Salt Heat Treatment and Passivation to Improve the Corrosion Resistance of Nitinol (Ni-Ti)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
2.2. Experimental Consideration
- Factors such as shape or size that can affect surface finish, such as proper grinding of areas of high curvature, were considered.
- Test reports for official corrosion potential tests were conducted in accordance with ASTM F2129. For example, the test report included corrosion/static potential, breakdown potential, and polarization curves and reviewed any deviations from the ASTM F2129 standard (e.g., ASTM G5: Standard Reference Test for Potential Dynamic Bipolar Polarization Measurements) test setups that did not meet the criteria described in Methods.
- Results were evaluated against acceptance criteria, and acceptance criteria for formal corrosion testing applied legally marketed materials with a clinically good history of use (i.e., no history of corrosion-related fractures or adverse events related to nickel release).
- Alternatively, although data linking in vitro corrosion tests directly to in vivo corrosion results are lacking, conservative guidelines have been used to establish acceptance criteria.
2.3. Heat Treatment
2.4. Scratch Experiment
2.5. Corrosion Testing
2.6. Acceptance Criteria
3. Results
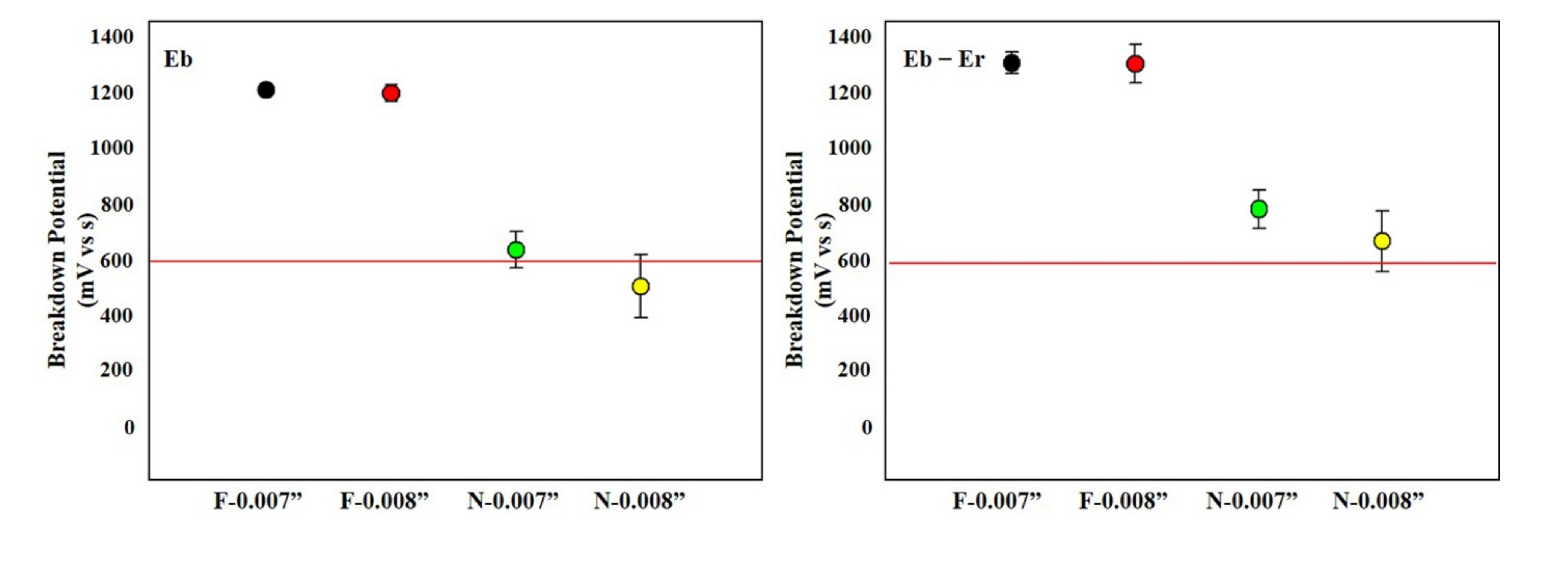
3.1. Cyclic Potentiodynamic Test
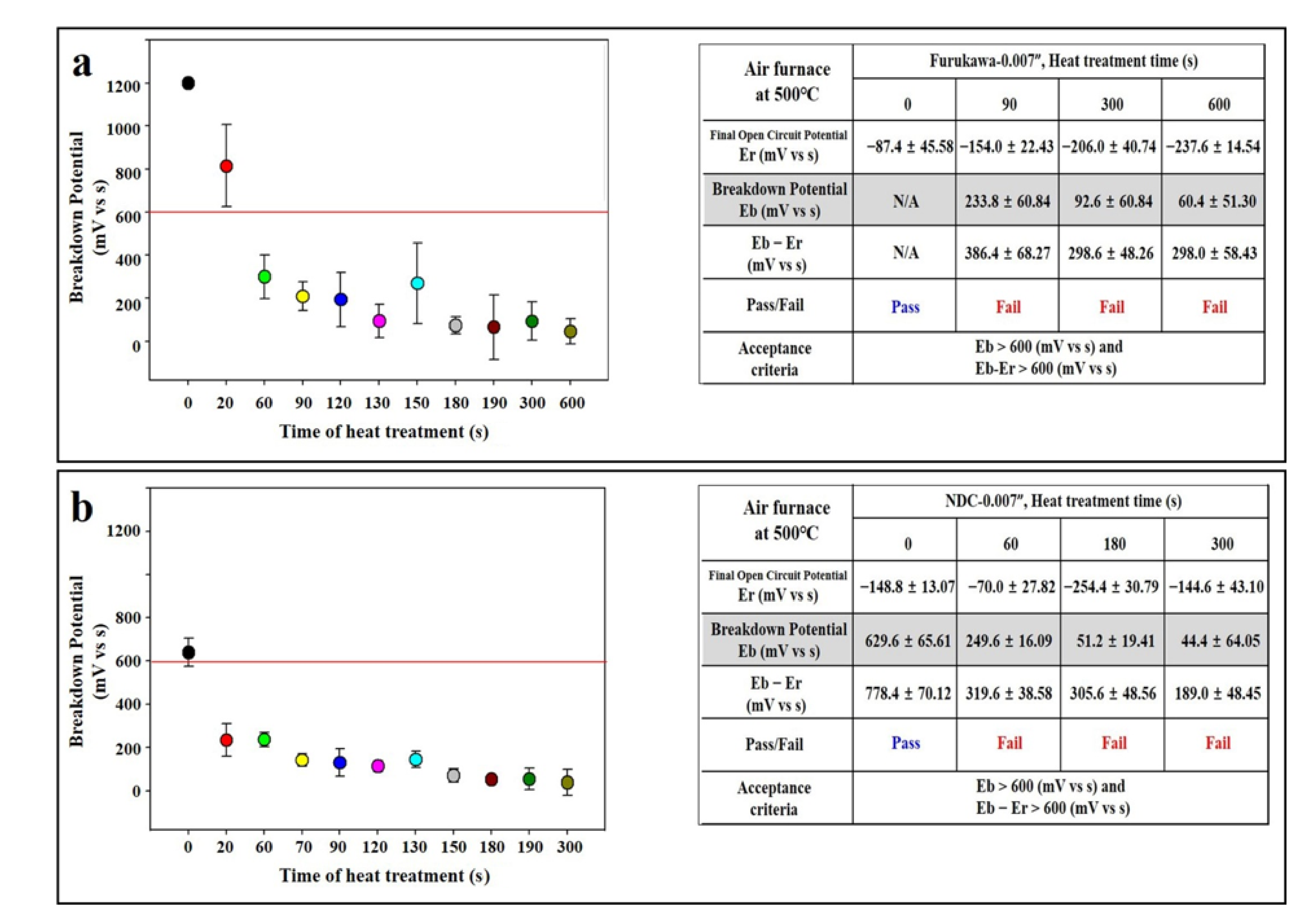
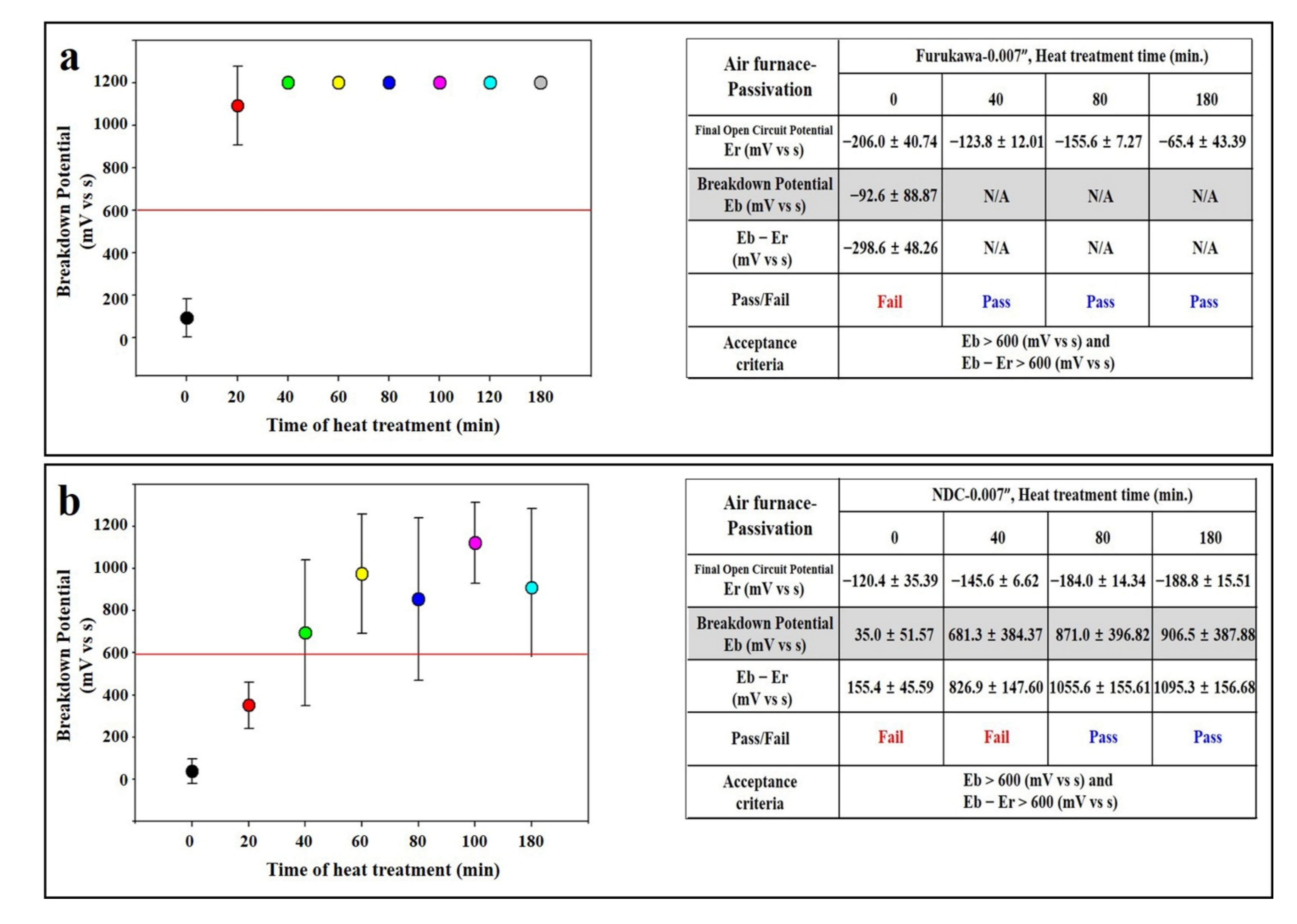
3.2. Heat Treatment of Air Furnace
3.3. Heat Treatment of Salt Bath
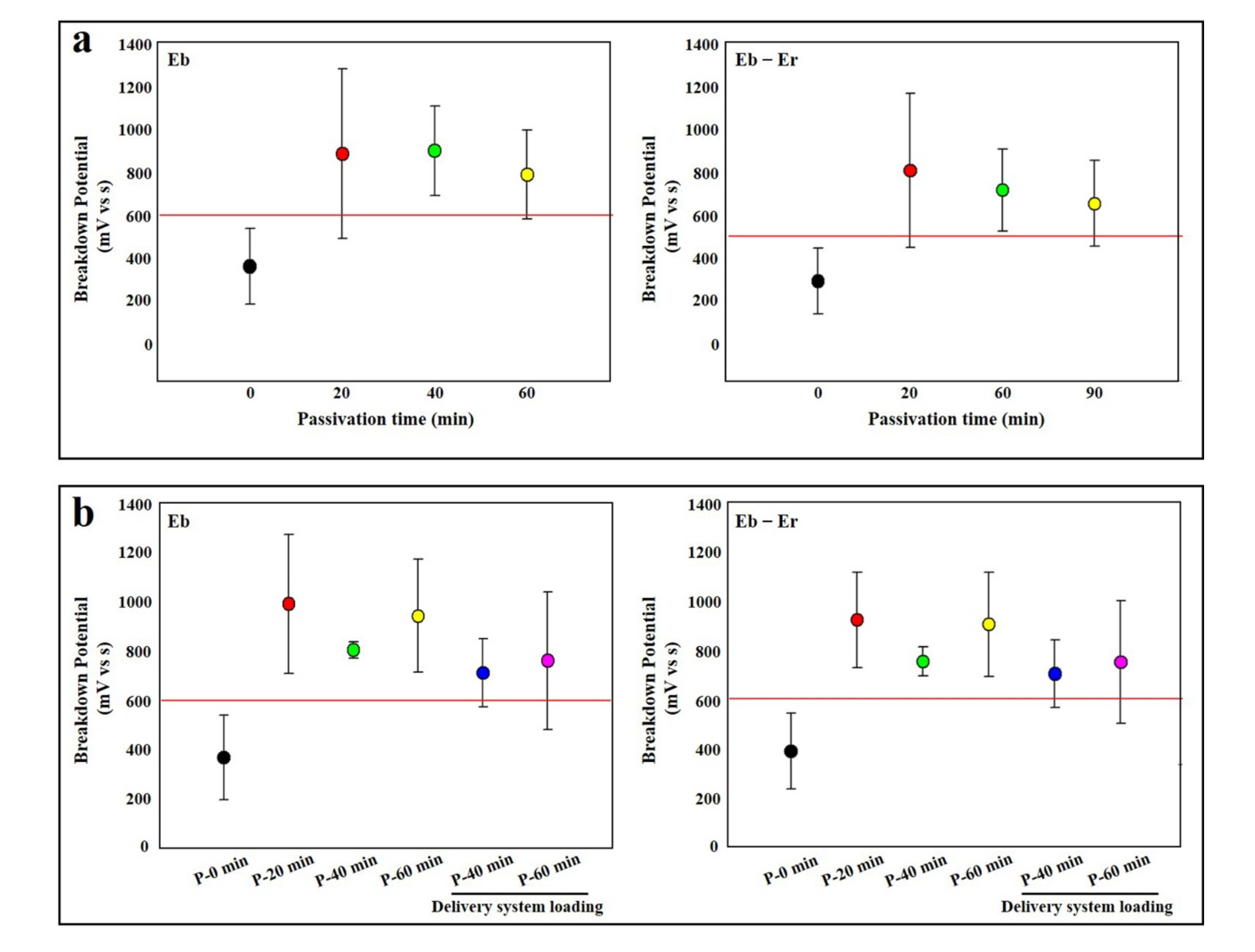
3.4. Passivation Experiment of Nitinol Wire
3.5. Passivation Experiment of Stent
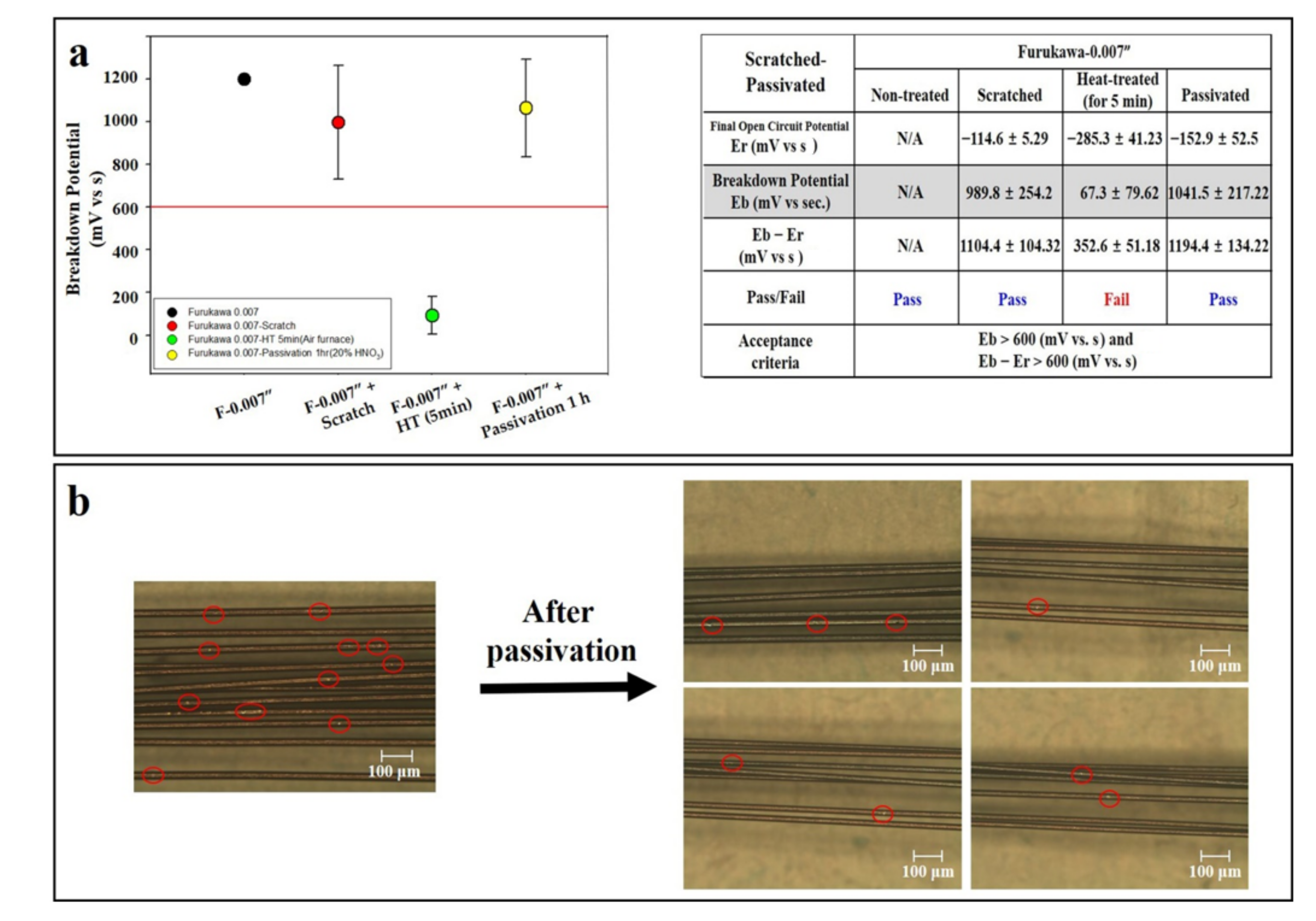
3.6. Scratch Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duerig, T.W.; Pelton, D.W.; Stockel, D. The utility of superelasticity in medicine. Biomed. Mater. Eng. 1996, 6, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Inbar, O.; Amsalem, Y. Cerebral nitinol stenting in progressive stroke and in crescendo TIAs. J. Neurol. Surg. A. Cent. Eur. Neurosur. 2015, 76, 499–507. [Google Scholar]
- Greenbaum, A.B.; Khan, J.M.; Rogers, T.; Babaliaros, V.C.; Eng, M.H.K.; Wang, D.D.; Parone, G.; Lederman, R.J. First-in-human transcatheter pledget-assisted suture tricuspid annuloplasty for severe tricuspid insufficiency. Catheter. Cardiovasc. Interv. 2021, 97, E130–E134. [Google Scholar] [CrossRef] [PubMed]
- Scali, M.; Breedveld, P.; Dodou, D. Experimental evaluation of a self-propelling bio-inspired needle in single- and multi-layered phantoms. Sci. Rep. 2019, 9, 19988. [Google Scholar] [CrossRef]
- Lombardo, L.; Toni, G.; Mazzanti, V.; Mollica, F.; Spedicato, G.A.; Siciliani, G. The mechanical behavior of as received and retrieved nickel titanium orthodontic archwires. Prog. Orthod. 2019, 20, 1. [Google Scholar] [CrossRef] [Green Version]
- Kuntz, M.L.; Vadori, R.; Khan, M.I. Review of Superelastic Differential Force Archwires for Producing Ideal Orthodontic Forces: An Advanced Technology Potentially Applicable to Orthognathic Surgery and Orthopedics. Curr. Osteoporos Rep. 2018, 16, 380–386. [Google Scholar] [CrossRef]
- Senkutvan, R.S.; Jacob, S.; Charles, A.; Vadgaonkar, V.; Tekade, S.J.; Gangurde, P. Evaluation of nickel ion release from various orthodontic arch wires: An in vitro study. J. Int. Soc. Prev. Community Dent. 2014, 4, 12–16. [Google Scholar]
- Petoumenou, E.; Arndt, M.; Keilig, L.; Reimann, S.; Hoederath, H.; Eliades, T.; Jager, A.; Bourauel, C. Nickel concentration in the saliva of patients with nickel-titanium orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 59–65. [Google Scholar] [CrossRef]
- Chakravarthi, S.; Padmanabhan, S.; Chitharanjan, A.B. Allergy and orthodontics. J. Orthod. Sci. 2012, 1, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Janas, A.; Milewski, K.; Buszman, P.P.; Nowakowski, P.; Jelonek, M.; Orlik, B.; Krauze, A.; Samborski, S.; Beaudry, D.; Lecelrc, G.; et al. Safety and biocompatibility of a novel self-expanding nitinol carotid stent with hybrid cell design in a porcine model of neointimal hyperplasia. Kardiol. Pol. 2015, 73, 240–245. [Google Scholar] [CrossRef]
- Chang, S.H.; Lin, P.T.; Tsai, C.W. High-temperature martensitic transformation of CuNiHfTiZr high- entropy alloys. Sci. Rep. 2019, 9, 19598. [Google Scholar] [CrossRef]
- Taylor, S.L.; Ibeh, A.J.; Jakus, A.E.; Shah, R.N.; Dunand, D.C. NiTi-Nb micro-trusses fabricated via extrusion-based 3D-printing of powders and transient-liquid-phase sintering. Acta. Biomater. 2018, 76, 359–370. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, S.; Zhang, Y. Phase Transformation, Twinning, and Detwinning of NiTi Shape-Memory Alloy Subject to a Shock Wave Based on Molecular-Dynamics Simulation. Materials 2018, 11, 2334. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Xu, Y.; He, X.; Li, C.; Zhu, M. Progress in biocompatibility and surface modification of nickel titanium shape memory alloys. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2018, 32, 1091–1095. [Google Scholar]
- Lee, J.I.; Tsuchiya, K.; Tasaki, W.; Oh, H.S.; Sawaguchi, T.; Murakami, H.; Hiroto, T.; Matsushita, Y.; Park, E.S. A strategy of designing high-entropy alloys with high-temperature shape memory effect. Sci. Rep. 2019, 9, 13140. [Google Scholar] [CrossRef]
- Mahtabi, M.J.; Shamsaei, N.; Mitchell, M.R. Fatigue of Nitinol: The state-of-the-art and ongoing challenges. J. Mech. Behav. Biomed. Mater. 2015, 50, 228–254. [Google Scholar] [CrossRef]
- Cattaneo, G.; Brauner, C.; Siekmeyer, G.; Ding, A.; Bauer, S.; Wohlschlogel, M.; Lang, L.; Hierlemann, T.; Akimov, M.; Schlensak, C.; et al. In vitro investigation of chemical properties and biocompatibility of neurovascular braided implants. J. Mater. Sci. Mater. Med. 2019, 30, 67. [Google Scholar] [CrossRef]
- Taheri Andani, M.; Saedi, S.; Turabi, A.S.; Karamooz, M.R.; Haberland, C.; Karaca, H.E.; Elahinia, M. Mechanical and shape memory properties of porous Ni50.1Ti49.9 alloys manufactured by selective laser melting. J. Mech. Behav. Biomed. Mater. 2017, 68, 224–231. [Google Scholar] [CrossRef]
- Cisse, O.; Savadogo, O.; Wu, M.; Yahia, L. Effect of surface treatment of NiTi alloy on its corrosion behavior in Hanks’ solution. J. Biomed. Mater. Res. 2002, 61, 339–345. [Google Scholar] [CrossRef]
- Shabalovskaya, S.A.; Anderegg, J.W.; Undisz, A.; Rettenmayr, M.; Rondelli, G.C. Corrosion resistance, chemistry, and mechanical aspects of Nitinol surfaces formed in hydrogen peroxide solutions. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1490–1499. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- ASTM International. Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices; ASTM Standard F2129-04; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar]
- ASTM International. Standard Test Method for Pitting and Crevice Corrosion of Metallic Surgical Implant Materials; ASTM Standard F746-87; (Reapproved 1999); ASTM International: West Conshohocken, PA, USA, 1987. [Google Scholar]
- Corbett, R.A. Laboratroy corrosion testing of medical implants. In In Proceedings of the Materials and Processes for Medical Devices Conference, St. Paul, MN, USA, 25–27 August 2004; Shrivastave, S., Ed.; ASM International: Materials Park, OH, USA, 2004; pp. 166–171. [Google Scholar]
- Rosenbloom, S.N.; Corbett, R.A. An Assessment of ASTM F 2129 Electrochemical Testing of Small Medical Implants-Lessons Learned. In Proceedings of the CORROSION 2007, Houston, TX, USA, 11–15 March 2007. [Google Scholar]
- ASTM International. Standard Test Method for conducting cyclic potentiodynamic polarization measurements to determine the corrosion susceptibility of small implant devices. In Annualbook of ASTM Standards; ASTM F2129-01; ASTM International: Conshohocken, PA, USA, 2003. [Google Scholar]
- Chaudhari, R.; Vora, J.J.; Patel, V.; Lopez de Lacalle, I.N.; Parikh, D.M. Surface analysis of wire electrical discharge machining processed shape-momory alloys. Materials 2020, 13, 530. [Google Scholar] [CrossRef] [Green Version]
- Haider, W.; Munroe, N.; Tek, V.; Pulletikurthi, C.; Gill, P.K.; Pandya, S. Surface modifications of nitinol. J. Long Term. Eff. Med. Implant 2009, 19, 113–122. [Google Scholar] [CrossRef]
- Shabalovskaya, S.; Anderegg, J.; Van Humbeeck, J. Critical overview of Nitinol surfaces and their modifications for medical applications. Acta Biomater. 2008, 4, 447–467. [Google Scholar] [CrossRef]
- Bates, M.C.; Campbell, J.R.; Campbell, J.E. Late complication of stent fragmentation related to the “lever-arm effect”. J. Endovasc. Ther. 2008, 15, 224–230. [Google Scholar] [CrossRef]
- Sukuroglu, E.E.; Sukuroglu, S.; Akar, K.; Totik, Y.; Efeoglu, I.; Arslan, E. The effect of TiO2 coating on biological NiTi alloys after micro-arc oxidation treatment for corrosion resistance. Proc. Inst. Mech. Eng. H. 2017, 231, 699–704. [Google Scholar] [CrossRef]
- Haider, W.; Munroe, N. Assessment of corrosion resistance and metal ion leaching of nitinol alloys. J. Mater. Eng. Perfor. 2011, 20, 812–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, I.; Kim, B.-H.; Kim, D.-G.; Sohn, I.-B.; Yang, S.-W. Salt Heat Treatment and Passivation to Improve the Corrosion Resistance of Nitinol (Ni-Ti). Materials 2021, 14, 7789. https://doi.org/10.3390/ma14247789
Bae I, Kim B-H, Kim D-G, Sohn I-B, Yang S-W. Salt Heat Treatment and Passivation to Improve the Corrosion Resistance of Nitinol (Ni-Ti). Materials. 2021; 14(24):7789. https://doi.org/10.3390/ma14247789
Chicago/Turabian StyleBae, Inho, Byung-Hoon Kim, Dong-Gon Kim, Ik-Bu Sohn, and Seong-Won Yang. 2021. "Salt Heat Treatment and Passivation to Improve the Corrosion Resistance of Nitinol (Ni-Ti)" Materials 14, no. 24: 7789. https://doi.org/10.3390/ma14247789
APA StyleBae, I., Kim, B.-H., Kim, D.-G., Sohn, I.-B., & Yang, S.-W. (2021). Salt Heat Treatment and Passivation to Improve the Corrosion Resistance of Nitinol (Ni-Ti). Materials, 14(24), 7789. https://doi.org/10.3390/ma14247789




