Metal-Formate Framework Stiffening and Its Relevance to Phase Transition Mechanism
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. X-ray Diffraction
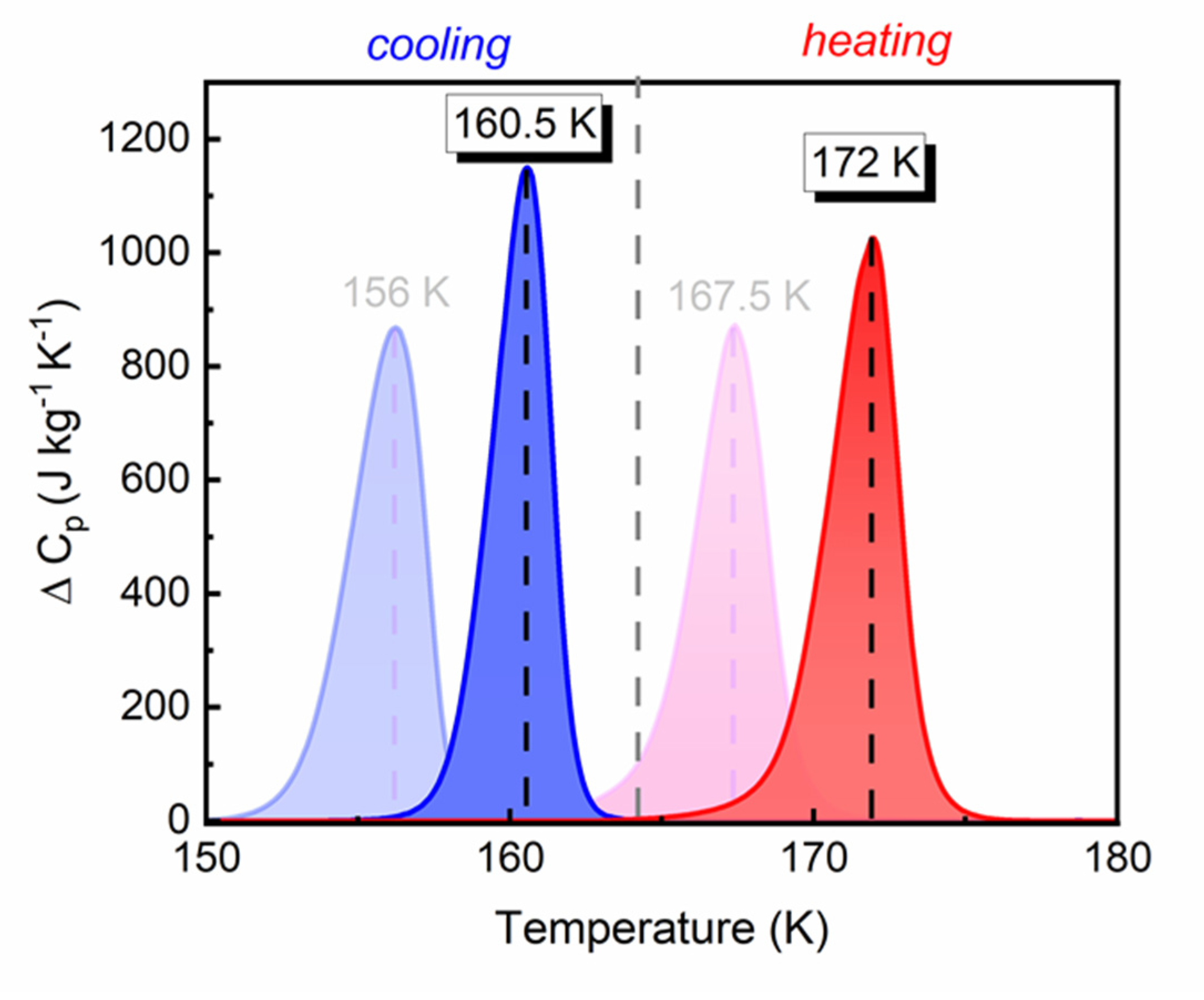
3.2. Thermal Properties
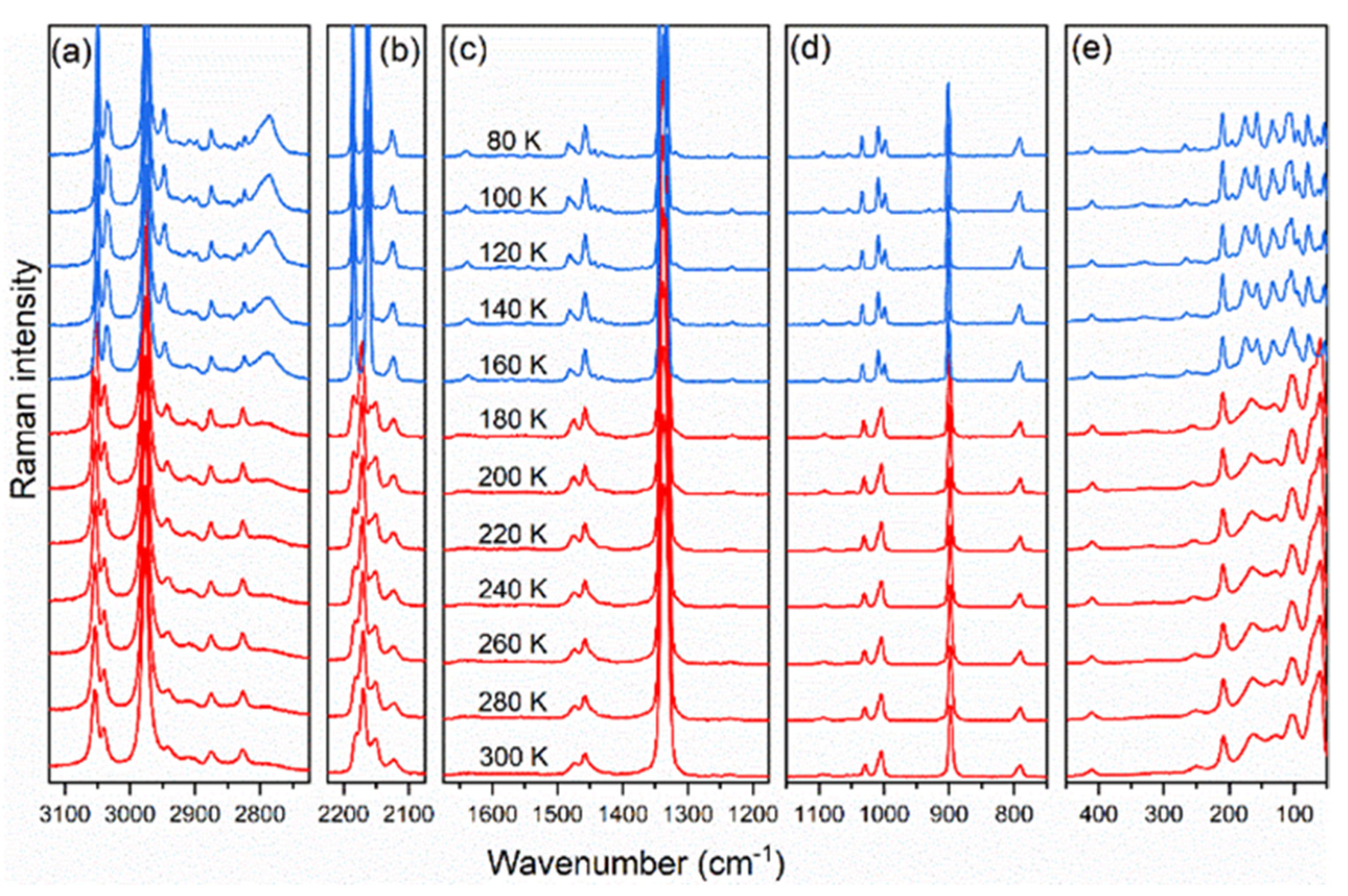
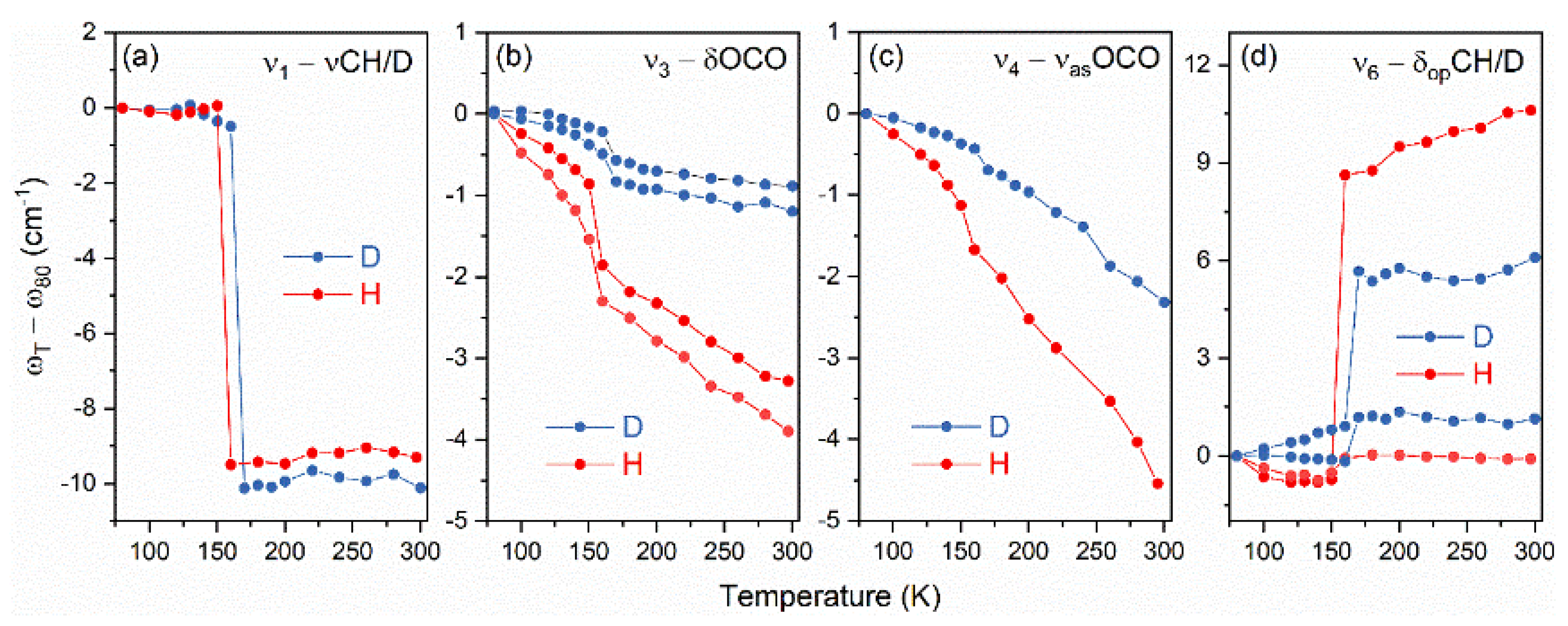
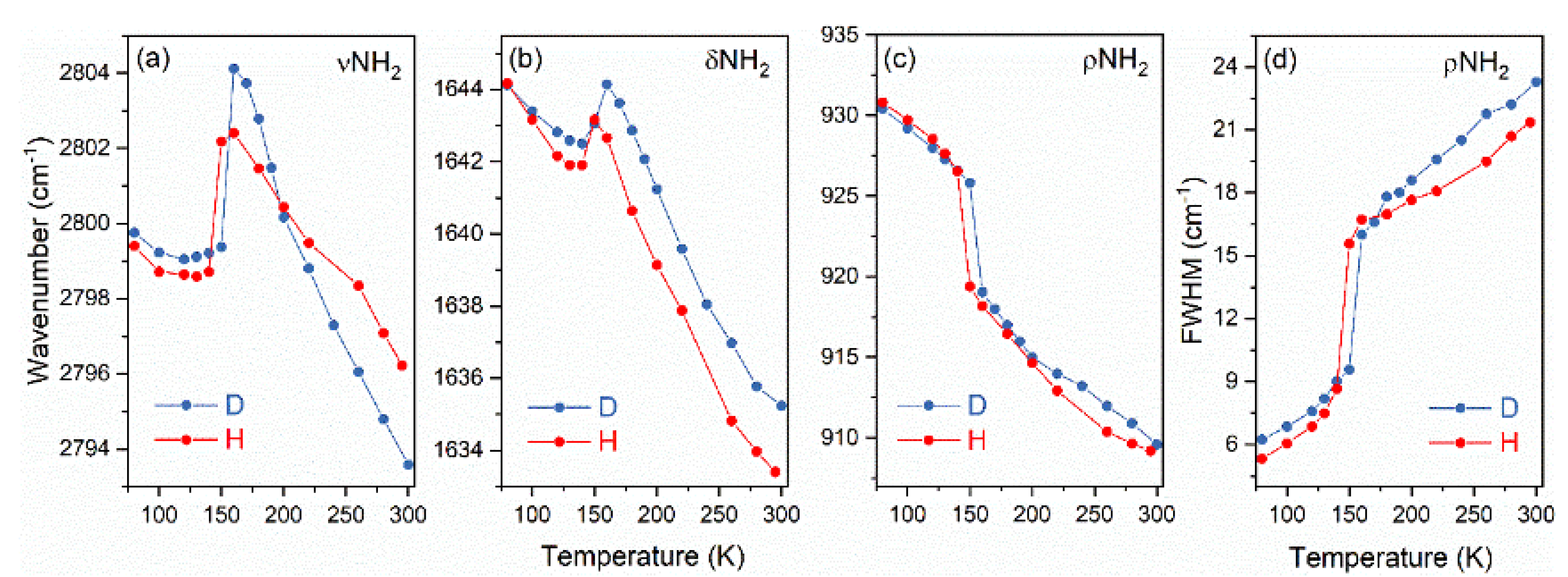
3.3. Raman and IR Spectroscopies
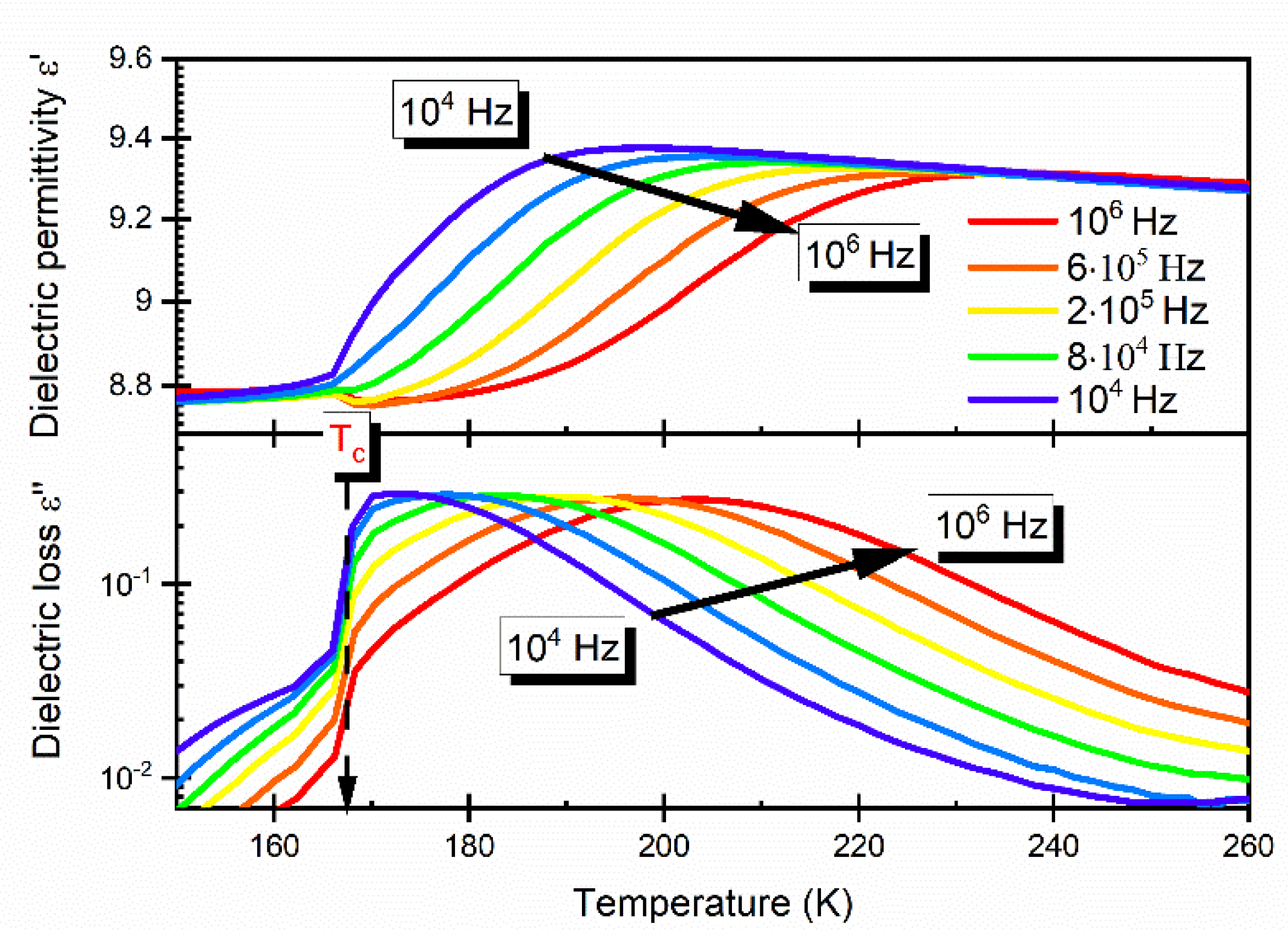
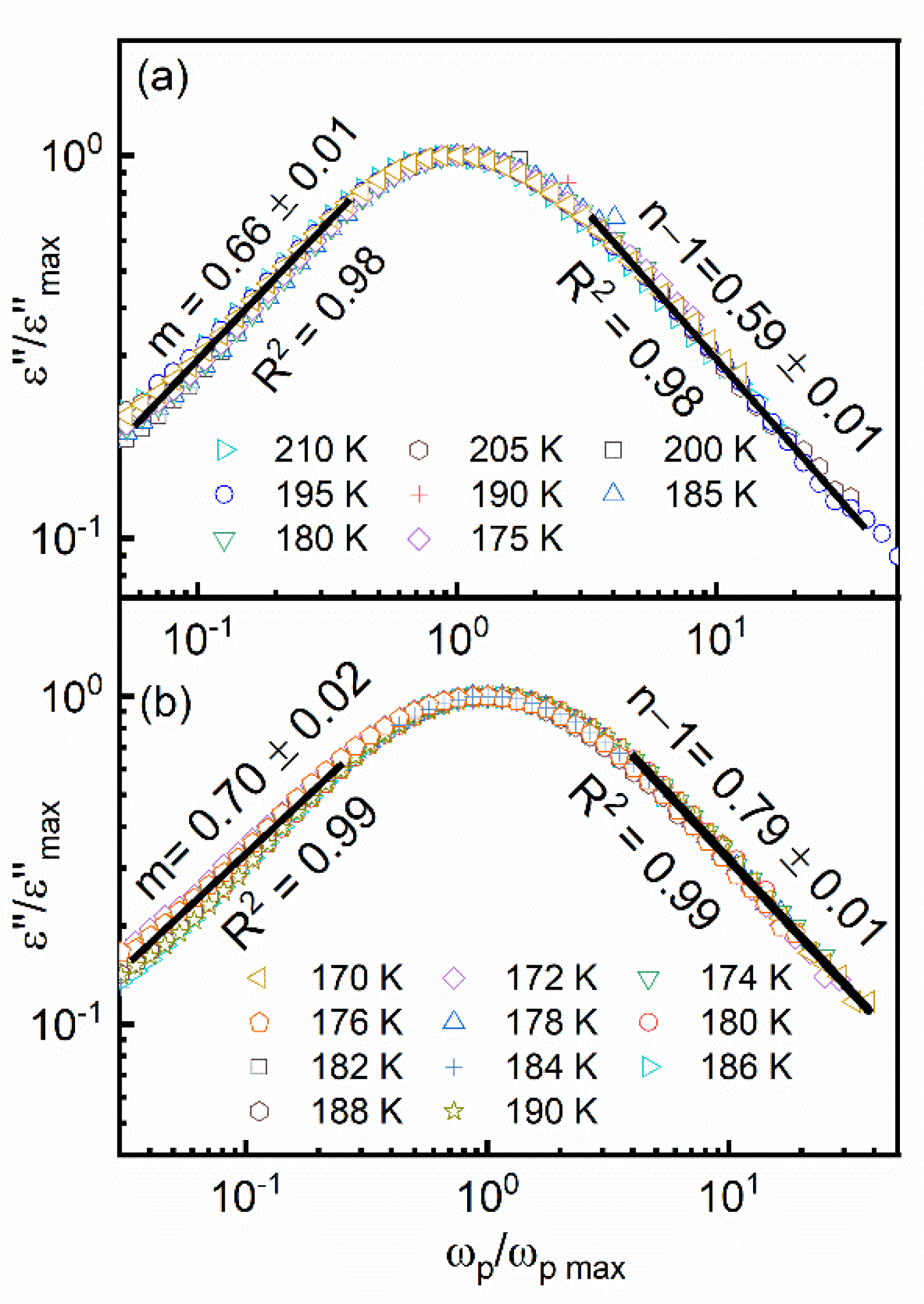
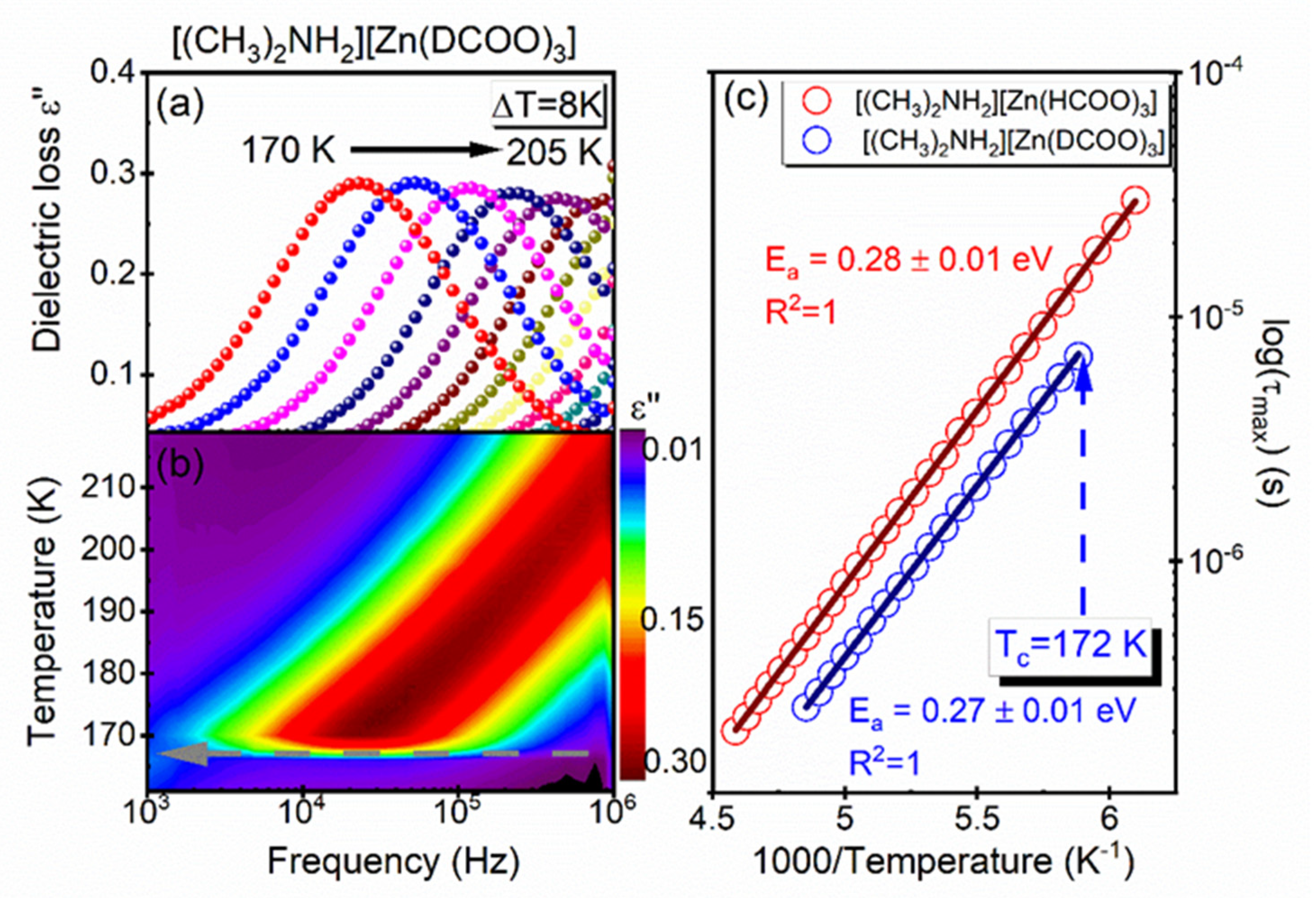
3.4. Dielectric Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ptak, M.; Sieradzki, A.; Šimėnas, M.; Maczka, M. Molecular spectroscopy of hybrid organic–inorganic perovskites and related compounds. Coord. Chem. Rev. 2021, 448, 214180. [Google Scholar] [CrossRef]
- Keskin, S.; Kizilel, S. Biomedical Applications of Metal Organic Frameworks. Ind. Eng. Chem. Res. 2011, 50, 1799–1812. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Asaji, T.; Yoshitake, S.; Ito, Y.; Fujimori, H. Phase transition and cationic motion in the perovskite formate framework [(CH3)2NH2][Mg(HCOO)3]. J. Mol. Struct. 2014, 1076, 719–723. [Google Scholar] [CrossRef]
- Besara, T.; Jain, P.; Dalal, N.S.; Kuhns, P.L.; Reyes, A.P.; Kroto, H.W.; Cheetham, A.K. Mechanism of the order–disorder phase transition, and glassy behavior in the metal-organic framework [(CH3)2NH2]Zn(HCOO)3. Proc. Natl. Acad. Sci. USA 2011, 108, 6828–6832. [Google Scholar] [CrossRef]
- Clune, A.; Harms, N.; O’Neal, K.R.; Hughey, K.; Smith, K.A.; Obeysekera, D.; Haddock, J.; Dalal, N.S.; Yang, J.; Liu, Z.; et al. Developing the Pressure-Temperature-Magnetic Field Phase Diagram of Multiferroic [(CH3)2NH2]Mn(HCOO)3. Inorg. Chem. 2020, 59, 10083–10090. [Google Scholar] [CrossRef] [PubMed]
- Hughey, K.D.; Clune, A.J.; Yokosuk, M.O.; Li, J.; Abhyankar, N.; Ding, X.; Dalal, N.S.; Xiang, H.; Smirnov, D.; Singleton, J.; et al. Structure-Property Relations in Multiferroic [(CH3)2NH2] M(HCOO)3 (M = Mn, Co, Ni). Inorg. Chem. 2018, 57, 11569–11577. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Ramachandran, V.; Clark, R.J.; Hai, D.Z.; Toby, B.H.; Dalal, N.S.; Kroto, H.W.; Cheetham, A.K. Multiferroic behavior associated with an order-disorder hydrogen bonding transition in metal-organic frameworks (MOFs) with the perovskite ABX 3 architecture. J. Am. Chem. Soc. 2009, 131, 13625–13627. [Google Scholar] [CrossRef] [PubMed]
- Mączka, M.; Gągor, A.; Hermanowicz, K.; Sieradzki, A.; MacAlik, L.; Pikul, A. Structural, magnetic and phonon properties of Cr(III)-doped perovskite metal formate framework [(CH3)2NH2][Mn(HCOO)3]. J. Solid State Chem. 2016, 237, 150–158. [Google Scholar] [CrossRef]
- Mączka, M.; Almeida da Silva, T.; Paraguassu, W.; Pereira Da Silva, K. Raman scattering studies of pressure-induced phase transitions in perovskite formates [(CH3)2NH2][Mg(HCOO)3] and [(CH3)2NH2][Cd(HCOO)3]. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 156, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Mączka, M.; Gągor, A.; Macalik, B.; Pikul, A.; Ptak, M.; Hanuza, J. Order–Disorder Transition and Weak Ferromagnetism in the Perovskite Metal Formate Frameworks of [(CH3)2NH2][M(HCOO)3] and [(CH3)2ND2][M(HCOO)3] (M = Ni, Mn). Inorg. Chem. 2014, 53, 457–467. [Google Scholar] [CrossRef]
- Ramakrishna, S.K.; Kundu, K.; Bindra, J.K.; Locicero, S.A.; Talham, D.R.; Reyes, A.P.; Fu, R.; Dalal, N.S. Probing the Dielectric Transition and Molecular Dynamics in the Metal–Organic Framework [(CH3)2NH2]Mg(HCOO)3 Using High Resolution NMR. J. Phys. Chem. C 2021, 125, 3441–3450. [Google Scholar] [CrossRef]
- Sánchez-Andújar, M.; Gómez-Aguirre, L.C.; Pato Doldán, B.; Yáñez-Vilar, S.; Artiaga, R.; Llamas-Saiz, A.L.; Manna, R.S.; Schnelle, F.; Lang, M.; Ritter, F.; et al. First-order structural transition in the multiferroic perovskite-like formate [(CH3)2NH2][Mn(HCOO)3]. CrystEngComm 2014, 16, 3558–3566. [Google Scholar] [CrossRef]
- Sieradzki, A.; Pawlus, S.; Tripathy, S.N.; Gagor, A.; Ciupa, A.; Maczka, M.; Paluch, M. Dielectric relaxation behavior in antiferroelectric metal organic framework [(CH3)2NH2][FeIIIFeII(HCOO)6] single crystals. Phys. Chem. Chem. Phys. 2016, 18, 8462–8467. [Google Scholar] [CrossRef][Green Version]
- Szymborska-Małek, K.; Trzebiatowska-Gusowska, M.; Maczka, M.; Gagor, A. Temperature-dependent IR and Raman studies of metal-organic frameworks [(CH3)2NH2][M(HCOO)3], M = Mg and Cd. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 159, 35–41. [Google Scholar] [CrossRef]
- Śanchez-Andujar, M.; Presedo, S.; Ýã Nez-Vilar, S.; Castro-García, S.; Shamir, J.; Senaris-Rodriguez, M.A. Characterization of the order-disorder dielectric transition in the hybrid organic-inorganic perovskite-like formate Mn(HCOO)3[(CH3)2NH2]. Inorg. Chem. 2010, 49, 1510–1516. [Google Scholar] [CrossRef]
- Trzebiatowska, M.; Mączka, M.; Ptak, M.; Giriunas, L.; Balciunas, S.; Simenas, M.; Klose, D.; Banys, J. Spectroscopic Study of Structural Phase Transition and Dynamic Effects in a [(CH3)2NH2][Cd(N3)3] Hybrid Perovskite Framework. J. Phys. Chem. C 2019, 123, 11840–11849. [Google Scholar] [CrossRef]
- Yadav, R.; Swain, D.; Bhat, H.L.; Elizabeth, S. Order-disorder phase transition and multiferroic behaviour in a metal organic framework compound (CH3)2NH2Co(HCOO)3. J. Appl. Phys. 2016, 119, 064103. [Google Scholar] [CrossRef]
- Šimenas, M.; Balčiunas, S.; Ciupa, A.; Vilčiauskas, L.; Jablonskas, D.; Kinka, M.; Sieradzki, A.; Samulionis, V.; Maczka, M.; Banys, J. Elucidation of dipolar dynamics and the nature of structural phases in the [(CH3)2NH2][Zn(HCOO)3] hybrid perovskite framework. J. Mater. Chem. C 2019, 7, 6779–6785. [Google Scholar] [CrossRef]
- Jain, P.; Dalal, N.S.; Toby, B.H.; Kroto, H.W.; Cheetham, A.K. Order-disorder antiferroelectric phase transition in a hybrid inorganic-organic framework with the perovskite architecture. J. Am. Chem. Soc. 2008, 130, 10450–10451. [Google Scholar] [CrossRef]
- Svane, K.L.; Forse, A.C.; Grey, C.P.; Kieslich, G.; Cheetham, A.K.; Walsh, A.; Butler, K.T. How Strong Is the Hydrogen Bond in Hybrid Perovskites? J. Phys. Chem. Lett. 2017, 8, 6154–6159. [Google Scholar] [CrossRef]
- Abhyankar, N.; Kweon, J.J.; Orio, M.; Bertaina, S.; Lee, M.; Choi, E.S.; Fu, R.; Dalal, N.S. Understanding Ferroelectricity in the Pb-Free Perovskite-Like Metal–Organic Framework [(CH3)2NH2]Zn(HCOO)3: Dielectric, 2D NMR, and Theoretical Studies. J. Phys. Chem. C 2017, 121, 6314–6322. [Google Scholar] [CrossRef]
- Šimenas, M.; Ciupa, A.; Maczka, M.; Pöppl, A.; Banys, J. EPR Study of Structural Phase Transition in Manganese-Doped [(CH3)2NH2][Zn(HCOO)3]. J. Phys. Chem. C 2015, 119, 24522–24528. [Google Scholar] [CrossRef]
- Peksa, P.; Trzmiel, J.; Fedoruk, K.; Gagor, A.; Šimenas, M.; Ciupa, A.; Pawlus, S.; Banys, J.; Maczka, M.; Sieradzki, A. Impact of the Copper-Induced Local Framework Deformation on the Mechanism of Structural Phase Transition in [(CH3)2NH2][Zn(HCOO)3] Hybrid Metal-Formate Perovskite. J. Phys. Chem. C 2019, 123, 23594–23603. [Google Scholar] [CrossRef]
- Šimenas, M.; Ptak, M.; Khan, A.H.; Dagys, L.; Balevičius, V.; Bertmer, M.; Völkel, G.; Maczka, M.; Pöppl, A.; Banys, J. Spectroscopic Study of [(CH3)2NH2][Zn(HCOO)3] Hybrid Perovskite Containing Different Nitrogen Isotopes. J. Phys. Chem. C 2018, 122, 10284–10292. [Google Scholar] [CrossRef]
- Šimenas, M.; Ciupa, A.; Usevičius, G.; Aidas, K.; Klose, D.; Jeschke, G.; Mączka, M.; Völkel, G.; Pöppl, A.; Banys, J. Electron paramagnetic resonance of a copper doped [(CH3)2NH2][Zn(HCOO)3] hybrid perovskite framework. Phys. Chem. Chem. Phys. 2018, 20, 12097–12105. [Google Scholar] [CrossRef]
- Šimėnas, M.; Macalik, L.; Aidas, K.; Kalendra, V.; Klose, D.; Jeschke, G.; Mączka, M.; Völkel, G.; Banys, J.; Pöppl, A. Pulse EPR and ENDOR Study of Manganese Doped [(CH3)2NH2][Zn(HCOO)3] Hybrid Perovskite Framework. J. Phys. Chem. C 2017, 121, 27225–27232. [Google Scholar] [CrossRef]
- Asaji, T.; Ashitomi, K. Phase transition and cationic motion in a metal-organic perovskite, dimethylammonium zinc formate [(CH3)2NH2][Zn(HCOO)3]. J. Phys. Chem. C 2013, 117, 10185–10190. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, R.G. Ferroelectric metal-organic frameworks. Chem. Rev. 2012, 112, 1163–1195. [Google Scholar] [CrossRef]
- Duncan, H.D.; Dove, M.T.; Keen, D.A.; Phillips, A.E. Local structure of the metal-organic perovskite dimethylammonium manganese(ii) formate. Dalt. Trans. 2016, 45, 4380–4391. [Google Scholar] [CrossRef]
- Mączka, M.; Gągor, A.; Ptak, M.; Paraguassu, W.; Da Silva, T.A.; Sieradzki, A.; Pikul, A. Phase Transitions and Coexistence of Magnetic and Electric Orders in the Methylhydrazinium Metal Formate Frameworks. Chem. Mater. 2017, 29, 2264–2275. [Google Scholar] [CrossRef]
- Maczka, M.; Kadlubański, P.; Freire, P.T.C.; Macalik, B.; Paraguassu, W.; Hermanowicz, K.; Hanuza, J. Temperature-and pressure-induced phase transitions in the metal formate framework of [ND4][Zn(DCOO)3] and [NH4][Zn(HCOO)3]. Inorg. Chem. 2014, 53, 9615–9624. [Google Scholar] [CrossRef] [PubMed]
- Mączka, M.; Ptak, M.; Macalik, L. Infrared and Raman studies of phase transitions in metal–organic frameworks of [(CH3)2NH2][M(HCOO)3] with M = Zn, Fe. Vib. Spectrosc. 2014, 71, 98–104. [Google Scholar] [CrossRef]
- Jonscher, A.K. Relaxation in low-loss dielectrics. J. Mol. Liq. 2000, 86, 259–268. [Google Scholar] [CrossRef]
- Jonscher, A.K. Dielectric relaxation in solids. J. Phys. D Appl. Phys. 1999, 32, R57. [Google Scholar] [CrossRef]
- Stanislavsky, A.; Weron, K.; Trzmiel, J. Subordination model of anomalous diffusion leading to the two-power-law relaxation responses. EPL 2010, 91, 40003. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peksa, P.; Trzmiel, J.; Ptak, M.; Ciupa-Litwa, A.; Sieradzki, A. Metal-Formate Framework Stiffening and Its Relevance to Phase Transition Mechanism. Materials 2021, 14, 6150. https://doi.org/10.3390/ma14206150
Peksa P, Trzmiel J, Ptak M, Ciupa-Litwa A, Sieradzki A. Metal-Formate Framework Stiffening and Its Relevance to Phase Transition Mechanism. Materials. 2021; 14(20):6150. https://doi.org/10.3390/ma14206150
Chicago/Turabian StylePeksa, Paulina, Justyna Trzmiel, Maciej Ptak, Aneta Ciupa-Litwa, and Adam Sieradzki. 2021. "Metal-Formate Framework Stiffening and Its Relevance to Phase Transition Mechanism" Materials 14, no. 20: 6150. https://doi.org/10.3390/ma14206150
APA StylePeksa, P., Trzmiel, J., Ptak, M., Ciupa-Litwa, A., & Sieradzki, A. (2021). Metal-Formate Framework Stiffening and Its Relevance to Phase Transition Mechanism. Materials, 14(20), 6150. https://doi.org/10.3390/ma14206150






