Effect of Ceramic Formation on the Emission of Eu3+ and Nd3+ Ions in Double Perovskites
Abstract
:1. Introduction
2. Experimental
2.1. Samples Preparation
2.2. Research Techniques
3. Results and Discussion
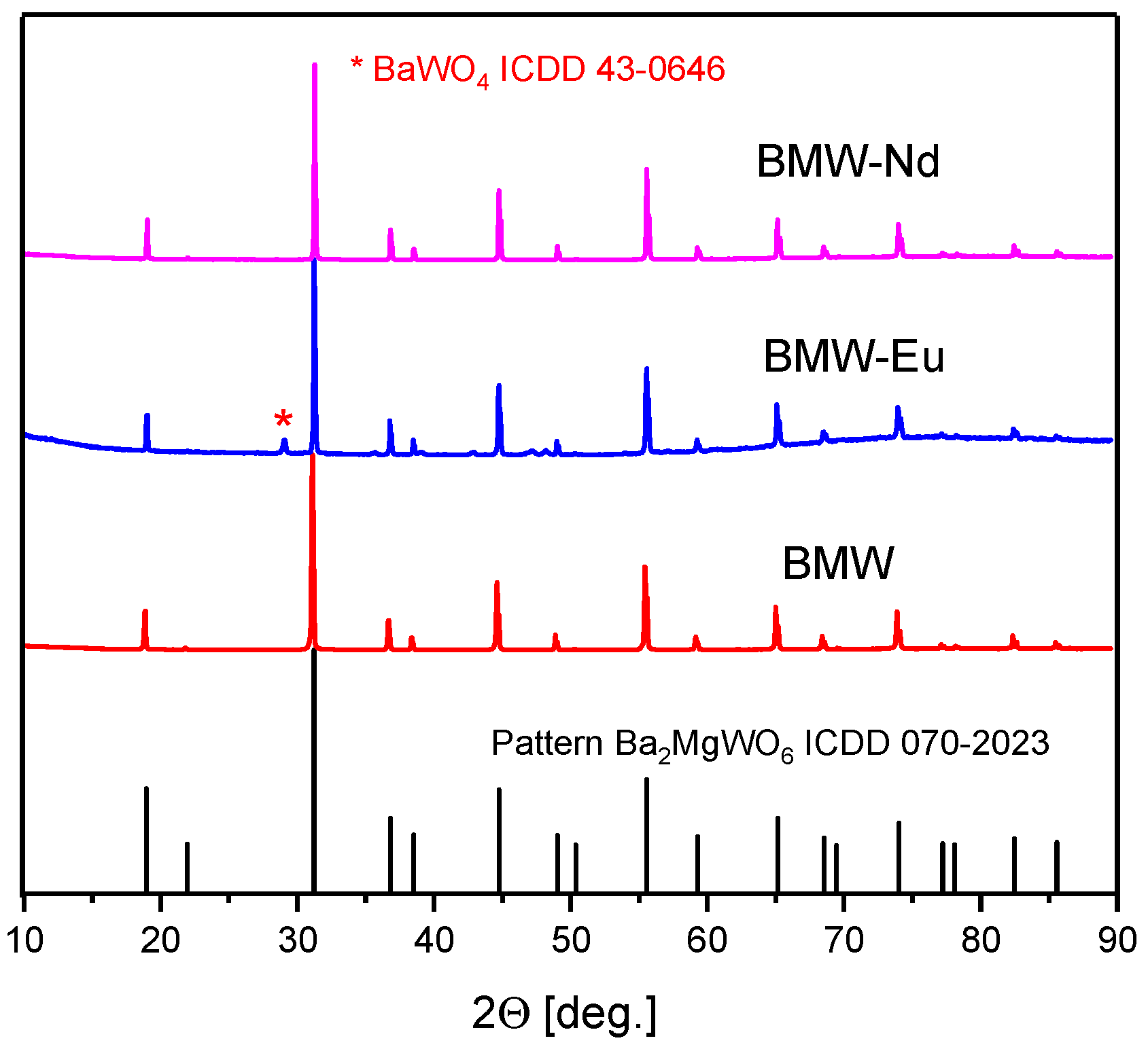
3.1. BMW Powders Characterizations
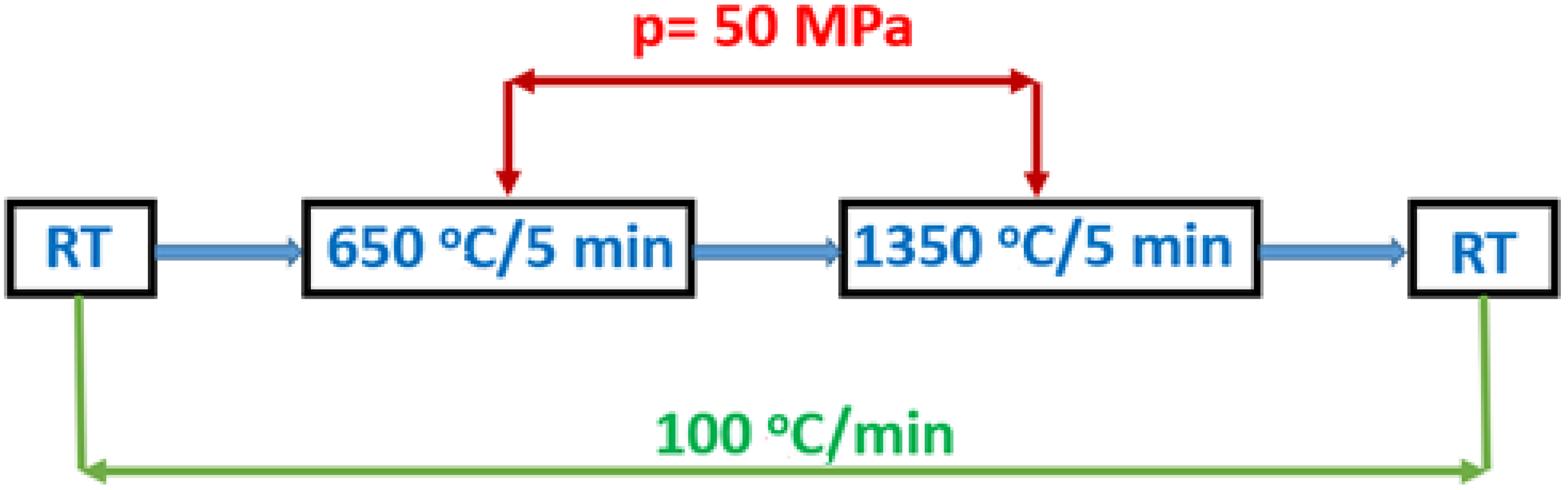
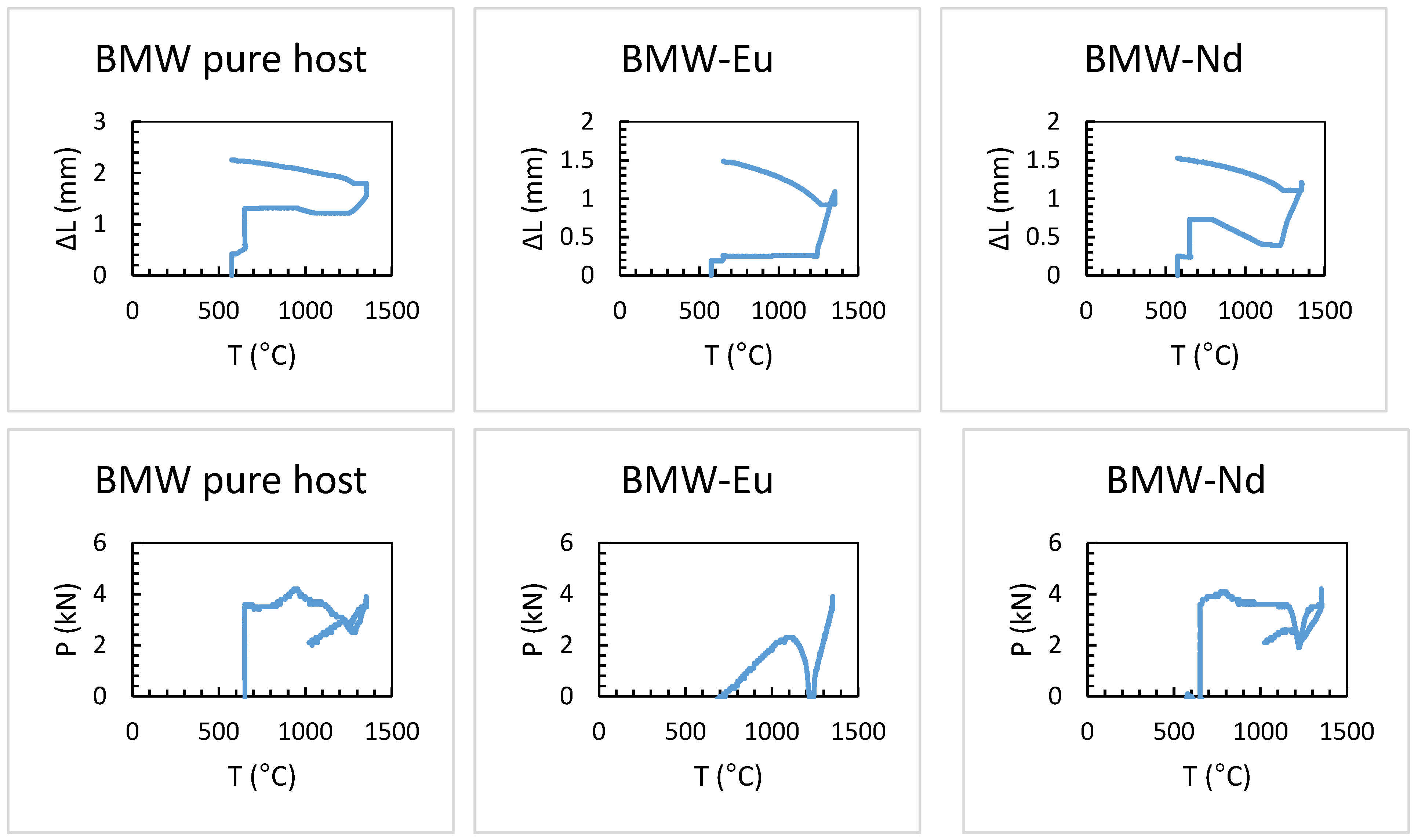
3.2. Study of BMW Sintering by SPS
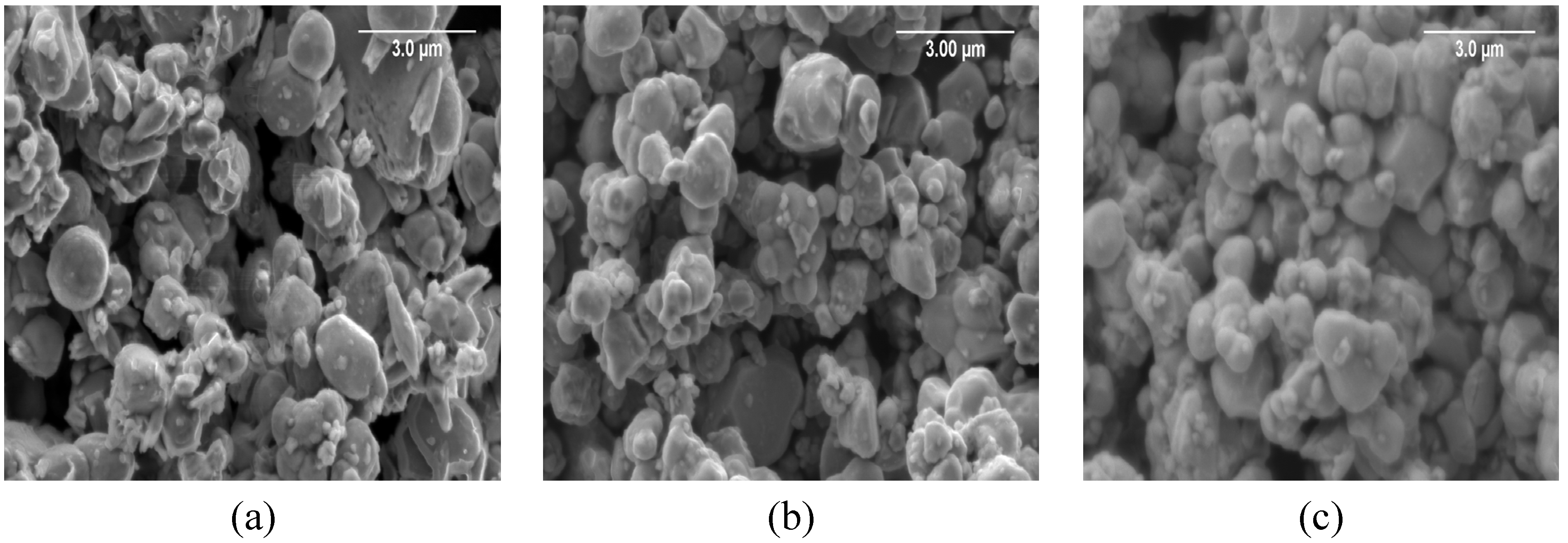
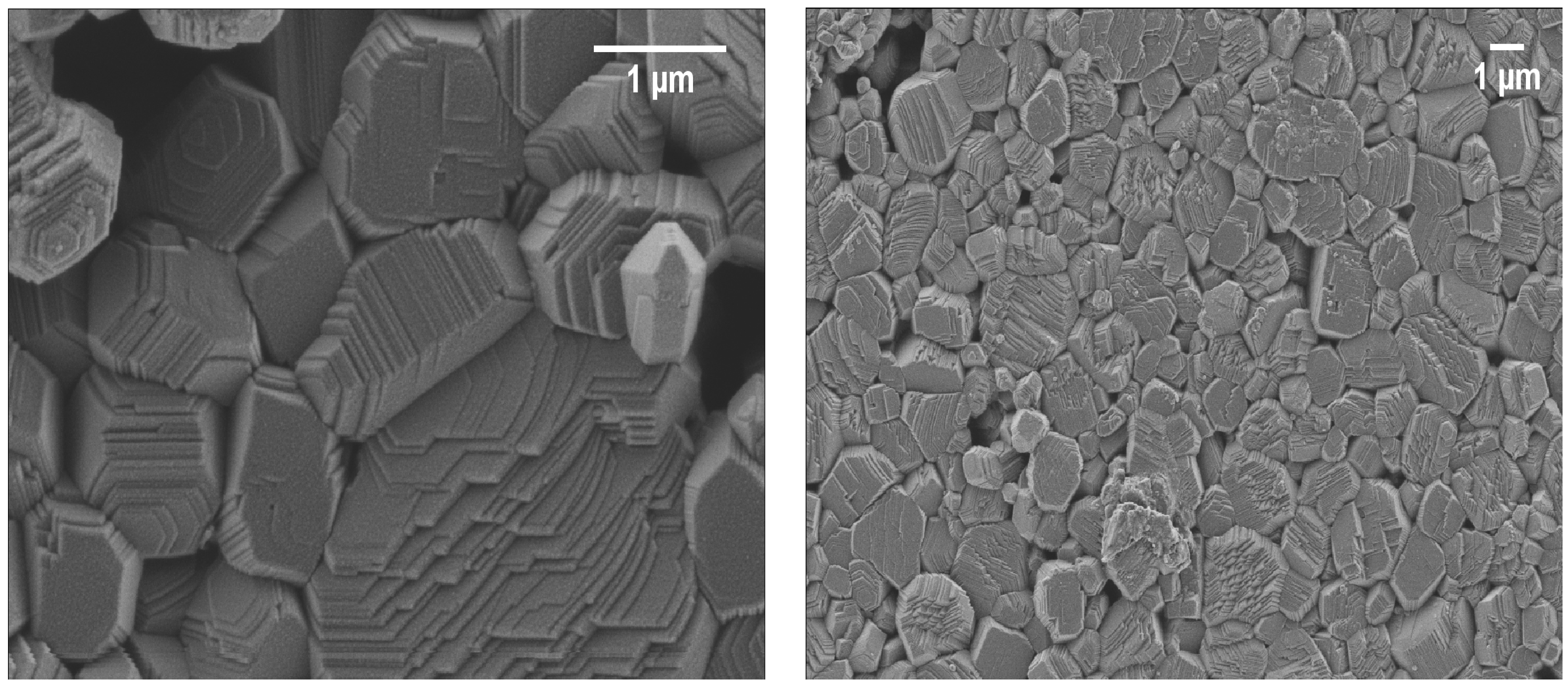
3.3. Microstructural Features of BMW Ceramics
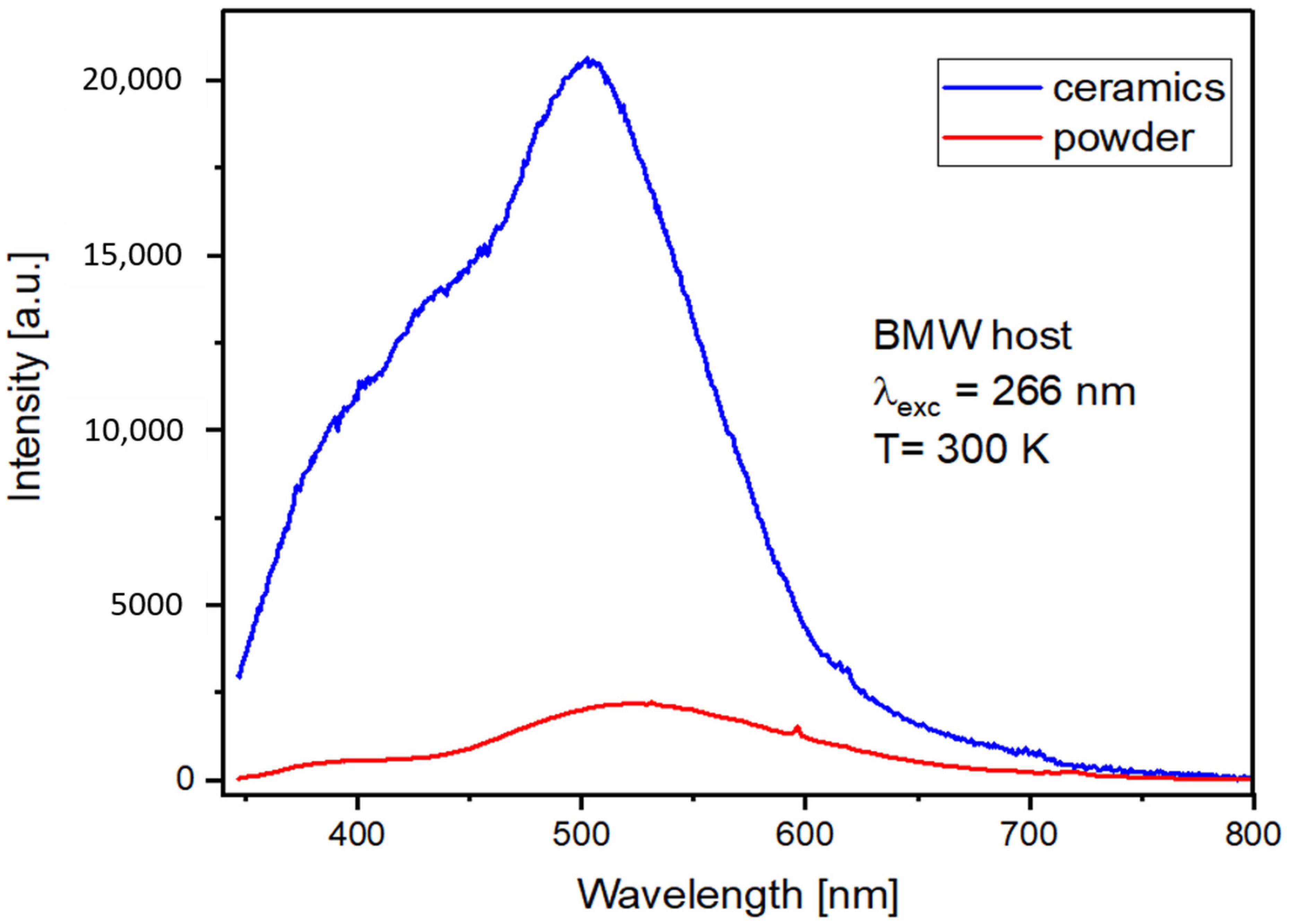
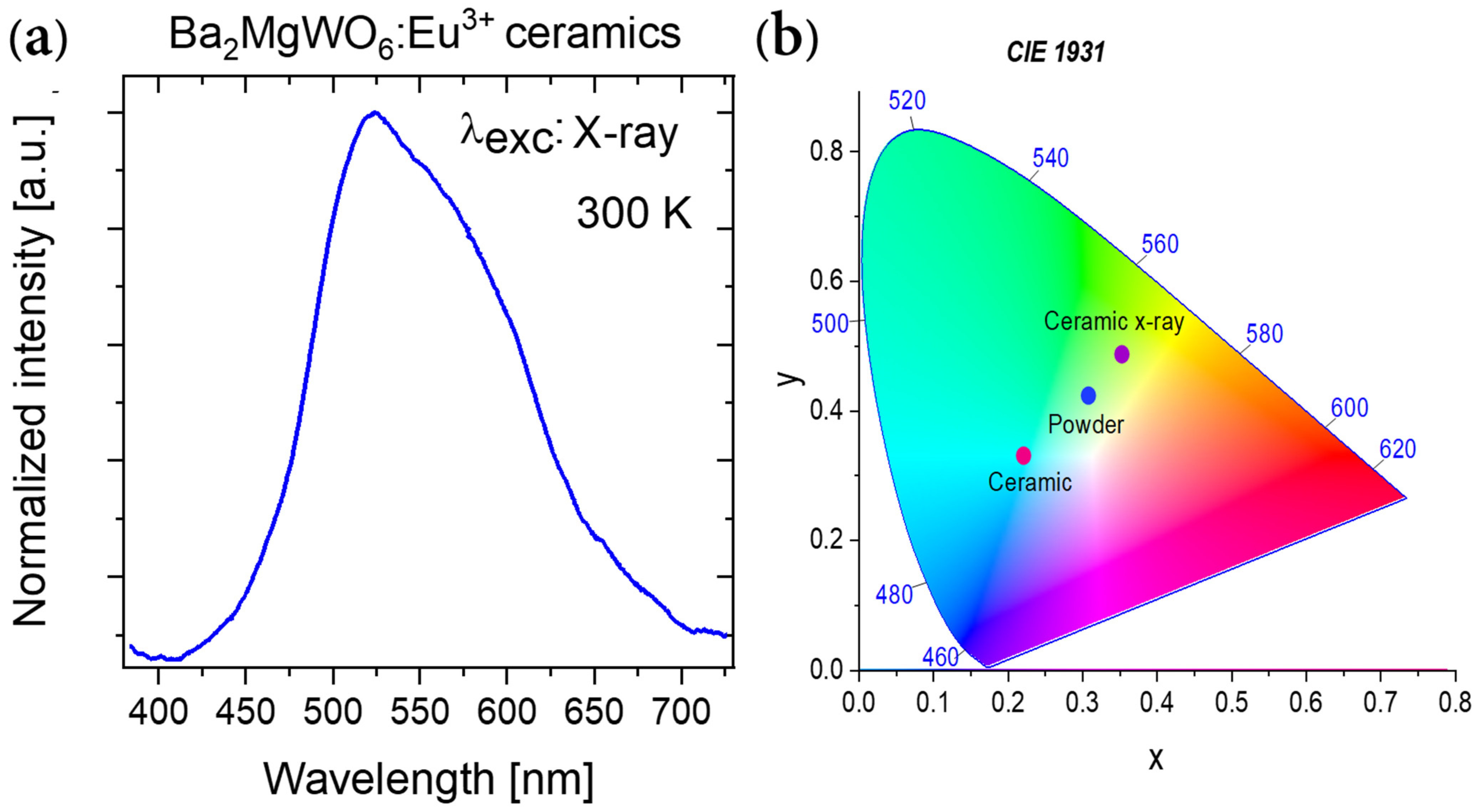
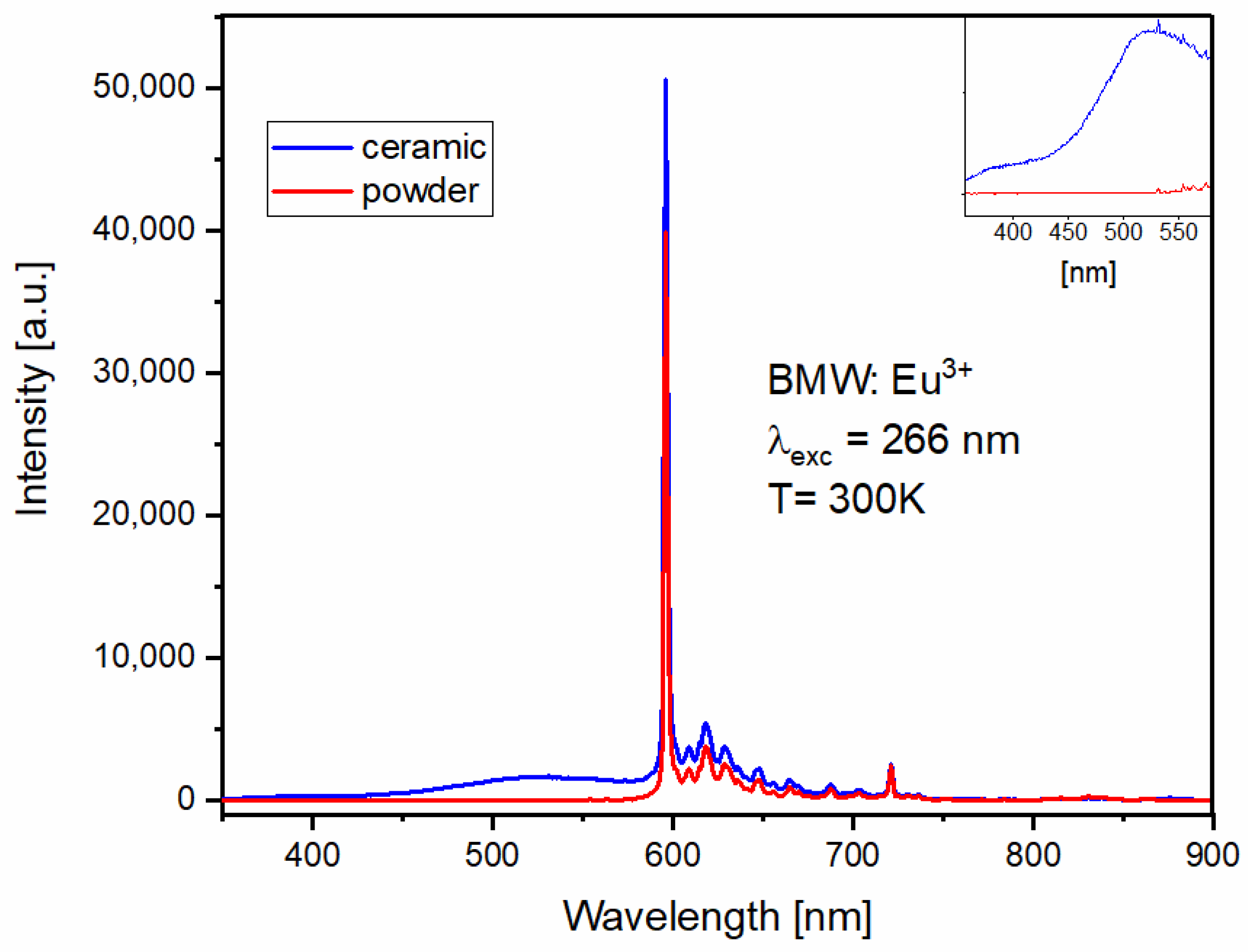
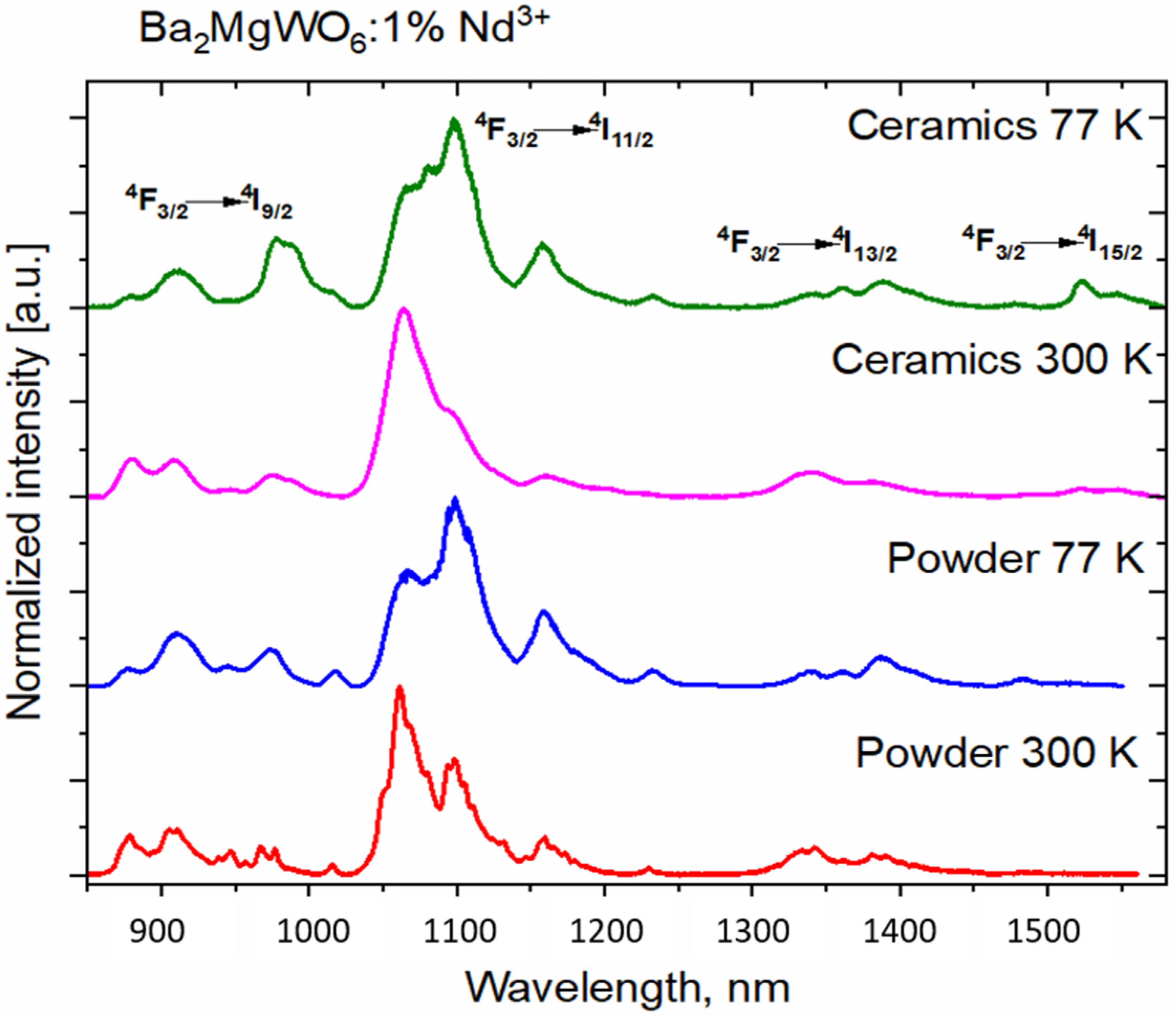
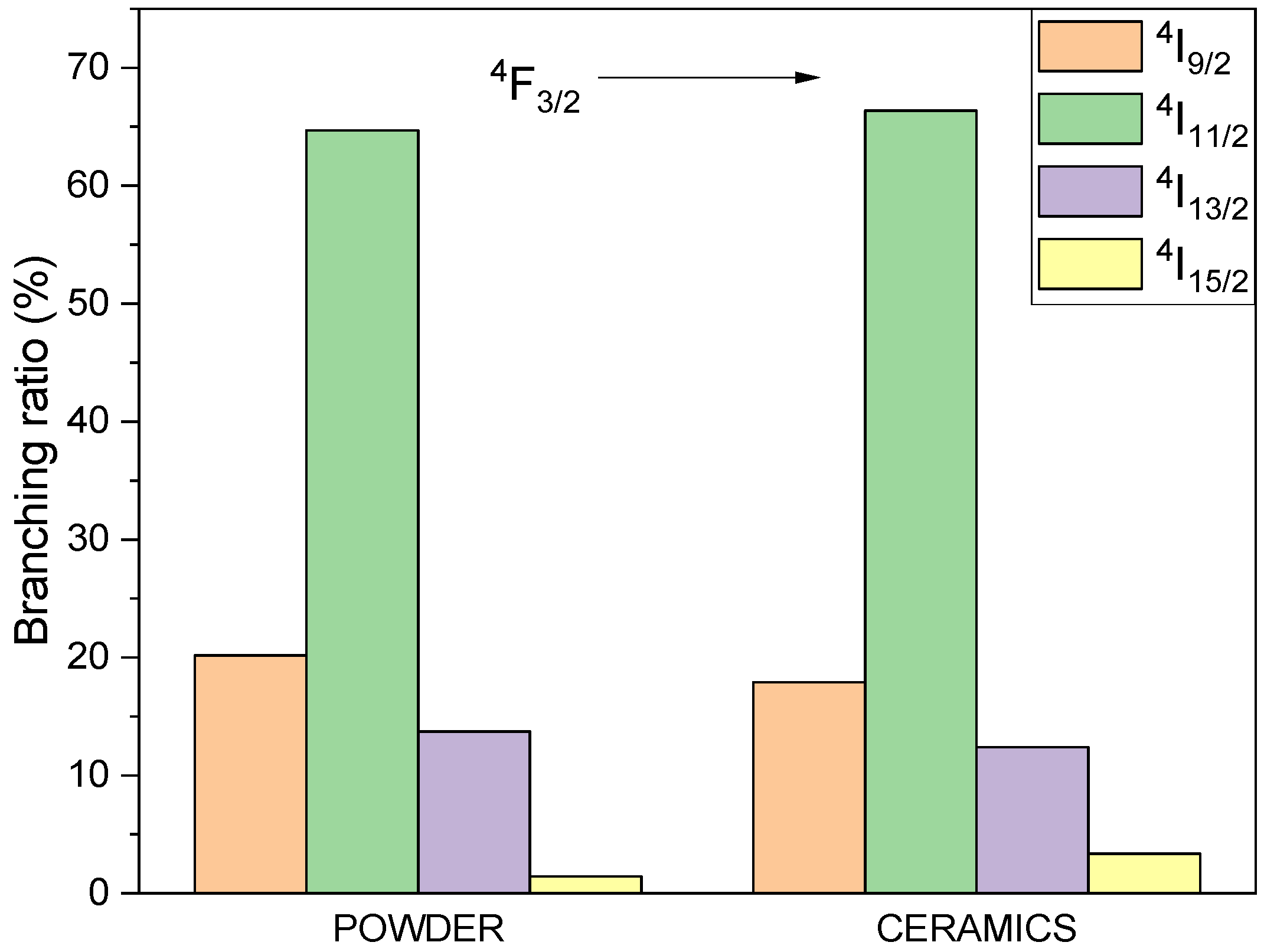
3.4. Spectroscopic Properties of BMW Double Perovskites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, R.; Shin, D.S.; Jang, K.; Guo, Y.; Noh, H.M.; Moon, B.K.; Choi, B.C.; Jeong, J.H.; Yi, S.S. Luminescence and thermal-quenching properties of Dy3+-doped Ba2CaWO6 phosphors. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 125, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Sreeja, E.; Mohan, R.P.; Gopi, S.; Joseph, C.; Unnikrishnan, N.; Biju, P. Structural and photoluminescence properties of UV-excited Er3+ doped Ba2CaWO6 yellowish-green phosphors. Phys. B Condens. Matter 2019, 555, 74–80. [Google Scholar] [CrossRef]
- Lapa, C.M.; Vasconcelos, I.B.; Santos, E.J.P.; Yadava, Y.P. Production, sintering characterization of Ba2MgWO6 ceramics for ceramic components for planar RTD applications. In Proceedings-Electrochemical Society; Electrochemical Society: Pennington, NJ, USA, 2004; pp. 325–330. [Google Scholar]
- Chen, Y.-C.; Wang, Y.-N.; Syu, R.-Y. Effect of sintering temperature on microstructures and microwave dielectric properties of Ba2MgWO6 ceramics. J. Mater. Sci. Mater. Electron. 2016, 27, 4259–4264. [Google Scholar] [CrossRef]
- Leonardo, Y.P.Y.; de Aguiar1, L.A.R.; Lapa, C.M.; Ferreira, R.A.S.; Aguiar, J.A.; da Silva, C.L.; de Souza, D.P.F. Production, sintering and microstructural characteristics of Ba2MgWO6 ceramics. Mater. Sci. Forum 2005, 498–499, 523–528. [Google Scholar]
- Miniajluk, N.; Boulesteix, R.; Dereń, P. Spark plasma sintering of double perovskite Ba2MgWO6 doped with Ce3+: Part I—Structural and microstructural characterizations. Ceram. Int. 2020, 46, 7602–7608. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, J.; Wang, J.; Shi, F.; Qi, Z.-M. Lattice vibrational characteristics, crystal structure and dielectric properties of Ba2MgWO6 microwave dielectric ceramic. Ceram. Int. 2021, 47, 17784–17788. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Yue, Z.; Gui, Z. Microstructure and microwave dielectric properties of (1-x)ZnNb2O6-x ZnTa2O6 ceramics. Dev. Dielectr. Mater. Electron. Devices 2012, 18, 109–115. [Google Scholar]
- Stefańska, D.; Bondzior, B.; Vu, T.; Miniajluk-Gaweł, N.; Dereń, P. The influence of morphology and Eu3+ concentration on luminescence and temperature sensing behavior of Ba2MgWO6 double perovskite as a potential optical thermometer. J. Alloys Compd. 2020, 842, 155742. [Google Scholar] [CrossRef]
- Bondzior, B.; Stefańska, D.; Vũ, T.; Miniajluk-Gaweł, N.; Dereń, P. Red luminescence with controlled rise time in La2MgTiO6: Eu3+. J. Alloys Compd. 2021, 852, 157074. [Google Scholar] [CrossRef]
- Vu, T.; Bondzior, B.; Stefańska, D.; Dereń, P. Influence of temperature on near-infrared luminescence, energy transfer mechanism and the temperature sensing ability of La2MgTiO6: Nd3+ double perovskites. Sens. Actuators A Phys. 2021, 317, 112453. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, W.; Su, Y.; Chen, Z.; Chen, X.; Ma, Y.; Bai, L.; Xiao, W.; Liu, T.; Zhu, H.; et al. Low-dose real-time X-ray imaging with nontoxic double perovskite scintillators. Light Sci. Appl. 2020, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Sun, M.; Lu, Q.; Wu, T.; Huang, B. High energy X-ray radiation sensitive scintillating materials for medical imaging, cancer diagnosis and therapy. Nano Energy 2021, 79, 105437. [Google Scholar] [CrossRef]
- Xu, J.; Shi, Y.; Xie, J.; Lei, F. Fabrication, microstructure, and luminescent properties of Ce3+-doped Lu3Al5O12 (Ce: LuAG) transparent ceramics by low-temperature vacuum sintering. J. Am. Ceram. Soc. 2013, 96, 1930–1936. [Google Scholar] [CrossRef]
- Li, H.-L.; Liu, X.-J.; Huang, L.-P. Fabrication of transparent cerium-doped lutetium aluminum garnet (LuAG:Ce) ceramics by a solid-state reaction method. J. Am. Ceram. Soc. 2005, 88, 3226–3228. [Google Scholar] [CrossRef]
- Arai, M.; Mizoi, K.; Fujimoto, Y.; Koshimizu, M.; Nakauchi, D.; Yanagida, T.; Asai, K. Tl2NaYCl6: A new self-activated scintillator possessing an elpasolite structure. J. Mater. Sci. Mater. Electron. 2021, 32, 7906–7912. [Google Scholar] [CrossRef]
- Roy, P.; Waghmare, V.; Maiti, T. Environmentally friendly BaxSr2-xTiFeO6double perovskite with enhanced thermopower for high temperature thermoelectric power generation. RSC Adv. 2016, 6, 54636–54643. [Google Scholar] [CrossRef]
- Nguyen, H.-D.; Doan, T.T.; Vu, T.H.Q.; Bondzior, B.; Deren, P.J.; Velpula, R.T.; Nguyen, H.P.T.; Luu, A.T.; Nguyen, Q.H.N. Deep red fluoride dots-in-nanoparticles for high color quality micro white light-emitting diodes. Opt. Express 2020, 28, 26189–26199. [Google Scholar] [CrossRef]
- Kosyanov, D.; Yavetskiy, R.P.; Tolmachev, A.; Vornovskikh, A.; Pogodaev, A.; Gridasova, E.; Shichalin, O.; Kaidalova, T.; Kuryavyi, V. Fabrication of highly-doped Nd3+:YAG transparent ceramics by reactive SPS. Ceram. Int. 2018, 44, 23145–23149. [Google Scholar] [CrossRef]
- Kosyanov, D.; Vornovskikh, A.; Zakharenko, A.; Gridasova, E.; Yavetskiy, R.; Dobrotvorskaya, M.; Tolmachev, A.; Shichalin, O.; Papynov, E.; Ustinov, A.; et al. Influence of sintering parameters on transparency of reactive SPSed Nd3+:YAG ceramics. Opt. Mater. 2021, 112, 110760. [Google Scholar] [CrossRef]
- Morita, K.; Kim, B.-N.; Yoshida, H.; Hiraga, K. Spark-plasma-sintering condition optimization for producing transparent MgAl2O4 spinel polycrystal. J. Am. Ceram. Soc. 2009, 92, 1208–1216. [Google Scholar] [CrossRef]
- Boulesteix, R.; Maître, A.; Lemański, K.; Dereń, P.J. Structural and spectroscopic properties of MgAl2O4:Nd3+ transparent ceramics fabricated by using two-step Spark Plasma Sintering. J. Alloys Compd. 2017, 722, 358–364. [Google Scholar] [CrossRef]
- Suárez, M.; Fernández, A.; Menéndez, J.L.; Torrecillas, R. Transparent yttrium aluminium garnet obtained by spark plasma sintering of lyophilized gels. J. Nanomater. 2009. [Google Scholar] [CrossRef]
- Vu, T.H.Q.; Bondzior, B.; Stefańska, D.; Miniajluk-Gaweł, N.; Winiarski, M.J.; Dereń, P.J. Changing the sensitivity and operating range of the Ba2MgWO6:Eu3+ optical thermometer by applying the distinct synthesis routes. Sci. Rep. 2021. [Google Scholar] [CrossRef]
- Bode, J.; Van Oosterhout, A. Defect luminescence of ordered perovskites A2BWO6. J. Lumin 1975, 10, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Blasse, G.; Grabmaier, B.C. Luminescence Materials; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Kaminskii, A.A. Crystalline Lasers: Physical Processes and Operating Schemes; CRC Press: New York, NY, USA, 1996. [Google Scholar]



| Samples | Apparent Density δapp [g/cm3] | Relative Density δ [%] | Open Porosity Πo [%] | Grain Size of Powder [μm] | Grain Size of Ceramic [μm] |
|---|---|---|---|---|---|
| BMW host | 6.62 | 91.99 | 4.29 | 0.5–2 | 1.5–3 |
| BMW-Eu | 6.09 | 84.57 | 6.43 | 0.5–1.7 | 0.4–1.4 |
| BMW-Nd | 6.60 | 91.72 | 4.88 | 0.7–1.6 | 1–1.8 |
| BMW:1% Nd3+ Sample/Temperature | Spectroscopic Quality Parameter (SQP) |
|---|---|
| Powder/300 K | 0.65 |
| Ceramics/300 K | 1.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miniajluk-Gaweł, N.; Bondzior, B.; Lemański, K.; Vu, T.H.Q.; Stefańska, D.; Boulesteix, R.; Dereń, P.J. Effect of Ceramic Formation on the Emission of Eu3+ and Nd3+ Ions in Double Perovskites. Materials 2021, 14, 5996. https://doi.org/10.3390/ma14205996
Miniajluk-Gaweł N, Bondzior B, Lemański K, Vu THQ, Stefańska D, Boulesteix R, Dereń PJ. Effect of Ceramic Formation on the Emission of Eu3+ and Nd3+ Ions in Double Perovskites. Materials. 2021; 14(20):5996. https://doi.org/10.3390/ma14205996
Chicago/Turabian StyleMiniajluk-Gaweł, Natalia, Bartosz Bondzior, Karol Lemański, Thi Hong Quan Vu, Dagmara Stefańska, Remy Boulesteix, and Przemysław Jacek Dereń. 2021. "Effect of Ceramic Formation on the Emission of Eu3+ and Nd3+ Ions in Double Perovskites" Materials 14, no. 20: 5996. https://doi.org/10.3390/ma14205996
APA StyleMiniajluk-Gaweł, N., Bondzior, B., Lemański, K., Vu, T. H. Q., Stefańska, D., Boulesteix, R., & Dereń, P. J. (2021). Effect of Ceramic Formation on the Emission of Eu3+ and Nd3+ Ions in Double Perovskites. Materials, 14(20), 5996. https://doi.org/10.3390/ma14205996










