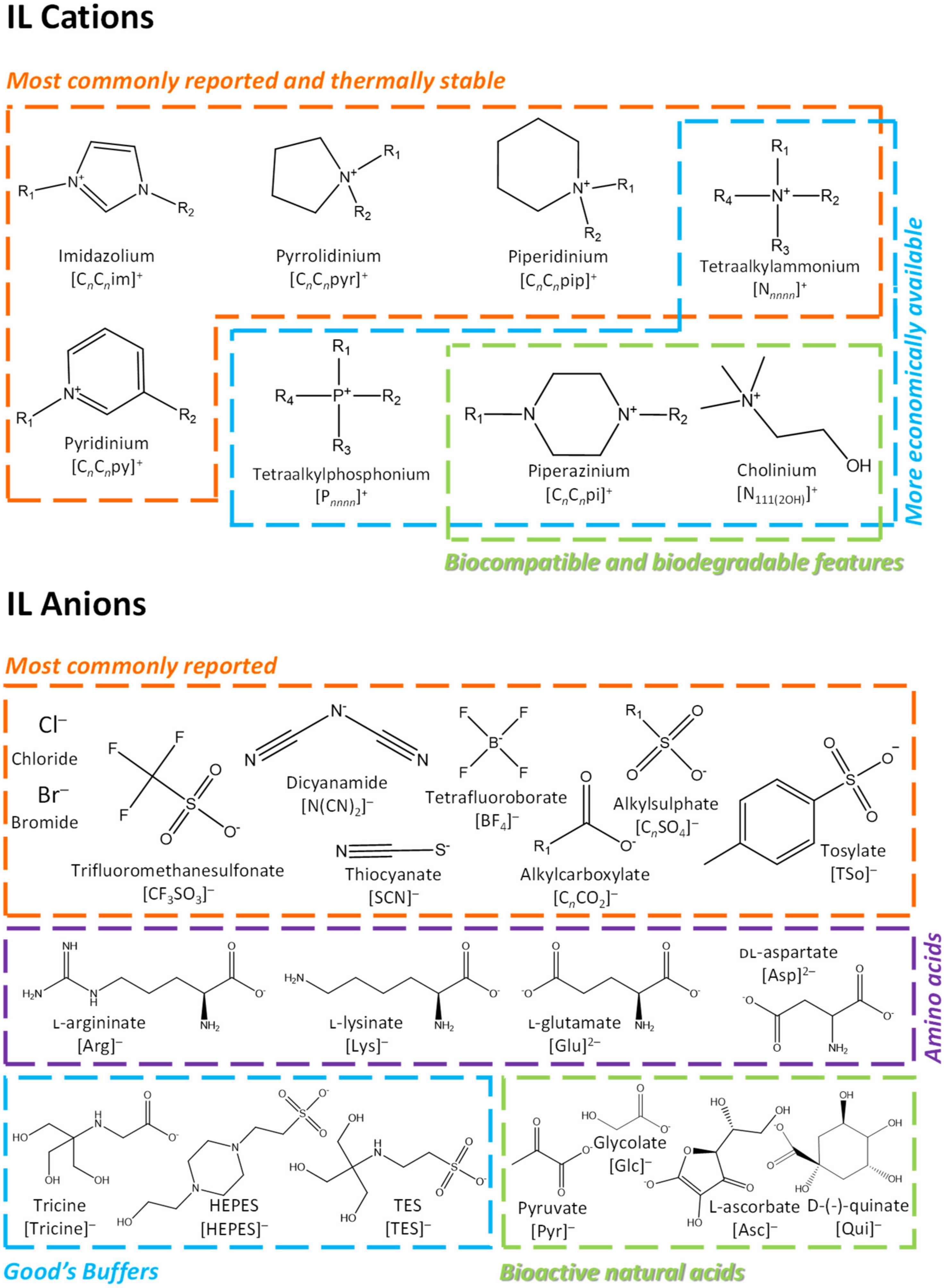
:1. Introduction
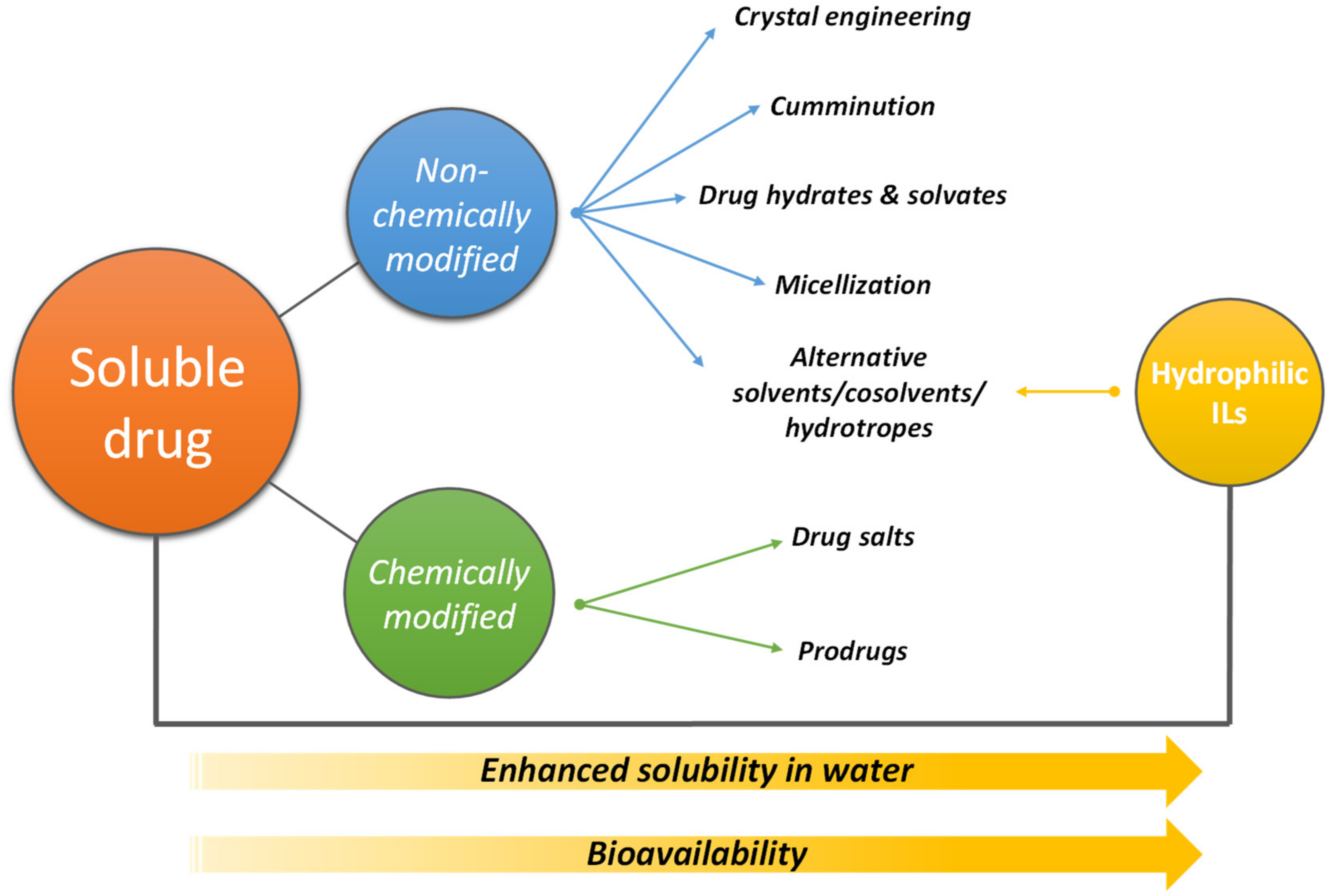
2. ILs Used to Improve the Solubility (Bioavailability) of Pharmaceuticals
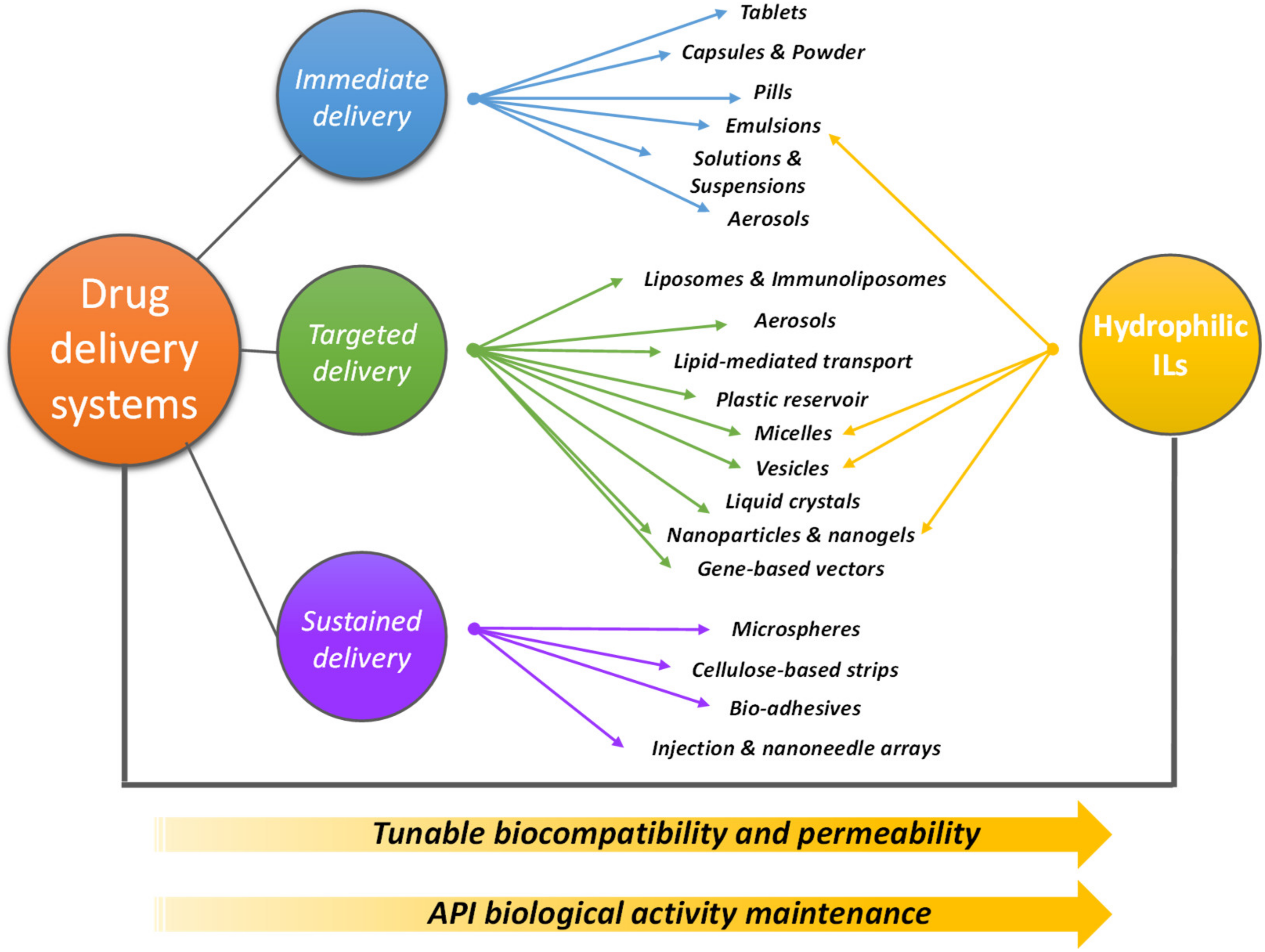
3. IL-Based Drug Delivery Systems
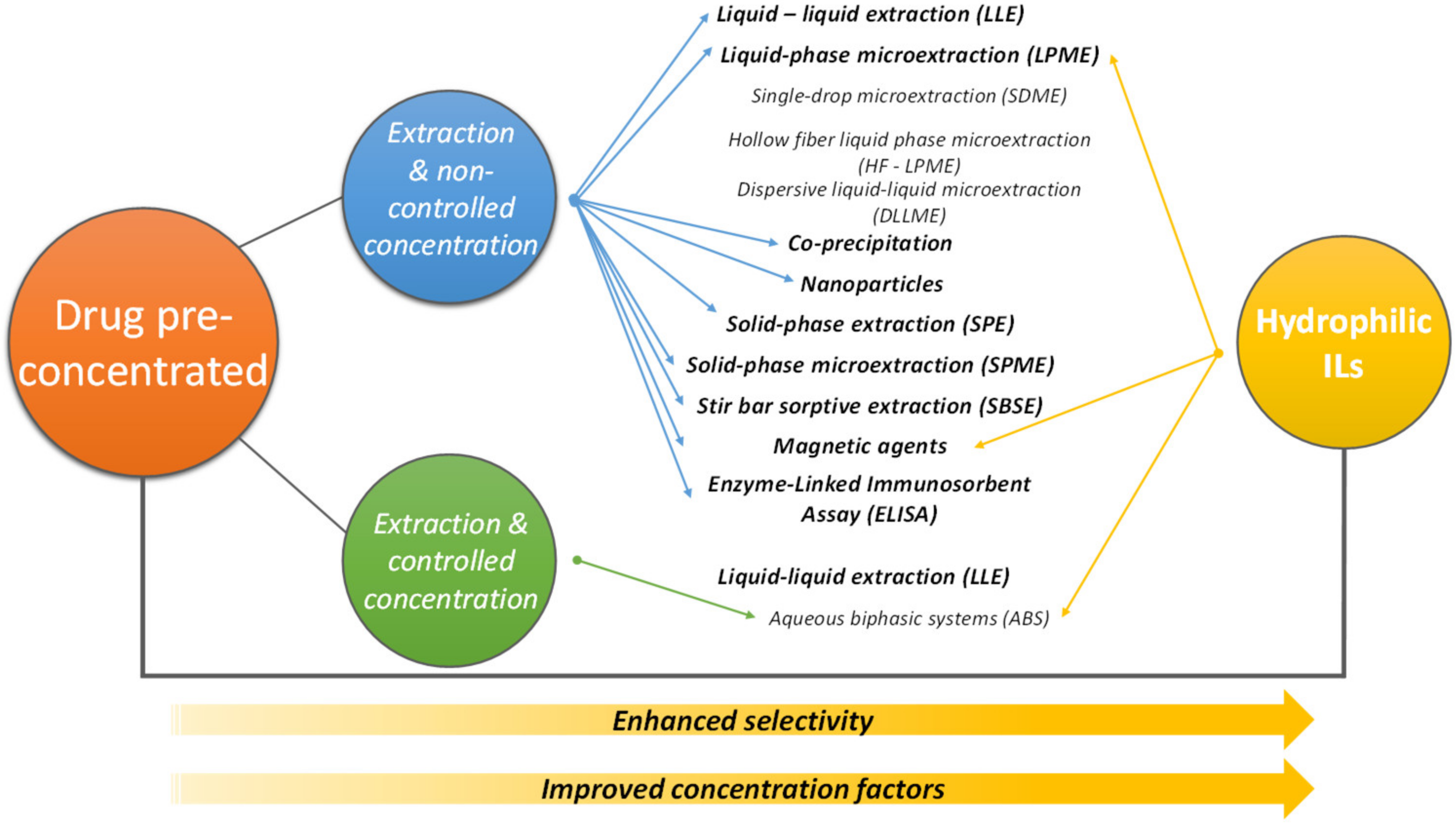
4. Pretreatment/Concentration of Pharmaceuticals to Improve Analytical Analysis
4.1. IL-Based ABS
4.2. Other Techniques
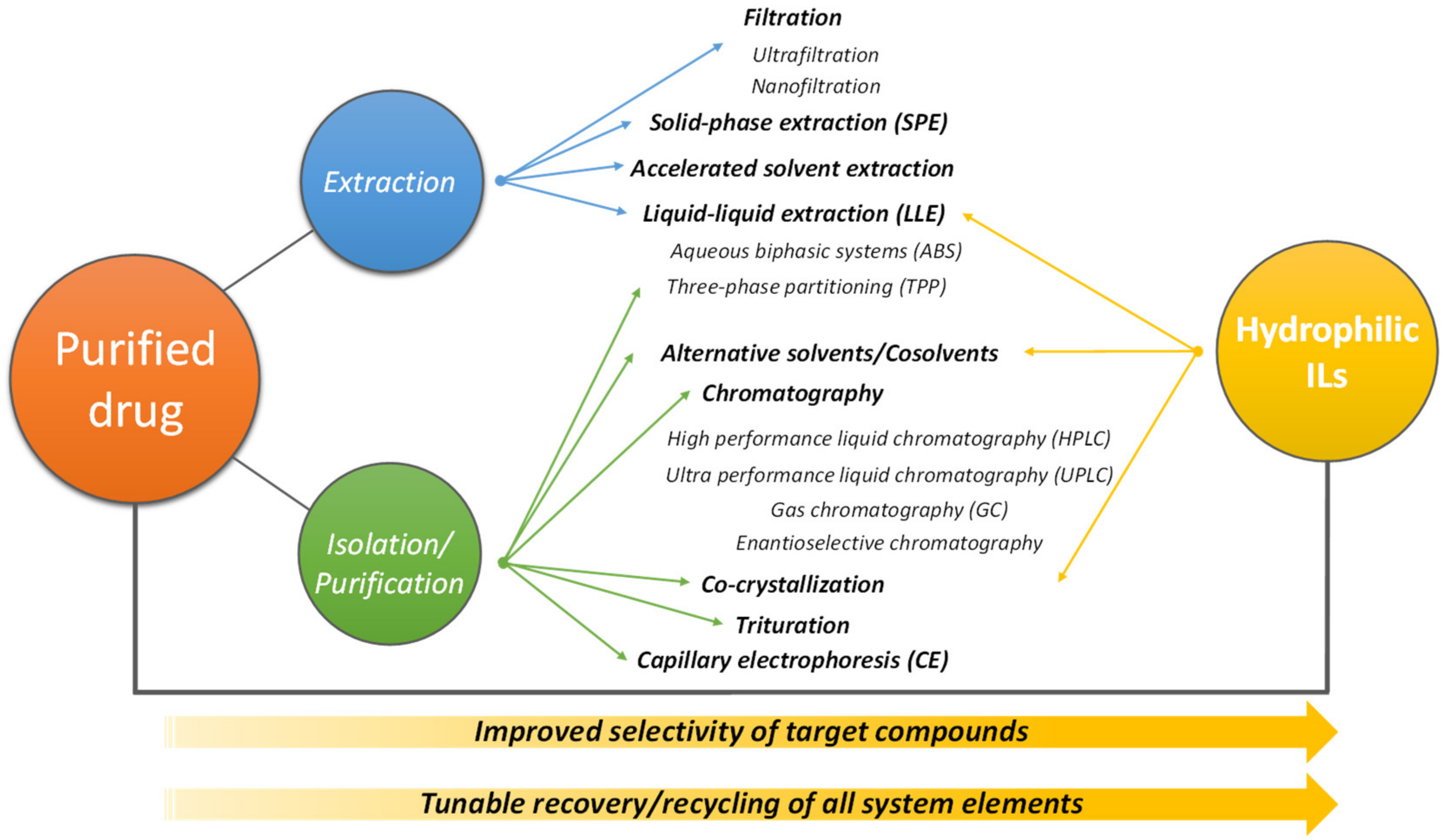
5. Recovery and Purification of Pharmaceuticals Using IL-Based Systems
5.1. IL-Based ABS
5.2. Other Techniques
6. Key Factors in Choosing Hydrophilic ILs in Fields Involving Pharmaceuticals
6.1. Cytotoxicity
6.2. Environmental Risks
6.3. IL Recovery and Reuse
6.4. Cost
7. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| [CnCar]+ | Carnitine alkyl ester |
| [CnC1C1im]+ | 1-alkyl-2,3-dimethylimidazolium |
| [CnC1im]+ | 1-alkyl-3-methylimidazolium |
| [CnC1im]+ | 1-alkyl-3-butylimidazolium |
| [CnC1pip]+ | 1-alkyl-1-methylpiperidinium |
| [CnC1pyr]+ | 1-alkyl-1-methylpyrrolidinium |
| [CnNH3]+ | N-alkylammonium |
| [C1C1C1C1guan]+ | 1,1,3,3-tetramethylguanidinium |
| [C4(Cnim)2]2+ | 1,1’-(butane-1,4-diyl)bis(3-alkylimidazolium) |
| [C7H7C1im]+ | 1-benzyl-3-methylimidazolium |
| [Nnnnn]+ | tetraalkylammonium |
| [N000(2OH)]+ | 2-hydroxyethylammonium |
| [N00(2OH)(2OH)]+ | bis(2-hydroxyethyl) ammonium |
| [N11n(2OH)]+ | N-Alkyl-N,N-dimethyl-N-(2-hydroxyethyl)ammonium |
| [N111(2OH)]+ | N,N,N-trimethyl-N-(2-hydroxyethyl)ammonium (cholinium) |
| [Pnnnn]+ | Tetraalkylphosphonium |
| [Pnnn1]+ | Trialkylmethylphosphonium |
| [P666,14]+ | Trihexyltetradecyl phosphonium |
| [OHCnC1im]+ | 1-hydroxyalkyl-3-methylimidazolium |
| [BF4]– | Tetrafluoroborate |
| [Bic]– | Bicarbonate |
| [CF3CO2]– | Trifluoroacetate |
| [CF3SO3]– | Trifluoromethanesulfonate |
| [CnCO2]– | Alkylcarboxylate |
| [C1SO4]– | Methylsulfate |
| [C1SO3]– | Methylsulfonate |
| [Gal]– | Gallate |
| [Ger]– | Geranate |
| [Glt]– | Glutarate |
| [Ibu]– | Ibuprofenate |
| [Lev]– | Levulinate |
| [NPA]– | Naphthoate |
| [NTf2]– | Bis(trifluoromethylsulfonyl)amide |
| [N(CN)2]– | Dicyanamide |
| [Ole]– | Oleate |
| [PF6]– | Hexafluorophosphate |
| [Sal]– | Salicylate |
| [SCN]– | Thiocyanate |
| [Suc]– | Succinate |
| [TEMPO-OSO3]– | 2,2,6,6-tetramethyl-1-piperidinyloxyl-4-sulfate |
| [TsO]– | Tosylate |
| [Trp]– | Triptophanate |
| [Van]– | Vanillate |
| [(CH3O)2PO2]– | Dimethyl phosphate |
| ABS | Aqueous biphasic systems |
| API | Active pharmaceutical ingredient |
| BPA | Bisphenol A |
| Br– | Bromide |
| CAF | Caffeine |
| CBZ | Carbamazepine |
| CE | Capillary electrophoresis |
| CF | Concentration factor |
| Cl– | Chloride |
| CMC | Critical micelle concentration |
| DAD | Diode array detector |
| DLLME | Dispersive liquid-liquid microextraction |
| EC50 | The effective concentration resulting in a 50% reduction of processes |
| EE2 | 17α-ethinylestradiol |
| FD | Fluorescence detection |
| FESEM | Field emission scanning microscopy |
| FQ | Fluoroquinolones |
| GC | Gas chromatography |
| HPLC | High performance liquid chromatography |
| IL | Ionic liquid |
| LC | Liquid chromatography |
| LLE | Liquid-liquid extraction |
| MS | Mass spectrometry |
| NMR | Nuclear magnetic resonance spectroscopy |
| NSAID | Non-steroidal anti-inflammatory drug |
| OECD | Organization for Economic Cooperation and Development |
| PEG | Polyethylene glycol |
| PIL | Polymerizable biobased ionic liquid |
| PPG | Polypropylene glycol |
| SAIL | Surface active ionic liquids |
| SPE | Solid-phase extraction |
| TL | Tie-line |
| TPP | Three-phase partitioning |
| TSIL | Task-specific ionic liquid |
| UV | Ultraviolet detector |
| VOC | Volatile organic compound |
| WWTP | Wastewater treatment plant |
References
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Seddon, K.R. Ionic liquids for clean technology. J. Chem. Technol. Biotechnol. 1997, 68, 351–356. [Google Scholar] [CrossRef]
- Freire, M.G.; Claudio, A.F.M.; Araujo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Canongia Lopes, J.N.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Kumar, A.; Chauhan, S.; Chauhan, S.M.S. Chemical and biochemical transformations in ionic liquids. Tetrahedron 2005, 61, 1015–1060. [Google Scholar] [CrossRef]
- Sheldon, R. Catalytic reactions in ionic liquids. Chem. Commun. 2001, 23, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.T.; Han, S.J.; Livingston, A.G. The effect of ionic liquids on product yield and catalyst stability. Chem. Eng. Sci. 2006, 61, 1338–1341. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, S.Q.; Ma, P.S. Use of ionic liquids as ’green’ solvents for extractions. J. Chem. Technol. Biotechnol. 2005, 80, 1089–1096. [Google Scholar] [CrossRef]
- Bowlas, C.J.; Bruce, D.W.; Seddon, K.R. Liquid-crystalline ionic liquids. Chem. Commun. 1996, 14, 1625–1626. [Google Scholar] [CrossRef]
- Endres, F.; El Abedin, S.Z. Air and water stable ionic liquids in physical chemistry. Phys. Chem. Chem. Phys. 2006, 8, 2101–2116. [Google Scholar] [CrossRef]
- Gordon, C.M.; Holbrey, J.D.; Kennedy, A.R.; Seddon, K.R. Ionic liquid crystals: Hexafluorophosphate salts. J. Mater. Chem. 1998, 8, 2627–2636. [Google Scholar] [CrossRef]
- Hussey, C.L. The Electrochemistry of Room-Temperature Haloaluminate Molten Salts. In Chemistry of Nonaqueous Solutions: Current Progress, 4th ed.; Mamantov, G., Popov, A.I., Eds.; VCH: New York, NY, USA, 1994; pp. 227–276. [Google Scholar]
- Ventura, S.P.M.; Santos, L.D.F.; Saraiva, J.A.; Coutinho, J.A.P. Ionic liquids microemulsions: The key to Candida antarctica lipase B superactivity. Green Chem. 2012, 14, 1620–1625. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; e Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Passos, H.; Freire, M.G.; Coutinho, J.A.P. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef] [Green Version]
- Cláudio, A.F.M.; Ferreira, A.M.; Freire, M.G.; Coutinho, J.A.P. Enhanced extraction of caffeine from guaraná seeds using aqueous solutions of ionic liquids. Green Chem. 2013, 15, 2002–2010. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Neves, M.C.; Shimizu, K.; Canongia Lopes, J.N.; Freire, M.G.; Coutinho, J.A.P. The magic of aqueous solutions of ionic liquids: Ionic liquids as a powerful class of catanionic hydrotropes. Green Chem. 2015, 17, 3948–3963. [Google Scholar] [CrossRef]
- Gras, M.; Duclos, L.; Schaeffer, N.; Mogilireddy, V.; Svecova, L.; Chaînet, E.; Billard, I.; Papaiconomou, N. A Comparison of Cobalt and Platinum Extraction in Hydrophobic and Hydrophilic Ionic Liquids: Implication for Proton Exchange Membrane Fuel Cell Recycling. ACS Sustain. Chem. Eng. 2020, 8, 15865–15874. [Google Scholar] [CrossRef]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef]
- Costa, A.; Forte, A.; Zalewska, K.; Tiago, G.; Petrovski, Z.; Branco, L.C. Novel biocompatible ionic liquids based on gluconate anion. Green. Chem. Lett. Rev. 2015, 8, 8–12. [Google Scholar] [CrossRef]
- Tao, D.-J.; Cheng, Z.; Chen, F.-F.; Li, Z.-M.; Hu, N.; Chen, X.-S. Synthesis and Thermophysical Properties of Biocompatible Cholinium-Based Amino Acid Ionic Liquids. J. Chem. Eng. Data 2013, 58, 1542–1548. [Google Scholar] [CrossRef]
- Mondal, D.; Sharma, M.; Quental, M.V.; Tavares, A.P.M.; Prasad, K.; Freire, M.G. Suitability of bio-based ionic liquids for the extraction and purification of IgG antibodies. Green Chem. 2016, 18, 6071–6081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quental, M.V.; Pedro, A.Q.; Pereira, P.; Sharma, M.; Queiroz, J.A.; Coutinho, J.A.P.; Sousa, F.; Freire, M.G. Integrated Extraction-Preservation Strategies for RNA Using Biobased Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 9439–9448. [Google Scholar] [CrossRef]
- Carvalho, P.J.; Ventura, S.P.M.; Batista, M.L.S.; Schroder, B.; Goncalves, F.; Esperanca, J.; Mutelet, F.; Coutinho, J.A.P. Understanding the impact of the central atom on the ionic liquid behavior: Phosphonium vs. ammonium cations. J. Chem. Phys. 2014, 140, 064505. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.P.M.; Silva, F.A.E.; Goncalves, A.M.M.; Pereira, J.L.; Goncalves, F.; Coutinho, J.A.P. Ecotoxicity analysis of cholinium-based ionic liquids to Vibrio fischeri marine bacteria. Ecotoxicol. Environ. Saf. 2014, 102, 48–54. [Google Scholar] [CrossRef]
- Attri, P.; Venkatesu, P. Ammonium ionic liquids as convenient co-solvents for the structure and stability of succinylated Con A. J. Chem. Thermodyn. 2012, 52, 78–88. [Google Scholar] [CrossRef]
- Passos, H.; Ferreira, A.R.; Cláudio, A.F.M.; Coutinho, J.A.P.; Freire, M.G. Characterization of aqueous biphasic systems composed of ionic liquids and a citrate-based biodegradable salt. Biochem. Eng. J. 2012, 67, 68–76. [Google Scholar] [CrossRef]
- Pereira, M.M.; Pedro, S.N.; Quental, M.V.; Lima, Á.S.; Coutinho, J.A.P.; Freire, M.G. Enhanced extraction of bovine serum albumin with aqueous biphasic systems of phosphonium- and ammonium-based ionic liquids. J. Biotechnol. 2015, 206, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Malhotra, S.V. Study on the potential anti-cancer activity of phosphonium and ammonium-based ionic liquids. Bioorganic Med. Chem. Lett. 2009, 19, 4643–4646. [Google Scholar] [CrossRef]
- Stojanovic, A.; Keppler, B.K.; Morgenbesser, C.; Kogelnig, D.; Krachler, R. Quaternary ammonium and phosphonium ionic liquids in chemical and environmental engineering. In Ionic Liquids: Theory, Properties, New Approaches, 1st ed.; Kokorin, A., Ed.; INTECH Open Access Publisher: Rijeka, Croatia, 2011; pp. 657–680. [Google Scholar] [CrossRef] [Green Version]
- Couling, D.J.; Bernot, R.J.; Docherty, K.M.; Dixon, J.K.; Maginn, E.J. Assessing the factors responsible for ionic liquid toxicity to aquatic organisms via quantitative structure-property relationship modeling. Green Chem. 2006, 8, 82–90. [Google Scholar] [CrossRef]
- Taha, M.; Quental, M.V.; Correia, I.; Freire, M.G.; Coutinho, J.A.P. Extraction and stability of bovine serum albumin (BSA) using cholinium-based Good’s buffers ionic liquids. Process Biochem. 2015, 50, 1158–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deive, F.J.; Ruivo, D.; Rodrigues, J.V.; Gomes, C.M.; Sanroman, M.A.; Rebelo, L.P.N.; Esperanca, J.M.S.S.; Rodriguez, A. On the hunt for truly biocompatible ionic liquids for lipase-catalyzed reactions. RSC Adv. 2015, 5, 3386–3389. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Pei, Y.; Wang, J.; He, M. Design of environmentally friendly ionic liquid aqueous two-phase systems for the efficient and high activity extraction of proteins. Green Chem. 2012, 14, 2941–2950. [Google Scholar] [CrossRef]
- Vijayaraghavan, R.; Izgorodin, A.; Ganesh, V.; Surianarayanan, M.; MacFarlane, D.R. Long-Term Structural and Chemical Stability of DNA in Hydrated Ionic Liquids. Angew. Chem. Int. Ed. 2010, 49, 1631–1633. [Google Scholar] [CrossRef]
- Zhao, H. DNA stability in ionic liquids and deep eutectic solvents. J. Chem. Technol. Biotechnol. 2015, 90, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Vrikkis, R.M.; Fraser, K.J.; Fujita, K.; MacFarlane, D.R.; Elliott, G.D. Biocompatible Ionic Liquids: A New Approach for Stabilizing Proteins in Liquid Formulation. J. Biomech. Eng. 2009, 131, 074514. [Google Scholar] [CrossRef] [PubMed]
- Sintra, T.E.; Luís, A.; Rocha, S.N.; Lobo Ferreira, A.I.M.C.; Gonçalves, F.; Santos, L.M.N.B.F.; Neves, B.M.; Freire, M.G.; Ventura, S.P.M.; Coutinho, J.A.P. Enhancing the Antioxidant Characteristics of Phenolic Acids by Their Conversion into Cholinium Salts. ACS Sustain. Chem. Eng. 2015, 3, 2558–2565. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.F.B.; Vicente, F.; Santos-Ebinuma, V.C.; Araújo, J.M.; Pessoa, A.; Freire, M.G.; Coutinho, J.A.P. Extraction of tetracycline from fermentation broth using aqueous two-phase systems composed of polyethylene glycol and cholinium-based salts. Process Biochem. 2013, 48, 716–722. [Google Scholar] [CrossRef]
- Shahriari, S.; Tome, L.C.; Araujo, J.M.M.; Rebelo, L.P.N.; Coutinho, J.A.P.; Marrucho, I.M.; Freire, M.G. Aqueous biphasic systems: A benign route using cholinium-based ionic liquids. RSC Adv. 2013, 3, 1835–1843. [Google Scholar] [CrossRef]
- Capela, E.V.; Santiago, A.E.; Rufino, A.F.C.S.; Tavares, A.P.M.; Pereira, M.M.; Mohamadou, A.; Aires-Barros, M.R.; Coutinho, J.A.P.; Azevedo, A.M.; Freire, M.G. Sustainable strategies based on glycine–betaine analogue ionic liquids for the recovery of monoclonal antibodies from cell culture supernatants. Green Chem. 2019, 21, 5671–5682. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Morais, E.S.; Leite, A.C.; Mohamadou, A.; Holmbom, B.; Holmbom, T.; Neves, B.M.; Coutinho, J.A.P.; Freire, M.G.; Silvestre, A.J.D. Enhanced extraction and biological activity of 7-hydroxymatairesinol obtained from Norway spruce knots using aqueous solutions of ionic liquids. Green Chem. 2017, 19, 2626–2635. [Google Scholar] [CrossRef]
- Parajó, J.J.; Macário, I.P.E.; De Gaetano, Y.; Dupont, L.; Salgado, J.; Pereira, J.L.; Gonçalves, F.J.M.; Mohamadou, A.; Ventura, S.P.M. Glycine-betaine-derived ionic liquids: Synthesis, characterization and ecotoxicological evaluation. Ecotoxicol. Environ. Saf. 2019, 184, 109580. [Google Scholar] [CrossRef]
- Weaver, K.D.; Kim, H.J.; Sun, J.Z.; MacFarlane, D.R.; Elliott, G.D. Cyto-toxicity and biocompatibility of a family of choline phosphate ionic liquids designed for pharmaceutical applications. Green Chem. 2010, 12, 507–513. [Google Scholar] [CrossRef]
- Seddon, K.R. Ionic Liquids: Designer solvents? In The International George Papatheodorou Symposium: Proceedings; Institute of Chemical Engineering and High Temperature Chemical Processes: Patras, Greece, 1999. [Google Scholar]
- Smith, K.B.; Bridson, R.H.; Leeke, G.A. Solubilities of Pharmaceutical Compounds in Ionic Liquids. J. Chem. Eng. Data 2011, 56, 2039–2043. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Kamiya, N.; Goto, M. Ionic liquid based microemulsion with pharmaceutically accepted components: Formulation and potential applications. J. Colloid Interface Sci. 2010, 352, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Tamura, M.; Tahara, Y.; Kamiya, N.; Goto, M. Ionic liquid-in-oil microemulsion as a potential carrier of sparingly soluble drug: Characterization and cytotoxicity evaluation. Int. J. Pharm. 2010, 400, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Stoimenovski, J.; MacFarlane, D.R.; Bica, K.; Rogers, R.D. Crystalline vs. Ionic Liquid Salt Forms of Active Pharmaceutical Ingredients: A Position Paper. Pharm. Res. 2010, 27, 521–526. [Google Scholar] [CrossRef]
- McCrary, P.D.; Beasley, P.A.; Gurau, G.; Narita, A.; Barber, P.S.; Cojocaru, O.A.; Rogers, R.D. Drug specific, tuning of an ionic liquid’s hydrophilic-lipophilic balance to improve water solubility of poorly soluble active pharmaceutical ingredients. New J. Chem. 2013, 37, 2196–2202. [Google Scholar] [CrossRef]
- Wypych, G. (Ed.) Solvent use in various industries. In Handbook of Solvents, 2nd ed.; ChemTec Publishing: Oxford, UK, 2014; pp. 1–261. [Google Scholar] [CrossRef]
- Lieberman, H.; Murti Vemuri, N. Chapter 32—Chemical and Physicochemical Approaches to Solve Formulation Problems. In The Practice of Medicinal Chemistry, 4th ed.; Wermuth, C.G., Aldous, D., Raboisson, P., Rognan, D., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 767–791. [Google Scholar] [CrossRef]
- Kunz, W.; Holmberg, K.; Zemb, T. Hydrotropes. Curr. Opin. Colloid Interface Sci. 2016, 22, 99–107. [Google Scholar] [CrossRef]
- Soares, B.; Silvestre, A.J.D.; Rodrigues Pinto, P.C.; Freire, C.S.R.; Coutinho, J.A.P. Hydrotropy and Cosolvency in Lignin Solubilization with Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 12485–12493. [Google Scholar] [CrossRef]
- Feng, B.Y.; Shoichet, B.K. A detergent-based assay for the detection of promiscuous inhibitors. Nat. Protoc. 2006, 1, 550–553. [Google Scholar] [CrossRef]
- Owen, S.C.; Doak, A.K.; Wassam, P.; Shoichet, M.S.; Shoichet, B.K. Colloidal Aggregation Affects the Efficacy of Anticancer Drugs in Cell Culture. ACS Chem. Biol. 2012, 7, 1429–1435. [Google Scholar] [CrossRef]
- Ryan, A.J.; Gray, N.M.; Lowe, P.N.; Chung, C.W. Effect of detergent on “promiscuous” inhibitors. J. Med. Chem. 2003, 46, 3448–3451. [Google Scholar] [CrossRef]
- Mizuuchi, H.; Jaitely, V.; Murdan, S.; Florence, A.T. Room temperature ionic liquids and their mixtures: Potential pharmaceutical solvents. Eur. J. Pharm. Sci. 2008, 33, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Sintra, T.E.; Shimizu, K.; Ventura, S.P.M.; Shimizu, S.; Canongia Lopes, J.N.; Coutinho, J.A.P. Enhanced dissolution of ibuprofen using ionic liquids as catanionic hydrotropes. Phys. Chem. Chem. Phys. 2018, 20, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Abranches, D.O.; Benfica, J.; Soares, B.P.; Ferreira, A.M.; Sintra, T.E.; Shimizu, S.; Coutinho, J.A.P. The impact of the counterion in the performance of ionic hydrotropes. Chem. Commun. 2021, 57, 2951–2954. [Google Scholar] [CrossRef] [PubMed]
- Sales, I.; Abranches, D.O.; Costa, P.; Sintra, T.E.; Ventura, S.P.M.; Mattedi, S.; Coutinho, J.A.P.; Freire, M.G.; Pinho, S.P. Enhancing Artemisinin Solubility in Aqueous Solutions: Searching for Hydrotropes based on Ionic Liquids. Fluid Phase Equilib. 2021, 534, 112961. [Google Scholar] [CrossRef]
- Barzegar-Jalali, M.; Jouyban, A.; Shekaari, H.; Martinez, F.; Mirheydari, S.N. The effect of 1-hexyl-3-methylimidazolium bromide ionic liquid as a co-solvent on the aqueous solubility of lamotrigine at T = (293.2–313.2) K. J. Chem. Thermodyn. 2019, 133, 261–271. [Google Scholar] [CrossRef]
- Mehrdad, A.; Miri, A.H. Aqueous solubility of acetaminophen in the presence of 1-hexyl-3-methyl imidazolium bromide, ionic liquid as co-solvent. Fluid Phase Equilib. 2016, 425, 51–56. [Google Scholar] [CrossRef]
- Alawi, M.A.; Hamdan, I.I.; Sallam, A.A.; Heshmeh, N.A. Solubility enhancement of glibenclamide in choline–tryptophan ionic liquid: Preparation, characterization and mechanism of solubilization. J. Mol. Liq. 2015, 212, 629–634. [Google Scholar] [CrossRef]
- Faria, R.A.; Bogel-Łukasik, E. Solubilities of pharmaceutical and bioactive compounds in trihexyl(tetradecyl)phosphonium chloride ionic liquid. Fluid Phase Equilib. 2015, 397, 18–25. [Google Scholar] [CrossRef]
- Faria, R.A.; da Ponte, M.N.; Bogel-Łukasik, E. Solubility studies on the system of trihexyl(tetradecyl)phosphonium bis[(trifluoromethyl)sulfonyl]amide) ionic liquid and pharmaceutical and bioactive compounds. Fluid Phase Equilib. 2015, 385, 1–9. [Google Scholar] [CrossRef]
- Melo, C.I.; Bogel-Lukasik, R.; da Ponte, M.N.; Bogel-Lukasik, E. Ammonium ionic liquids as green solvents for drugs. Fluid Phase Equilib. 2013, 338, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Pernak, J.; Smiglak, M.; Griffin, S.T.; Hough, W.L.; Wilson, T.B.; Pernak, A.; Zabielska-Matejuk, J.; Fojutowski, A.; Kita, K.; Rogers, R.D. Long alkyl chain quaternary ammonium-based ionic liquids and potential applications. Green Chem. 2006, 8, 798–806. [Google Scholar] [CrossRef]
- Wellens, S.; Goovaerts, R.; Moller, C.; Luyten, J.; Thijs, B.; Binnemans, K. A continuous ionic liquid extraction process for the separation of cobalt from nickel. Green Chem. 2013, 15, 3160–3164. [Google Scholar] [CrossRef]
- Sintra, T.E.; Abranches, D.O.; Benfica, J.; Soares, B.P.; Ventura, S.P.M.; Coutinho, J.A.P. Cholinium-based ionic liquids as bioinspired hydrotropes to tackle solubility challenges in drug formulation. Eur. J. Pharm. Biopharm. 2021, 164, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Häckl, K.; Mühlbauer, A.; Ontiveros, J.F.; Marinkovic, S.; Estrine, B.; Kunz, W.; Nardello-Rataj, V. Carnitine alkyl ester bromides as novel biosourced ionic liquids, cationic hydrotropes and surfactants. J. Colloid Interface Sci. 2018, 511, 165–173. [Google Scholar] [CrossRef]
- Lemke, A.; Kiderlen, A.F.; Kayser, O. Amphotericin B. Appl. Microbiol. Biotechnol. 2005, 68, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Mellaerts, R.; Mols, R.; Jammaer, J.A.G.; Aerts, C.A.; Annaert, P.; Van Humbeeck, J.; Van den Mooter, G.; Augustijns, P.; Martens, J.A. Increasing the oral bioavailability of the poorly water soluble drug itraconazole with ordered mesoporous silica. Eur. J. Pharm. Biopharm. 2008, 69, 223–230. [Google Scholar] [CrossRef]
- Abd Elbary, A.A.; Salem, H.F.; Maher, M.E. In vitro and in vivo Evaluation of Glibenclamide using Surface Solid Dispersion (SSD) Approach. Br. J. Pharmacol. Toxicol. 2011, 2, 51–62. [Google Scholar]
- Elkordy, A.A.; Jatto, A.; Essa, E. In situ controlled crystallization as a tool to improve the dissolution of Glibenclamide. Int. J. Pharm. 2012, 428, 118–120. [Google Scholar] [CrossRef]
- Remko, M. Theoretical study of molecular structure, pKa, lipophilicity, solubility, absorption, and polar surface area of some hypoglycemic agents. J. Mol. Struct. Theochem. 2009, 897, 73–82. [Google Scholar] [CrossRef]
- Yalkowsky, S.H.; Roseman, T.J. Techniques of Solubilization of Drugs; M. Dekker: New York, NY, USA, 1981. [Google Scholar]
- Lavik, E.B.; Kuppermann, B.D.; Humayun, M.S. Drug Delivery. Available online: http://kinampark.com/PL/files/Lavik%2C%20Drug%20delivery%20in%20Translational%20Basic%20Science.pdf (accessed on 21 April 2021).
- Khan, A.B.; Ali, M.; Malik, N.A.; Ali, A.; Patel, R. Role of 1-methyl-3-octylimidazolium chloride in the micellization behavior of amphiphilic drug amitriptyline hydrochloride. Colloids Surf. B Biointerfaces 2013, 112, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Khan, A.B.; Dohare, N.; Ali, M.M.; Rajor, H.K. Mixed Micellization and Interfacial Properties of Ionic Liquid-Type Imidazolium Gemini Surfactant with Amphiphilic Drug Amitriptyline Hydrochloride and its Thermodynamics. J. Surfactants Deterg. 2015, 18, 719–728. [Google Scholar] [CrossRef]
- Dandpat, S.S.; Sarkar, M. Investigating the molecular and aggregated states of a drug molecule rutaecarpine using spectroscopy, microscopy, crystallography and computational studies. Phys. Chem. Chem. Phys. 2015, 17, 13992–14002. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.; Kaur, R.; Aswal, V.K.; Mahajan, R.K. Composition and Concentration Gradient Induced Structural Transition from Micelles to Vesicles in the Mixed System of Ionic Liquid-Diclofenac Sodium. Langmuir 2016, 32, 6638–6647. [Google Scholar] [CrossRef] [PubMed]
- Kuchlyan, J.; Kundu, N.; Sarkar, N. Ionic liquids in microemulsions: Formulation and characterization. Curr. Opin. Colloid Interface Sci. 2016, 25, 27–38. [Google Scholar] [CrossRef]
- Rao, V.G.; Banerjee, C.; Ghosh, S.; Mandal, S.; Kuchlyan, J.; Sarkar, N. A Step toward the Development of High-Temperature Stable Ionic Liquid-in-Oil Microemulsions Containing Double-Chain Anionic Surface Active Ionic Liquid. J. Phys. Chem. B 2013, 117, 7472–7480. [Google Scholar] [CrossRef]
- Rao, V.G.; Mandal, S.; Ghosh, S.; Banerjee, C.; Sarkar, N. Ionic Liquid-in-Oil Microemulsions Composed of Double Chain Surface Active Ionic Liquid as a Surfactant: Temperature Dependent Solvent and Rotational Relaxation Dynamics of Coumarin-153 in [Py][TF2N]/[C4mim][AOT]/Benzene Microemulsions. J. Phys. Chem. B 2012, 116, 8210–8221. [Google Scholar] [CrossRef]
- Kumar, S.S.D.; Mahadevan, S.; Vijayaraghavan, R.; Mandal, A.B.; MacFarlane, D.R. Curcumin loaded poly(2-hydroxyethyl methacrylate) nanoparticles from gelled ionic liquid—In vitro cytotoxicity and anti-cancer activity in SKOV-3 cells. Eur. J. Pharm. Sci. 2014, 51, 34–44. [Google Scholar] [CrossRef]
- Mahkam, M.; Pakravan, A. Synthesis and Characterization of pH-Sensitive Positive-charge Silica Nanoparticles for Oral Anionic Drug Delivery. J. Chin. Chem. Soc. 2013, 60, 293–296. [Google Scholar] [CrossRef]
- Meng, L.J.; Niu, L.Y.; Li, L.; Lu, Q.H.; Fei, Z.F.; Dyson, P.J. Gold Nanoparticles Grown on Ionic Liquid-Functionalized Single-Walled Carbon Nanotubes: New Materials for Photothermal Therapy. Chem. Eur. J. 2012, 18, 13314–13319. [Google Scholar] [CrossRef]
- Rasouli, S.; Davaran, S.; Rasouli, F.; Mahkam, M.; Salehi, R. Synthesis, characterization and pH-controllable methotrexate release from biocompatible polymer/silica nanocomposite for anticancer drug delivery. Drug Deliv. 2014, 21, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Rasouli, S.; Davaran, S.; Rasouli, F.; Mahkam, M.; Salehi, R. Positively charged functionalized silica nanoparticles as nontoxic carriers for triggered anticancer drug release. Des. Monomers Polym. 2014, 17, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Neouze, M.A.; Le Bideau, J.; Leroux, F.; Vioux, A. A route to heat resistant solid membranes with performances of liquid electrolytes. Chem. Commun. 2005, 8, 1082–1084. [Google Scholar] [CrossRef]
- Bica, K.; Rodriguez, H.; Gurau, G.; Cojocaru, O.A.; Riisager, A.; Fehrmann, R.; Rogers, R.D. Pharmaceutically active ionic liquids with solids handling, enhanced thermal stability, and fast release. Chem. Commun. 2012, 48, 5422–5424. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Q.Y.; Kong, M.Y.; Qu, L.L.; Geng, Z.R.; Wang, Z.L. An ionic liquid-modified nano-vehicle to construct nano-models of catalase to target mitochondria. J. Mater. Chem. 2012, 22, 20299–20304. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Q.Y.; Li, X.; Gao, J. Functional ionic liquids induced the formation of mitochondria targeted fluorescent core-shell ellipsoidal nanoparticles with anticancer properties. Colloids Surf. B Biointerfaces 2012, 98, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Mukesh, C.; Bhatt, J.; Prasad, K. A Polymerizable Bioionic Liquid Based Nanogel: A New Nanocarrier for an Anticancer Drug. Macromol. Chem. Phys. 2014, 215, 1498–1504. [Google Scholar] [CrossRef]
- Viau, L.; Tourne-Peteilh, C.; Devoisselle, J.M.; Vioux, A. Ionogels as drug delivery system: One-step sol-gel synthesis using imidazolium ibuprofenate ionic liquid. Chem. Commun. 2010, 46, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Mondal, D.; Sharma, M.; Freire, M.G.; Mukesh, C.; Bhatt, J. Stimuli responsive ion gels based on polysaccharides and other polymers prepared using ionic liquids and deep eutectic solvents. Carbohydr. Polym. 2018, 180, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Winther-Jensen, O.; Vijayaraghavan, R.; Sun, J.Z.; Winther-Jensen, B.; MacFarlane, D.R. Self polymerising ionic liquid gel. Chem. Commun. 2009, 21, 3041–3043. [Google Scholar] [CrossRef] [PubMed]
- Zakrewsky, M.; Lovejoy, K.S.; Kern, T.L.; Miller, T.E.; Le, V.; Nagy, A.; Goumas, A.M.; Iyer, R.S.; Del Sesto, R.E.; Koppisch, A.T.; et al. Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc. Natl. Acad. Sci. USA 2014, 111, 13313–13318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, R.; Tokuda, M.; Suzuki, T.; Minami, H. Preparation of Poly(ionic liquid) Hollow Particles with Switchable Permeability. Langmuir 2016, 32, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.X.; Yin, T.X.; Tao, X.Y.; Shen, W.G. Light induced micelle to vesicle transition in an aqueous solution of a surface active ionic liquid. RSC Adv. 2015, 5, 75806–75809. [Google Scholar] [CrossRef]
- Mahkam, M.; Latifpour, A.; Rafi, A.A.; Gheshlaghi, L.M.; Takfallah, A. Preparation of Montmorillonite-pH-Sensitive Positive Charges Nanocomposites as a Drug Delivery System. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 32–37. [Google Scholar] [CrossRef]
- Williams, H.D.; Sahbaz, Y.; Ford, L.; Nguyen, T.H.; Scammells, P.J.; Porter, C.J.H. Ionic liquids provide unique opportunities for oral drug delivery: Structure optimization and in vivo evidence of utility. Chem. Commun. 2014, 50, 1688–1690. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Tahara, Y.; Tamura, M.; Kamiya, N.; Goto, M. Ionic liquid-assisted transdermal delivery of sparingly soluble drugs. Chem. Commun. 2010, 46, 1452–1454. [Google Scholar] [CrossRef]
- Dobler, D.; Schmidts, T.; Klingenhofer, I.; Runkel, F. Ionic liquids as ingredients in topical drug delivery systems. Int. J. Pharm. 2013, 441, 620–627. [Google Scholar] [CrossRef]
- Gao, Y.N.; Han, S.B.; Han, B.X.; Li, G.Z.; Shen, D.; Li, Z.H.; Du, J.M.; Hou, W.G.; Zhang, G.Y. TX-100/water/1-butyl-3-methylimidazolium hexafluorophosphate microemulsions. Langmuir 2005, 21, 5681–5684. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Eli, W.J.; Li, G. FTIR study of Tween80/1-butyl-3-methylimidazolium hexafluorophosphate/toluene microemulsions. Colloid Polym. Sci. 2009, 287, 871–876. [Google Scholar] [CrossRef]
- De Nicola, A.; Hezaveh, S.; Zhao, Y.; Kawakatsu, T.; Roccatano, D.; Milano, G. Micellar drug nanocarriers and biomembranes: How do they interact? Phys. Chem. Chem. Phys. 2014, 16, 5093–5105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Kataoka, K.; Kwon, G.S.; Yokoyama, M.; Okano, T.; Sakurai, Y. Block-copolymer micelles as vehicles for drug delivery. J. Control. Release 1993, 24, 119–132. [Google Scholar] [CrossRef]
- Rapoport, N. Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog. Polym. Sci. 2007, 32, 962–990. [Google Scholar] [CrossRef]
- Rosler, A.; Vandermeulen, G.W.M.; Klok, H.A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Del. Rev. 2012, 64, 270–279. [Google Scholar] [CrossRef]
- Geng, F.; Liu, J.; Zheng, L.Q.; Yu, L.; Li, Z.; Li, G.Z.; Tung, C.H. Micelle Formation of Long-Chain Imidazolium Ionic Liquids in Aqueous Solution Measured by Isothermal Titration Microcalorimetry. J. Chem. Eng. Data 2010, 55, 147–151. [Google Scholar] [CrossRef]
- Pal, A.; Yadav, A. Binding interactions of anesthetic drug with surface active ionic liquid. J. Mol. Liq. 2016, 222, 471–479. [Google Scholar] [CrossRef]
- Kundu, N.; Banik, D.; Roy, A.; Kuchlyan, J.; Sarkar, N. Modulation of the aggregation properties of sodium deoxycholate in presence of hydrophilic imidazolium based ionic liquid: Water dynamics study to probe the structural alteration of the aggregates. Phys. Chem. Chem. Phys. 2015, 17, 25216–25227. [Google Scholar] [CrossRef]
- Ohno, H.; Fujita, K.; Kohno, Y. Is seven the minimum number of water molecules per ion pair for assured biological activity in ionic liquid-water mixtures? Phys. Chem. Chem. Phys. 2015, 17, 14454–14460. [Google Scholar] [CrossRef]
- Marrucho, I.M.; Freire, M.G. Aqueous Biphasic Systems Based on Ionic Liquids for Extraction, Concentration and Purification Approaches. In Ionic Liquids for Better Separation Processes, 1st ed.; Rodríguez, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 91–119. [Google Scholar] [CrossRef]
- Poole, C.F.; Lenca, N. Green sample-preparation methods using room-temperature ionic liquids for the chromatographic analysis of organic compounds. Trends Anal. Chem. 2015, 71, 144–156. [Google Scholar] [CrossRef]
- Ho, T.D.; Zhang, C.; Hantao, L.W.; Anderson, J.L. Ionic Liquids in Analytical Chemistry: Fundamentals, Advances, and Perspectives. Anal. Chem. 2014, 86, 262–285. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Trujillo-Rodríguez, M.J.; Pino, V.; Anderson, J.L. Non-conventional solvents in liquid phase microextraction and aqueous biphasic systems. J. Chromatogr. A 2017, 1500, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Rodríguez, M.J.; Nan, H.; Varona, M.; Emaus, M.N.; Souza, I.D.; Anderson, J.L. Advances of Ionic Liquids in Analytical Chemistry. Anal. Chem. 2019, 91, 505–531. [Google Scholar] [CrossRef] [PubMed]
- McQueen, L.; Lai, D. Ionic Liquid Aqueous Two-Phase Systems From a Pharmaceutical Perspective. Front. Chem. 2019, 7, 135. [Google Scholar] [CrossRef] [Green Version]
- Rykowska, I.; Ziemblińska, J.; Nowak, I. Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: A review. J. Mol. Liq. 2018, 259, 319–339. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Rocío-Bautista, P.; Pino, V.; Afonso, A.M. Ionic liquids in dispersive liquid-liquid microextraction. Trends Anal. Chem. 2013, 51, 87–106. [Google Scholar] [CrossRef]
- Gutowski, K.E.; Broker, G.A.; Willauer, H.D.; Huddleston, J.G.; Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Controlling the Aqueous Miscibility of Ionic Liquids: Aqueous Biphasic Systems of Water-Miscible Ionic Liquids and Water-Structuring Salts for Recycle, Metathesis, and Separations. J. Am. Chem. Soc. 2003, 125, 6632–6633. [Google Scholar] [CrossRef]
- Dinis, T.B.V.; Passos, H.; Lima, D.L.D.; Esteves, V.I.; Coutinho, J.A.P.; Freire, M.G. One-step extraction and concentration of estrogens for an adequate monitoring of wastewater using ionic-liquid-based aqueous biphasic systems. Green Chem. 2015, 17, 2570–2579. [Google Scholar] [CrossRef]
- Dinis, T.B.V.; Passos, H.; Lima, D.L.D.; Sousa, A.C.A.; Coutinho, J.A.P.; Esteves, V.I.; Freire, M.G. Simultaneous extraction and concentration of water pollution tracers using ionic-liquid-based systems. J. Chromatogr. A 2017, 1599, 69–77. [Google Scholar] [CrossRef]
- Noorashikin, M.S.; Mohamad, S.; Abas, M.R. Extraction and determination of parabens in water samples using an aqueous two-phase system of ionic liquid and salts with beta-cyclodextrin as the modifier coupled with high performance liquid chromatography. Anal. Methods 2014, 6, 419–425. [Google Scholar] [CrossRef]
- Passos, H.; Sousa, A.C.A.; Ramiro Pastorinho, M.; Nogueira, A.J.A.; Rebelo, L.P.N.; Coutinho, J.A.P.; Freire, M.G. Ionic-liquid-based aqueous biphasic systems for improved detection of bisphenol A in human fluids. Anal. Methods 2012, 4, 2664–2667. [Google Scholar] [CrossRef]
- Asenjo, J.A.; Andrews, B.A. Aqueous two-phase systems for protein separation: A perspective. J. Chromatogr. A 2011, 1218, 8826–8835. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.D.; Coelho, M.A.Z.; Rebelo, L.P.N.; Marrucho, I.M. Ionic Liquids as Additives for Extraction of Saponins and Polyphenols from Mate (Ilex paraguariensis) and Tea (Camellia sinensis). Ind. Eng. Chem. Res. 2013, 52, 12146–12153. [Google Scholar] [CrossRef]
- He, C.Y.; Li, S.H.; Liu, H.W.; Li, K.; Liu, F. Extraction of testosterone and epitestosterone in human urine using aqueous two-phase systems of ionic liquid and salt. J. Chromatogr. A 2005, 1082, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Yu, Y.L.; Wang, J.H. Extraction of proteins from biological fluids by use of an ionic liquid/aqueous two-phase system. Chem. Eur. J. 2007, 13, 2130–2137. [Google Scholar] [CrossRef]
- Flieger, J.; Czajkowska-Żelazko, A. Aqueous two phase system based on ionic liquid for isolation of quinine from human plasma sample. Food Chem. 2015, 166, 150–157. [Google Scholar] [CrossRef]
- Almeida, H.F.D.; Freire, M.G.; Marrucho, I.M. Improved monitoring of aqueous samples by the preconcentration of active pharmaceutical ingredients using ionic-liquid-based systems. Green Chem. 2017, 19, 4651–4659. [Google Scholar] [CrossRef]
- Li, S.; He, C.; Liu, H.; Li, K.; Liu, F. Ionic liquid-based aqueous two-phase system, a sample pretreatment procedure prior to high-performance liquid chromatography of opium alkaloids. J. Chromatogr. B 2005, 826, 58–62. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Kang, W.; Li, C.; Yan, Y.; Pan, J.; Xie, X. Phase equilibrium and macrolide antibiotics partitioning in real water samples using a two-phase system composed of the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate and an aqueous solution of an inorganic salt. Microchim. Acta 2010, 169, 15–22. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Xie, X.-Q.; Li, C.-X. Extraction of trace acetylspiramycin in real aqueous environments using aqueous two-phase system of ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate and phosphate. Cent. Eur. J. Chem. 2010, 8, 1185–1191. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Yu, C.-L.; Yan, Y.-S.; Xie, X.-Q. Extraction and determination of chloramphenicol in feed water, milk, and honey samples using an ionic liquid/sodium citrate aqueous two-phase system coupled with high-performance liquid chromatography. Anal. Bioanal. Chem. 2011, 399, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, X.; Tu, G.; Zhang, S.; Zhang, P. Novel aqueous biphasic system based on ionic liquid for the simultaneous extraction of seven active pharmaceutical ingredients in aquatic environment. Environ. Sci. Pollut. Res. 2021, 28, 17853–17864. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, M.G.; Svinyarov, I. Analysis of acetylcholinesterase inhibitors by extraction in choline saccharinate aqueous biphasic systems. J. Chromatogr. A 2018, 1559, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Antep, H.M.; Mumcu, T.; Bostanci, K.; Bozkurt, S.S.; Merdivan, M. Ultrasound-assisted surfactant/ionic liquid aqueous two-phase system extraction prior to high performance liquid chromatography for the determination of tetracyclines in milk and honey samples. Turk. J. Chem. 2017, 41, 955–966. [Google Scholar] [CrossRef]
- Yu, W.; Li, K.; Liu, Z.; Zhang, H.; Jin, X. Novelty aqueous two-phase extraction system based on ionic liquid for determination of sulfonamides in blood coupled with high-performance liquid chromatography. Microchem. J. 2018, 136, 263–269. [Google Scholar] [CrossRef]
- Sajid, M. Magnetic ionic liquids in analytical sample preparation: A literature review. Trends Anal. Chem. 2019, 113, 210–223. [Google Scholar] [CrossRef]
- Yao, T.; Yao, S. Magnetic ionic liquid aqueous two-phase system coupled with high performance liquid chromatography: A rapid approach for determination of chloramphenicol in water environment. J. Chromatogr. A 2017, 1481, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, L.-R.; Song, H.; Yohannes, A.; Liang, S.; Yao, S. Extraction in cholinium-based magnetic ionic liquid aqueous two-phase system for the determination of berberine hydrochloride in Rhizoma coptidis. RSC Adv. 2018, 8, 25201–25209. [Google Scholar] [CrossRef] [Green Version]
- Guardia, M.D.L.; Garrigues, S. Chapter 1: Past, Present and Future of Green Analytical Chemistry. In Challenges in Green Analytical Chemistry, 2nd ed.; Justyna, P.-W., Jacek, N., Eds.; Royal Society of Chemistry: London, UK, 2020; pp. 1–18. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Xu, B.; Li, X.; Jin, R.; Zhang, H.; Song, D. Magnetic ionic liquid-based dispersive liquid–liquid microextraction for the determination of triazine herbicides in vegetable oils by liquid chromatography. J. Chromatogr. A 2014, 1373, 9–16. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, L.; Su, A.; Zhang, H. Dispersive liquid–liquid microextraction of phenolic compounds from vegetable oils using a magnetic ionic liquid. J. Sep. Sci. 2017, 40, 3130–3137. [Google Scholar] [CrossRef]
- Wang, H.; Gao, J.; Yu, N.; Qu, J.; Fang, F.; Wang, H.; Wang, M.; Wang, X. Development of a novel naphthoic acid ionic liquid and its application in “no-organic solvent microextraction” for determination of triclosan and methyltriclosan in human fluids and the method optimization by central composite design. Talanta 2016, 154, 381–391. [Google Scholar] [CrossRef]
- Gao, J.; Wang, H.; Qu, J.; Wang, H.; Wang, X. Development and optimization of a naphthoic acid-based ionic liquid as a “non-organic solvent microextraction” for the determination of tetracycline antibiotics in milk and chicken eggs. Food Chem. 2017, 215, 138–148. [Google Scholar] [CrossRef]
- Kaynaker, M.; Antep, M.; Merdivan, M. Determination of Tetracyclines in Milk, Eggs and Honey Using in-situ Ionic Liquid Based Dispersive Liquid–Liquid Microextraction. J. Anal. Chem. 2018, 73, 23–29. [Google Scholar] [CrossRef]
- López-Darias, J.; Pino, V.; Ayala, J.H.; Afonso, A.M. In-situ ionic liquid-dispersive liquid-liquid microextraction method to determine endocrine disrupting phenols in seawaters and industrial effluents. Microchim. Acta 2011, 174, 213. [Google Scholar] [CrossRef]
- Vicente-Martínez, Y.; Caravaca, M.; Soto-Meca, A. Determination of Very Low Concentration of Bisphenol A in Toys and Baby Pacifiers Using Dispersive Liquid–Liquid Microextraction by In Situ Ionic Liquid Formation and High-Performance Liquid Chromatography. Pharmaceuticals 2020, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fan, C.; Kong, D.; Tang, G.; Zhang, W.; Dong, H.; Liang, Y.; Wang, D.; Cao, Y. Synthesis and application of imidazolium-based ionic liquids as extraction solvent for pretreatment of triazole fungicides in water samples. Anal. Bioanal. Chem. 2018, 410, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Du, K. Simultaneous determination of sulfonamides in milk: In-situ magnetic ionic liquid dispersive liquid-liquid microextraction coupled with HPLC. Food Chem. 2020, 331, 127342. [Google Scholar] [CrossRef]
- Zhao, R.-S.; Wang, X.; Sun, J.; Wang, S.-S.; Yuan, J.-P.; Wang, X.-K. Trace determination of triclosan and triclocarban in environmental water samples with ionic liquid dispersive liquid-phase microextraction prior to HPLC–ESI-MS–MS. Anal. Bioanal. Chem. 2010, 397, 1627–1633. [Google Scholar] [CrossRef]
- Freire, M.G.; Neves, C.M.S.S.; Marrucho, I.M.; Coutinho, J.A.P.; Fernandes, A.M. Hydrolysis of Tetrafluoroborate and Hexafluorophosphate Counter Ions in Imidazolium-Based Ionic Liquids. J. Phys. Chem. A 2010, 114, 3744–3749. [Google Scholar] [CrossRef]
- Kunz, W.; Häckl, K. The hype with ionic liquids as solvents. Chem. Phys. Lett. 2016, 661, 6–12. [Google Scholar] [CrossRef]
- Shu, Y.; Gao, M.; Wang, X.; Song, R.; Lu, J.; Chen, X. Separation of curcuminoids using ionic liquid based aqueous two-phase system coupled with in situ dispersive liquid–liquid microextraction. Talanta 2016, 149, 6–12. [Google Scholar] [CrossRef]
- Slater, C.S.; Savelski, M.J.; Hesketh, R.P.; Frey, E. The Selection and Reduction of Organic Solvents in Pharmaceutical Manufacture. In Proceedings of the American Chemical Society 10th Green Chemistry and Engineering Conference, Washington, DC, USA, 26–30 June 2006. [Google Scholar]
- Drug Companies Must Adopt Green Chemistry. Available online: https://www.nature.com/news/industrial-research-drug-companies-must-adopt-green-chemistry-1.19992 (accessed on 21 April 2021).
- Almeida, H.F.D.; Marrucho, I.M.; Freire, M.G. Removal of Nonsteroidal Anti-Inflammatory Drugs from Aqueous Environments with Reusable Ionic-Liquid-Based Systems. ACS Sustain. Chem. Eng. 2017, 5, 2428–2436. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Jiang, X.Y.; Yu, J.G.; Tang, K.W. Enantioseparation of mandelic acid enantiomers in ionic liquid aqueous two-phase extraction systems. Chem. Pap. 2014, 68, 465–471. [Google Scholar] [CrossRef]
- Cull, S.G.; Holbrey, J.D.; Vargas-Mora, V.; Seddon, K.R.; Lye, G.J. Room-temperature ionic liquids as replacements for organic solvents in multiphase bioprocess operations. Biotechnol. Bioeng. 2000, 69, 227–233. [Google Scholar] [CrossRef]
- Manic, M.S.; da Ponte, M.N.; Najdanovic-Visak, V. Recovery of erythromycin from aqueous solutions with an ionic liquid and high-pressure carbon dioxide. Chem. Eng. J. 2011, 171, 904–911. [Google Scholar] [CrossRef]
- E Silva, F.A.; Sintra, T.; Ventura, S.P.M.; Coutinho, J.A.P. Recovery of paracetamol from pharmaceutical wastes. Sep. Purif. Technol. 2014, 122, 315–322. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.R.; Richard, P.L.; Ward, A.J.; van de Water, L.G.A.; Masters, A.F.; Maschmeyer, T. Facile synthesis of ionic liquids possessing chiral carboxylates. Tetrahedron Lett. 2006, 47, 7367–7370. [Google Scholar] [CrossRef]
- Petkovic, M.; Ferguson, J.L.; Gunaratne, H.Q.N.; Ferreira, R.; Leitao, M.C.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Novel biocompatible cholinium-based ionic liquids-toxicity and biodegradability. Green Chem. 2010, 12, 643–649. [Google Scholar] [CrossRef]
- Dimitrijević, A.; Ignjatović, L.; Tot, A.; Vraneš, M.; Zec, N.; Gadžurić, S.; Trtić-Petrović, T. Simultaneous extraction of pesticides of different polarity applying aqueous biphasic systems based on ionic liquids. J. Mol. Liq. 2017, 243, 646–653. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Faustino, V.F.M.; Mondal, D.; Coutinho, J.A.P.; Freire, M.G. Improving the extraction and purification of immunoglobulin G by the use of ionic liquids as adjuvants in aqueous biphasic systems. J. Biotechnol. 2016, 236, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinis, T.B.V.; Neves, C.M.S.S.; Barbosa, L.; Coutinho, J.A.P.; Freire, M.G. 3. Aqueous biphasic systems formed by cholinium-based ionic liquids and mixtures of polymers. In Ionic Liquids: Synthesis, Properties, Technologies and Applications, 1st ed.; Fehrmann, R., Santini, C., Eds.; Walter de Gruyter: Berlin, Germany; Boston, MA, USA, 2019; pp. 29–53. [Google Scholar] [CrossRef]
- Pereira, J.F.B.; Magri, A.; Quental, M.V.; Gonzalez-Miquel, M.; Freire, M.G.; Coutinho, J.A.P. Alkaloids as Alternative Probes To Characterize the Relative Hydrophobicity of Aqueous Biphasic Systems. ACS Sustain. Chem. Eng. 2016, 4, 1512–1520. [Google Scholar] [CrossRef] [Green Version]
- Marić, S.; Jocić, A.; Krstić, A.; Momčilović, M.; Ignjatović, L.; Dimitrijević, A. Poloxamer-based aqueous biphasic systems in designing an integrated extraction platform for the valorization of pharmaceutical waste. Sep. Purif. Technol. 2021, 275, 119101. [Google Scholar] [CrossRef]
- Álvarez, M.S.; Esperança, J.M.S.S.; Deive, F.J.; Sanromán, M.Á.; Rodríguez, A. A biocompatible stepping stone for the removal of emerging contaminants. Sep. Purif. Technol. 2015, 153, 91–98. [Google Scholar] [CrossRef]
- Domínguez-Pérez, M.; Tomé, L.I.N.; Freire, M.G.; Marrucho, I.M.; Cabeza, O.; Coutinho, J.A.P. (Extraction of biomolecules using) aqueous biphasic systems formed by ionic liquids and aminoacids. Sep. Purif. Technol. 2010, 72, 85–91. [Google Scholar] [CrossRef]
- De Sousa, K.M.; Lima, T.S.P.; de Souza, R.L.; Nerli, B.B.; Pereira, M.M.; Soares, C.M.F.; Lima, Á.S. Liquid-liquid equilibrium data for the ternary system based on ionic liquid + organic solvents + water at 298 K and atmospheric pressure applied in antidepressant partitioning. Sep. Purif. Technol. 2021, 278, 119532. [Google Scholar] [CrossRef]
- Buarque, F.S.; Barreto, V.S.; Soares, C.M.F.; Souza, R.L.; Pereira, M.M.; Lima, Á.S. Selective extraction of female hormones using aqueous two-phase system composed of double protic ionic liquid + acetonitrile. Fluid Phase Equilib. 2020, 508, 112443. [Google Scholar] [CrossRef]
- E Silva, F.A.; Caban, M.; Kholany, M.; Stepnowski, P.; Coutinho, J.A.P.; Ventura, S.P.M. Recovery of Nonsteroidal Anti-Inflammatory Drugs from Wastes Using Ionic-Liquid-Based Three-Phase Partitioning Systems. ACS Sustain. Chem. Eng. 2018, 6, 4574–4585. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Yang, C.; Lai, C.; Zhang, C.; Liu, Y. Advantages and challenges of Tween 80 surfactant-enhanced technologies for the remediation of soils contaminated with hydrophobic organic compounds. Chem. Eng. J. 2017, 314, 98–113. [Google Scholar] [CrossRef]
- Gao, Z.; Rohani, S.; Gong, J.; Wang, J. Recent Developments in the Crystallization Process: Toward the Pharmaceutical Industry. Engineering 2017, 3, 343–353. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Kelley, S.P.; Myerson, A.S.; Rogers, R.D. Hydrophobic vs. hydrophilic ionic liquid separations strategies in support of continuous pharmaceutical manufacturing. RSC Adv. 2013, 3, 10019–10026. [Google Scholar] [CrossRef]
- Kroon, M.C.; van Spronsen, J.; Peters, C.J.; Sheldon, R.A.; Witkamp, G.J. Recovery of pure products from ionic liquids using supercritical carbon dioxide as a co-solvent in extractions or as an anti-solvent in precipitations. Green Chem. 2006, 8, 246–249. [Google Scholar] [CrossRef]
- Weber, C.C.; Kunov-Kruse, A.J.; Rogers, R.D.; Myerson, A.S. Manipulation of ionic liquid anion-solute-antisolvent interactions for the purification of acetaminophen. Chem. Commun. 2015, 51, 4294–4297. [Google Scholar] [CrossRef]
- Rasenack, N.; Muller, B.W. Properties of ibuprofen crystallized under various conditions: A comparative study. Drug Dev. Ind. Pharm. 2002, 28, 1077–1089. [Google Scholar] [CrossRef]
- Tang, W.W.; Mo, H.P.; Zhang, M.T.; Parkin, S.; Gong, J.B.; Wang, J.K.; Li, T.L. Persistent Self-Association of Solute Molecules in Solution. J. Phys. Chem. B 2017, 121, 10118–10124. [Google Scholar] [CrossRef]
- Jaitely, V.; Karatas, A.; Florence, A.T. Water-immiscible room temperature ionic liquids (RTILs) as drug reservoirs for controlled release. Int. J. Pharm. 2008, 354, 168–173. [Google Scholar] [CrossRef]
- Egorova, K.S.; Seitkalieva, M.M.; Posvyatenko, A.V.; Ananikov, V.P. An unexpected increase of toxicity of amino acid-containing ionic liquids. Toxicol. Res. 2015, 4, 152–159. [Google Scholar] [CrossRef]
- Malhotra, S.V.; Kumar, V. A profile of the in vitro anti-tumor activity of imidazolium-based ionic liquids. Bioorgan. Med. Chem. Lett. 2010, 20, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Rengstl, D.; Kraus, B.; Van Vorst, M.; Elliott, G.D.; Kunz, W. Effect of choline carboxylate ionic liquids on biological membranes. Colloids Surf. B Biointerfaces 2014, 123, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Stepnowski, P.; Skladanowski, A.C.; Ludwiczak, A.; Laczynska, E. Evaluating the cytotoxicity of ionic liquids using human cell line HeLa. Hum. Exp. Toxicol. 2004, 23, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.R.P.; Paredes, X.; Cristino, A.F.; Santos, F.J.V.; Queirós, C.S.G.P. Ionic Liquids—A Review of Their Toxicity to Living Organisms. Int. J. Mol. Sci. 2021, 22, 5612. [Google Scholar] [CrossRef]
- Kumari, P.; Pillai, V.V.S.; Benedetto, A. Mechanisms of action of ionic liquids on living cells: The state of the art. Biophys. Rev. 2020, 12, 1187–1215. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ohlin, C.A.; Lu, Q.; Fei, Z.; Hu, J.; Dyson, P.J. Cytotoxicity of ionic liquids and precursor compounds towards human cell line HeLa. Green Chem. 2007, 9, 1191–1197. [Google Scholar] [CrossRef]
- Li, X.-Y.; Jing, C.-Q.; Lei, W.-L.; Li, J.; Wang, J.-J. Apoptosis caused by imidazolium-based ionic liquids in PC12 cells. Ecotoxicol. Environ. Saf. 2012, 83, 102–107. [Google Scholar] [CrossRef] [PubMed]
- García-Lorenzo, A.; Tojo, E.; Tojo, J.; Teijeira, M.; Rodríguez-Berrocal, F.J.; González, M.P.; Martínez-Zorzano, V.S. Cytotoxicity of selected imidazolium-derived ionic liquids in the human Caco-2 cell line. Sub-structural toxicological interpretation through a QSAR study. Green Chem. 2008, 10, 508–516. [Google Scholar] [CrossRef]
- Samorì, C.; Malferrari, D.; Valbonesi, P.; Montecavalli, A.; Moretti, F.; Galletti, P.; Sartor, G.; Tagliavini, E.; Fabbri, E.; Pasteris, A. Introduction of oxygenated side chain into imidazolium ionic liquids: Evaluation of the effects at different biological organization levels. Ecotoxicol. Environ. Saf. 2010, 73, 1456–1464. [Google Scholar] [CrossRef]
- Stolte, S.; Arning, J.; Bottin-Weber, U.; Müller, A.; Pitner, W.-R.; Welz-Biermann, U.; Jastorff, B.; Ranke, J. Effects of different head groups and functionalised side chains on the cytotoxicity of ionic liquids. Green Chem. 2007, 9, 760–767. [Google Scholar] [CrossRef]
- Stolte, S.; Arning, J.; Bottin-Weber, U.; Matzke, M.; Stock, F.; Thiele, K.; Uerdingen, M.; Welz-Biermann, U.; Jastorff, B.; Ranke, J. Anion effects on the cytotoxicity of ionic liquids. Green Chem. 2006, 8, 621–629. [Google Scholar] [CrossRef]
- Gouveia, W.; Jorge, T.F.; Martins, S.; Meireles, M.; Carolino, M.; Cruz, C.; Almeida, T.V.; Araújo, M.E.M. Toxicity of ionic liquids prepared from biomaterials. Chemosphere 2014, 104, 51–56. [Google Scholar] [CrossRef]
- Mazid, R.R.; Divisekera, U.; Yang, W.J.; Ranganathan, V.; MacFarlane, D.R.; Cortez-Jugo, C.; Cheng, W.L. Biological stability and activity of siRNA in ionic liquids. Chem. Commun. 2014, 50, 13457–13460. [Google Scholar] [CrossRef]
- Banerjee, A.; Ibsen, K.; Brown, T.; Chen, R.; Agatemor, C.; Mitragotri, S. Ionic liquids for oral insulin delivery. Proc. Natl. Acad. Sci. USA 2018, 115, 7296. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Xia, S.; Wang, Q.; Ma, P. Predicting the Toxicity of Ionic Liquids in Leukemia Rat Cell Line by the Quantitative Structure–Activity Relationship Method Using Topological Indexes. Ind. Eng. Chem. Res. 2012, 51, 13897–13901. [Google Scholar] [CrossRef]
- Yu, M.; Li, S.-M.; Li, X.-Y.; Zhang, B.-J.; Wang, J.-J. Acute effects of 1-octyl-3-methylimidazolium bromide ionic liquid on the antioxidant enzyme system of mouse liver. Ecotoxicol. Environ. Saf. 2008, 71, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, A.; Wright, M.C. Ionic Liquids: New Emerging Pollutants, Similarities with Perfluorinated Alkyl Substances (PFASs). Environ. Sci. Technol. 2019, 53, 10539–10541. [Google Scholar] [CrossRef] [PubMed]
- Probert, P.M.; Leitch, A.C.; Dunn, M.P.; Meyer, S.K.; Palmer, J.M.; Abdelghany, T.M.; Lakey, A.F.; Cooke, M.P.; Talbot, H.; Wills, C.; et al. Identification of a xenobiotic as a potential environmental trigger in primary biliary cholangitis. J. Hepatol. 2018, 69, 1123–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitch, A.C.; Abdelghany, T.M.; Charlton, A.; Grigalyte, J.; Oakley, F.; Borthwick, L.A.; Reed, L.; Knox, A.; Reilly, W.J.; Agius, L.; et al. Renal injury and hepatic effects from the methylimidazolium ionic liquid M8OI in mouse. Ecotoxicol. Environ. Saf. 2020, 202, 110902. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.I.; Gonçalves, A.M.M.; Pereira, J.L.; Figueiredo, B.F.H.T.; e Silva, F.A.; Coutinho, J.A.P.; Ventura, S.P.M.; Gonçalves, F. Environmental safety of cholinium-based ionic liquids: Assessing structure–ecotoxicity relationships. Green Chem. 2015, 17, 4657–4668. [Google Scholar] [CrossRef]
- Pretti, C.; Chiappe, C.; Pieraccini, D.; Gregori, M.; Abramo, F.; Monni, G.; Intorre, L. Acute toxicity of ionic liquids to the zebrafish (Danio rerio). Green Chem. 2006, 8, 238–240. [Google Scholar] [CrossRef]
- Siedlecka, E.M.; Czerwicka, M.; Neumann, J.; Stepnowski, P.; Fernández, J.F.; Thöming, J. Ionic Liquids: Methods of Degradation and Recovery. In Ionic Liquids: Theory, Properties, New Approaches; Kokorin, A., Ed.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Mehrkesh, A.; Karunanithi, A.T. Life-Cycle Perspectives on Aquatic Ecotoxicity of Common Ionic Liquids. Environ. Sci. Technol. 2016, 50, 6814–6821. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Singh, N.; Prasad, K. Multi-tasking hydrated ionic liquids as sustainable media for the processing of waste human hair: A biorefinery approach. Green Chem. 2019, 21, 3328–3333. [Google Scholar] [CrossRef]
- Gaber, Y.; Törnvall, U.; Kumar, M.A.; Amin, M.A.; Hatti-Kaul, R. HPLC-EAT (Environmental Assessment Tool): A tool for profiling safety, health and environmental impacts of liquid chromatography methods. Green Chem. 2011, 13, 2021–2025. [Google Scholar] [CrossRef]
- Ostadjoo, S.; Berton, P.; Shamshina, J.L.; Rogers, R.D. Scaling-Up Ionic Liquid-Based Technologies: How Much Do We Care About Their Toxicity? Prima Facie Information on 1-Ethyl-3-Methylimidazolium Acetate. Toxicol. Sci. 2018, 161, 249–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Sui, H.; Jia, Z.; Yang, Z.; He, L.; Li, X. Recovery and purification of ionic liquids from solutions: A review. RSC Adv. 2018, 8, 32832–32864. [Google Scholar] [CrossRef] [Green Version]
- Torres-Acosta, M.A.; Pereira, J.F.B.; Freire, M.G.; Aguilar-Yáñez, J.M.; Coutinho, J.A.P.; Titchener-Hooker, N.J.; Rito-Palomares, M. Economic evaluation of the primary recovery of tetracycline with traditional and novel aqueous two-phase systems. Sep. Purif. Technol. 2018, 203, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Ionic Liquids Market Size By Application (Catalysis/Synthesis, Food, Paper & Pulp, Electronics, Biotechnology, Automotive, Pharmaceuticals), Industry Analysis Report, Regional Outlook, Application Potential, Price Trends, Competitive Market Share & Forecast, 2015–2022. Available online: https://www.gminsights.com/industry-analysis/ionic-liquids-market-report (accessed on 21 April 2021).

| Pharmaceutical/Compound | Hydrophilic IL | Solubility in Water (mg/L) | Maximum Solubility in IL Aqueous Solution (mg/L) | Operating Conditions | Ref. | |
|---|---|---|---|---|---|---|
| T (K) | wILa | |||||
| Albendazole | [C4C1im][BF4] | 5.31 × 10−1 | 3.10 × 102 | 298 | 0.33 | [58] |
| [C6C1im][BF4] | 1.26 × 103 | |||||
| [C6C1im]Cl | 2.11 × 103 | |||||
| Vanillin | [C4C1im][TsO] | 1.11 × 104 | 4.46 × 105 | 303 | 0.5 | [17] |
| [C4C1im]Cl | 3.75 × 105 | 0.8 | ||||
| [C2C1im][N(CN)2] | 3.95 × 105 | 0.5 | ||||
| [C4C1im]Cl | 1.34 × 105 | 298 | 0.3 | [71] | ||
| [C4Car]Br | 6.09 × 104 | 0.23 | ||||
| [C6Car]Br | 9.43 × 104 | 0.25 | ||||
| Gallic acid | [C4C1im]Cl | 1.44 × 104 | 2.88 × 105 | 303 | 0.5 | [17] |
| [C4C1im][N(CN)2] | 3.30 × 105 | 0.5 | ||||
| Ibuprofen | [C4C1im][N(CN)2] | 3.76 × 101 | 4.47 × 103 | 303 | 0.2 | [59] |
| [C4C1im][TsO] | 2.01 × 103 | 0.3 | ||||
| [C4C1im][SCN] | 2.29 × 103 | 0.2 | ||||
| [N111(2OH)][Van] | 1.88 × 104 | 0.8 | [70] | |||
| [N111(2OH)][Gal] | 1.71 × 104 | 0.65 | ||||
| [N111(2OH)][Sal] | 2.26 × 105 | 0.78 | ||||
| Artemisinin | [C4C1im][N(CN)2] | 6.18 × 101 | 2.85 × 104 | 303 | 0.9 | [61] |
| [C4C1im][TsO] | 4.14 × 103 | 0.6 | ||||
| [C4C1im][SCN] | 1.91 × 104 | 0.8 | ||||
| Amphotericin B | [C6NH3][C1CO2] | 2.00 × 10−1 [72] | 2.50 × 102 | 298 | N.R.b | [50] |
| Itraconazole | [m-PEG350-NH3][C5CO2] | 1.00 × 10−6 [73] | 1.00 × 102 | N.R.b | ||
| Glibenclamide | [N111(2OH)][Trp] | (1.50–2.40) × 101 [74,75,76] | 9.89×103 | 310 | 0.07 | [64] |
| Lamotrigine | [C6C1im]Br | 1.70 × 102 [77] | 5.51 × 108 | 313 | 0.8 | [62] |
| Acetaminophen | [C6C1im]Br | 2.49 × 104 | 8.91 × 104 | 313 | 0.15 | [63] |
| Naproxen | [N111(2OH)][Van] | 3.19 × 101 [60] | 1.78 × 104 | 303 | 0.6 | [70] |
| [N111(2OH)][Gal] | 2.00 × 104 | 0.6 | ||||
| [N111(2OH)][Sal] | 1.35 × 103 | 0.78 | ||||
| Pharmaceutical/Compound | Hydrophilic IL | Drug Delivery Strategy | Ref. |
|---|---|---|---|
| Acyclovir | [C1C1im][(CH3O)2PO2] | (Tween-80 + Span-20) + IL microemulsions | [104] |
| [C1C1im][(CH3O)2PO2]; [C2C1im][BF4] | (Tween-80 + Span-20) + IL microemulsions | [47] | |
| Methotrexate | [C1C1im][(CH3O)2PO2]; [C2C1im][BF4] | (Tween-80 + Span-20) + IL microemulsions | [47] |
| Dantrolene sodium | [C1C1im][(CH3O)2PO2]; [C2C1im][BF4] | (Tween-80 + Span-20) + IL microemulsions | [47] |
| Lidocaine hydrochloride | [C12C1im]Cl; [C14C1im]Cl | micellar-based systems | [114] |
| Rutaecarpine | [C12C1im]Br | shaped iongels | [81] |
| Ibuprofen | [C4C1im][Ibu] | silica-based iongels | [96] |
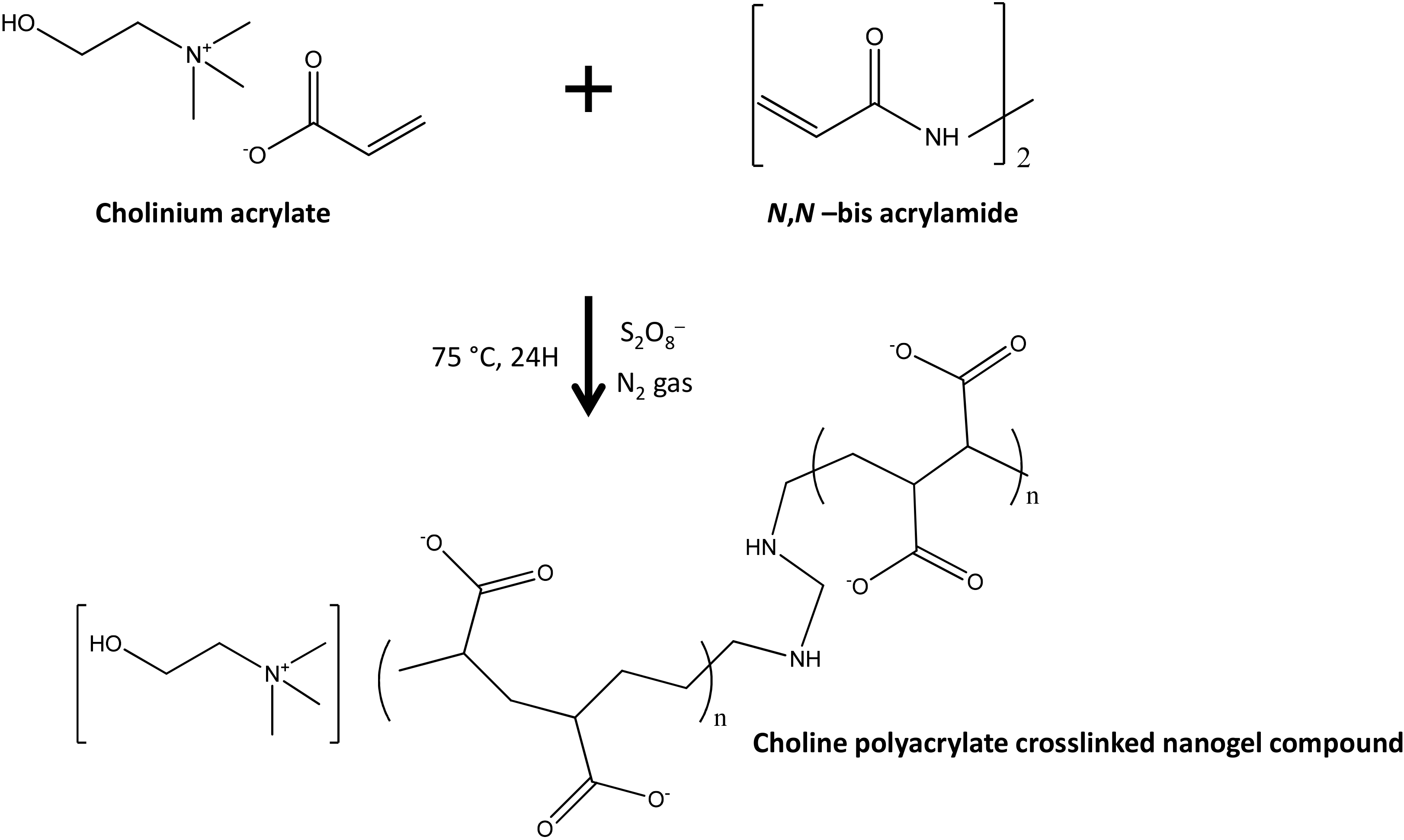
| 5-Fluorouracil | Cholinium Polyacrylate crosslinked structures | stimuli-responsive nanogels | [95] |
| Mannitol | [N111(2OH)][Ger] | Transdermal drug delivery | [99] |
| Cefadroxil | [N111(2OH)][Ger] | Transdermal drug delivery | [99] |
| Ceftazidime | [N111(2OH)][Ger] | Topical formulation | [99] |
| Pharmaceutical/Compound | Hydrophilic ILs | Sample to Be Analyzed | CF (-Fold) | System Components | Ref. |
|---|---|---|---|---|---|
| IL-based ABS | |||||
| Testosterone | [C4C1im]Cl | human urine | 10 | IL + K2HPO4 | [132] |
| Epitestosterone | [C4C1im]Cl | human urine | 10 | IL + K2HPO4 | [132] |
| Serum albumin | [C4C1im]Cl | human urine | 20 | IL + K2HPO4 | [133] |
| BPA | [N111(2OH)]Cl | synthetic urine | 100 | IL + K3PO4 | [129] |
| [C2C1im]Cl | synthetic urine | 100 | IL + K3PO4 | [129] | |
| EE2 | [C4C1im][N(CN)2] | standard | 1000 | IL + KNaC4H4O6 | [126] |
| Quinine | [C4C1im]Cl | human plasma | N.R.a | IL + K2HPO4 | [134] |
| Caffeine | [N4444]Cl | pretreated wastewater effluent | 28,595 b | IL + K3C6H5O7 | [127] |
| Carbamazepine | [N4444]Cl | pretreated wastewater effluent | 8259 b | IL + K3C6H5O7 | [127] |
| Ciprofloxacin | [N4444]Cl | pretreated wastewater effluent | 1000 | IL + K3C6H5O7 | [135] |
| Diclofenac | [N4444]Cl | pretreated wastewater effluent | 1000 | IL + K3C6H5O7 | [135] |
| [N4444]Cl | environmental water samples | 1230.8 | IL + Na2C4H4O5 | [140] | |
| Codeine | [C4C1im]Cl | Pericarpium papaveris | N.R.a | IL + K2HPO4 | [136] |
| Papaverine | [C4C1im]Cl | Pericarpium papaveris | N.R.a | IL + K2HPO4 | [136] |
| Azithromycin | [C4C1im][BF4] | Environmental water samples | N.R.a | IL + Na2CO3 | [137] |
| Mydecamycin | [C4C1im][BF4] | Environmental water samples | N.R.a | IL + NaH2PO4 | [137] |
| Acetylspiramycin | [C4C1im][BF4] | Environmental water samples | 10 | IL + NaH2PO4 | [138] |
| Chloramphenicol | [C4C1im][BF4] | Honey, milk and water samples | 22.5 | IL + Na3C6H5O7 | [139] |
| [N4444]Cl | Environmental water samples | 1216 | IL + Na2C4H4O5 | [140] | |
| [C1C1C1C1guan][TEMPO-OSO3] | Environmental water samples | 147.2 | IL + K3PO4 | [145] | |
| Indomethacin | [N4444]Cl | Environmental water samples | 1238 | IL + Na2C4H4O5 | [140] |
| Ibuprofen | [N4444]Cl | Environmental water samples | 1228 | IL + Na2C4H4O5 | [140] |
| Ketoprofen | [N4444]Cl | Environmental water samples | 1230 | IL + Na2C4H4O5 | [140] |
| Flurbiprofen | [N4444]Cl | Environmental water samples | 1218 | IL + Na2C4H4O5 | [140] |
| Acethylcholinesterase inhibitors | [N111(2OH)][Sac] | Tablet and human urine | 153 | IL + Na2CO3 | [141] |
| Tetracycline | [aC1im]Cl | Milk and honey | N.R.a | IL + K2HPO4 + Triton X-100 | [142] |
| Sulfonamides | [C6C1im]Cl | Human plasma | N.R.a | IL + K2HPO4 + SDS | [143] |
| Berberine hydrochloride | [N115(2OH)][TEMPO-OSO3] | Rhizoma coptidis | 127.68 | IL + K3PO4 | [146] |
| IL-DLLME | |||||
| BPA | [C6C1im][FeCl4] | Vegetable oils | N.R.a | Extraction Solvent: IL Dispersant: acetone Magnetic aid: Fe3O4 | [149] |
| 4-nonylphenol | [C6C1im][FeCl4] | Vegetable oils | N.R.a | Extraction solvent: IL Dispersant: acetone Magnetic aid: Fe3O4 | [149] |
| Triclosan | [C4C1im][BF4] + [C4C1im][NPA] | Human serum and urine | N.R.a | Extraction solvent: [C8C1im][PF6] Dispersant: ([C4C1im][BF4] + [C4C1im][NPA]) Ion exchange reagent: NH4PF6 | [150] |
| Methyltriclosan | [C4C1im][BF4] + [C4C1im][NPA] | Human serum and urine | N.R.a | Extraction solvent: [C8C1im][PF6] Dispersant: ([C4C1im][BF4] + [C4C1im][NPA]) Ion exchange reagent: NH4PF6 | [150] |
| Tetracycline | [C2C1im][BF4] + [C4C1im][NPA] | Milk and eggs | N.R.a | Extraction solvent: [C6C1im][PF6] Dispersant: ([C4C1im][BF4] + [C4C1im][NPA]) Ion exchange reagent: NH4PF6 | [151] |
| Oxytetracycline | [C2C1im][BF4] + [C4C1im][NPA] | Milk and eggs | N.R.a | Extraction solvent: [C6C1im][PF6] Dispersant: ([C4C1im][BF4] + [C4C1im][NPA]) Ion exchange reagent: NH4PF6 | [151] |
| Chlorotetracycline | [C2C1im][BF4] + [C4C1im][NPA] | Milk and eggs | N.R.a | Extraction solvent: [C6C1im][PF6] Dispersant: ([C4C1im][BF4] + [C4C1im][NPA]) Ion exchange reagent: NH4PF6 | [151] |
| Doxycycline | [C2C1im][BF4] + [C4C1im][NPA] | Milk and eggs | N.R.a | Extraction solvent: [C6C1im][PF6] Dispersant: ([C4C1im][BF4] + [C4C1im][NPA]) Ion exchange reagent: NH4PF6 | [151] |
| in situ IL-DLLME | |||||
| Tetracycline | [C7H7C1im]Cl | Milk, honey, egg | 25 | Extraction solvent: IL Ion exchange reagent: NH4PF6 | [152] |
| Methacycline | [C7H7C1im]Cl | Milk, honey, egg | 98 | Extraction solvent: IL Ion exchange reagent: NH4PF6 | [152] |
| Chlortetracycline | [C7H7C1im]Cl | Milk, honey, egg | 60 | Extraction solvent: IL Ion exchange reagent: NH4PF6 | [152] |
| Doxycycline | [C7H7C1im]Cl | Milk, honey, egg | 56 | Extraction solvent: IL Ion exchange reagent: NH4PF6 | [152] |
| BPA | [C4C1im]Cl | Environmental water samples and effluents | 130–149 | Extraction solvent: IL Ion exchange reagent: LiNTf2 | [153] |
| [C8C1im]Cl | Toys and pacifiers | 299 | Extraction solvent: IL Non-stick agent: X-100 Ion exchange reagent: LiNTf2 | [154] | |
| 4-cumylphenol | [C4C1im]Cl | Environmental water samples and effluents | 965–1037 | Extraction solvent: IL Ion exchange reagent: LiNTf2 | [153] |
| 4-tert-Butylphenol | [C4C1im]Cl | Environmental water samples and effluents | 354–410 | Extraction solvent: IL Ion exchange reagent: LiNTf2 | [153] |
| 4-Octylphenol | [C4C1im]Cl | Environmental water samples and effluents | 402–463 | Extraction solvent: IL Ion exchange reagent: LiNTf2 | [153] |
| 4-tert-Octylphenol | [C4C1im]Cl | Environmental water samples and effluents | 891–967 | Extraction solvent: IL Ion exchange reagent: LiNTf2 | [153] |
| 4-n-Nonylphenol | [C4C1im]Cl | Environmental water samples and effluents | 682–762 | Extraction solvent: IL Ion exchange reagent: LiNTf2 | [153] |
| Myclobutanil | [C4C4im]Br | Environmental water samples | 323 | Extraction solvent: IL Ion exchange reagent: LiNTf2 Magnetic aid: Fe3O4 | [155] |
| Tebuconazole | [C4C4im]Br | Environmental water samples | 211 | Extraction solvent: IL Ion exchange reagent: LiNTf2 Magnetic aid: Fe3O4 | [155] |
| Cyproconazole | [C4C4im]Br | Environmental water samples | 187 | Extraction solvent: IL Ion exchange reagent: LiNTf2 Magnetic aid: Fe3O4 | [155] |
| Prothioconazole | [C4C4im]Br | Environmental water samples | 247 | Extraction solvent: IL Ion exchange reagent: LiNTf2 Magnetic aid: Fe3O4 | [155] |
| Sulfamethazine | [C4C1im-TEMPO]Cl | Milk | 44.3 | Extraction solvent: IL Ion exchange reagent: KPF6 | [156] |
| Sulfamonomethoxine | [C4C1im-TEMPO]Cl | Milk | 47.0 | Extraction solvent: IL Ion exchange reagent: KPF6 | [156] |
| Sulfadiazine | [C4C1im-TEMPO]Cl | Milk | 46.5 | Extraction solvent: IL Ion exchange reagent: KPF6 | [156] |
| Sulfamerazine | [C4C1im-TEMPO]Cl | Milk | 42.4 | Extraction solvent: IL Ion exchange reagent: KPF6 | [156] |
| Sulfamethizole | [C4C1im-TEMPO]Cl | Milk | 43.9 | Extraction solvent: IL Ion exchange reagent: KPF6 | [156] |
| Pharmaceutical | Hydrophilic IL | Pharmaceutical Source | Recovery/Purification Method | Operating Conditions | Ref. | |
|---|---|---|---|---|---|---|
| T (K) | System Components | |||||
| Tetracycline | [N111(2OH)]Cl | standard | ABS | 298 | IL + K3PO4 | [40] |
| [N111(2OH)][Ace] | standard | ABS | 298 | IL + K3PO4 | [40] | |
| [N111(2OH)][Lev] | standard | ABS | 298 | IL +K3PO4 | [40] | |
| [N111(2OH)][Glt] | standard | ABS | 298 | IL + K3PO4 | [40] | |
| [N111(2OH)][Suc] | standard | ABS | 298 | IL + K3PO4 | [40] | |
| [N111(2OH)]Cl | fermentation broth | ABS | 298 | IL + K3PO4 | [39] | |
| [N111(2OH)][Bic] | fermentation broth | ABS | 298 | IL + K3PO4 | [39] | |
| Ciprofloxacin (and its hydrochloride salt) | [C4C1im][CF3SO3] | standard | ABS | 298 | IL + lysine | [177] |
| Ibuprofen | [C4C1im]Cl | pills | TPP + precipitation with antisolvent | 298 | IL + K3C6H5O7/C6H8O7 (pH = 7) | [180] |
| [P4441][C1SO4] | standard | ABS + precipitation with antisolvent | 298 | IL + Al2(SO4)3 | [163] | |
| [N111(2OH)]Cl | standard | ABS | 298, 333 | IL + Tween 80 | [176] | |
| [C4C1im][SCN] | standard | Crystallization with antisolvent | --- | IL aqueous solution | [59] | |
| Naproxen | [C4C1im]Cl | pills | TPP + precipitation with antisolvent | 298 | IL + K3C6H5O7/C6H8O7 (pH = 7) | [180] |
| [P4441][C1SO4] | standard | ABS + precipitation with antisolvent | 298 | IL + Al2(SO4)3 | [163] | |
| Ketoprofen | [C4C1im]Cl | pills | TPP + precipitation with antisolvent | 298 | IL + K3C6H5O7/C6H8O7 (pH = 7) | [180] |
| [P4441][C1SO4] | standard | ABS + precipitation with antisolvent | 298 | IL + Al2(SO4)3 | [163] | |
| Paracetamol | [N2222]Br | pills | ABS | 298 | IL + K3C6H5O7/C6H8O7 (pH = 7) or K2CO3 | [167] |
| [N4444]Br | pills | ABS | 298 | IL + K3C6H5O7/C6H8O7 (pH = 7) or K2CO3 | [167] | |
| [C4C1im]Cl | pills | ABS +ABS | 298 | IL + Pluronic PE 6200 | [175] | |
| [C2C1im][C1CO2]x[NTf2]1-x | standard | Crystallization with anti-solvent | 298 | IL | [185] | |
| Diclofenac | [P4441][C1SO4] | standard | ABS + precipitation with antisolvent | 298 | IL + Al2(SO4)3 | [163] |
| [N111(2OH)]Cl | standard | ABS | 298, 333 | IL + Tween 80 | [176] | |
| Fluoxetine hydrochloride | [C2C1im][C1CO2] | standard | ABS | 298 | IL + 1,3-dioxolane | [178] |
| Paroxetine hydrochloride | [C2C1im][C1CO2] | standard | ABS | 298 | IL + 1,3-dioxolane | [178] |
| Sertraline hydrochloride | [C2C1im][C1CO2] | standard | ABS | 298 | IL + 1,3-dioxolane | [178] |
| 17β-estradiol | ([N000(2OH)][C1CO2] + [N00(2OH)(2OH)][C1CO2]), ([N000(2OH)][C1CO2] + [N00(2OH)(2OH)][C3CO2]) | standard | ABS | 298 | IL mixture + acetonitrile | [179] |
| estriol | ([N000(2OH)][C1CO2] + [N00(2OH)(2OH)][C1CO2]), ([N000(2OH)][C1CO2] + [N00(2OH)(2OH)][C3CO2]) | standard | ABS | 298 | IL mixture + acetonitrile | [179] |
| EE2 | ([N000(2OH)][C1CO2] + [N00(2OH)(2OH)][C1CO2]), ([N000(2OH)][C1CO2] + [N00(2OH)(2OH)][C3CO2]) | standard | ABS | 298 | IL mixture + acetonitrile | [179] |
| progesterone | ([N000(2OH)][C1CO2] + [N00(2OH)(2OH)][C1CO2]), ([N000(2OH)][C1CO2] + [N00(2OH)(2OH)][C3CO2]) | standard | ABS | 298 | IL mixture + acetonitrile | [179] |
| Intermediate amine of the aliskiren synthesis | [C2C1im][C1CO2] | Reaction mixtures | LLE | 294 | IL + ethyl acetate | [183] |
| methyl-(Z)-α-acetamido cinnamate | [C4C1im][BF4] | standard | Crystallization with anti-solvent or thermal shift | 278–338 | IL + CO2 | [184] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinis, T.B.V.; e Silva, F.A.; Sousa, F.; Freire, M.G. Advances Brought by Hydrophilic Ionic Liquids in Fields Involving Pharmaceuticals. Materials 2021, 14, 6231. https://doi.org/10.3390/ma14216231
Dinis TBV, e Silva FA, Sousa F, Freire MG. Advances Brought by Hydrophilic Ionic Liquids in Fields Involving Pharmaceuticals. Materials. 2021; 14(21):6231. https://doi.org/10.3390/ma14216231
Chicago/Turabian StyleDinis, Teresa B. V., Francisca A. e Silva, Fani Sousa, and Mara G. Freire. 2021. "Advances Brought by Hydrophilic Ionic Liquids in Fields Involving Pharmaceuticals" Materials 14, no. 21: 6231. https://doi.org/10.3390/ma14216231
APA StyleDinis, T. B. V., e Silva, F. A., Sousa, F., & Freire, M. G. (2021). Advances Brought by Hydrophilic Ionic Liquids in Fields Involving Pharmaceuticals. Materials, 14(21), 6231. https://doi.org/10.3390/ma14216231








