High-Temperature Corrosion of APS- and HVOF-Coated Nickel-Based Super Alloy under Air Oxidation and Melted Salt Domains
Abstract
:1. Introduction
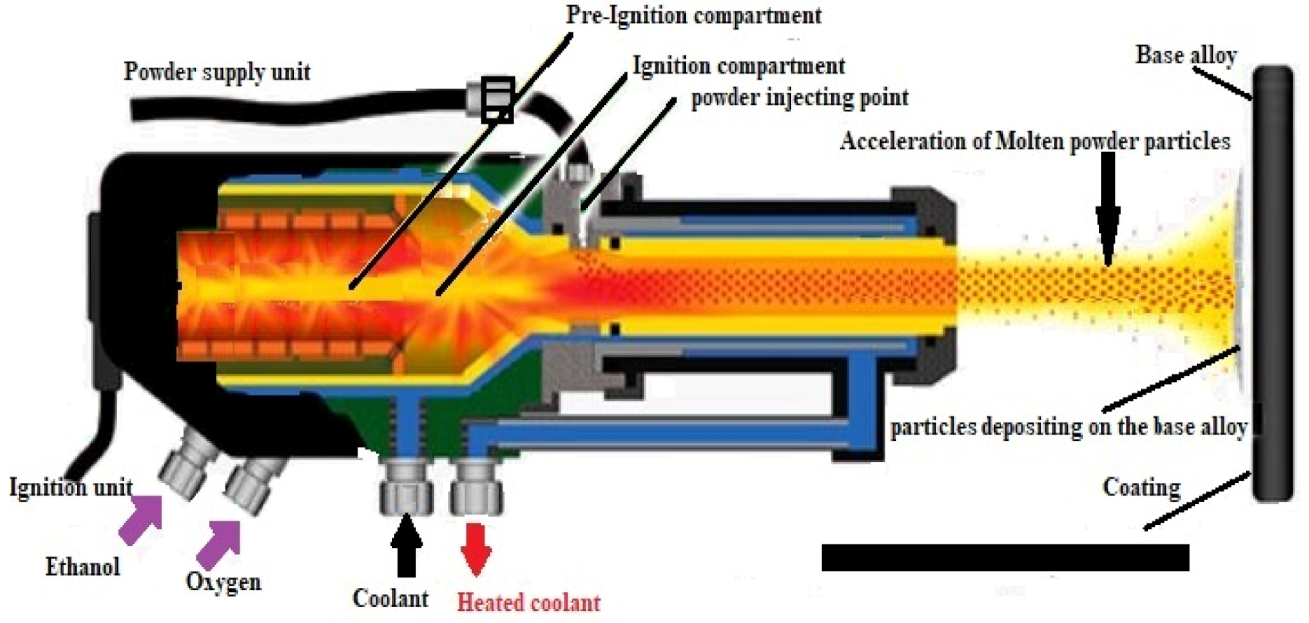
2. Experimental Procedure
2.1. Characterization of Coatings
2.1.1. Bond Strength Testing
2.1.2. Pictorial Examination
2.1.3. Mass Gain Analysis
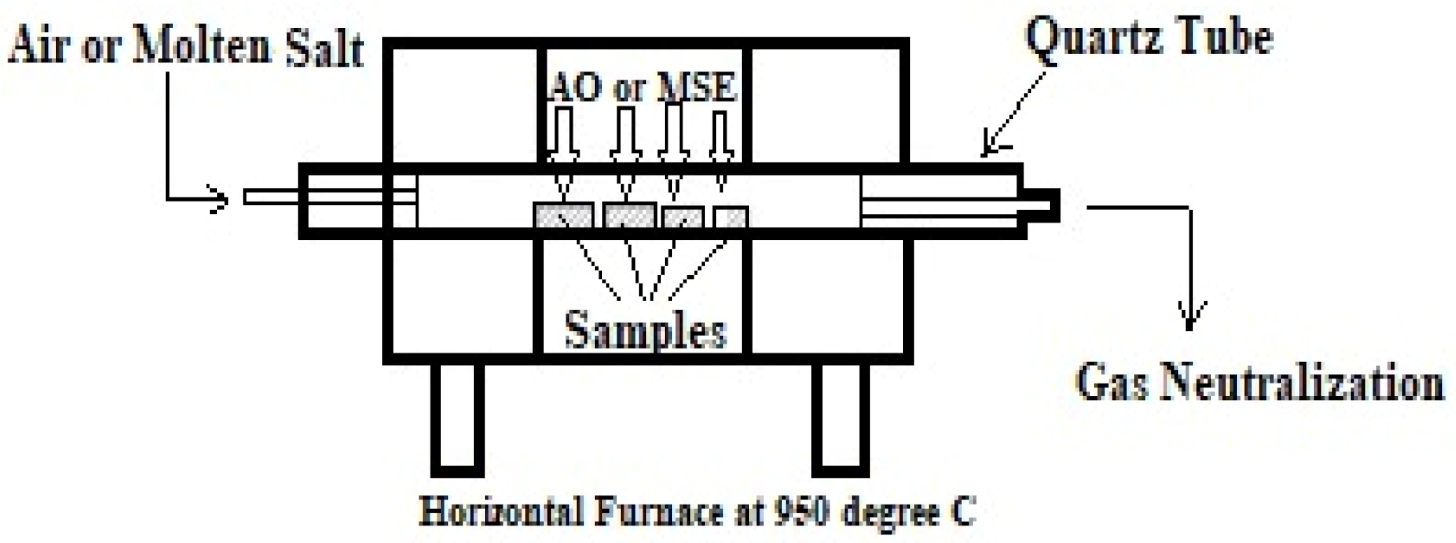
2.1.4. Cyclic High-Temperature Corrosion Test
2.1.5. Micro-Hardness Testing
3. Results and Discussion of Air Plasma Spray Coating
3.1. Microhardness (Hv) of the Base Metal and Depositions (APS and HVOF)
3.2. Adhesion/Bond Strength of the Deposits
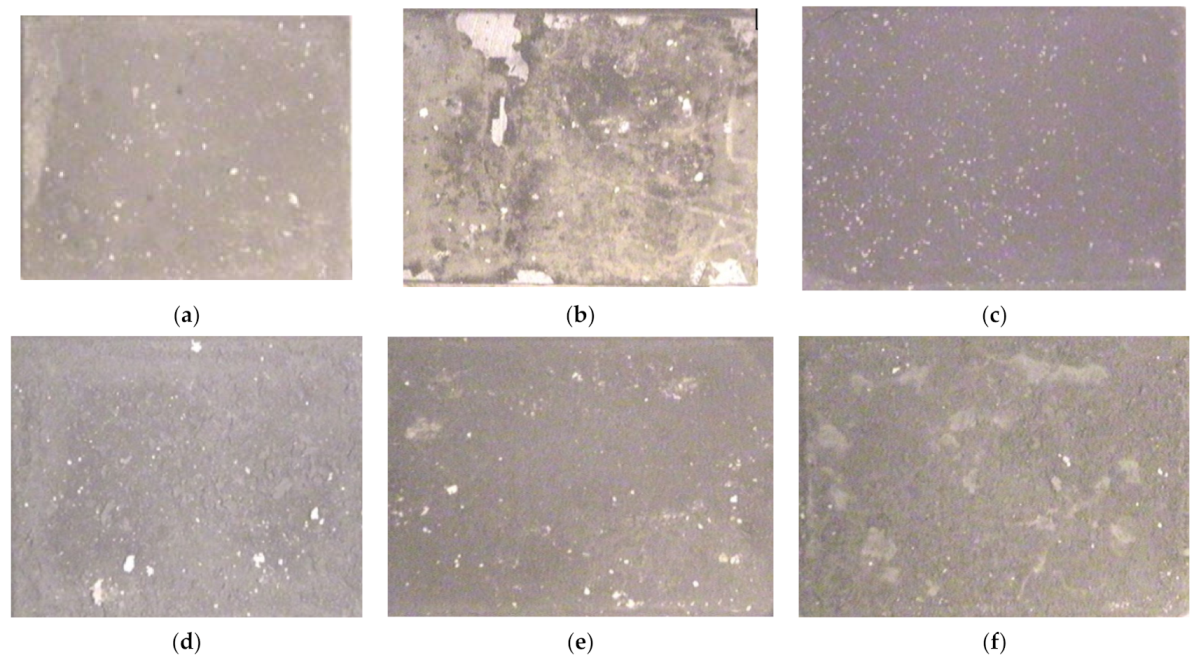
3.3. Pictorial Examination
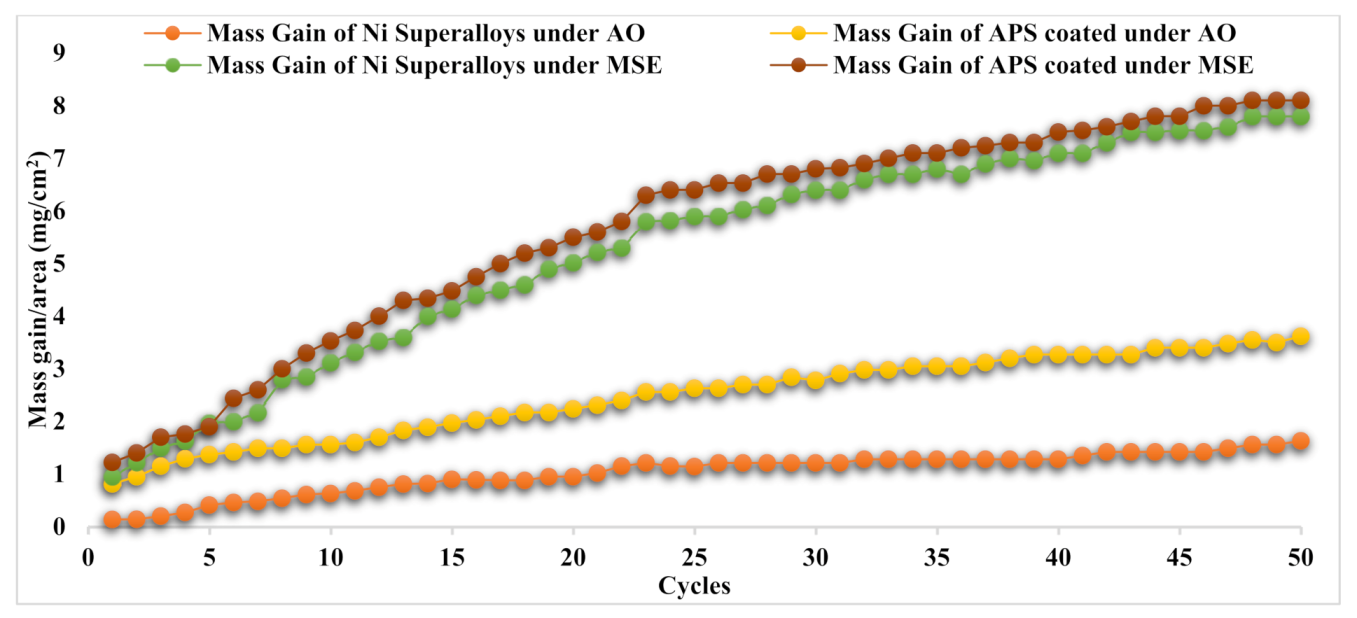
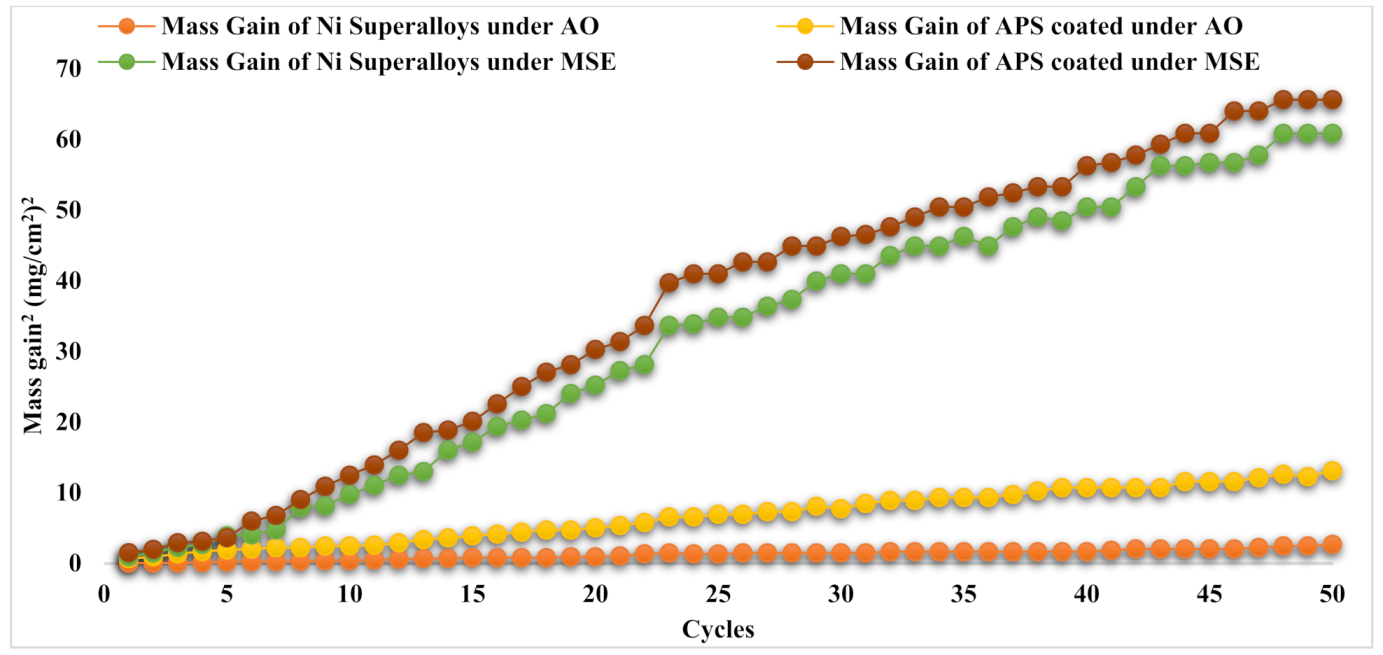
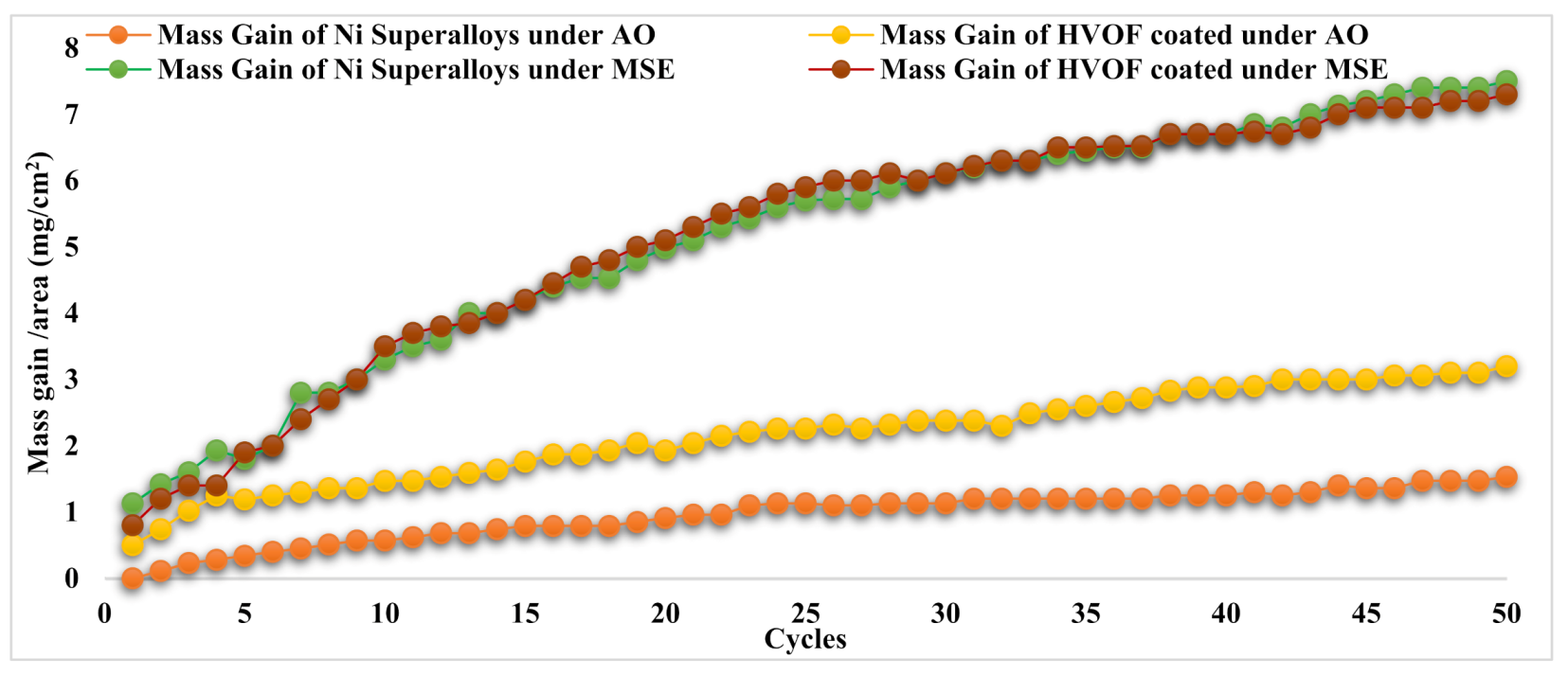
3.4. Oxidation Kinetics
3.5. Discussion of HT Corrosion on APS and HVOF Depositions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirzadi, A.; Jackson, S. Structural Alloys for Power Plants; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780857092380. [Google Scholar]
- Asnavandi, M.; Kahram, M.; Rezaei, M.; Rezakhani, D. Fire-Side Corrosion: A Case Study of Failed Tubes of a Fossil Fuel Boiler. Int. J. Corros. 2017, 2017, 7367046. [Google Scholar] [CrossRef]
- Kamal, S.; Jayaganthan, R.; Prakash, S. Evaluation of cyclic hot corrosion behaviour of detonation gun sprayed Cr3C2-25%NiCr coatings on nickel- and iron-based superalloys. Surf. Coat. Technol. 2009, 203, 1004–1013. [Google Scholar] [CrossRef]
- Eliaz, N.; Shemesh, G.; Latanision, R.M. Hot corrosion in gas turbine components. Eng. Fail. Anal. 2002, 9, 31–43. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, M.; Handa, A. Combating hot corrosion of boiler tubes—A study. Eng. Fail. Anal. 2018, 94, 379–395. [Google Scholar] [CrossRef]
- Hu, H.; Nie, X.; Ma, Y. Corrosion and Surface Treatment of Magnesium Alloys. In Magnesium Alloys—Properties in Solid and Liquid States; InTech: London, UK, 2014. [Google Scholar]
- Singh, A.; Sharma, V.; Mittal, S.; Pandey, G.; Mudgal, D.; Gupta, P. An overview of problems and solutions for components subjected to fireside of boilers. Int. J. Ind. Chem. 2018, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, G.; Banerjee, S.; Raja, V.S.; Joshi, S.V. Hot corrosion behavior of plasma sprayed powder-solution precursor hybrid thermal barrier coatings. Surf. Coat. Technol. 2018, 349, 452–461. [Google Scholar] [CrossRef]
- Cerullo, N.; Lomonaco, G. Corrosion issues in high temperature gas-cooled reactor (HTR) systems. In Nuclear Corrosion Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2012; pp. 731–772. ISBN 9781845697655. [Google Scholar]
- Wang, B.Q. Dependence of erosion–corrosion on carbide/metal matrix proportion for HVOF Cr3C2–NiCr coatings. Surf. Eng. 1998, 14, 165–169. [Google Scholar] [CrossRef]
- Shukla, V.N.; Trivedi, H.; Kumar, H.; Yadav, A. Surface Engineering Analysis of D-Gun Sprayed Cermet Coating in Aggressive Environment. Mater. Today Proc. 2017, 4, 10212–10215. [Google Scholar] [CrossRef]
- Kutz, M. Handbook of Environmental Degradation of Materials; Elsevier Science: Amsterdam, The Netherlands, 2018; ISBN 9780323524728. [Google Scholar]
- Garcia, E.; Lee, H.; Sampath, S. Phase and microstructure evolution in plasma sprayed Yb2Si2O7 coatings. J. Eur. Ceram. Soc. 2019, 39, 1477–1486. [Google Scholar] [CrossRef]
- Liu, X.; He, D.; Zhou, Z.; Wang, G.; Wang, Z.; Guo, X. Effect of post-heat treatment on the microstructure of micro-plasma sprayed hydroxyapatite coatings. Surf. Coat. Technol. 2019, 367, 225–230. [Google Scholar] [CrossRef]
- Zhao, S.Q.; Dong, J.X.; Zhang, M.C.; Xie, X.S. Oxidation behaviors of new Ni-based superalloy at 950 °C and 1000 °C. Rare Met. Mater. Eng. 2005, 34, 208–211. [Google Scholar]
- Yin, B.; Liu, G.; Zhou, H.; Chen, J.; Yan, F. Sliding Wear Behavior of HVOF-sprayed Cr3C2–NiCr/CeO2 Composite Coatings at Elevated Temperature up to 800 °C. Tribol. Lett. 2010, 37, 463–475. [Google Scholar] [CrossRef]
- Hong, H.; Zhao, Y.; Jin, H. Proposed Partial Repowering of a Coal-Fired Power Plant Using Low-Grade Solar Thermal Energy. Int. J. Thermodyn. 2011, 14, 21–28. [Google Scholar]
- Budinski, K.G. Engineering Materials: Properties and Selection; Prentice Hall: Englewood Cliffs, NJ, USA, 1996. [Google Scholar]
- Ghosh, D.; Mitra, S.K. Plasma sprayed Cr3C2–Ni–Cr coating for oxidation protection of 2·25Cr–1Mo steel. Surf. Eng. 2015, 31, 342–348. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, B.; Kim, H.; Lee, C. Isothermal oxidation of air plasma spray NiCrAlY bond coatings. Surf. Coat. Technol. 2002, 150, 297–308. [Google Scholar] [CrossRef]
- Bhatia, R.; Singh, H.; Sidhu, B.S. Hot Corrosion Studies of HVOF-Sprayed Coating on T-91 Boiler Tube Steel at Different Operating Temperatures. J. Mater. Eng. Perform. 2014, 23, 493–505. [Google Scholar] [CrossRef]
- Prakash, S.; Singh, S. Effects of MgO and CaO on hot corrosion of Fe base superalloy Superfer 800H in Na2SO4-60%V2O5 environment. Br. Corros. J. 2002, 37, 56–62. [Google Scholar] [CrossRef]
- Sharma, A.; Kant Verma, D.; Kumaran, S. Effect of post weld heat treatment on microstructure and mechanical properties of Hot Wire GTA welded joints of SA213 T91 steel. Mater. Today Proc. 2018, 5, 8049–8056. [Google Scholar] [CrossRef]
- Somasundaram, B.; Kadoli, R.; Ramesh, M.R. Hot Corrosion Behaviour of HVOF Sprayed (Cr3C2–35% NiCr) + 5% Si Coatings in the Presence of Na2SO4–60% V2O5 at 700 °C. Trans. Indian Inst. Met. 2015, 68, 257–268. [Google Scholar] [CrossRef]
- Bala, N.; Singh, H.; Prakash, S.; Karthikeyan, J. Investigations on the Behavior of HVOF and Cold Sprayed Ni-20Cr Coating on T22 Boiler Steel in Actual Boiler Environment. J. Therm. Spray Technol. 2012, 21, 144–158. [Google Scholar] [CrossRef]
- Hong, S.; Wu, Y.; Li, G.; Wang, B.; Gao, W.; Ying, G. Microstructural characteristics of high-velocity oxygen-fuel (HVOF) sprayed nickel-based alloy coating. J. Alloys Compd. 2013, 581, 398–403. [Google Scholar] [CrossRef]
- Paul, S.; Harvey, M.D.F. Corrosion Testing of Ni Alloy HVOF Coatings in High Temperature Environments for Biomass Applications. J. Therm. Spray Technol. 2013, 22, 316–327. [Google Scholar] [CrossRef]
- Ramesh, M.R.; Prakash, S.; Nath, S.K.; Sapra, P.K.; Krishnamurthy, N. Evaluation of Thermocyclic Oxidation Behavior of HVOF-Sprayed NiCrFeSiB Coatings on Boiler Tube Steels. J. Therm. Spray Technol. 2011, 20, 992–1000. [Google Scholar] [CrossRef]
- Houdková, Š.; Česánek, Z.; Smazalová, E.; Lukáč, F. The High-Temperature Wear and Oxidation Behavior of CrC-Based HVOF Coatings. J. Therm. Spray Technol. 2018, 27, 179–195. [Google Scholar] [CrossRef]
- Yu, B.; Li, Y.; Nie, Y.; Mei, H. High temperature oxidation behavior of a novel cobalt-nickel-base superalloy. J. Alloys Compd. 2018, 765, 1148–1157. [Google Scholar] [CrossRef]
- Kamal, S.; Jayaganthan, R.; Prakash, S. High temperature cyclic oxidation and hot corrosion behaviours of superalloys at 900 °C. Bull. Mater. Sci. 2010, 33, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Singh, H.; Prakash, S. High-temperature behavior of a high-velocity oxy-fuel sprayed Cr3C2-NiCr coating. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2012, 43, 2979–2993. [Google Scholar] [CrossRef]
- Sidhu, T.S.; Prakash, S.; Agrawal, R.D. Hot corrosion studies of HVOF sprayed Cr3C2–NiCr and Ni–20Cr coatings on nickel-based superalloy at 900 °C. Surf. Coat. Technol. 2006, 201, 792–800. [Google Scholar] [CrossRef]
- ASTM C633-13(2017). Standard Test Method for Adhesion or Cohesion Strength of Thermal Spray Coatings; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Yilbas, B.S.; Khalid, M.; Abdul-Aleem, B.J. Corrosion Behavior of HVOF Coated Sheets. J. Therm. Spray Technol. 2003, 12, 572–575. [Google Scholar] [CrossRef]
- ASTM E384-17. Standard Test Method for Microindentation Hardness of Materials; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Hawthorne, H.; Arsenault, B.; Immarigeon, J.; Legoux, J.; Parameswaran, V. Comparison of slurry and dry erosion behaviour of some HVOF thermal sprayed coatings. Wear 1999, 225–229, 825–834. [Google Scholar] [CrossRef]
- Yunus, M.; Alsoufi, M.S. Multi-output optimization of tribological characteristics control factors of thermally sprayed industrial ceramic coatings using hybrid Taguchi-grey relation analysis. Friction 2016, 4, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Fontana, M.G. High-Temperature Corrosion (Corrosion Engineering), 3rd ed.; Tata McGraw-Hill Book Company: New York, NY, USA, 1987. [Google Scholar]
- Sreenivas, K.S.; Radhakrishnan, V.M. Oxidation and hot corrosion behaviour of Nimonic-75 superalloy. Indian J. Eng. Mater. Sci. 1998, 5, 295–301. [Google Scholar]
- Fryburg, G.C.; Kohl, F.J.; Stearns, C.A.; Fielder, W.L. Chemical Reactions Involved in the Initiation of Hot Corrosion of IN-738. J. Electrochem. Soc. 1984, 131, 2985. [Google Scholar] [CrossRef]
- Crawmer, D.C. Coating development for HVOF process using design of experiments. In Proceedings of the 13th International Thermal Spray Conference, Orlando, FL, USA, 25 May–5 June 1992. [Google Scholar]




| Base Metal and Coating Powder | Chemical Composition (Wt %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Cr | C | Fe | Mo | Co | Nb | Al | Ti | Mn | Si | |
| Alloy 80A base metal | Bal. | 19.33 | 0.074 | 0.3 | - | 0.2 | Nil | 1.43 | 2.38 | 0.6 | 0.1 |
| Cr3C2-25NiCr | 20.60 | Bal. | 9.6 | 0.15 | - | - | - | - | - | - | - |
| Process Parameter | APS | HVOF |
|---|---|---|
| Argon (Ar) flow rate (lpm) | 55 | - |
| Hydrogen (H2) flow rate (lpm) | 12 | - |
| Argon carrier flow rate (lpm) | 3.2 | 8 |
| O2 flow rate (lpm) | - | 840 |
| Jet fuel flow rate (lpm) | - | 20 |
| Ar, H2 and Ar carrier pressure (MPa) | 0.5,0.5 & 0.5 | - |
| O2, fuel and Ar carrier pressure (MPa) | - | 1, 0.7 & 0.25 |
| Powder inputting rate (gm/s) | 0.66 | 0.58 |
| Current (Amps) | 600 | - |
| Voltage (Volts) | 80 | - |
| Deposition angle | 90° | 90° |
| Stand-off distance (mm) | 130 | 365 |
| Specimen | Whole MG (mg/cm2) | KP (MG/Area)2/Cycle, g2/cm4/s1 |
|---|---|---|
| Undeposited Ni superalloy under AO | 1.46 | 1.08 × 10−6 |
| Undeposited Ni superalloy under MSE | 7.46 | 2.78 × 10−5 |
| APS-coated Ni superalloy under AO | 3.35 | 5.03 × 10−6 |
| APS-coated Ni superalloy under MSE | 7.57 | 2.62 × 10−5 |
| HVOF-coated Ni superalloy under AO | 3.09 | 4.36 × 10−6 |
| HVOF-coated Ni superalloy under MSE | 7.30 | 2.5 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnaser, I.A.; Yunus, M.; Alfattani, R.; Alamro, T. High-Temperature Corrosion of APS- and HVOF-Coated Nickel-Based Super Alloy under Air Oxidation and Melted Salt Domains. Materials 2021, 14, 5119. https://doi.org/10.3390/ma14185119
Alnaser IA, Yunus M, Alfattani R, Alamro T. High-Temperature Corrosion of APS- and HVOF-Coated Nickel-Based Super Alloy under Air Oxidation and Melted Salt Domains. Materials. 2021; 14(18):5119. https://doi.org/10.3390/ma14185119
Chicago/Turabian StyleAlnaser, Ibrahim A., Mohammed Yunus, Rami Alfattani, and Turki Alamro. 2021. "High-Temperature Corrosion of APS- and HVOF-Coated Nickel-Based Super Alloy under Air Oxidation and Melted Salt Domains" Materials 14, no. 18: 5119. https://doi.org/10.3390/ma14185119
APA StyleAlnaser, I. A., Yunus, M., Alfattani, R., & Alamro, T. (2021). High-Temperature Corrosion of APS- and HVOF-Coated Nickel-Based Super Alloy under Air Oxidation and Melted Salt Domains. Materials, 14(18), 5119. https://doi.org/10.3390/ma14185119






