The Effect of Zn and Zn–WO3 Composites Nano-Coatings Deposition on Hardness and Corrosion Resistance in Steel Substrate
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
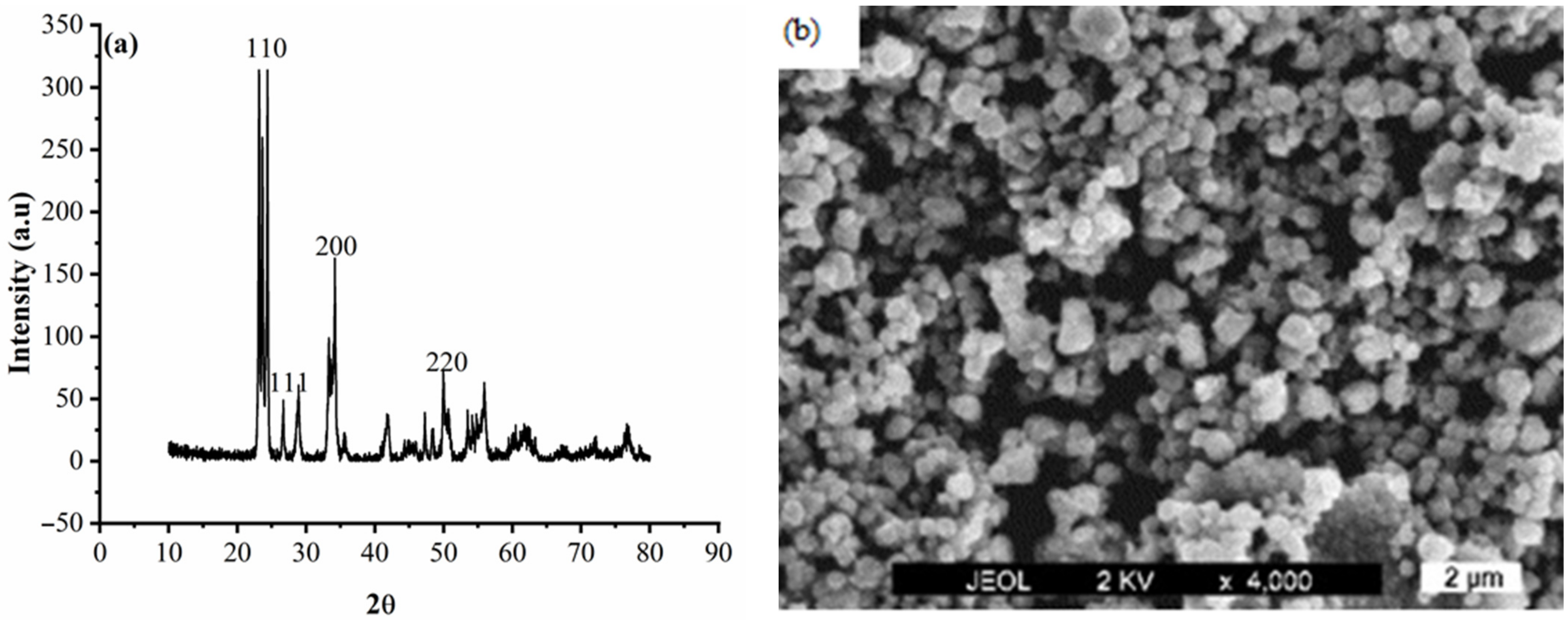
3.1. Particle Characterization
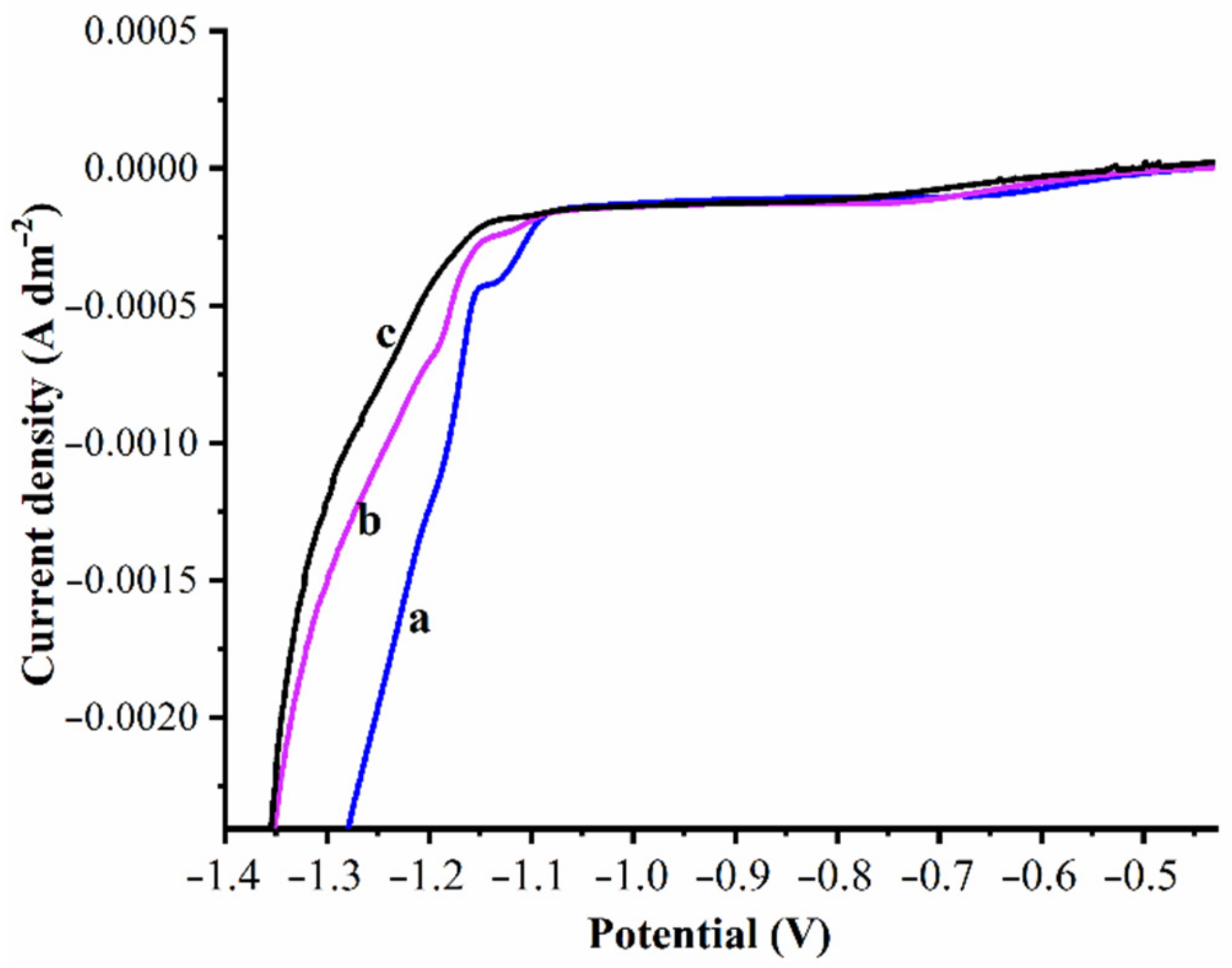
3.2. Electrodeposition
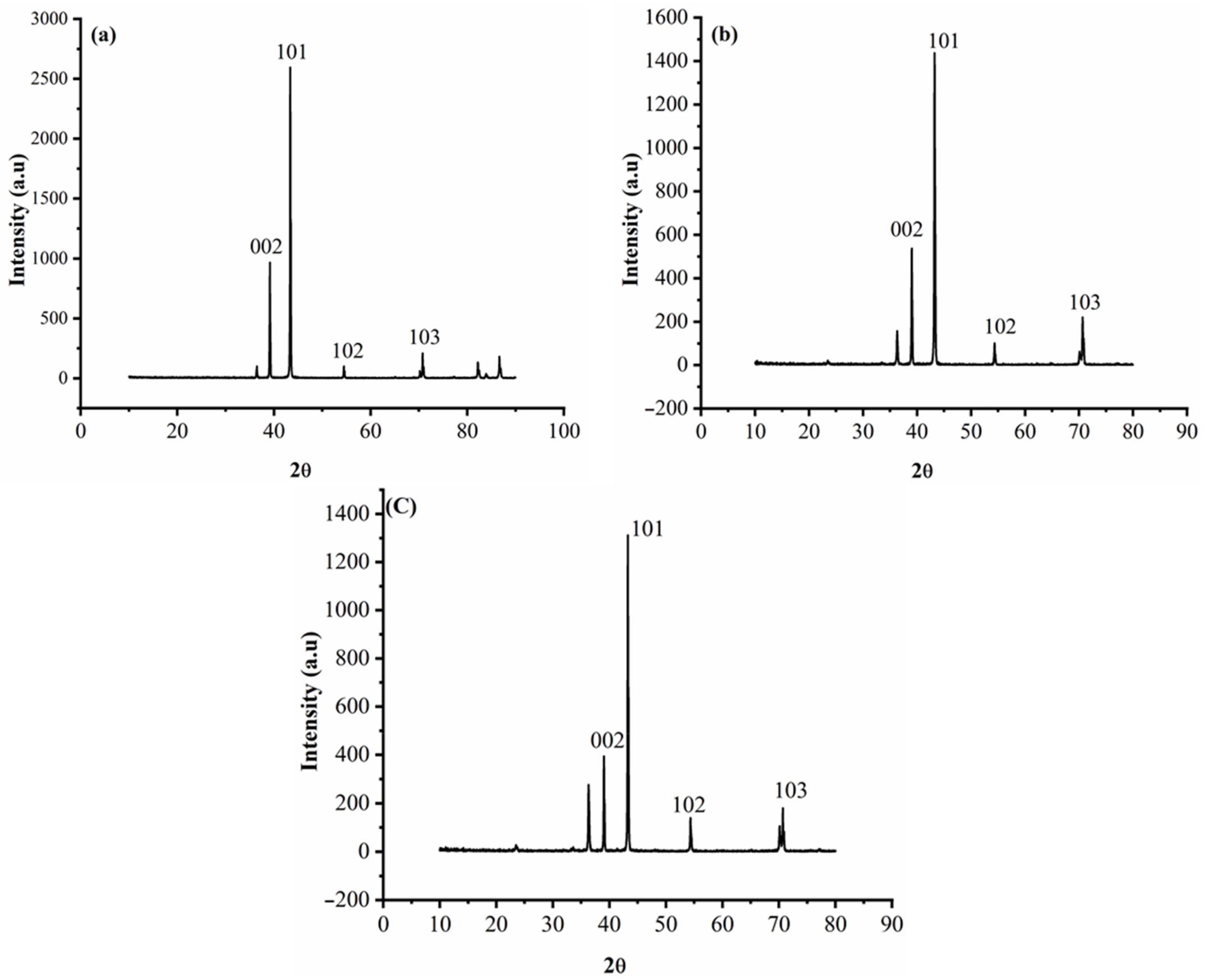
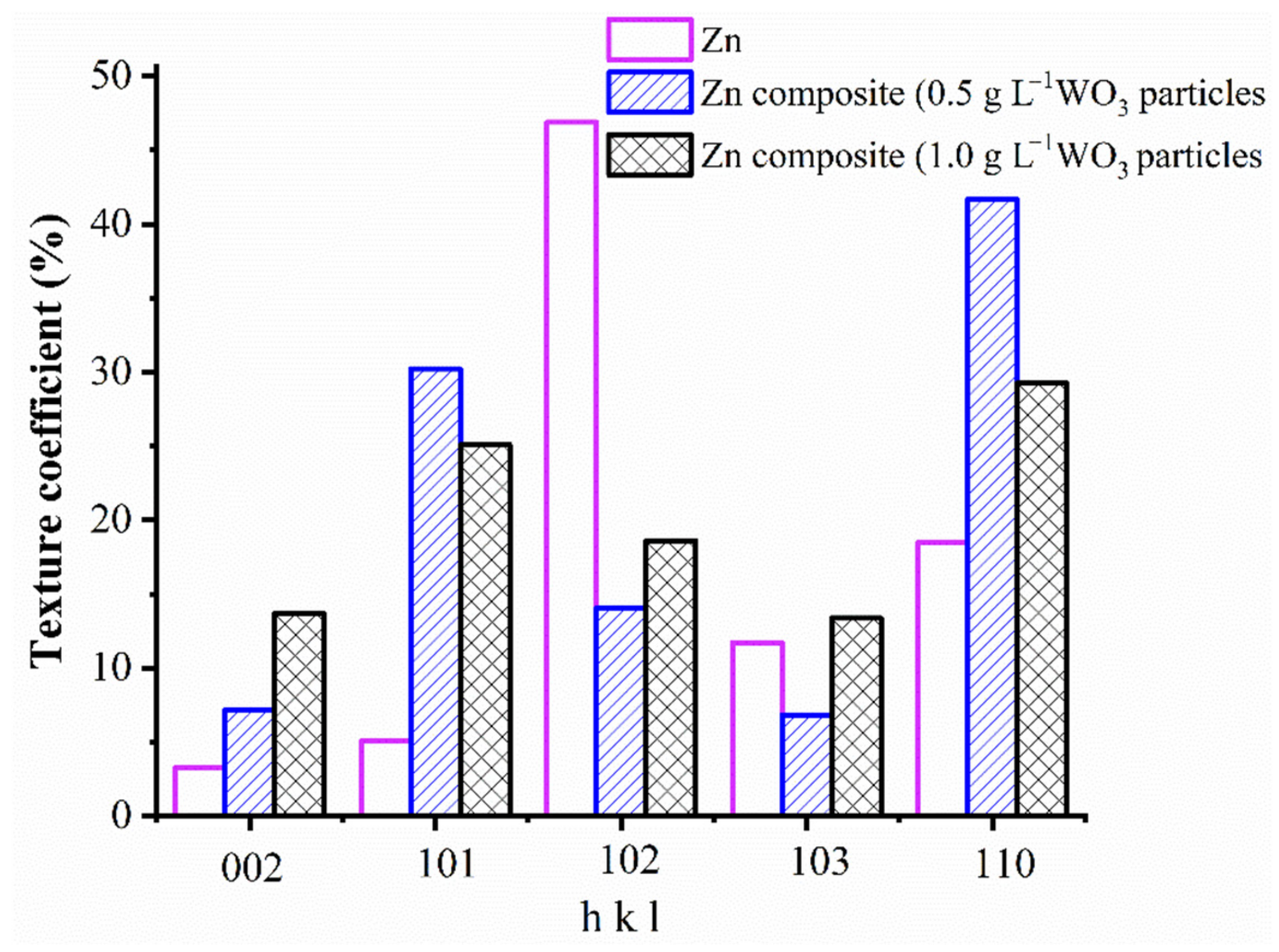
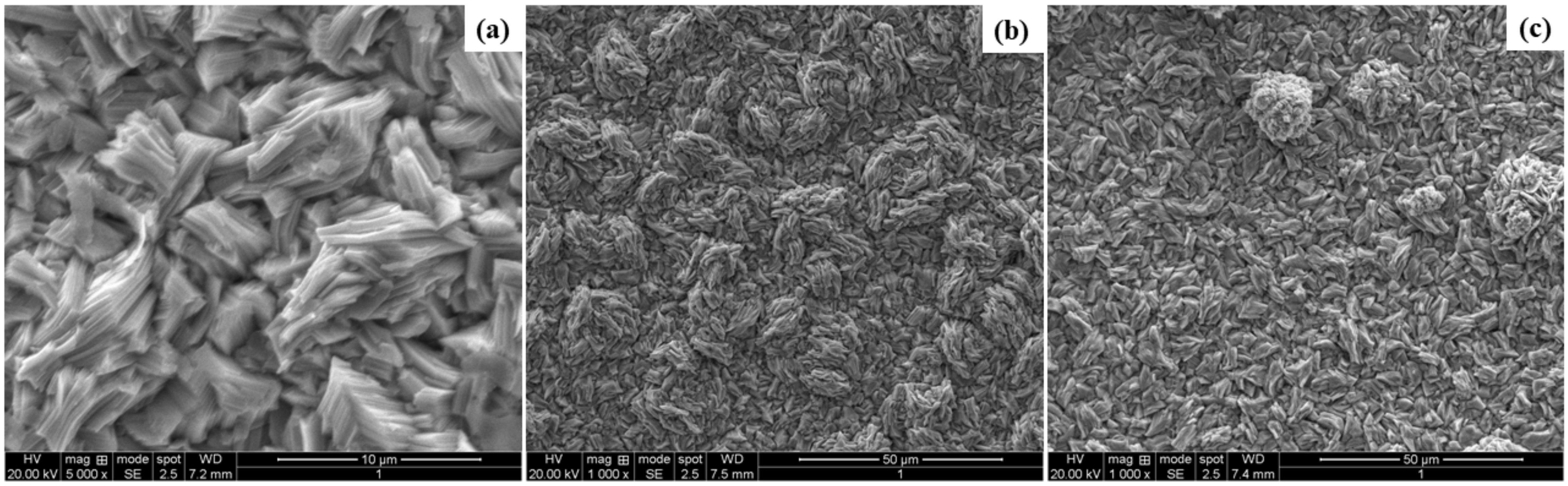
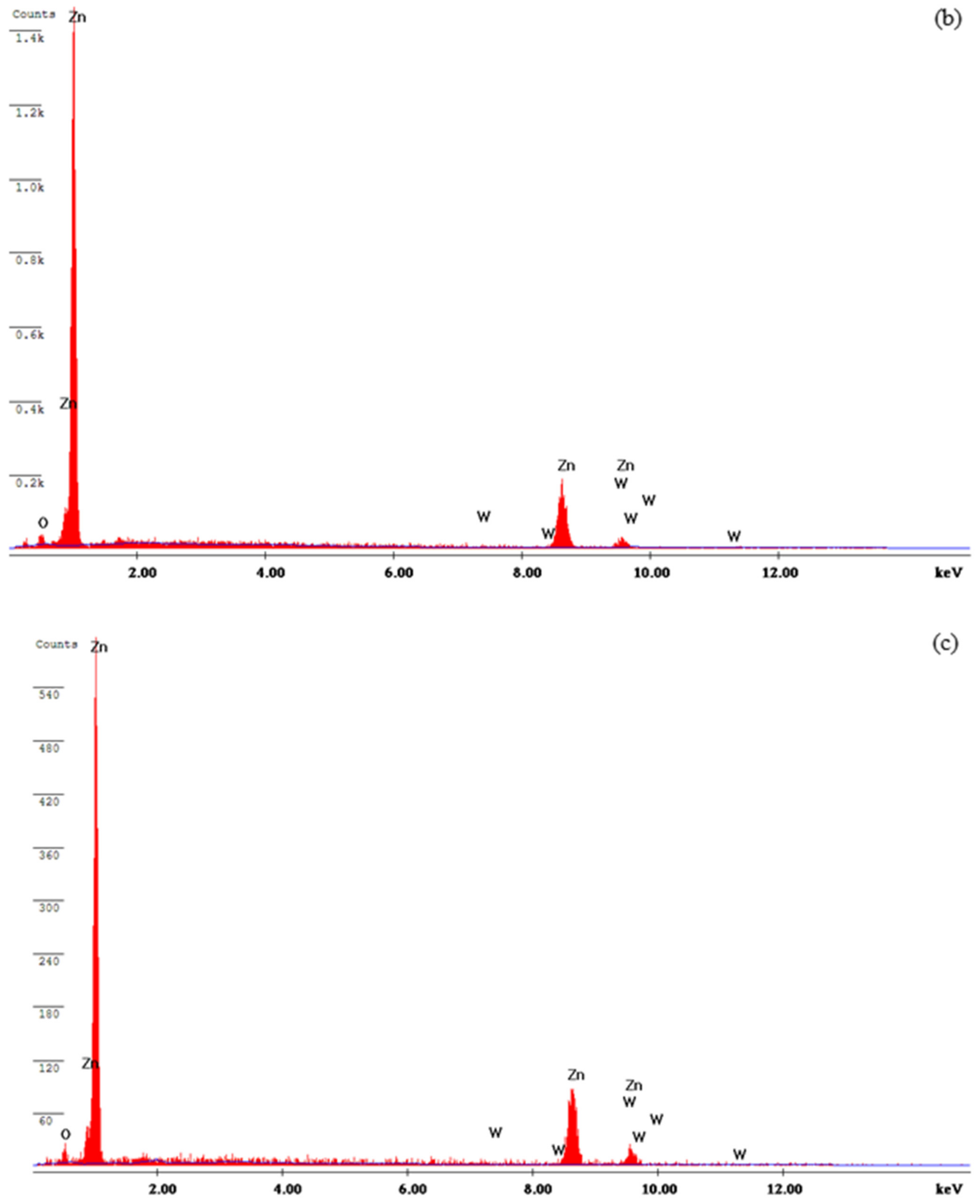
3.3. Characterization of the Deposits
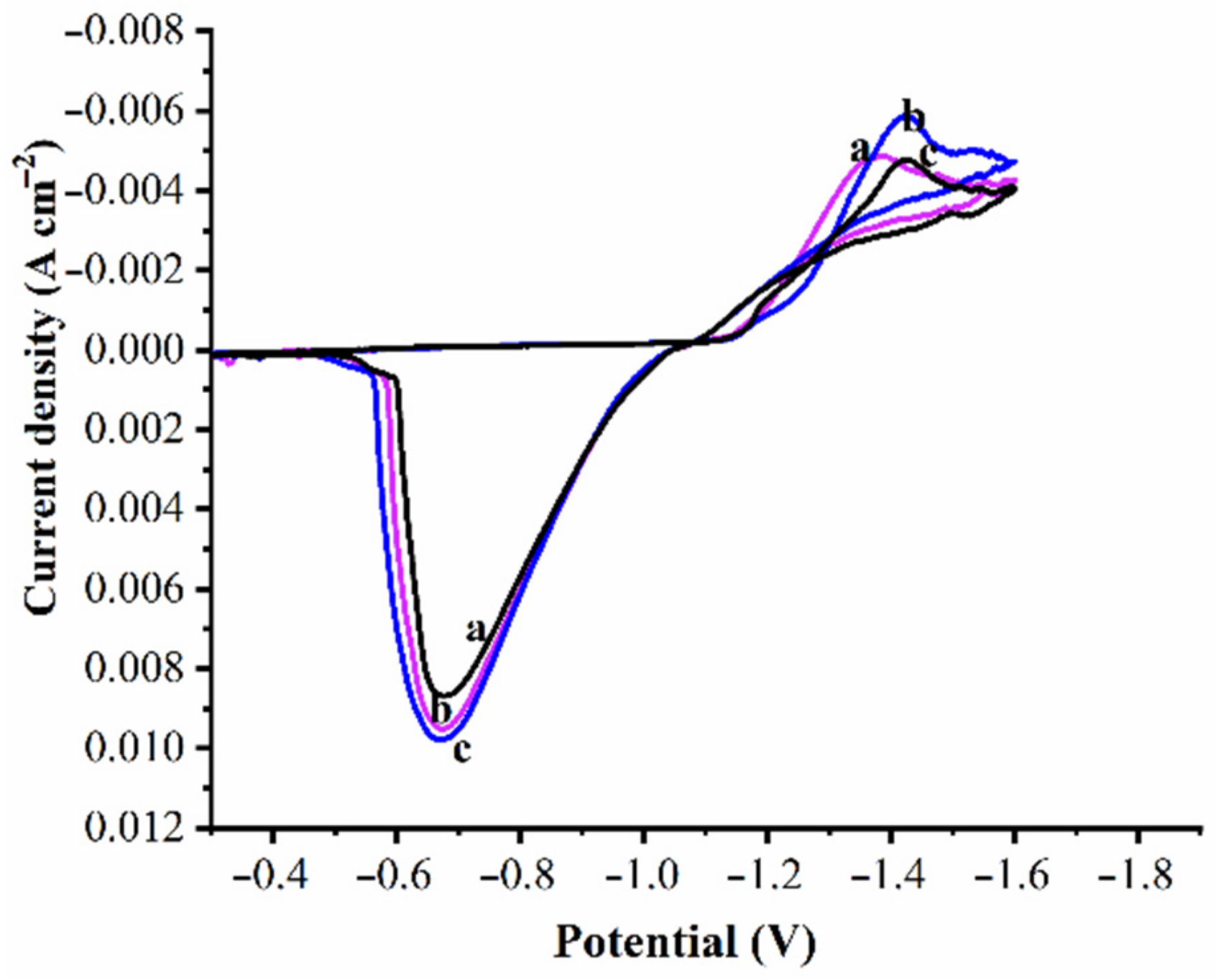
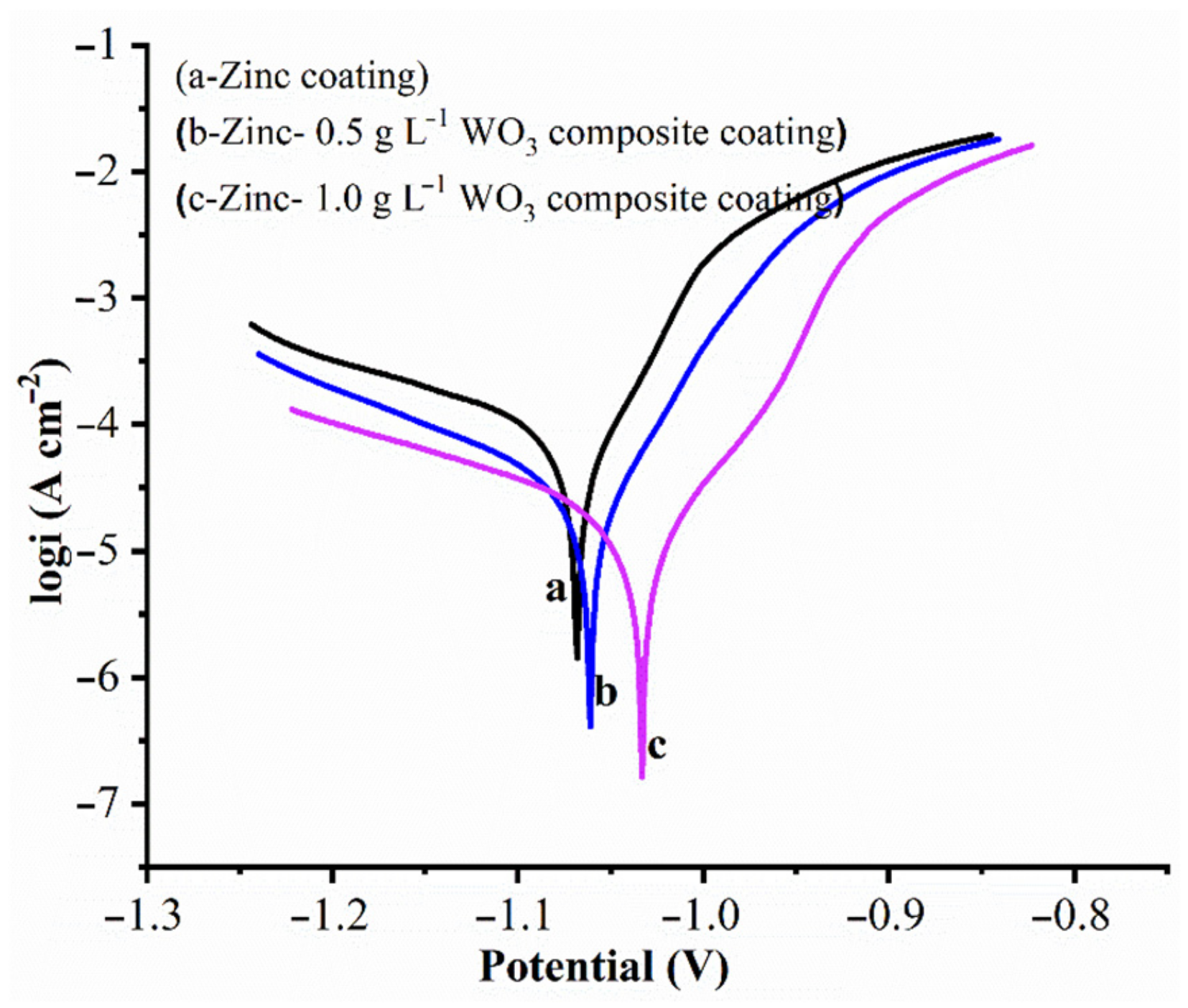
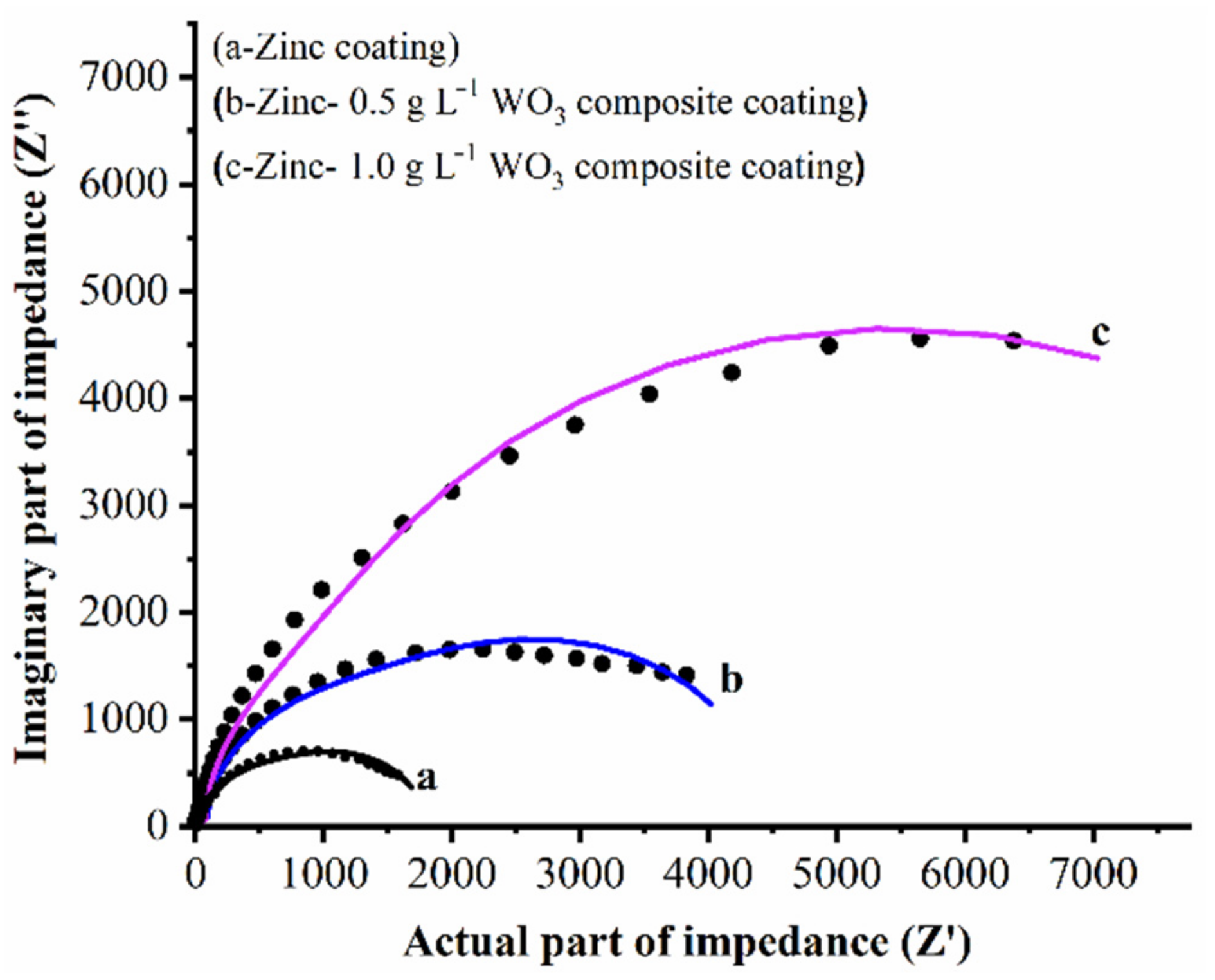
3.4. Corrosion Behavior of Coatings
4. Conclusions
- The pure Zn and Zn–WO3 composites are electrodeposited successfully on mild steel specimens.
- The Zn bath solution with 1 g/L of WO3 particles greatly influences the deposition process.
- The WO3 particles were present and incorporated in the Zn matrix, which was observed from SEM analysis.
- The incorporated WO3 particles changed the surface morphology of Zn deposits from a coarse grain to smaller grain size.
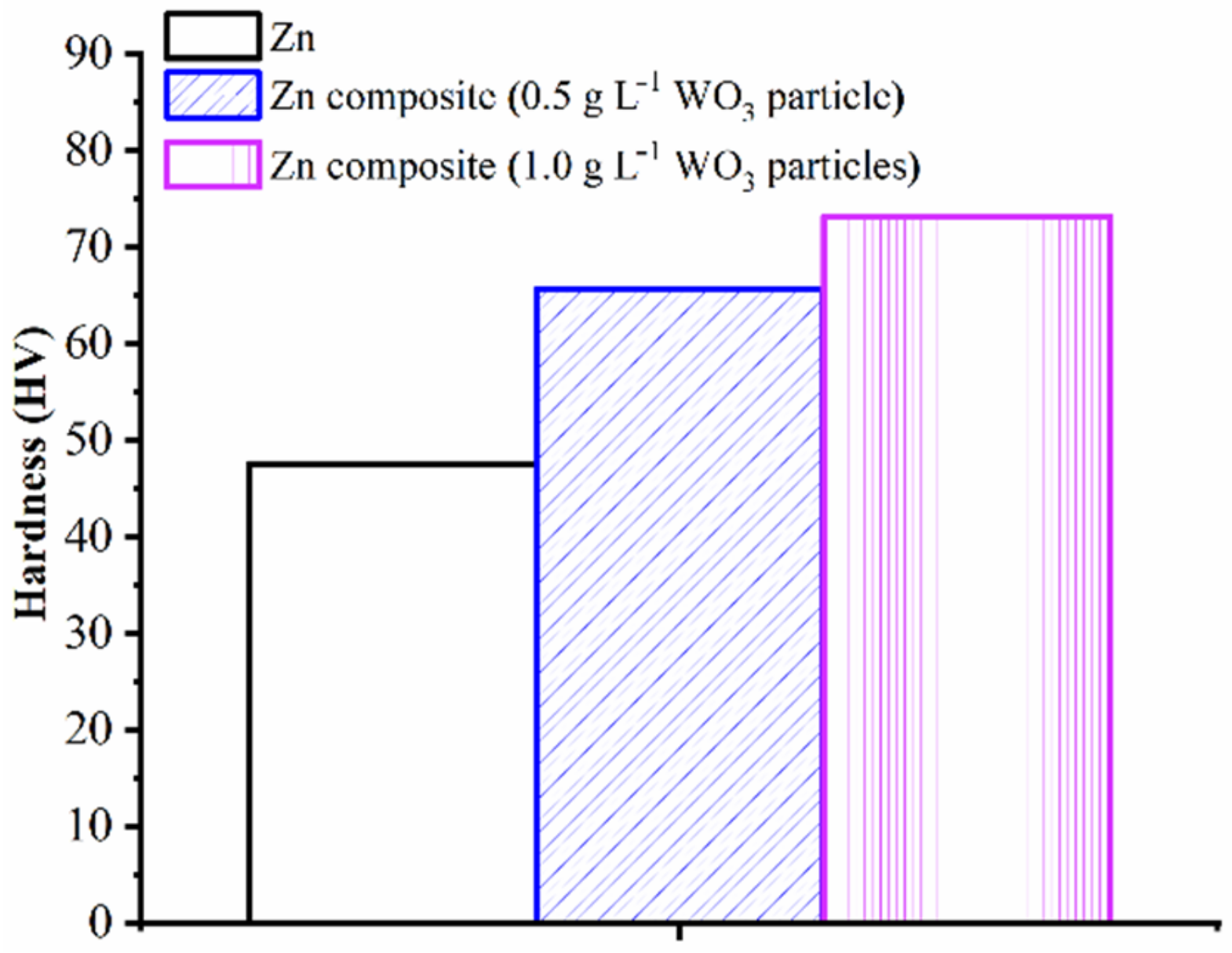
- The microhardness of Zn and Zn–WO3 of 0.5 g/L and Zn–WO3 of 1 g/L composite electrodeposits are found equal to 47.6, 65.6 and 73.2 HV, respectively. An increase in WO3 particle concentration (from 0.5 g/L and 1.0 g/L) tends to increase the micro-hardness by 11.58% on electrodeposits. Electrodepositing WO3 particles of 1 g/L composites resulted in a 53.78% increase in microhardness compared to bare Zn coating deposits.
- The corrosion rate of Zn and Zn–WO3 of 0.5 g/L and Zn–WO3 of 1 g/L composite electrodeposits is found equal to 32.69, 8.229, 2.803 Å min−1, respectively. The better corrosion resistance with composite coatings is attributed to the WO3 particles, which act as a physical barrier to the corrosion phenomenon.
- Zn–WO3 composite coatings can be economically coated onto steel parts (tanks, containers, boilers, etc.) and for those applications that require higher corrosion resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, S.; Kumar, M.P. Corrosion Resistance Methods for Stainless Steel: A Review. In New Challenges and Industrial Applications for Corrosion Prevention and Control; IGI Global: Hershey, PA, USA, 2020; pp. 208–225. [Google Scholar] [CrossRef]
- Patel, R.D.; Bhavsar, S.N. Experimental investigation during end milling of AISI D2 tool steel using AlCrN coated tool. Mater. Today-Proc. 2020, 22, 2647–2656. [Google Scholar] [CrossRef]
- Shreyas, P.; Panda, B.; Vishwanatha, A.D. Embrittlement of hot-dip galvanized steel: A review. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2021; Volume 2317, p. 020038. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, S.; Lai, F.; Qin, H.; Huang, L.; Li, X. Effect of quenching temperature on microstructure and rolling contact fatigue behavior of 17Cr2Ni2MoVNb steel. Metals 2018, 8, 735. [Google Scholar] [CrossRef]
- Abbas, M.; Shafiee, M. An overview of maintenance management strategies for corroded steel structures in extreme marine environments. Mar. Struct. 2020, 71, 102718. [Google Scholar] [CrossRef]
- Kumar, K.; Goyal, D.; Banwait, S.S. Effect of key parameters on fretting behaviour of wire rope: A review. Arch. Comput. Method E 2020, 27, 549–561. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, Z.; Zheng, H.; Wang, X.; Gao, J.; Wu, X.; Han, E.; Yang, S.; Huang, P. Corrosion fatigue model of austenitic stainless steels used in pressurized water reactor nuclear power plants. J. Nucl. Mater. 2020, 541, 152407. [Google Scholar] [CrossRef]
- Zhang, P.; Kang, L.; Wang, J.; Guo, J.; Hu, S.; Ling, Y. Mechanical properties and explosive spalling behavior of steel-fiber-reinforced concrete exposed to high temperature—a review. Appl. Sci. 2020, 10, 2324. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Y.; Yu, J.; Zhang, Y.; Zhao, Y.; Yuan, Q. Microstructure evolution and corrosion behavior of dissimilar 304/430 stainless steel welded joints. J. Manuf. Process. 2020, 50, 183–191. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, S.; Li, R. Comparative investigation of the effect of corrosion on the mechanical properties of different parts of thin-walled steel. Thin Wall. Struct. 2020, 146, 106450. [Google Scholar] [CrossRef]
- Li, C.Q.; Yang, W.; Shi, W. Corrosion effect of ferrous metals on degradation and remaining service life of infrastructure using pipe fracture as example. Struct. Infrastruct. Eng. 2020, 16, 583–598. [Google Scholar] [CrossRef]
- Kim, T.; Lee, Y.J.; Sanyal, S.; Woo, J.W.; Choi, I.H.; Yi, J. Mechanism of corrosion in porcelain insulators and its effect on the lifetime. Appl. Sci. 2020, 10, 423. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-resistant high-entropy alloys: A review. Metals 2017, 7, 43. [Google Scholar] [CrossRef]
- Koch, G.H.; Brongers, M.P.; Thompson, N.G.; Virmani, Y.P.; Payer, J.H. Corrosion Cost and Preventive Strategies in the United States; U.S. Federal Highway Administration: Washington, DC, USA, 2001.
- Cramer, S.D.; Covino, B.S., Jr. ASM Handbook, Volume 13A—Corrosion: Fundamentals, Testing, and Protection; ASM International: Novelty, OH, USA, 2003. [Google Scholar]
- Wei, X.; Fu, D.; Chen, M.; Wu, W.; Wu, D.; Liu, C. Data mining to effect of key alloying elements on corrosion resistance of low alloy steels in sanya seawater environment alloying elements. J. Mater. Sci. Technol. 2021, 64, 222–232. [Google Scholar] [CrossRef]
- Yang, W.; Feng, W.; Liao, Z.; Yang, Y.; Miao, G.; Yu, B.; Pei, X. Protection of mild steel with molecular engineered epoxy nanocomposite coatings containing corrosion inhibitor functionalized nanoparticles. Surf. Coat. Technol. 2021, 406, 126639. [Google Scholar] [CrossRef]
- Díaz, I.; Cano, H.; Lopesino, P.; De la Fuente, D.; Chico, B.; Jiménez, J.A.; Medina, S.F.; Morcillo, M. Five-year atmospheric corrosion of Cu, Cr and Ni weathering steels in a wide range of environments. Corros. Sci. 2018, 141, 146–157. [Google Scholar] [CrossRef]
- Volovitch, P.; Vu, T.N.; Allély, C.; Aal, A.A.; Ogle, K. Understanding corrosion via corrosion product characterization: II. Role of alloying elements in improving the corrosion resistance of Zn–Al–Mg coatings on steel. Corros. Sci. 2011, 53, 2437–2445. [Google Scholar] [CrossRef]
- Sk, M.H.; Abdullah, A.M.; Ko, M.; Laycock, N.; Ingham, B.; Ryan, M.P.; Williams, D.E. Effect of Cr/Mo on the protectiveness of corrosion scales on carbon steel in sweet medium under high flow regime. ECS Trans. 2017, 80, 509. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Z.; Wang, Q.; Wang, Z.; Zhang, X.; Liu, Y. Role of passive film in dominating the electrochemical corrosion behavior of FeCrMoCBY amorphous coating. J. Alloys Compd. 2019, 811, 151962. [Google Scholar] [CrossRef]
- Zhang, Z.; Jing, H.; Xu, L.; Han, Y.; Zhao, L.; Zhou, C. Effects of nitrogen in shielding gas on microstructure evolution and localized corrosion behavior of duplex stainless steel welding joint. Appl. Surf. Sci. 2017, 404, 110–128. [Google Scholar] [CrossRef]
- Zhu, Y.; Poplawsky, J.D.; Li, S.; Unocic, R.R.; Bland, L.G.; Taylor, C.D.; Locke, J.S.; Marquis, E.A.; Frankel, G.S. Localized corrosion at nm-scale hardening precipitates in Al-Cu-Li alloys. Acta Mater. 2020, 189, 204–213. [Google Scholar] [CrossRef]
- Zhang, X.; Ba, Z.; Wang, Q.; Wu, Y.; Wang, Z.; Wang, Q. Uniform corrosion behavior of GZ51K alloy with long period stacking ordered structure for biomedical application. Corros. Sci. 2014, 88, 1–5. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Han, J.; Wu, H.C.; Yang, B.; Wang, X.T. Effect of sigma phase precipitation on the mechanical and wear properties of Z3CN20. 09M cast duplex stainless steel. Nucl. Eng. Des. 2013, 259, 1–7. [Google Scholar] [CrossRef]
- Ram Mohan Rao, K. Corrosion Resistance of High Entropy Alloys. In Coatings. Materials Forming, Machining and Tribology; Kumar, K., Babu, B.S., Davim, J.P., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, K.E.; Hong, S.J. Surface Analysis of Chamber Coating Materials Exposed to CF4/O2 Plasma. Coatings 2021, 11, 105. [Google Scholar] [CrossRef]
- Yung, T.Y.; Chen, T.C.; Tsai, K.C.; Lu, W.F.; Huang, J.Y.; Liu, T.Y. Thermal spray coatings of Al, ZnAl and inconel 625 alloys on SS304L for anti-saline corrosion. Coatings 2019, 9, 32. [Google Scholar] [CrossRef]
- Ji, R.; Liu, Y.; Xu, C.; Li, X.; Cai, B.; Zhang, Y. Novel method for the hybrid composite electroplating of the upstream pumping mechanical seal. Int. J. Adv. Manuf. Technol. 2017, 89, 1875–1886. [Google Scholar] [CrossRef]
- Patel, G.C.M.; Pradeep, N.B.; Harsha, H.M.; Shettigar, A.K. Experimental analysis and optimization of plasma spray parameters on microhardness and wear loss of Mo-Ni-Cr coated super duplex stainless steel. Aust. J. Mech. Eng. 2020, 1–13. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, J.; Chen, J.; Zhao, J.; Yu, Y.; Zhou, H. High temperature wear resistance of laser cladding NiCrBSi and NiCrBSi/WC-Ni composite coatings. Wear 2011, 270, 492–498. [Google Scholar] [CrossRef]
- Luo, X.; Smith, G.M.; Sampath, S. On the interplay between adhesion strength and tensile properties of thermal spray coated laminates—Part I: High velocity thermal spray coatings. J. Therm. Spray Technol. 2018, 27, 296–307. [Google Scholar] [CrossRef]
- Ang, A.S.M.; Berndt, C.C. A review of testing methods for thermal spray coatings. Int. Mater. Rev. 2014, 59, 179–223. [Google Scholar] [CrossRef]
- Nie, P.; Ojo, O.A.; Li, Z. Modeling analysis of laser cladding of a nickel-based superalloy. Surf. Coat. Technol. 2014, 258, 1048–1059. [Google Scholar] [CrossRef]
- Yao, F.; Fang, L. Thermal stress cycle simulation in laser cladding process of Ni-based coating on H13 steel. Coatings 2021, 11, 203. [Google Scholar] [CrossRef]
- Rottwinkel, B.; Pereira, A.; Alfred, I.; Noelke, C.; Wesling, V.; Kaierle, S. Turbine blade tip single crystalline clad deposition with applied remelting passes for well oriented volume extension. J. Laser Appl. 2017, 29, 022310. [Google Scholar] [CrossRef]
- Libório, M.S.; Praxedes, G.B.; Lima, L.L.F.; Nascimento, I.G.; Sousa, R.R.M.; Naeem, M.; Costa, T.H.; Alves, S.M.; Iqbal, J. Surface modification of M2 steel by combination of cathodic cage plasma deposition and magnetron sputtered MoS2-TiN multilayer coatings. Surf. Coat. Technol. 2020, 384, 125327. [Google Scholar] [CrossRef]
- Musil, J.; Kadlec, S. Reactive sputtering of TiN films at large substrate to target distances. Vacuum 1990, 40, 435–444. [Google Scholar] [CrossRef]
- Raman, P.; Shchelkanov, I.A.; McLain, J.; Ruzic, D.N. High power pulsed magnetron sputtering: A method to increase deposition rate. J. Vac. Sci. Technol. A 2015, 33, 031304. [Google Scholar] [CrossRef]
- Tozar, A. Investigating the hexadecylamine as a new nonionic surfactant candidate for electrodeposition of wear-resistant metal-matrix composites. Surf. Eng. 2020, 36, 990–999. [Google Scholar] [CrossRef]
- Schlesinger, M. Modern Electroplating; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Lotfi, N.; Aliofkhazraei, M.; Rahmani, H.; Darband, G.B. Zinc–nickel alloy electrodeposition: Characterization, properties, multilayers and composites. Prot. Met. Phys. Chem. Surf. 2018, 54, 1102–1140. [Google Scholar] [CrossRef]
- Mohankumar, P.C.; Venkatesha, V.T. Surfactants effect on Zn–Si3N4 coating, electrochemical properties, and their corrosion behaviors. Ind. Eng. Chem. Res. 2013, 52, 12827–12837. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Luo, J.; Cheng, P.; Wang, Y.; Wang, H.; Ding, G. Microstructure of Cu-diamond composites with near-perfect interfaces prepared via electroplating and its thermal properties. Mater. Charact. 2019, 150, 199–206. [Google Scholar] [CrossRef]
- Xia, F.; Jia, W.; Ma, C.; Wang, J. Synthesis of Ni–TiN composites through ultrasonic pulse electrodeposition with excellent corrosion and wear resistance. Ceram. Int. 2018, 44, 766–773. [Google Scholar] [CrossRef]
- DeMasi-Marcin, J.T.; Gupta, D.K. Protective coatings in the gas turbine engine. Surf. Coat. Technol. 1994, 68, 1–9. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Li, G.; Li, C. Effects of zinc coating on interfacial microstructures and mechanical properties of aluminum/steel bimetallic composites. J. Alloys Compd. 2016, 678, 249–257. [Google Scholar] [CrossRef]
- Sriraman, K.R.; Brahimi, S.; Szpunar, J.A.; Osborne, J.H.; Yue, S. Tribocorrosion behavior of Zn, Zn–Ni, Cd and Cd–Ti electrodeposited on low carbon steel substrates. Surf. Coat. Technol. 2013, 224, 126–137. [Google Scholar] [CrossRef]
- Praveen Kumar, C.M.; Venkatesha, T.V.; Vathsala, K.; Nayana, K.O. Electrodeposition and corrosion behavior of Zn–Ni and Zn–Ni–Fe2O3 coatings. J. Coat. Technol. Res. 2012, 9, 71–77. [Google Scholar] [CrossRef]
- Burduhos-Nergis, D.P.; Vizureanu, P.; Sandu, A.V.; Bejinariu, C. Evaluation of the corrosion resistance of phosphate coatings deposited on the surface of the carbon steel used for carabiners manufacturing. Appl. Sci. 2020, 10, 2753. [Google Scholar] [CrossRef]
- Sajjadnejad, M.; Mozafari, A.; Omidvar, H.; Javanbakht, M. Preparation and corrosion resistance of pulse electrodeposited Zn and Zn–SiC nanocomposite coatings. Appl. Surf. Sci. 2014, 300, 1–7. [Google Scholar] [CrossRef]
- Sajjadnejad, M.; Ghorbani, M.; Afshar, A. Microstructure-corrosion resistance relationship of direct and pulse current electrodeposited Zn-TiO2 nanocomposite coatings. Ceram. Int. 2015, 41, 217–224. [Google Scholar] [CrossRef]
- Gao, W.; Cao, D.; Jin, Y.; Zhou, X.; Cheng, G.; Wang, Y. Microstructure and properties of Cu-Sn-Zn-TiO2 nano-composite coatings on mild steel. Surf. Coat. Technol. 2018, 350, 801–806. [Google Scholar] [CrossRef]
- Fayomi, O.S.I.; Popoola, A.P.I.; Kanyane, L.R.; Monyai, T. Development of reinforced in-situ anti-corrosion and wear Zn-TiO2/ZnTiB2 coatings on mild steel. Results Phys. 2017, 7, 644–650. [Google Scholar] [CrossRef]
- Miller, R.J.; Adeleye, A.S.; Page, H.M.; Kui, L.; Lenihan, H.S.; Keller, A.A. Nano and traditional copper and zinc antifouling coatings: Metal release and impact on marine sessile invertebrate communities. J. Nanopart. Res. 2020, 22, 1–15. [Google Scholar] [CrossRef]
- Hegyi, A.; Dico, C.; Constantinescu, H.; Baeră, C. Influence of hot-dip galvanizing of reinforcement on the kinetics and thermodynamics of corrosion process in concrete. Procedia Eng. 2017, 181, 226–233. [Google Scholar] [CrossRef]
- Baruwa, A.D.; Akinlabi, E.T.; Oladijo, O.P. Surface coating processes: From conventional to the advanced methods: A short review. In Advances in Manufacturing Engineering, Lecture Notes in Mechanical Engineering; Emamian, S.S., Awang, M., Yusof, F., Eds.; Spring: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Zotov, S.V. Analysis of galvanized coatings applied on general purpose wire. Solid State Phenom. 2020, 299, 827–832. [Google Scholar] [CrossRef]
- Roventi, G.; Bellezze, T.; Fratesi, R. Electrodeposition of Zn–SiC nanocomposite coatings. J. Appl. Electrochem. 2013, 43, 839–846. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Do, D.T.; Vu, X.H.; Dang, D.V.; Nguyen, D.C. ZnO nanoplates surfaced-decorated by WO3 nanorods for NH3 gas sensing application. Adv. Nat. Sci.-Nanosci. Nanotechnol. 2016, 7, 015004. [Google Scholar] [CrossRef]
- Demir, K.Ç. Corrosion behavior of electrodeposited WO3 thin films. Ceram. Int. 2020, 46, 4358–4364. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2003; Volume 529, ISBN 9781439814611. [Google Scholar]
- Zhao, Q.; Fang, Y.; Qiao, K.; Wei, W.; Yao, Y.; Gao, Y. Printing of WO3/ITO nanocomposite electrochromic smart windows. Sol. Energy Mater. Sol. Cells 2019, 194, 95–102. [Google Scholar] [CrossRef]
- Lin, Z.; Xie, P.; Zhan, R.; Chen, D.; She, J.; Deng, S.; Xu, N.; Chen, J. Defect-enhanced field emission from WO3 nanowires for flat-panel X-ray sources. ACS Appl. Nano Mater. 2019, 2, 5206–5213. [Google Scholar] [CrossRef]
- Ho, G.W.; Chua, K.J.; Siow, D.R. Metal loaded WO3 particles for comparative studies of photocatalysis and electrolysis solar hydrogen production. Chem. Eng. J. 2012, 181, 661–666. [Google Scholar] [CrossRef]
- Goyal, A.; Pouya, H.S.; Ganjian, E.; Olubanwo, A.O.; Khorami, M. Predicting the corrosion rate of steel in cathodically protected concrete using potential shift. Constr. Build. Mater. 2019, 194, 344–349. [Google Scholar] [CrossRef]
- Daniyan, A.A.; Umoru, L.E.; Popoola, A.P.I.; Fayomi, O.S.I. Comparative studies of microstructural, tribological and corrosion properties of Zn-TiO2 and Zn-TiO2-WO3 nano-composite coatings. Results Phys. 2017, 7, 3222–3229. [Google Scholar] [CrossRef]
- Deepa, K.; Arthoba Nayaka, Y. Synthesis of CuO micro and nanoparticles as composite additives for corrosion resistant Zn-composite coatings on mild steel. Inorg. Nano-Met. Chem. 2020, 50, 354–360. [Google Scholar] [CrossRef]
- Pouraliakbar, H.; Jandaghi, M.R.; Baygi, S.J.M.; Khalaj, G. Microanalysis of crystallographic characteristics and structural transformations in SPDed Al–Mn–Si alloy by dual-straining. J. Alloys Compd. 2017, 696, 1189–1198. [Google Scholar] [CrossRef]
- Bach, L.G.; Nguyen, N.G.; Ho, V.T.T. Enhanced light scattering by preferred orientation control of Ga doped ZnO films prepared through MOCVD. Int. J. Photoenergy 2016, 2016, 1217576. [Google Scholar] [CrossRef]
- Shirani, A.; Momenzadeh, M.; Sanjabi, S. Surfactant effect on electrochemical behavior of Co–TiO2 nanocomposite coatings. Surf. Coat. Technol. 2012, 206, 2870–2876. [Google Scholar] [CrossRef]
- Wu, N.L.; Wang, S.Y.; Rusakova, I.A. Inhibition of crystallite growth in the sol-gel synthesis of nanocrystalline metal oxides. Science 1999, 285, 1375–1377. [Google Scholar] [CrossRef]
- Daniyan, A.A.; Umoru, L.E.; Fayomi, O.S.I.; Popoola, A.P.I. Structural evolution, optoelectrical and corrosion properties of electrodeposited WO3 integration on Zn-TiO2 electrolyte for defence super application. Def. Technol. 2018, 14, 396–402. [Google Scholar] [CrossRef]
- Liang, Y.C.; Chang, C.W. Improvement of ethanol gas-sensing responses of ZnO–WO3 composite nanorods through annealing induced local phase transformation. Nanomaterials 2019, 9, 669. [Google Scholar] [CrossRef]
- Praveen, B.M.; Venkatesha, T.V. Electrodeposition and properties of Zn-nanosized TiO2 composite coatings. Appl. Surf. Sci. 2008, 254, 2418–2424. [Google Scholar] [CrossRef]
- Jabbar, A.; Yasin, G.; Khan, W.Q.; Anwar, M.Y.; Korai, R.M.; Nizam, M.N.; Muhyodin, G. Electrochemical deposition of nickel graphene composite coatings: Effect of deposition temperature on its surface morphology and corrosion resistance. RSC Adv. 2017, 7, 31100–31109. [Google Scholar] [CrossRef]
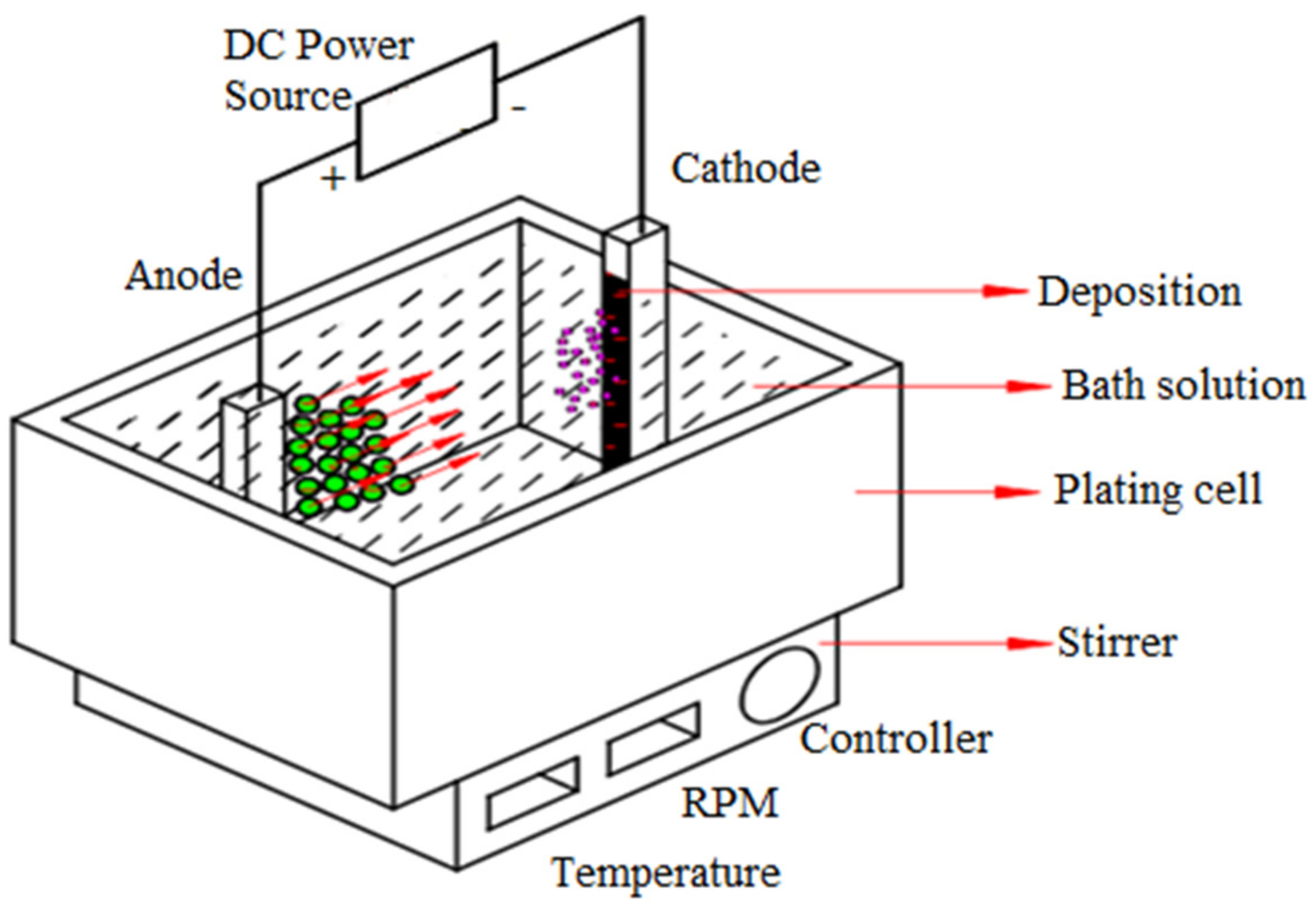

| Deposit | Bath Solution | ZnSO4 (g/L) | Na2SO4 (g/L) | H3BO3 (g/L) | CTAB (g/L) | WO3 (g/L) | Operating Parameters |
|---|---|---|---|---|---|---|---|
| I | Zn | 200 | 40 | 8 | 0.05 | - | Current density: 4A dm-2 |
| II | Zn composite | 200 | 40 | 8 | 0.05 | 0.5 | pH-3.0 |
| III | Zn composite | 200 | 40 | 8 | 0.05 | 1.0 | Anode—Zn metal Cathode—mild Steel Stirring rate—300 rpm Cathode—40 mm × 40 mm × 1 mm Dimension Plating time—20 min |
| Samples | βa (V−1) | βc (V−1) | Ecorr (V) | icorr (A) | Corrosion Rate (Å min−1) |
|---|---|---|---|---|---|
| Zn | 10.912 | 4.417 | −1.068 | 1.148 × 10−4 | 32.69 ± 0.27 |
| Zn–WO3 (0.5 g L−1) | 17.583 | 5.771 | −1.061 | 2.890 × 10−5 | 8.229 ± 0.22 |
| Zn–WO3 (1.0 g L−1) | 26.995 | 4.611 | −1.033 | 9.844 × 10−6 | 2.803 ± 0.19 |
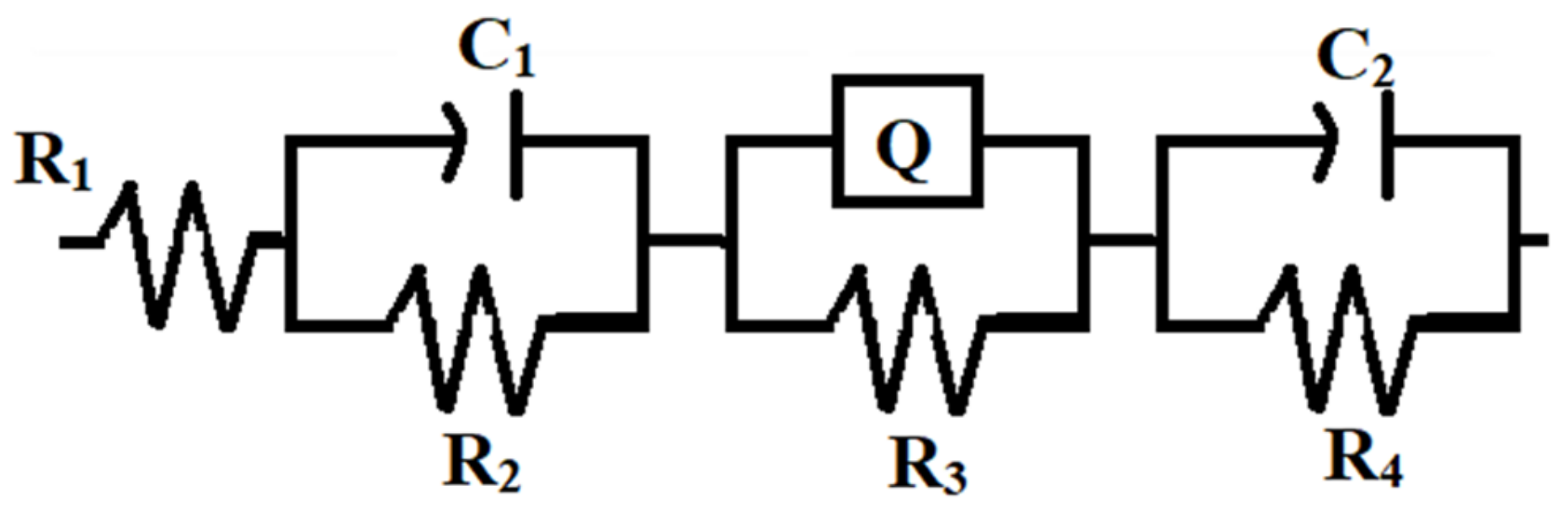
| Samples | R1 (Ωcm2) | R2 (Ωcm2) | C1 (F) | R3 (Ωcm2) | Q | R4 (Ωcm2) | C2 (F) | Rp (Ωcm2) |
|---|---|---|---|---|---|---|---|---|
| Zn coating | 9.014 × 10−4 | 76.50 | 5.626 × 10−7 | 537.6 | 3.586 × 10−6 | 139.5 | 4.323 × 10−4 | 753.6 |
| Zn–WO3 (0.5 g L−1) | 3.093 × 10−11 | 85.70 | 3.195 × 10−7 | 1733 | 14.86 × 10−6 | 920.7 | 3.123 × 10−6 | 2739.4 |
| Zn–WO3 (1.0 g L−1) | 4.769 × 10−4 | 624.2 | 0.714 × 10−6 | 3292 | 10.65 × 10−6 | 52.00 | 6.828 × 10−7 | 3968.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, C.M.P.; Chandrashekarappa, M.P.G.; Kulkarni, R.M.; Pimenov, D.Y.; Giasin, K. The Effect of Zn and Zn–WO3 Composites Nano-Coatings Deposition on Hardness and Corrosion Resistance in Steel Substrate. Materials 2021, 14, 2253. https://doi.org/10.3390/ma14092253
Kumar CMP, Chandrashekarappa MPG, Kulkarni RM, Pimenov DY, Giasin K. The Effect of Zn and Zn–WO3 Composites Nano-Coatings Deposition on Hardness and Corrosion Resistance in Steel Substrate. Materials. 2021; 14(9):2253. https://doi.org/10.3390/ma14092253
Chicago/Turabian StyleKumar, Channagiri Mohankumar Praveen, Manjunath Patel Gowdru Chandrashekarappa, Raviraj Mahabaleshwar Kulkarni, Danil Yurievich Pimenov, and Khaled Giasin. 2021. "The Effect of Zn and Zn–WO3 Composites Nano-Coatings Deposition on Hardness and Corrosion Resistance in Steel Substrate" Materials 14, no. 9: 2253. https://doi.org/10.3390/ma14092253
APA StyleKumar, C. M. P., Chandrashekarappa, M. P. G., Kulkarni, R. M., Pimenov, D. Y., & Giasin, K. (2021). The Effect of Zn and Zn–WO3 Composites Nano-Coatings Deposition on Hardness and Corrosion Resistance in Steel Substrate. Materials, 14(9), 2253. https://doi.org/10.3390/ma14092253









