Effect of TiO2 Concentration on Microstructure and Properties of Composite Cu–Sn–TiO2 Coatings Obtained by Electrodeposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrodeposition of Composite Coatings
2.2. Morphology and Structural Analysis
2.3. Antibacterial Performance
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Electrodeposition of Cu–Sn–TiO2 Composite Coatings
- (i)
- according to the Guglielmi model of the electrochemical deposition of composite coatings [40], TiO2 nanoparticles can physically adsorb on the electrode surface during electrolysis, thereby reducing the active electrode area involved in the electrochemical process. This leads to a decrease in the current response at an applied potential;
- (ii)
- since diffusion is the limiting stage of the discharge of copper(II) ions in acidic electrolytes, TiO2 particles dispersed in the electrolyte could prevent diffusion and migration of copper and stannous ions from the bulk solution to the electrode surface.
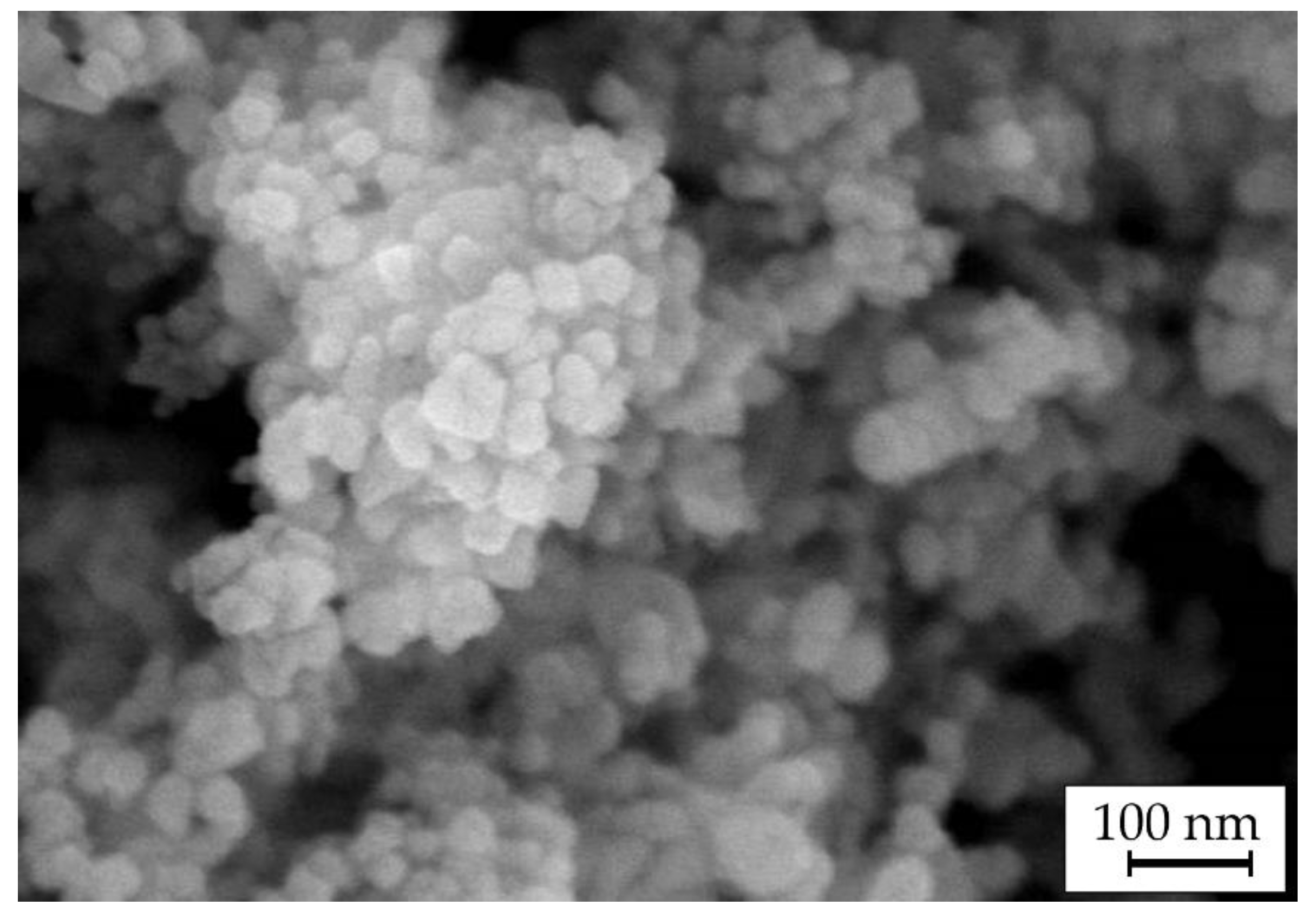
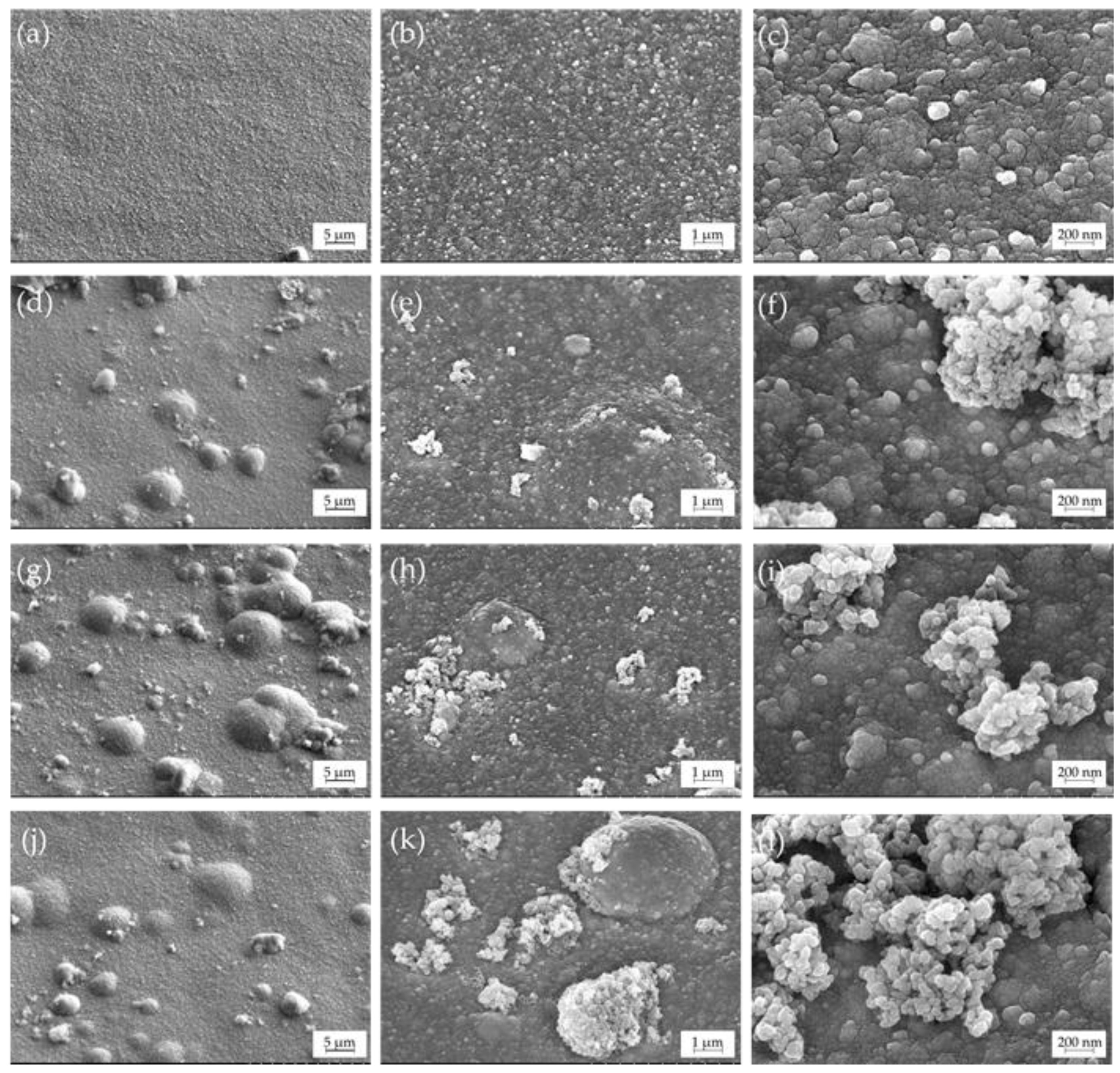
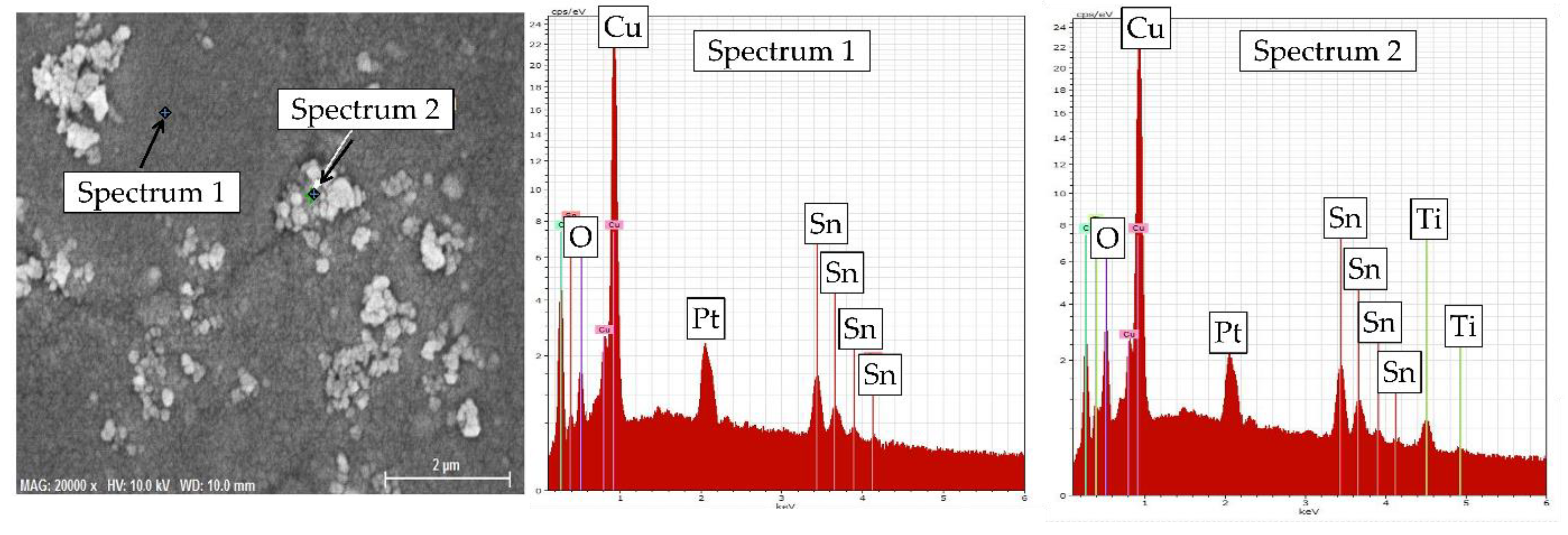
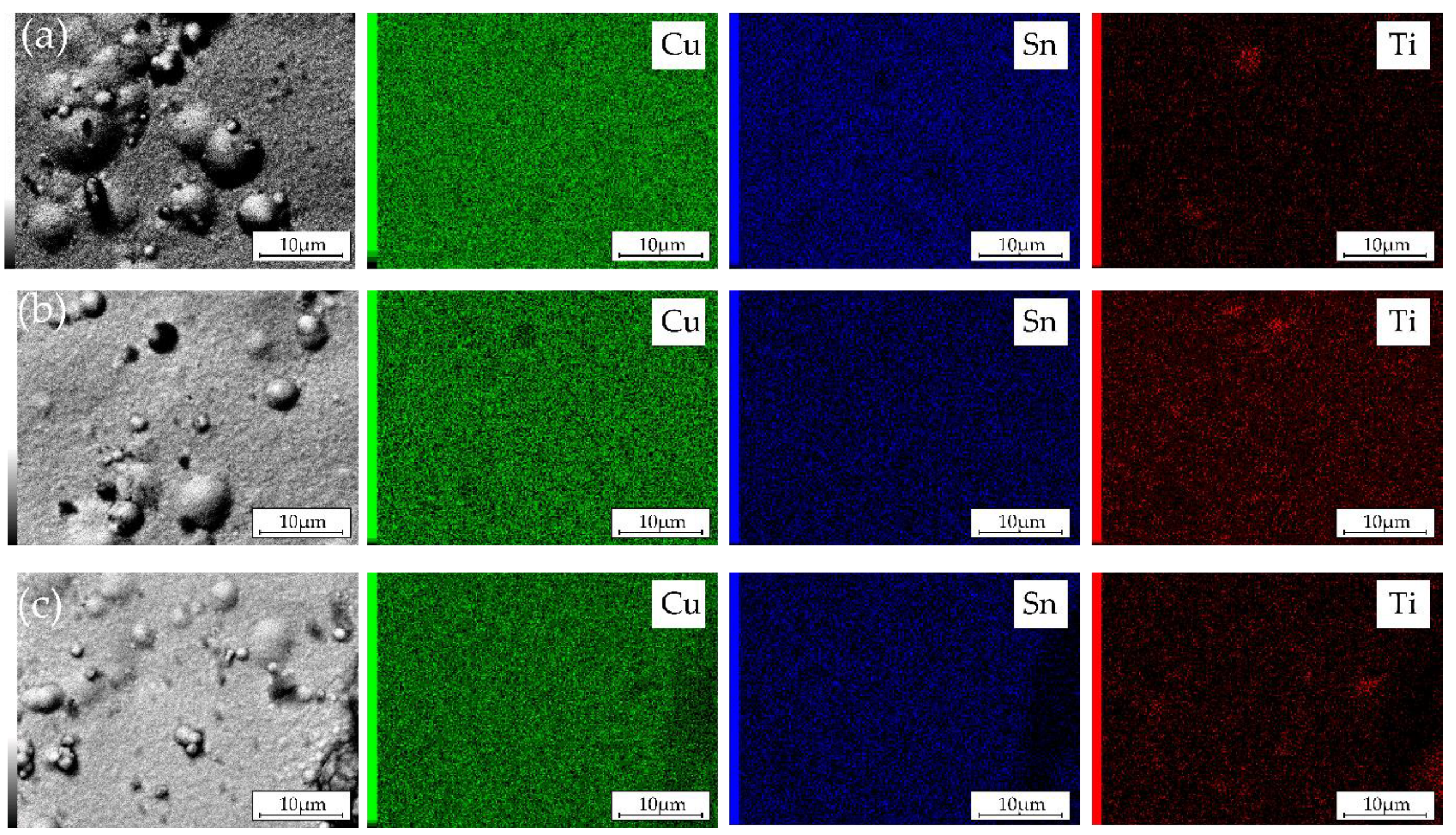
3.2. Microstructure and Elemental Composition
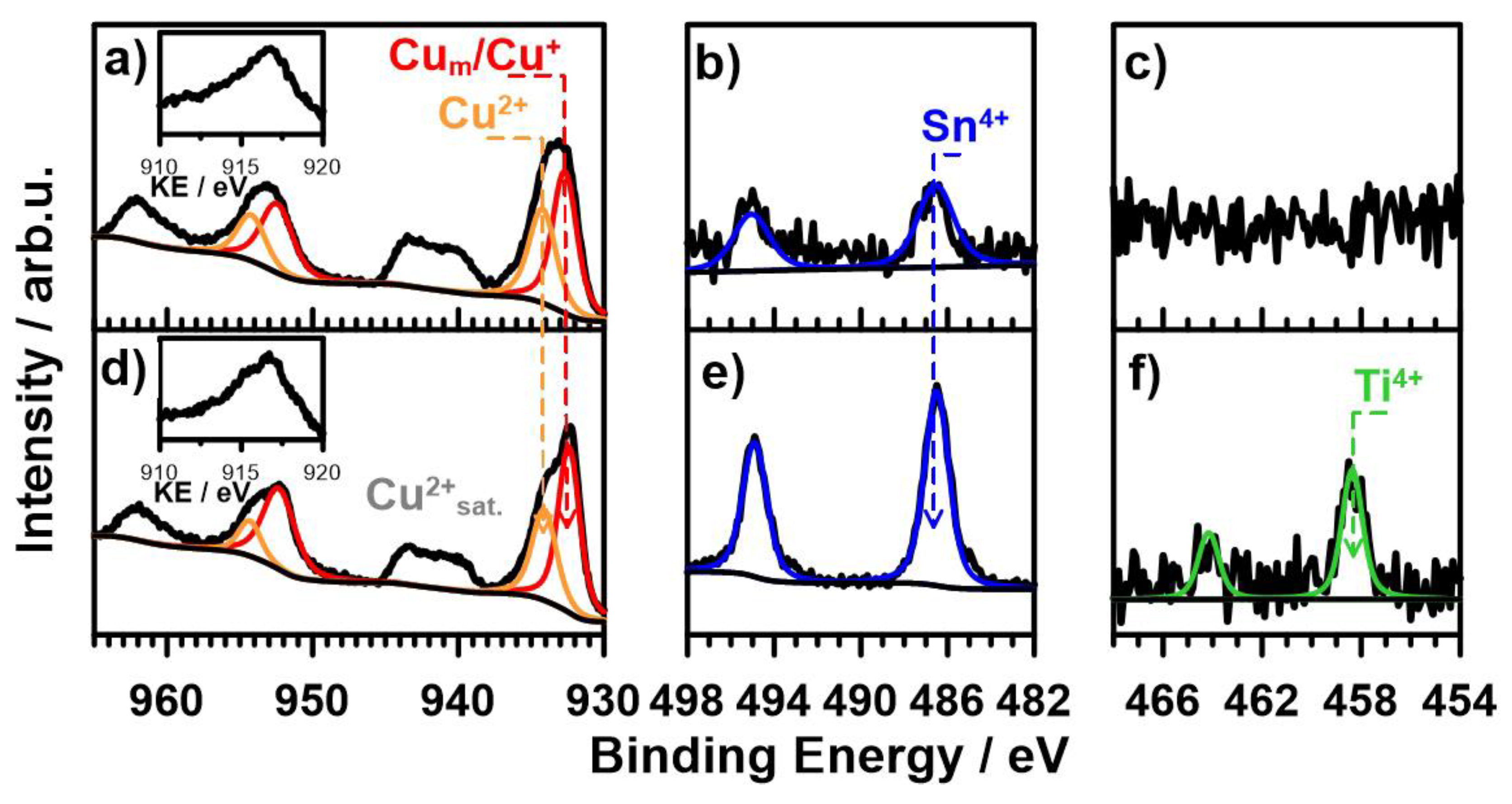
3.3. XRD and XPS Analysis
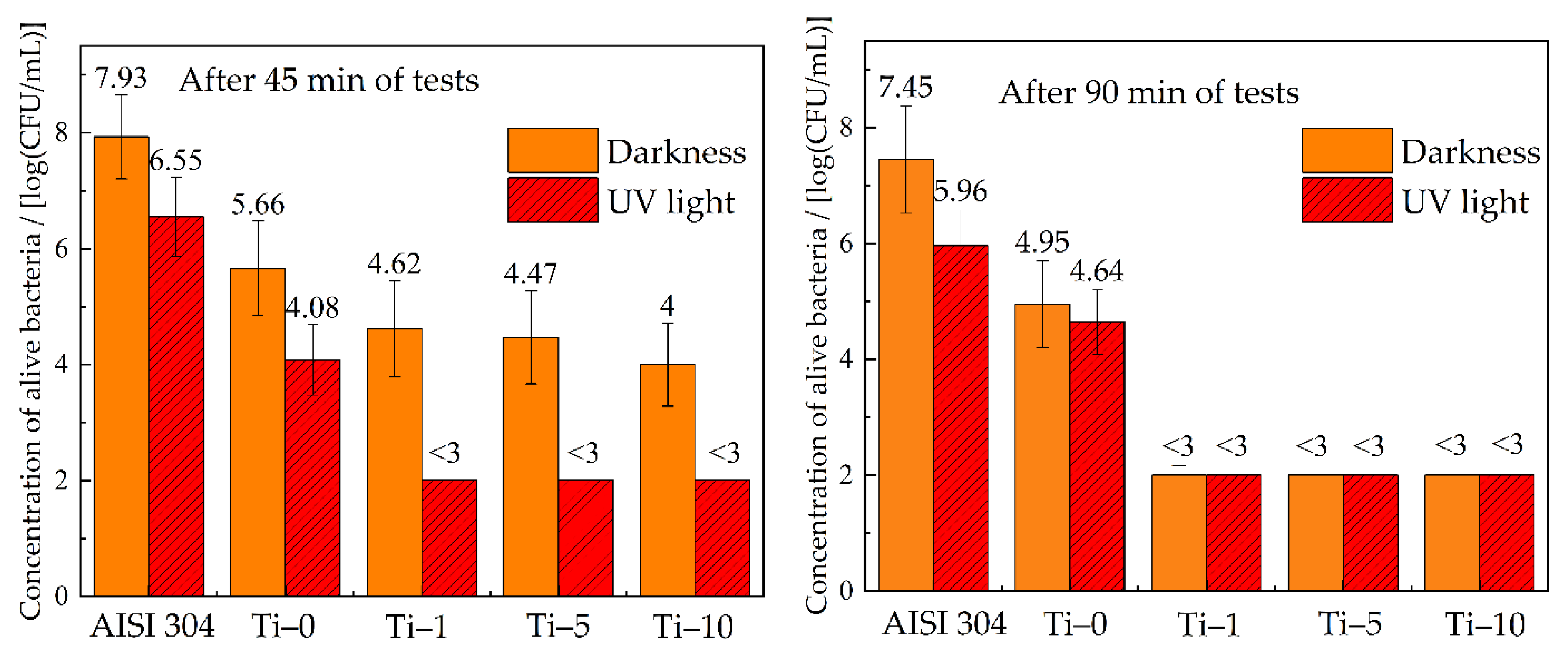
3.4. Antibacterial Performance
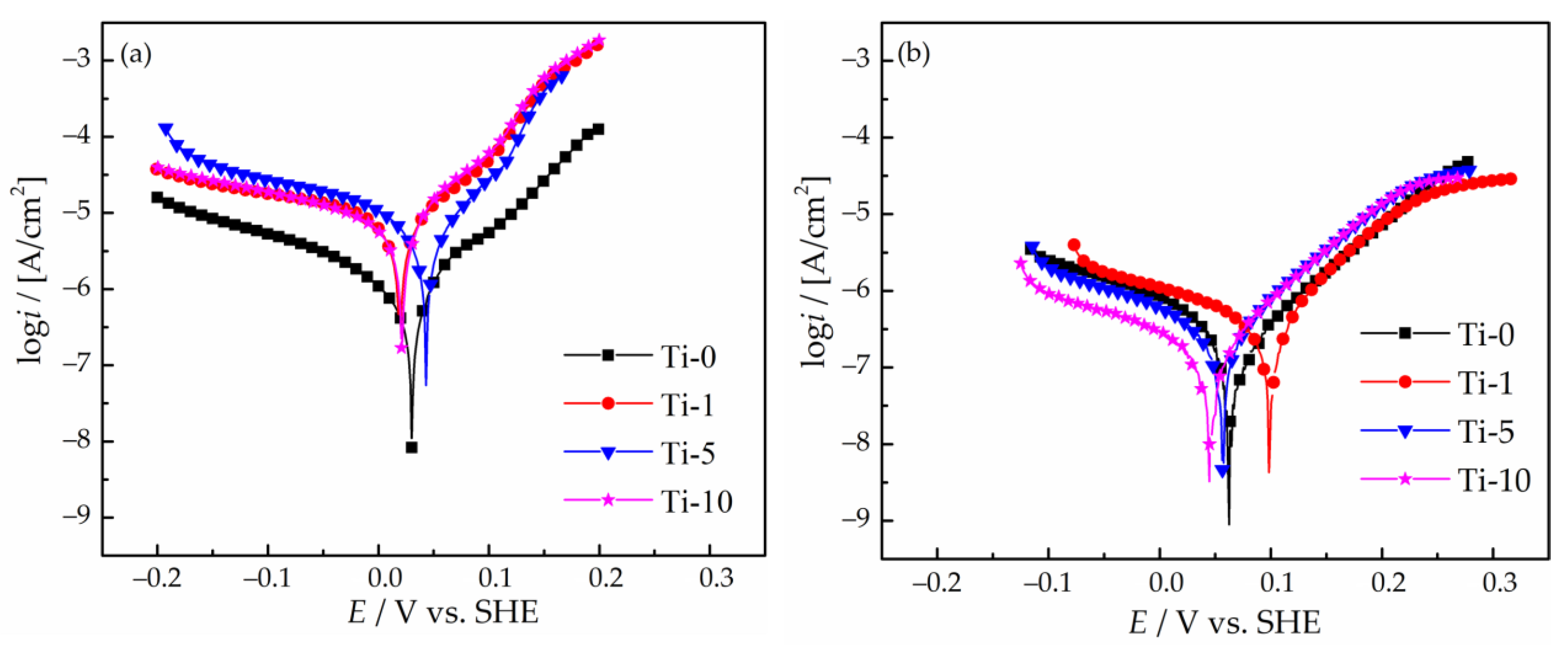
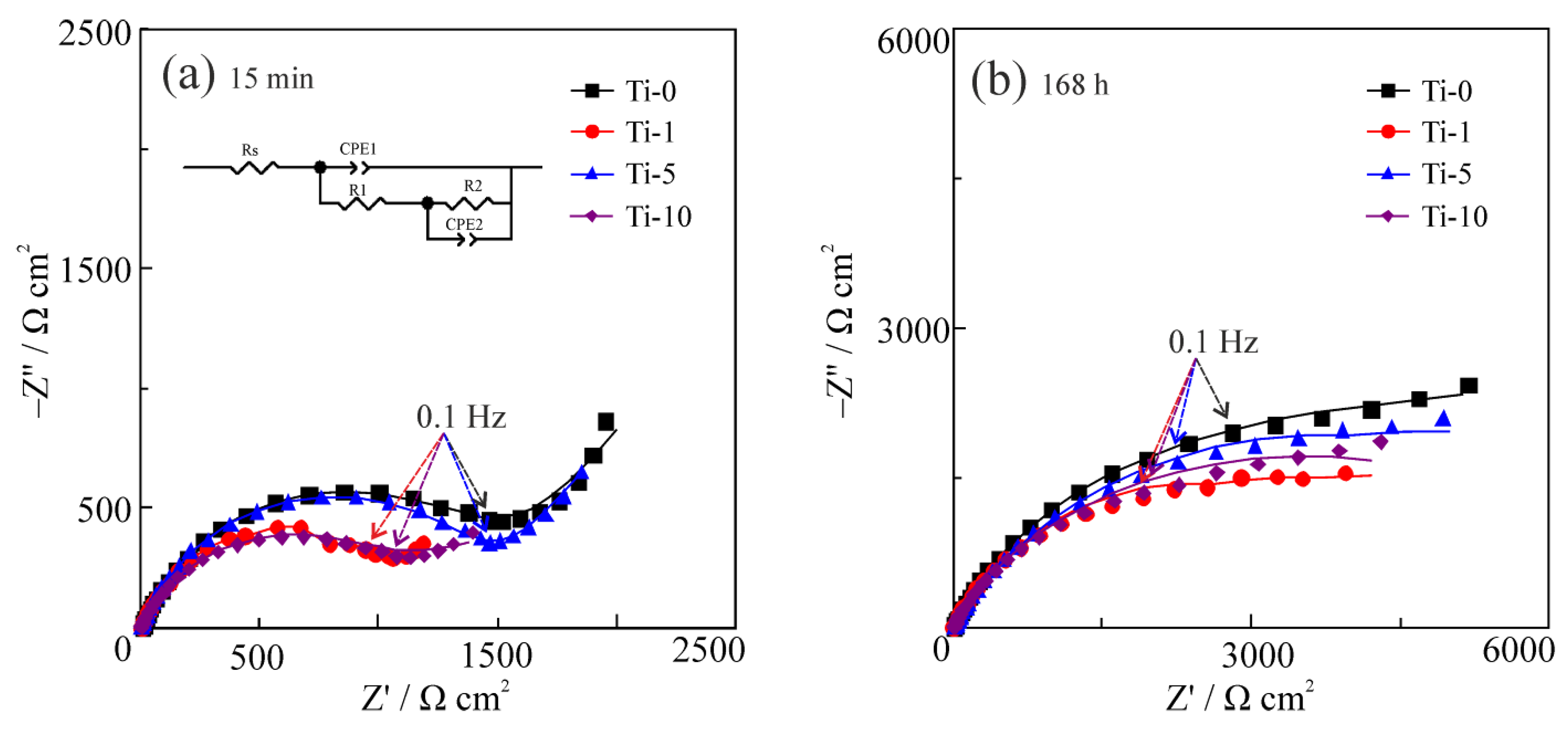
3.5. Corrosion Properties
4. Conclusions
- The results of linear polarization and chronopotentiometry experiments revealed that the introduction of 1–10 g/L of TiO2 particles into the sulfate electrolyte of the Cu–Sn deposition leads to a significant decrease in the cathodic current density, mainly due to reduced active electrode area involved in the electrochemical process.
- SEM/EDX data revealed that Cu–Sn–TiO2 composite coatings obtained from sulfuric acid electrolyte are characterized by a more inhomogeneous structure than Cu–Sn coatings obtained under the same electrolysis conditions. It was found that TiO2 particles are embedded in the alloy matrix mostly in the form of agglomerates with sizes from 100 to 700 nm.
- Electrolysis in the potentiodynamic regime allowed to effectively control the chemical composition of the metal matrix, which contained 10.8–11.5 wt.% Sn. The highest average amount of embed TiO2 nanoparticles (1.7 wt.%) was observed in the coating obtained from the electrolyte containing 5 g/L TiO2.
- Introduction of TiO2 significantly improved antibacterial properties of the composites. All formed structures have pronounced bactericidal properties in relation to the strain of bacteria E. coli. This improvement was connected to the corrosion resistance of the formed composites. The best corrosion performance among composites was shown by the Ti-5 coating with corrosion resistance comparable to the Ti-0 coating.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bright, K.R.; Boone, S.A.; Gerba, C.P. Occurrence of Bacteria and Viruses on Elementary Classroom Surfaces and the Potential Role of Classroom Hygiene in the Spread of Infectious Diseases. J. Sch. Nurs. 2010, 26, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.A.; Watt, P.M.; Boone, S.A.; Gerba, C.P. Occurrence of bacteria and biochemical markers on public surfaces. Int. J. Environ. Health Res. 2005, 15, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Boone, S.A.; Gerba, C.P. Significance of Fomites in the Spread of Respiratory and Enteric Viral Disease. Appl. Environ. Microbiol. 2007, 73, 1687–1696. [Google Scholar] [CrossRef] [Green Version]
- Mikolay, A.; Huggett, S.; Tikana, L.; Grass, G.; Braun, J.; Nies, D.H. Survival of bacteria on metallic copper surfaces in a hospital trial. Appl. Microbiol. Biotechnol. 2010, 87, 1875–1879. [Google Scholar] [CrossRef]
- Dalecki, A.G.; Crawford, C.L.; Wolschendorf, F. Copper and Antibiotics: Discovery, Modes of Action, and Opportunities for Medicinal Applications. In Advances in Microbial Physiology; Elsevier Ltd.: Oxford, UK, 2017; pp. 193–260. [Google Scholar]
- Grass, G.; Rensing, C.; Solioz, M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [Green Version]
- Walkowicz, M.; Osuch, P.; Smyrak, B.; Knych, T.; Rudnik, E.; Cieniek, Ł.; Różańska, A.; Chmielarczyk, A.; Romaniszyn, D.; Bulanda, M. Impact of oxidation of copper and its alloys in laboratory-simulated conditions on their antimicrobial efficiency. Corros. Sci. 2018, 140, 321–332. [Google Scholar] [CrossRef]
- Weaver, L.; Michels, H.T.; Keevil, C.W. Survival of Clostridium difficile on copper and steel: Futuristic options for hospital hygiene. J. Hosp. Infect. 2008, 68, 145–151. [Google Scholar] [CrossRef]
- Różańska, A.; Chmielarczyk, A.; Romaniszyn, D.; Sroka-Oleksiak, A.; Bulanda, M.; Walkowicz, M.; Osuch, P.; Knych, T. Antimicrobial Properties of Selected Copper Alloys on Staphylococcus aureus and Escherichia coli in Different Simulations of Environmental Conditions: With vs. without Organic Contamination. Int. J. Environ. Res. Public Health 2017, 14, 813. [Google Scholar] [CrossRef] [Green Version]
- Montero, D.A.; Arellano, C.; Pardo, M.; Vera, R.; Gálvez, R.; Cifuentes, M.; Berasain, M.A.; Gómez, M.; Ramírez, C.; Vidal, R.M. Antimicrobial properties of a novel copper-based composite coating with potential for use in healthcare facilities. Antimicrob. Resist. Infect. Control 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Chang, T.; Butina, K.; Herting, G.; Rajarao, G.K.; Richter-Dahlfors, A.; Blomberg, E.; Odnevall Wallinder, I.; Leygraf, C. The interplay between atmospheric corrosion and antimicrobial efficiency of Cu and Cu5Zn5Al1Sn during simulated high-touch conditions. Corros. Sci. 2021, 185, 109433. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Huang, H.; Fan, C.; Li, M.; Nie, H.-L.; Wang, F.-B.; Wang, H.; Wang, R.; Xia, J.; Zheng, X.; Zuo, X.; et al. COVID-19: A Call for Physical Scientists and Engineers. ACS Nano 2020, 14, 3747–3754. [Google Scholar] [CrossRef] [Green Version]
- Hutasoit, N.; Kennedy, B.; Hamilton, S.; Luttick, A.; Rahman Rashid, R.A.; Palanisamy, S. Sars-CoV-2 (COVID-19) inactivation capability of copper-coated touch surface fabricated by cold-spray technology. Manuf. Lett. 2020, 25, 93–97. [Google Scholar] [CrossRef]
- Chang, T.; Sepati, M.; Herting, G.; Leygraf, C.; Rajarao, G.K.; Butina, K.; Richter-Dahlfors, A.; Blomberg, E.; Odnevall Wallinder, I. A novel methodology to study antimicrobial properties of high-touch surfaces used for indoor hygiene applications—A study on Cu metal. PLoS ONE 2021, 16, e0247081. [Google Scholar] [CrossRef]
- Poggio, C.; Colombo, M.; Arciola, C.R.; Greggi, T.; Scribante, A.; Dagna, A. Copper-Alloy Surfaces and Cleaning Regimens against the Spread of SARS-CoV-2 in Dentistry and Orthopedics. From Fomites to Anti-Infective Nanocoatings. Materials 2020, 13, 3244. [Google Scholar] [CrossRef] [PubMed]
- Gamburg, Y.D.; Zangari, G. Electrodeposition of Alloys. In Theory and Practice of Metal Electrodeposition; Springer: New York, NY, USA, 2011; pp. 205–232. [Google Scholar]
- Jung, M.; Lee, G.; Choi, J. Electrochemical plating of Cu-Sn alloy in non-cyanide solution to substitute for Ni undercoating layer. Electrochim. Acta 2017, 241, 229–236. [Google Scholar] [CrossRef]
- Kasach, A.A.; Kharitonov, D.S.; Romanovskii, V.I.; Kuz’menok, N.M.; Zharskii, I.M.; Kurilo, I.I. Electrodeposition of Cu-Sn Alloy from Oxalic Acid Electrolyte in the Presence of Amine-containing Surfactants. Russ. J. Appl. Chem. 2019, 92, 835–841. [Google Scholar] [CrossRef]
- Kasach, A.A.; Kharitonov, D.S.; Makarova, I.V.; Wrzesińska, A.; Zharskii, I.M.; Kurilo, I.I. Effect of thiourea on electrocrystallization of Cu–Sn alloys from sulphate electrolytes. Surf. Coat. Technol. 2020, 399, 126137. [Google Scholar] [CrossRef]
- Juškenas, R.; Mockus, Z.; Kanapeckaite, S.; Stalnionis, G.; Survila, A. XRD studies of the phase composition of the electrodeposited copper-rich Cu-Sn alloys. Electrochim. Acta 2006, 52, 928–935. [Google Scholar] [CrossRef]
- Kasach, A.A.; Kharitonov, D.S.; Radchenko, S.L.; Zharskii, I.M.; Kurilo, I.I. Effect of Parameters of Pulse Electrolysis on Electrodeposition of Copper–Tin Alloy from Sulfate Electrolyte. Russ. J. Electrochem. 2020, 56, 744–753. [Google Scholar] [CrossRef]
- Low, C.T.J.; Wills, R.G.A.; Walsh, F.C. Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surf. Coat. Technol. 2006, 201, 371–383. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W. Facile synthesis and electrochemical properties of a novel Ni-B/TiC composite coating via ultrasonic-assisted electrodeposition. Ultrason. Sonochem. 2020, 61, 104837. [Google Scholar] [CrossRef]
- Lixia, Y.; Zhenghui, L.; Ke, W.; Xiupeng, L.; Guixiang, W. Effect of TiO2 Sol on the Microstructure and Tribological Properties of Cu-Sn Coating. Rare Met. Mater. Eng. 2017, 46, 2801–2806. [Google Scholar] [CrossRef] [Green Version]
- Walsh, F.C.; Wang, S.; Zhou, N. The electrodeposition of composite coatings: Diversity, applications and challenges. Curr. Opin. Electrochem. 2020, 20, 8–19. [Google Scholar] [CrossRef]
- Walsh, F.C.; de Leon, C.P. A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: An established and diversifying technology. Trans. IMF 2014, 92, 83–98. [Google Scholar] [CrossRef] [Green Version]
- Cui, G.; Bi, Q.; Niu, M.; Yang, J.; Liu, W. The tribological properties of bronze-SiC-graphite composites under sea water condition. Tribol. Int. 2013, 60, 25–35. [Google Scholar] [CrossRef]
- Wang, X.H.; Ying, L.X.; Zhang, C.J.; Lv, X.P. Electrodeposition of Cu-Sn-Graphite-Al2O3 Composite Coatings and their Tribological Properties. In Proceedings of the Mechanical Engineering, Materials Science and Civil Engineering IV, Sanya, China, 19–20 November 2016; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2017; Volume 893, pp. 335–339. [Google Scholar]
- Ying, L.; Fu, Z.; Wu, K.; Wu, C.; Zhu, T.; Xie, Y.; Wang, G. Effect of TiO2 Sol and PTFE Emulsion on Properties of Cu–Sn Antiwear and Friction Reduction Coatings. Coatings 2019, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Kasach, A.A.; Kharitonov, D.S.; Wrzesińska, A.; Bobowska, I.; Predko, A.A.; Romanovskii, V.I.; Zharskii, I.M.; Kurilo, I.I. The Effect of Ultrasound Treatment on Physicochemical and Tribological Properties of Electrolytic Cu–Sn–TiO2 Coatings. Prot. Met. Phys. Chem. Surfaces 2020, 56, 385–391. [Google Scholar] [CrossRef]
- Gao, W.; Cao, D.; Jin, Y.; Zhou, X.; Cheng, G.; Wang, Y. Microstructure and properties of Cu-Sn-Zn-TiO2 nano-composite coatings on mild steel. Surf. Coat. Technol. 2018, 350, 801–806. [Google Scholar] [CrossRef] [Green Version]
- Danilov, F.I.; Tsurkan, A.V.; Vasil’eva, E.A.; Korniy, S.A.; Cheipesh, T.A.; Protsenko, V.S. Electrochemical synthesis and properties of iron–titanium dioxide composite coatings. Russ. J. Appl. Chem. 2017, 90, 1148–1153. [Google Scholar] [CrossRef]
- Danilov, F.I.; Kityk, A.A.; Shaiderov, D.A.; Bogdanov, D.A.; Korniy, S.A.; Protsenko, V.S. Electrodeposition of Ni–TiO2 Composite Coatings Using Electrolyte Based on a Deep Eutectic Solvent. Surf. Eng. Appl. Electrochem. 2019, 55, 138–149. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Bogdanov, D.A.; Korniy, S.A.; Kityk, A.A.; Baskevich, A.S.; Danilov, F.I. Application of a deep eutectic solvent to prepare nanocrystalline Ni and Ni/TiO2 coatings as electrocatalysts for the hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 24604–24616. [Google Scholar] [CrossRef]
- Wysocka, I.; Kowalska, E.; Ryl, J.; Nowaczyk, G.; Zielińska-Jurek, A. Morphology, Photocatalytic and Antimicrobial Properties of TiO2 Modified with Mono- and Bimetallic Copper, Platinum and Silver Nanoparticles. Nanomaterials 2019, 9, 1129. [Google Scholar] [CrossRef] [Green Version]
- Pyanko, A.V.; Makarova, I.V.; Kharitonov, D.S.; Makeeva, I.S.; Alisienok, O.A.; Chernik, A.A. Tin–Nickel–Titania Composite Coatings. Inorg. Mater. 2019, 55, 568–575. [Google Scholar] [CrossRef]
- Chang, T.; Maltseva, A.; Volovitch, P.; Odnevall Wallinder, I.; Leygraf, C. A mechanistic study of stratified patina evolution on Sn-bronze in chloride-rich atmospheres. Corros. Sci. 2020, 166, 108477. [Google Scholar] [CrossRef]
- Kharitonov, D.S.; Kasach, A.A.; Sergievich, D.S.; Wrzesińska, A.; Bobowska, I.; Darowicki, K.; Zielinski, A.; Ryl, J.; Kurilo, I.I. Ultrasonic-assisted electrodeposition of Cu-Sn-TiO2 nanocomposite coatings with enhanced antibacterial activity. Ultrason. Sonochem. 2021, 75, 105593. [Google Scholar] [CrossRef]
- Guglielmi, N. Kinetics of the Deposition of Inert Particles from Electrolytic Baths. J. Electrochem. Soc. 1972, 119, 1009. [Google Scholar] [CrossRef]
- Bengoa, L.N.; Pary, P.; Conconi, M.S.; Egli, W.A. Electrodeposition of Cu-Sn alloys from a methanesulfonic acid electrolyte containing benzyl alcohol. Electrochim. Acta 2017, 256, 211–219. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins Dos Santos, V.A.P.; Fernández-García, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchison, M.J.; Scully, J.R. Patina enrichment with SnO2 and its effect on soluble Cu cation release and passivity of high-purity Cu-Sn bronze in artificial perspiration. Electrochim. Acta 2018, 283, 806–817. [Google Scholar] [CrossRef]
- Hutchison, M.J.; Zhou, P.; Ogle, K.; Scully, J.R. Enhanced Electrochemical Cu Release from Commercial Cu-Sn Alloys: Fate of the Alloying Elements in Artificial Perspiration. Electrochim. Acta 2017, 241, 73–88. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, F.S.; Cinca, N.; Dosta, S.; Cano, I.G.; Guilemany, J.M.; Caires, C.S.A.; Lima, A.R.; Silva, C.M.; Oliveira, S.L.; Caires, A.R.L.; et al. Corrosion resistance and antibacterial properties of copper coating deposited by cold gas spray. Surf. Coat. Technol. 2019, 361, 292–301. [Google Scholar] [CrossRef]
- Tudela, I.; Zhang, Y.; Pal, M.; Kerr, I.; Cobley, A.J. Ultrasound-assisted electrodeposition of composite coatings with particles. Surf. Coat. Technol. 2014, 259, 363–373. [Google Scholar] [CrossRef]
- Bahadormanesh, B.; Dolati, A.; Ahmadi, M.R. Electrodeposition and characterization of Ni–Co/SiC nanocomposite coatings. J. Alloy. Compd. 2011, 509, 9406–9412. [Google Scholar] [CrossRef]




| Component | Contents in Bath, g/L | Purpose of Component |
|---|---|---|
| CuSO4⋅5H2O | 40 | Source of Cu2+ |
| SnSO4 | 40 | Source of Sn2+ |
| H2SO4 | 100 | Base electrolyte |
| Thiourea | 0.005 | Brightening additive |
| TiO2 | 0–10 | Second phase |
| Coating | Chemical Composition, wt.% | ||
|---|---|---|---|
| Cu | Sn | TiO2 | |
| Ti-0 | 88.6 ± 0.4 | 11.4 ± 0.4 | 0 |
| Ti-1 | 89.0 ± 0.5 | 10.8 ± 0.4 | 0.2 ± 0.1 |
| Ti-5 | 87.8 ± 0.6 | 11.5 ± 0.4 | 1.7 ± 0.2 |
| Ti-10 | 88.1 ± 0.5 | 11.3 ± 0.4 | 0.6 ± 0.1 |
| Coating | 3% NaCl | Bacterial Media | ||
|---|---|---|---|---|
| Ecorr/V | icorr/10–7 A/cm2 | Ecorr/V | icorr/10–8 A/cm2 | |
| Ti-0 | 0.030 ± 0.005 | 1.20 ± 0.11 | 0.064 ± 0.010 | 3.04 ± 0.12 |
| Ti-1 | 0.019 ± 0.004 | 10.71± 0.09 | 0.099 ± 0.009 | 5.78 ± 0.10 |
| Ti-5 | 0.043 ± 0.002 | 8.45 ± 2.07 | 0.057 ± 0.011 | 5.80 ± 0.24 |
| Ti-10 | 0.019 ± 0.004 | 9.62 ± 0.13 | 0.045 ± 0.010 | 3.15 ± 0.26 |
| Coating | R1, Ω∙cm2 | R2, Ω∙cm2 | Y1, 10–4 Ω–1 cm–2 sn | n1 | Y2, 10−2 Ω–1 cm–2 sn | n2 | R3, Ω∙cm2 | Rp, Ω∙cm2 |
|---|---|---|---|---|---|---|---|---|
| After 15 min of corrosion test | ||||||||
| Ti-0 | 10.58 ± 4.21 | 1850 ± 120 | 2.64 ± 1.10 | 0.74 ± 0.01 | 1.34 ± 0.20 | 0.90 ± 0.02 | 5010 ± 298 | 6860 |
| Ti-1 | 7.76 ± 5.53 | 1250 ± 225 | 4.26 ± 1.06 | 0.74 ± 0.02 | 2.12 ± 0.52 | 0.90 ± 0.04 | 2988 ± 327 | 4238 |
| Ti-5 | 8.12 ± 3.77 | 1600 ± 165 | 2.42 ± 0.99 | 0.74 ± 0.01 | 1.16 ± 0.17 | 0.75 ± 0.07 | 4510 ± 465 | 6110 |
| Ti-10 | 8.03 ± 2.96 | 1160 ± 144 | 4.54 ± 1.50 | 0.73 ± 0.03 | 1.22 ± 0.18 | 0.62 ± 0.03 | 2498 ± 143 | 3658 |
| After 168 h of corrosion test | ||||||||
| Ti-0 | 10.21 ± 1.22 | 4625 ± 465 | 2.22 ± 0.41 | 0.76 ± 0.04 | 1.40 ± 0.28 | 0.70 ± 0.06 | 6310 ± 631 | 10935 |
| Ti-1 | 4.18 ± 2.25 | 2852 ± 204 | 3.32 ± 1.03 | 0.79 ± 0.01 | 2.00 ± 0.92 | 0.69 ± 0.05 | 3818 ± 274 | 6670 |
| Ti-5 | 15.98 ± 5.04 | 3927 ± 134 | 3.20 ± 0.48 | 0.71 ± 0.05 | 1.20 ± 0.09 | 0.60 ± 0.02 | 6125 ± 551 | 10052 |
| Ti-10 | 12.94 ± 3.28 | 3766 ± 316 | 3.19 ± 1.16 | 0.71 ± 0.03 | 1.80 ± 0.66 | 0.64 ± 0.04 | 5652 ± 502 | 9891 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasach, A.A.; Kharytonau, D.S.; Paspelau, A.V.; Ryl, J.; Sergievich, D.S.; Zharskii, I.M.; Kurilo, I.I. Effect of TiO2 Concentration on Microstructure and Properties of Composite Cu–Sn–TiO2 Coatings Obtained by Electrodeposition. Materials 2021, 14, 6179. https://doi.org/10.3390/ma14206179
Kasach AA, Kharytonau DS, Paspelau AV, Ryl J, Sergievich DS, Zharskii IM, Kurilo II. Effect of TiO2 Concentration on Microstructure and Properties of Composite Cu–Sn–TiO2 Coatings Obtained by Electrodeposition. Materials. 2021; 14(20):6179. https://doi.org/10.3390/ma14206179
Chicago/Turabian StyleKasach, Aliaksandr A., Dzmitry S. Kharytonau, Andrei V. Paspelau, Jacek Ryl, Denis S. Sergievich, Ivan M. Zharskii, and Irina I. Kurilo. 2021. "Effect of TiO2 Concentration on Microstructure and Properties of Composite Cu–Sn–TiO2 Coatings Obtained by Electrodeposition" Materials 14, no. 20: 6179. https://doi.org/10.3390/ma14206179
APA StyleKasach, A. A., Kharytonau, D. S., Paspelau, A. V., Ryl, J., Sergievich, D. S., Zharskii, I. M., & Kurilo, I. I. (2021). Effect of TiO2 Concentration on Microstructure and Properties of Composite Cu–Sn–TiO2 Coatings Obtained by Electrodeposition. Materials, 14(20), 6179. https://doi.org/10.3390/ma14206179









