Corrosion Protective Film Formation on Mg Alloy AZ31 by Exposure to Dilute Selenite Solutions
Abstract
1. Introduction
2. Materials and Methods
3. Results
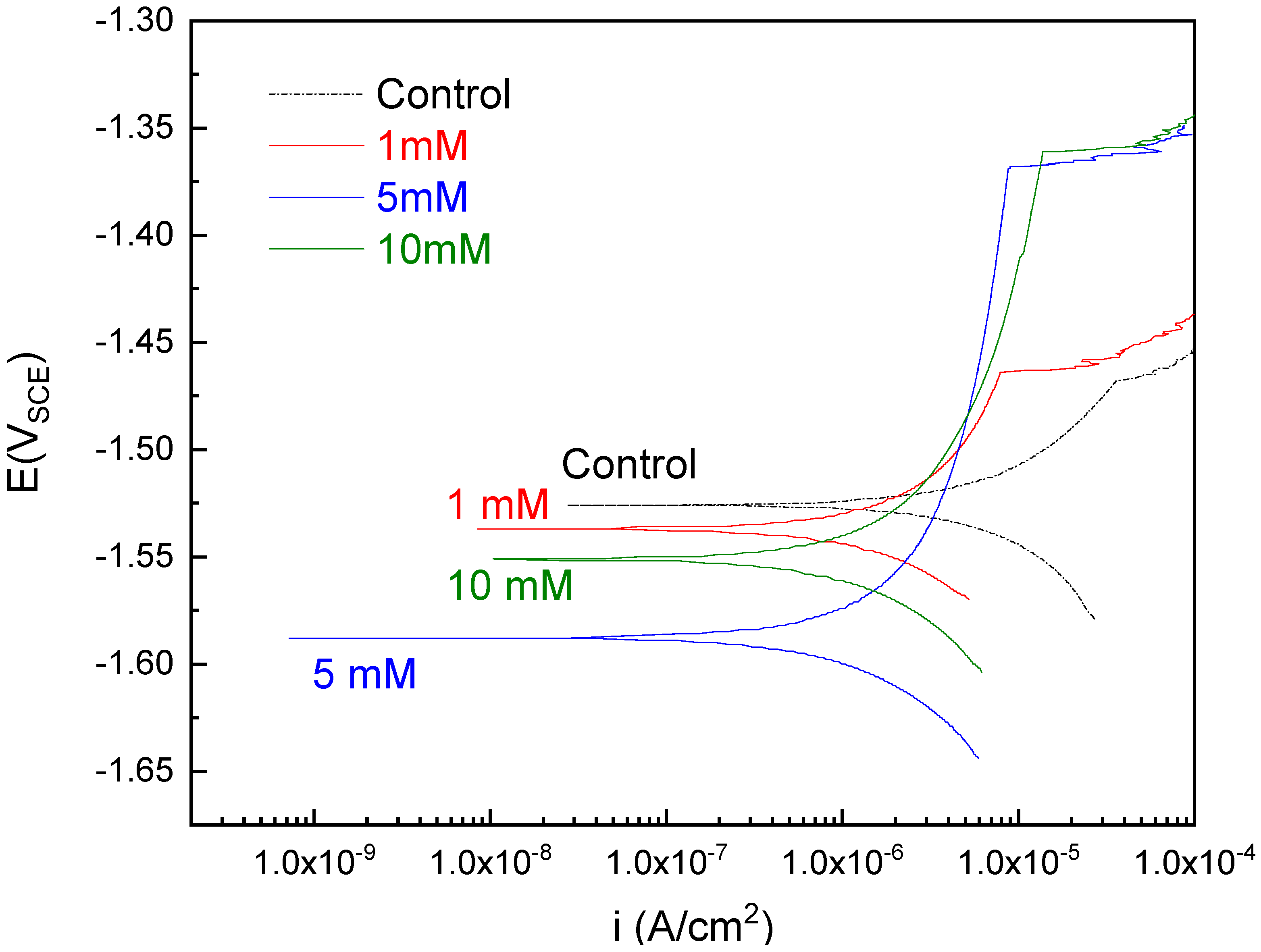
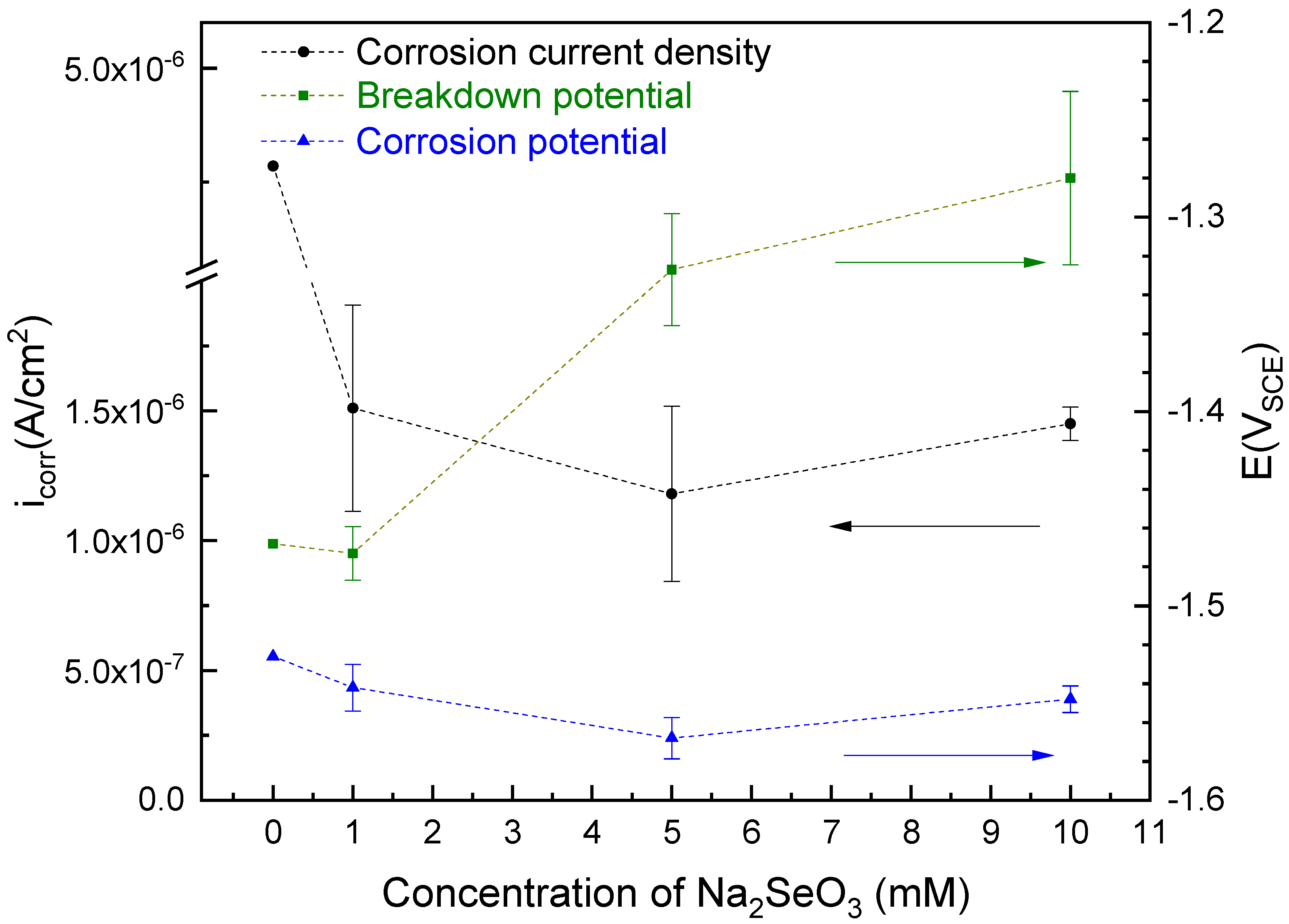
3.1. Electrochemical Testing
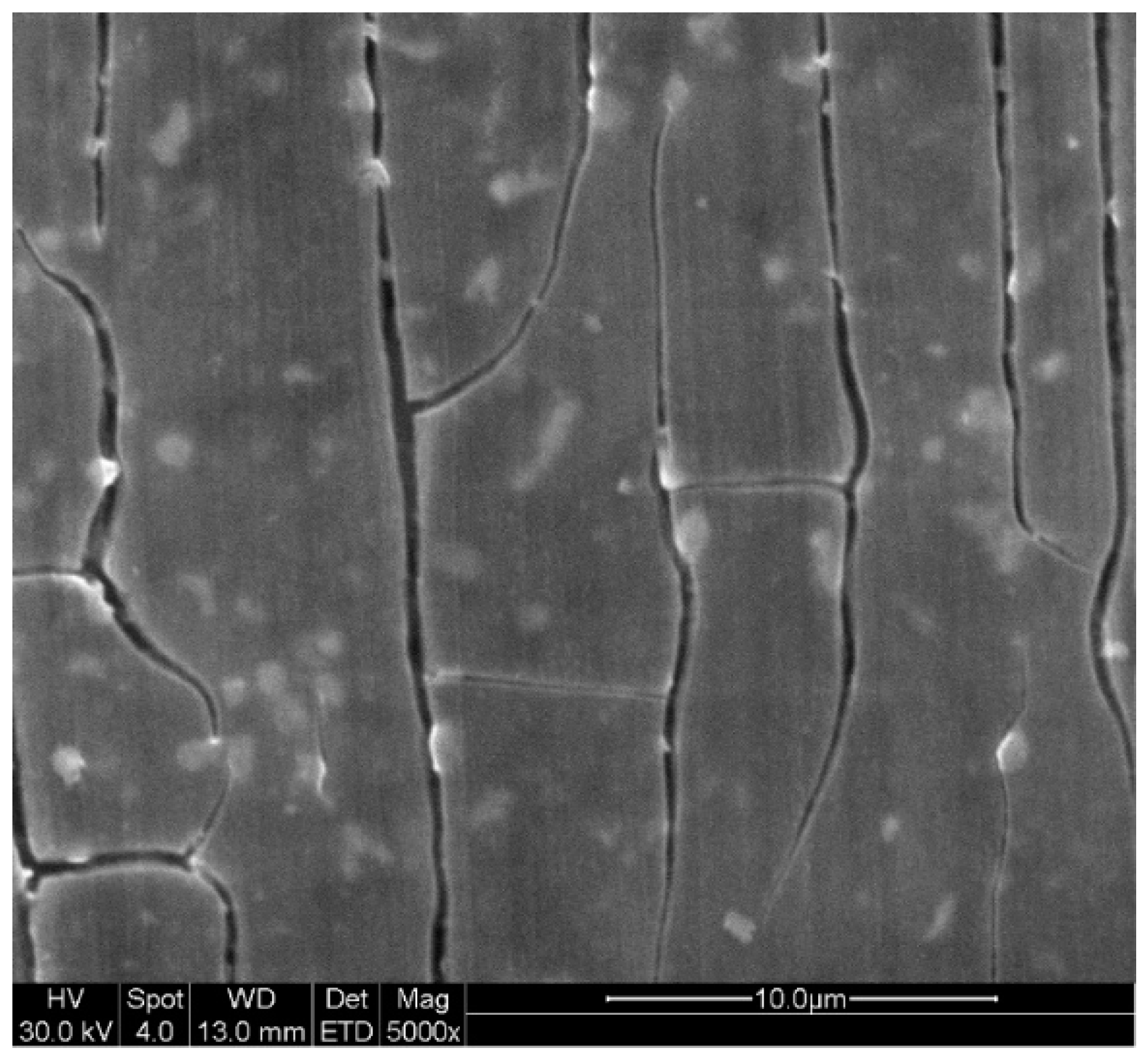
3.2. Surface Morphology and Characterization
4. Discussion
5. Conclusions
- The selenite protective film form on AZ31 upon exposure to selenite-bearing bath provides an approximate 5 times increment of corrosion resistance compared with control. A robust and apparently protective hydrated gel film was observed in SEM images collected after coating. The selenite coating from a higher concentration (5 mM and 10 mM) selenite decreases the likelihood of surface film breakdown.
- The protection mechanism of selenite coating was the interface reaction between Mg and coating bath that results in the reduction of selenite, formation of MgSeO3 hydrate, and polymerization of amorphous selenium to generate a Mg-Se mixed protection film.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Knörnschild, G.; Snihirova, D.V.; Taryba, M.G.; Zheludkevich, M.L.; Ferreira, M.G.S. Complex anticorrosion coating for ZK30 magnesium alloy. Electrochim. Acta 2009, 55, 131–141. [Google Scholar] [CrossRef]
- Song, G. Recent Progress in Corrosion and Protection of Magnesium Alloys. Adv. Eng. Mater. 2005, 7, 563–586. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ibrahim, H.; Amerinatanzi, A.; Elahinia, M. Biodegradable magnesium alloys. In Metals for Biomedical Devices; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–289. [Google Scholar]
- Furuya, H.; Kogiso, N.; Matunaga, S.; Senda, K. Applications of Magnesium Alloys for Aerospace Structure Systems. Mater. Sci. Forum 2000, 350–351, 341–348. [Google Scholar] [CrossRef]
- Xia, X.; Sun, W.; Luo, A.A.; Stone, D.S. Precipitation evolution and hardening in MgSmZnZr alloys. Acta Mater. 2016, 111, 335–347. [Google Scholar] [CrossRef]
- Zadeh, A.S.; Xia, X.; Luo, A.A.; Jakes, J.E.; Stone, D.S. Aging Behavior and Microstructural Evolution in Mg-3Nd-0.2Zn-0.5Zr Alloy. In Magnesium Technology 2013; Springer International Publishing: Berlin/Heidelberg, Germany, 2013; pp. 27–32. [Google Scholar]
- Thomas, S.; Medhekar, N.V.; Frankel, G.S.; Birbilis, N. Corrosion mechanism and hydrogen evolution on Mg. Curr. Opin. Solid State Mater. Sci. 2015, 19, 85–94. [Google Scholar] [CrossRef]
- Frankel, G.S.; Samaniego, A.; Birbilis, N. Evolution of hydrogen at dissolving magnesium surfaces. Corros. Sci. 2013, 70, 104–111. [Google Scholar] [CrossRef]
- Gusieva, K.; Davies, C.H.J.; Scully, J.R.; Birbilis, N. Corrosion of magnesium alloys: The role of alloying. Int. Mater. Rev. 2015, 60, 169–194. [Google Scholar] [CrossRef]
- Katzman, H.A.; Malouf, G.M.; Bauer, R.; Stupian, G.W. Corrosion-protective chromate coatings on aluminum. Appl. Surf. Sci. 1979, 2, 416–432. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Vaghefinazari, B.; Mei, D.; Petrauskas, R.P.; Höche, D.; Zheludkevich, M.L. Comprehensive screening of Mg corrosion inhibitors. Corros. Sci. 2017, 128, 224–240. [Google Scholar] [CrossRef]
- Dindodi, N.; Shetty, A.N. Stearate as a green corrosion inhibitor of magnesium alloy ZE41 in sulfate medium. Arab. J. Chem. 2014. [Google Scholar] [CrossRef]
- Feng, Z.; Hurley, B.; Zhu, M.; Yang, Z.; Hwang, J.; Buchheit, R. Corrosion Inhibition of AZ31 Mg Alloy by Aqueous Selenite (SeO 3 2−). J. Electrochem. Soc. 2019, 166, C520–C529. [Google Scholar] [CrossRef]
- Feng, Z.; Li, J.; Yang, Z.; Buchheit, R. The Effect of Vanadate, Phosphate, Fluoride Compounds on the Aqueous Corrosion of Magnesium Alloy AZ31 in Dilute Chloride Solutions. Materials 2020, 13, 1325. [Google Scholar] [CrossRef] [PubMed]
- Snihirova, D.; Taryba, M.; Lamaka, S.V.; Montemor, M.F. Corrosion inhibition synergies on a model Al-Cu-Mg sample studied by localized scanning electrochemical techniques. Corros. Sci. 2016, 112, 408–417. [Google Scholar] [CrossRef]
- Samaniego, A.; Birbilis, N.; Xia, X.; Frankel, G.S. Hydrogen Evolution During Anodic Polarization of Mg Alloyed with Li, Ca, or Fe. Corrosion 2015, 71, 224–233. [Google Scholar] [CrossRef]
- Zhu, Y.; Free, M.L.; Woollam, R.; Durnie, W. A review of surfactants as corrosion inhibitors and associated modeling. Prog. Mater. Sci. 2017, 90, 159–223. [Google Scholar] [CrossRef]
- Feng, Z. Corrosion inhibition study of AZ31 Mg alloy by Vanadate, Selenite and Phosphate. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2019. [Google Scholar]
- Pommiers-Belin, S.; Frayret, J.; Uhart, A.; Ledeuil, J.; Dupin, J.C.; Castetbon, A.; Potin-Gautier, M. Determination of the chemical mechanism of chromate conversion coating on magnesium alloys EV31A. Appl. Surf. Sci. 2014, 298, 199–207. [Google Scholar] [CrossRef]
- Schmutz, P.; Frankel, G.S. Influence of Dichromate Ions on Corrosion of Pure Aluminum and AA2024-T3 in NaCl Solution Studied by AFM Scratching. J. Electrochem. Soc. 1999, 146, 4461–4472. [Google Scholar] [CrossRef]
- Buchheit, R.G. Conversion coating science and technology is it evolving or is it stuck? Corros. Sci. A Retrosp. Curr. Status Honor Robert P Frankenthal 2002, 2002, 430–440. [Google Scholar]
- Buchheit, R.G.; Hughes, A.E. Chromate and Chromate-Free Conversion Coatings. In ASM Handbook Vol. 13A: Corrosion: Fundamentals, Testing and Protection; ASM International: Materials Park, OH, USA, 2003; pp. 720–735. [Google Scholar]
- Park, R.M.; Bena, J.F.; Stayner, L.T.; Smith, R.J.; Gibb, H.J.; Lees, P.S.J. Hexavalent chromium and lung cancer in the chromate industry: A quantitative risk assessment. Risk Anal. 2004, 24, 1099–1108. [Google Scholar] [CrossRef]
- Howlett, P.C.; Gramet, S.; Lin, J.; Efthimiadis, J.; Chen, X.B.; Birbilis, N.; Forsyth, M. Conversion coatings of Mg-alloy AZ91D using trihexyl(tetradecyl) phosphonium bis(trifluoromethanesulfonyl)amide ionic liquid. Sci. China Chem. 2012, 55, 1598–1607. [Google Scholar] [CrossRef]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. Review of corrosion-resistant conversion coatings for magnesium and its alloys. Corrosion 2011, 67, 035005-1–035005-16. [Google Scholar] [CrossRef]
- Saji, V.S. Review of rare-earth-based conversion coatings for magnesium and its alloys. J. Mater. Res. Technol. 2019, 8, 5012–5035. [Google Scholar] [CrossRef]
- Ardelean, H.; Frateur, I.; Marcus, P. Corrosion protection of magnesium alloys by cerium, zirconium and niobium-based conversion coatings. Corros. Sci. 2008, 50, 1907–1918. [Google Scholar] [CrossRef]
- Gao, H.F.; Tan, H.Q.; Li, J.; Wang, Y.Q.; Xun, J.Q. Synergistic effect of cerium conversion coating and phytic acid conversion coating on AZ31B magnesium alloy. Surf. Coatings Technol. 2012, 212, 32–36. [Google Scholar] [CrossRef]
- Abbasi, F.; Sarabi, A.A. Electrochemical Properties of Phosphate Coating on AZ31 Magnesium Alloy: Effect of Phosphating Process Parameters. Corrosion 2012, 68, 835–843. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Zhou, Y.; Ding, Y. The Preparation, Characterization and Formation Mechanism of a Calcium Phosphate Conversion Coating on Magnesium Alloy AZ91D. Materials 2018, 11, 908. [Google Scholar] [CrossRef]
- Guy, D.B.; Whitby, L. Process for protecting magnesium and its alloys against corrosion. U.S. Patent 1,961,030, 1934. [Google Scholar]
- Abdo, K.M. Sodium Selenate and Sodium Selenite. Natl. Toxicol. Rep. Toxic. Stud. 1994, Toxicity R, 127. [Google Scholar]
- OSHA Occupational Chemical Database | Occupational Safety and Health Administration. Available online: https://www.osha.gov/chemicaldata/ (accessed on 10 April 2020).
- Williams, G.; Ap Llwyd Dafydd, H.; Subramanian, R.; McMurray, H.N. The Influence of Chloride Ion Concentration on Passivity Breakdown in Magnesium. Corrosion 2017, 73, 471–481. [Google Scholar] [CrossRef]
- Feng, Z.; Hurley, B.; Buchheit, R. Corrosion Inhibition of Mg Alloy AZ31 By Selenite (SeO32−). Meet. Abstr. 2017, MA2017-02, 782. [Google Scholar] [CrossRef]
- Puranen, A.; Jonsson, M.; Dähn, R.; Cui, D. Reduction of selenite and selenate on anoxically corroded iron and the synergistic effect of uranyl reduction. J. Nucl. Mater. 2010, 406, 230–237. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Perry, D.L.; Phillips, S.L. Handbook of Inorganic Compounds; CRC Press: Boca Raton, FL, USA, 1995; ISBN 0849386713 9780849386718. [Google Scholar]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Olin, A.; Nolang, B.; Osadchii, E.G.; Ohman, L.-O.; Rosen, E. Chemical Thermodynamics of Selenium; Elsevier: Amsterdam, The Netherlands, 2005; p. 894. [Google Scholar]
- Sharmasarkar, S.; Reddy, K.J.; Vance, G.F. Preliminary Quantification of Metal Selenite Solubility in Aqueous Solutions. Chem. Geol. 1996, 132, 165–170. [Google Scholar] [CrossRef]
- Goldan, A.H.; Li, C.; Pennycook, S.J.; Schneider, J.; Blom, A.; Zhao, W. Molecular structure of vapor-deposited amorphous selenium. J. Appl. Phys. 2016, 120, 3840. [Google Scholar] [CrossRef]
- Keezer, R.C.; Bailey, M.W. The structure of liquid selenium from viscosity measurements. Mater. Res. Bull. 1967, 2, 185–192. [Google Scholar] [CrossRef]
- Koningsberger, D.C.; van Wolput, J.H.M.C.; Rieter, P.C.U. ESR measurements on the polymerization of liquid selenium. Chem. Phys. Lett. 1971, 8, 145–147. [Google Scholar] [CrossRef]
- Salman, S.A.; Akira, N.; Kuroda, K.; Okido, M. Deposition of Self-Assembled Monolayer on Vanadate Conversion Coated AZ31 Mg Alloy. Mater. Sci. Forum 2014, 783–786, 1482–1487. [Google Scholar]
- Feng, Z.; Hurley, B.; Li, J.; Buchheit, R. Corrosion Inhibition Study of Aqueous Vanadate on Mg Alloy AZ31. J. Electrochem. Soc. 2018, 165, C94–C102. [Google Scholar] [CrossRef]


| Chemicals | Industry Exposure Limit (mg/m3) |
|---|---|
| NaCl | 15 |
| Selenium and its compound | 0.2 |
| Chromate | 0.005 |
| Chemicals | Corrosion Current Density (A/cm2) | Rtot (Ω·cm2) |
|---|---|---|
| Control | 4.57 10−6 | 1921 |
| 1 mM Selenite | 1.51 10−6 4.0 10−7 | 7420 193 |
| 5 mM Selenite | 1.18 10−6 3.4 10−7 | 9907 1480 |
| 10 mM Selenite | 1.45 10−6 0.6 10−7 | 8583 940 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Z.; Xu, C.C.; Zhang, D.; Buchheit, R. Corrosion Protective Film Formation on Mg Alloy AZ31 by Exposure to Dilute Selenite Solutions. Materials 2021, 14, 286. https://doi.org/10.3390/ma14020286
Feng Z, Xu CC, Zhang D, Buchheit R. Corrosion Protective Film Formation on Mg Alloy AZ31 by Exposure to Dilute Selenite Solutions. Materials. 2021; 14(2):286. https://doi.org/10.3390/ma14020286
Chicago/Turabian StyleFeng, Zhiyuan, Charles C. Xu, Dadi Zhang, and Rudolph Buchheit. 2021. "Corrosion Protective Film Formation on Mg Alloy AZ31 by Exposure to Dilute Selenite Solutions" Materials 14, no. 2: 286. https://doi.org/10.3390/ma14020286
APA StyleFeng, Z., Xu, C. C., Zhang, D., & Buchheit, R. (2021). Corrosion Protective Film Formation on Mg Alloy AZ31 by Exposure to Dilute Selenite Solutions. Materials, 14(2), 286. https://doi.org/10.3390/ma14020286






