Abstract
In this study, the removal of nickel (Ni(II)) by adsorption from synthetically prepared solutions using natural bentonites (Lieskovec (L), Hliník nad Hronom (S), Jelšový Potok (JP), and Stará Kremnička (SK)) was investigated. All experiments were carried out under batch processing conditions, with the concentration of Ni(II), temperature, and time as the variables. The adsorption process was fast, approaching equilibrium within 30 min. The Langmuir maximum adsorption capacities of the four bentonite samples used were found to be 8.41, 12.24, 21.79, and 21.93 mg g–1, respectively. The results best fitted the pseudo-second-order kinetic model, with constant rates in a range of 0.0948–0.3153 g mg–1 min. The effect of temperature was investigated at temperatures of 20, 30, and 40 °C. Thermodynamic parameters, including standard enthalpy (ΔH0), Gibbs energy (ΔG0), and standard entropy (ΔS0), were calculated. The adsorption of Ni(II) by bentonite samples was an endothermic and spontaneous process. These results indicated that, of the bentonite samples used, the natural bentonites from JP and SK were most suitable for the removal of nickel from synthetically prepared solutions.
1. Introduction
The contamination of the environment with wastewater has received a great deal of attention in recent years. Wastewater contamination consists of fouling by heavy metal ions, which originate from various industrial activities; however, sometimes an increased concentration of heavy metals may also be found in drinking water sources. Heavy metals pose an extraordinary environmental risk, due to their ability to accumulate in living and nonliving organisms [1,2].
The group of heavy metals posing a potential risk to the environment also includes nickel. Nickel (Ni, atomic number: 28) is a silver-white heavy metal, occurring in oxidation states of −1, 0, +1, +2, +3, and +4. The most common and important oxidation state of Ni is +2. In nature, this occurs naturally in soils and surface waters (at less than 100 mg L–1) [3]. The source of nickel in wastewater is most often as waste from electroplating and battery production. Its other sources include metal mining, smelting, fossil fuel combustion, vehicle emissions, domestic, municipal, and industrial waste disposal, fertilizer applications, and organic fertilizers [4]. Although nickel (as Ni(II) complexes) is an essential element for plants and plays an important role in plant metabolism at increased concentrations (5–15 mg L–1 Ni, over one week), it causes chlorosis, necrosis, and leaf wilting [3]. As nickel is a ubiquitous metal, contact with nickel compounds (soluble or insoluble) cannot be eliminated. The most common skin allergies are dermatitis, while diseases such as cardiovascular and kidney diseases, lung fibrosis, and lung and upper respiratory cancer are also known. It is now confirmed that some nickel compounds are highly carcinogenic to humans, but the mechanism of carcinogenesis is unclear [5].
Ni(II) ions are also very important parts of rechargeable alkaline Zn–Co batteries. The electrochemical performance of the battery can be much higher via substitution of Ni(II) ions onto a Co3O4 electrode. The substitution ratios of Ni(II) ions can be different. The study [6] writes about the methods of doping the Co3O4 electrode with nickel ions. Cheap and effective electrocatalysis is crucial for hydrogen production via electrocatalytic water splitting. Nickel is used as a part of Ni/Gd2O3/NiO nanofibers, which have very high electrocatalytic performance. The core of this material is built by NiO, and the molecules of Ni/Gd2O3 are concentrated around the core. The details are summarized in the study [7].
As the concentration of nickel is constantly growing, it is important to know and explore all of its sources, so that technologies can be developed to reduce its concentration in the environment.
With the increasing concentration of toxic metals in wastewater and the stringent standards that determine the maximum concentration of heavy metals in wastewater, a great emphasis is placed on research into materials suitable for the cheap and efficient removal of heavy metals from wastewater. Several methods are known for removing toxic metals from water, such as ion exchange [8], chemical precipitation [4], membrane filtration [9], reverse osmosis [10], solvent extraction [11], electrochemical treatment [12], and adsorption [13]. The removal of metal ions by adsorption is of wide research interest, as it is a relatively cheap and easy-to-implement method [14]. Different materials may be used as adsorbents for metal ions and may be obtained from organic, biological, or mineral sources (e.g., agricultural waste, aquatic and terrestrial biomass, biochar [15] naturally occurring in soil and mineral deposits, and other locally available waste materials) [16].
Natural clays (particularly bentonite) have received significant attention for heavy metals adsorption from contaminated water due to their applicability. Bentonite has several advantageous properties as an adsorbent, including low cost, good selectivity, and ion exchange capacity and regenerability [17,18]. Bentonite is used for adsorption of Li [19], Cs [20], Fe(III) [21], Cd(II), Zn(II) [22], Cu(II), Pb(II), and Ni(II) [23]. The adsorption of Ni(II) was studied on Slovak bentonites [24,25], Iranian bentonites [26], nano-bentonites [27], fungal dead biomass composites with bentonite [28], hybridized bentonites [29], and other materials.
This research is devoted to the study of nickel adsorption on bentonites from selected Slovak deposits with different structures and chemical compositions, in order to compare their adsorption efficiencies as a function of different nickel concentrations. The influences of the temperature, time, and initial concentration of Ni(II) on the course of sorption were monitored, and for better illustration of Ni(II) adsorption on natural bentonites, thermodynamic parameters were also calculated.
2. Materials and Methods
2.1. Adsorbent Properties and Preparation
Four different bentonite samples were examined for the removal of Ni(II) from synthetically prepared aqueous solutions. All bentonite samples came from Central Slovakia, specifically from the Lieskovec deposit (L), the Jelšový Potok deposit (JP), the Stará Kremnička deposit (SK) (near the Jelšový Potok deposit), and the Hliník nad Hronom deposit (S).
All bentonite samples were used without further purification. Bentonite samples were sieved using a standard mesh sieve (<200 μm) and dried at 105 °C for approximately 2–3 h in Petri dishes in a drying oven. Then, the samples were transferred into polypropylene bags and stored in a desiccator until further use. The samples were tested for Ni(II) adsorption, without any pretreatment.
2.2. Synthetic Solutions
All chemicals used were of analytical reagent grade and were used without further purification. The aqueous solutions of nickel (NiSO4·7H2O, for analysis (CAS No. 10101-98-1) Acros Organics, NJ, USA) were prepared with water deionized via reverse osmosis (Demiwa, Watek, Czech Republic).
A certified reference material—aqueous Ni solution with a concentration of 1.0 g L–1 (matrix 2% HNO3; Slovak Metrology Institute, Bratislava, Slovakia)—was used for preparing standard metal ion solutions, which were used for AAS analysis.
2.3. Adsorption Experiments
The adsorption experiments were carried out in 250 mL Erlenmeyer flasks, by mixing 0.25 g of an adsorbent (weighed to four decimal places) with 50 mL of a nickel solution. The contents of the Erlenmeyer flasks were then shaken at a given temperature (20, 30, and 40 °C) in an ES–20/60 (bioSan, Riga, Latvia) orbital shaker at 200 rpm. The suspensions were centrifuged after 2 h or after the desired contact time in the case of time dependence experiments. Each adsorption experiment was performed in duplicate using two independent samples. Atomic absorption spectrometry was used for the determination of nickel content in the solutions. The limit of experimental error between duplicates was less than ±5%.
The adsorption percentage (Ads.%) was calculated by Equation (1):
where co is the initial Ni(II) concentration (mg L–1) and ct is the Ni(II) concentration left in aqueous solutions at time t (mg L–1).
The amount of the adsorbed Ni(II) per mass unit of the adsorbent at time qt (mg g–1) was calculated according to Equation (2):
where V is the volume of the aqueous phase (L) and m is the amount of the bentonite (g).
2.4. AAS Analysis
The Ni(II) concentrations before and after adsorption were measured using an atomic adsorption spectrometer AVANTA Σ (GBC Scientific, Melbourne, Australia) with acetylene–air flame atomization. The data were processed using GBC Avanta v.2.0 software. The working wavelengths were 341.5 nm for a Ni(II) concentration up to 20 mg L–1 and 351.5 nm for a Ni(II) concentration up to 300 mg L–1. Standard metal ion solutions were used for periodically checking the instrument response.
2.5. FTIR Analysis
For a more detailed qualitative analysis of the adsorption materials used, their IR spectra were measured at the Department of Hydrosilicates IIC SAS in Bratislava, Slovakia. The spectra in the mid-IR region (MIR, 4000–400 cm–1) were obtained on a NICOLET 6700 FT-IR spectrometer (Thermo Scientific, Waltham, MA, USA) equipped with a KBr beam splitter and a DTGS detector. Samples were measured by the KBr pressed disk technique, where 1 mg sample was homogenized with 200 mg KBr. The pellets were heated overnight at 130 °C in order to minimize the content of adsorbed water. Each spectrum was the average of 128 scans with a resolution of 4 cm–1. The Thermo Scientific OMNIC™ v.7.2 software package was used for the measurement and evaluation of the IR spectra.
3. Results and discussion
3.1. Characterization of the Adsorbents
3.1.1. Chemical Analysis and X-Ray Analysis
The chemical compositions of the used bentonite samples have been described in detail in previous works [24,25,30,31,32] and are shown in Table 1. The JP sample contained added Na2CO3, unlike other bentonite samples used. It was shown previously by X-ray analysis (Table 2) that montmorillonites (JP 86% [24], L 64% [25], S 47% [30], and SK 85% [30]) were the dominant component in all the samples. The samples also contained quartz, kaolinite, and biotite in smaller amounts. Opal-C (20%) was found in sample S [31].

Table 1.
Chemical analysis of bentonites [24,25,28,29,30,31,32].

Table 2.
Mineralogical compositions of bentonites [24,25,28,29,30,31,32].
3.1.2. IR Spectroscopy
FTIR spectroscopy in the MIR region was used for the structural characterization of the studied samples. The IR spectra (Figure 1) revealed the dominant component was smectite in all the samples. The presence of the smectite mineral montmorillonite was proved by the peak at 3628 cm–1, which corresponded to the stretching vibrations of the structural OH groups of montmorillonites. The bending vibration modes of the structural OH groups were observed at 915 cm–1 (AlAlOH), 845 cm–1 (AlMgOH), and 882 cm–1 (AlFeOH). The substitution of Fe by Al was observed mainly in the JP and SK samples. In the S and L samples, the intensity of the bending vibration mode of AlFeOH was negligible. The complex band near 1040 cm–1 and the shoulder near 1100 cm–1 corresponded to the stretching vibrations of Si–O groups. The bending modes of Al–O–Si and Si–O–Si were observed at 524 and 468 cm–1, respectively [33]. The bands near 3420 and 1630 cm–1 appeared due to the presence of molecular water [34]. In addition to the vibration bands of montmorillonites, the IR spectroscopy also revealed the presence of admixtures. The shoulder at 3698 cm–1 corresponded to the stretching vibration of kaolinite, observed mainly for sample L, although a weak shoulder was also observed in sample SK. The presence of another admixture proved the band near 794 cm–1 originated from the stretching vibrations of Si–O groups, from either quartz or silica. This band was observed in all the samples, but most intensively in sample S. The broad band near 1400 cm–1 in the IR spectra of the JP and SK samples also indicated the presence of carbonate [35,36,37].
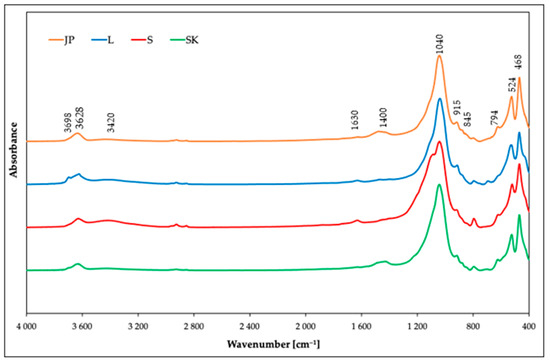
Figure 1.
IR spectra of the used bentonites before the adsorption of Ni(II) ions.
The IR spectra of samples after the adsorption were also measured (Figure S1). Differences in the spectra before and after sorption were only negligible, due to the unchanged structure of montmorillonites after the sorption of Ni(II). It can be observed only in the absence of vibration around 1400 cm–1.
3.2. Effect of Metal Concentrations
The effects of the initial Ni(II) concentration on the adsorption capacities of the used bentonite samples were investigated by varying the initial concentration of Ni(II) from 50 to 300 mg L–1 in solutions, at temperatures of 20, 30, and 40 °C. The amount of the samples (0.25 g per 50 mL of the solution) was constant, and the pH was not adjusted from its value of 5.85.
The results are presented in Figure 2, Figure 3 and Figure 4. In Figure 2, it can be seen that the equilibrium adsorption capacities of the bentonite samples toward Ni(II) increased while the adsorption percentages of Ni(II) (Ads. %) in Figure 3 showed an opposite trend.
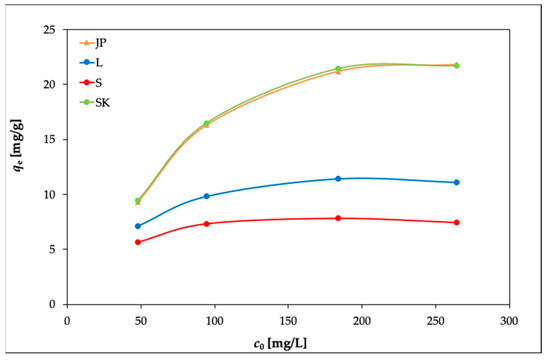
Figure 2.
Effects of the initial concentration of Ni(II) on the adsorption capacities of the bentonite samples (experimental conditions: 50 mL solution; 0.25 g bentonite; temperature, 20 °C).
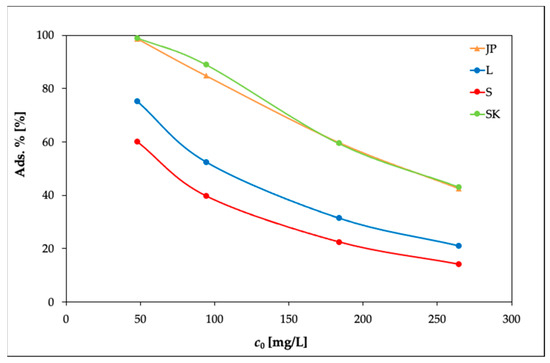
Figure 3.
Effects of the initial concentration of Ni(II) on the adsorption percentage of the bentonite samples (experimental conditions: 50 mL solution; 0.25 g bentonite; temperature, 20 °C).
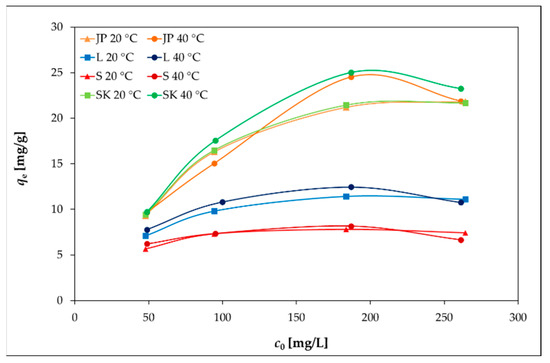
Figure 4.
Effects of the temperature and the initial concentration of Ni(II) on the adsorption capacities of bentonite samples.
However, at 40 °C (Figure 4), the removal efficiency slightly decreased with the increasing initial Ni(II) concentration, as the concentration gradient increased, leading to a higher probability of collision between Ni(II) and active adsorption sites on the bentonite and thereby increasing the adsorption capacity. With a further increase in the concentration of Ni(II), the active adsorption sites became saturated [38,39].
The elevated temperature caused only a slight increase in qe for all four bentonites, as can be seen in Figure 4.
3.3. Adsorption Isotherms
The adsorption data were described using the Freundlich and Langmuir adsorption models, which were applied in a linear form, according to Equations (3) and (4):
where qe is the amount of Ni(II) adsorbed per unit weight of bentonite at an equilibrium concentration (mg g–1), qm is the maximum adsorption capacity corresponding to the monolayer adsorption capacity (mg g–1), ce is the equilibrium concentration of Ni(II) in the solution (mg L–1), 1/n is an empirical parameter related to the intensity of adsorption (where for values in the range of 0.1 < 1/n < 1, adsorption is favorable), KF is the surface adsorption equilibrium constant (mg g–1) and b is the Langmuir coefficient which represents the equilibrium constant related to the adsorbate–adsorbent affinity (L mg–1).
The experimental data were plotted for Freundlich isotherms as log qe vs. log ce and for Langmuir isotherms as ce/qe vs. ce. The adsorption constants Kf and n (for Freundlich isotherms) and qm and b (for Langmuir isotherms) were calculated from these linear plots. The evaluation of isothermal equations was performed on the basis of correlation coefficient R2. Slightly improved results for the Ni(II) adsorption on the studied samples were provided by the Langmuir model (Figure 5) (R2 = 0.988–0.999) vs. the Freundlich model (R2 = 0.922–0.999). Table 3 shows the calculated results and R2. The values of qm were dependent on temperature; with the increasing temperature, qm slightly increased.
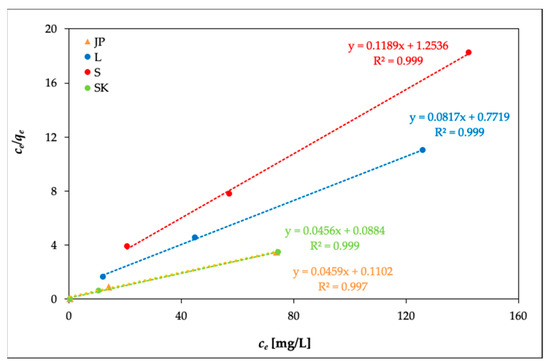
Figure 5.
Model of Langmuir isotherms (temperature: 20 °C).

Table 3.
Parameters of Langmuir and Freundlich isotherms.
The dimensionless separation factor RL defines the nature of adsorption in the Langmuir isotherm and can be calculated by Equation (5) [40]:
where b is the Langmuir equilibrium constant (L mg–1) and co is the initial concentration of Ni(II) (mg L–1). RL values reveal whether the adsorption is favorable (0 < RL < 1), unfavorable (RL > 1), linear (RL = 1), or irreversible (RL = 0) [41].
The RL values at concentrations of 50–300 mg L–1 Ni(II) for the used bentonites (Table 4) indicate that the Langmuir model is favorable as indicated by 0 < RL < 1; however, the Freundlich model also implies favorability as indicated by 0.1 < 1/n < 1.

Table 4.
RL values for bentonites at concentrations of 50–300 mg L–1 Ni(II).
The comparisons of the R2 values for both models showed that there were no significant differences between the Langmuir model and the Freundlich model. The Freundlich model is valid for heterogeneous surfaces, while the Langmuir model is preferred for monolayer adsorption onto a surface, on which the binding sites have an equivalent affinity for adsorption, with a finite number of identical sites [41].
The agreement of the experimental data with the Langmuir isotherm indicated a homogeneous adsorption process, which leads to monolayer binding.
Table 5 compares the maximum adsorption capacities of different natural and modified bentonites for Ni(II) removal reported in previous studies.

Table 5.
Comparison of the maximum adsorption capacities (qmax) of different natural and modified bentonites for the Ni(II) removal.
Among the natural materials, nano-bentonite showed the highest maximum adsorption capacity. The measured adsorption capacities of bentonites in this work towards Ni(II) are comparable to [43] or higher than the values reported by others [44].
3.4. Kinetic Studies
The effects of the contact time on the adsorption of Ni(II) by the JP and L bentonites were investigated at various time intervals (2–120 min), and the results are shown in Figure 6.
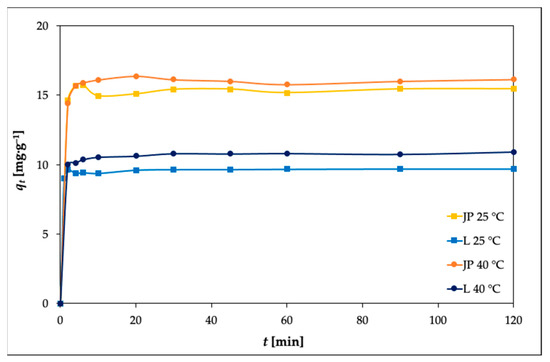
Figure 6.
Effects of adsorption time at 25 and 40 °C on the adsorption of Ni(II) by the JP and L bentonites (experimental conditions: 50 mL solution; 0.25 g adsorbent; c0 100 mg L–1; pH 5.85).
The adsorption rate was found to be rapid during the first 10 min of adsorption. This is in agreement with previous studies [23,45], where fast adsorption of metal ions has been reported.
A further increase in contact time did not result in further increase in the amount of Ni(II) adsorbed. To determine the order of the reaction, the experimental data were fit to a pseudo-first-order equation and a pseudo-second-order equation.
The pseudo-first-order equation can be expressed as shown in Equation (6):
where qe and qt are the adsorbed amounts of metal ions at equilibrium and at time t, respectively (mg g–1), t is the contact time (min), and k1 is the pseudo-first-order adsorption rate constant (L min–1).
The plot of ln(qe − qt) versus time (t) should be linear, and the rate constant k1 was obtained from the slope. The calculated values obtained from the linear plots did not agree with the experimental qe values, and the obtained R2 values were lower than the values of the pseudo-second-order kinetic model (Table 6), indicating that the adsorption of Ni(II) on bentonites JP and L did not follow the pseudo-first-order kinetic model.

Table 6.
Comparison of the experimental and calculated qe values and the adsorption rate constants for the pseudo-first-order and pseudo-second-order reaction kinetics of the Ni(II) adsorption on the JP and L bentonites.
The kinetics data were also fit to the pseudo-second-order equation (proposed by Ho and McKay [46]), expressed by Equation (7):
where k2 is the pseudo-second-order adsorption rate constant (g mg–1 min), calculated from the slope of the linear plot of t/qt against t.
Figure 7 illustrates the pseudo-second-order kinetics of Ni(II) adsorption onto bentonites JP and L at temperatures of 25 and 40 °C, respectively.
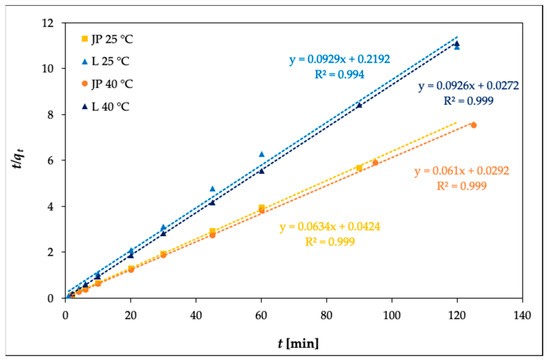
Figure 7.
Pseudo-second-order adsorption kinetics of Ni(II) on bentonites JP and L (c0 = 100 mg L–1).
From the data given in Table 6, R2 for the pseudo-first-order model (R2 = 0.1001–0.6706) are much smaller than those of the pseudo-second-order model (R2 = 0.9993–0.9999) for Ni(II) adsorption. The qe values obtained from the pseudo-second-order model were in good agreement with the experimental data. This means that the adsorption of Ni(II) is based on the assumption that the rate-limiting step may be chemisorption and that adsorption closely follows the pseudo-second-order kinetic model [41].
3.5. Thermodynamic Studies
The effect of the temperature on the adsorption of Ni(II) onto natural bentonites was studied at 20, 30, and 40 °C. It was found that the increase in temperature had a favorable effect on the adsorption process, as with increasing temperature the amount of Ni(II) adsorbed on the bentonite also increased.
The equilibrium constant Kc and the thermodynamic parameters (Gibbs free energy ΔG0 (kJ·mol–1), enthalpy ΔH0 (kJ·mol–1), and entropy change ΔS0 (J·mol−1·K−1))were calculated using Equations (8) and (9):
where T is the temperature (K), R is the ideal gas constant (8.314 J·mol−1·K−1), Kc is the ratio of the equilibrium concentration of the Ni(II) attached to bentonite to the equilibrium concentration in the solution and was determined from Langmuir adsorption isotherms, and the Langmuir constant b was calculated according to Milonjić [47]. The values of ΔH0 and ΔS0 were determined from the slopes and intercepts of the linear plots of ln Kc versus 1/T (Figure 8) and are shown in Table 7. The values of ΔG0 were calculated using Equation (9).
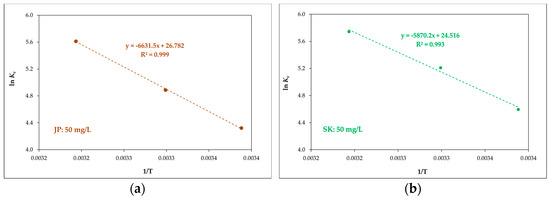
Figure 8.
Linear plots of Kc versus 1/T for JP (a) and SK (b) bentonite and for a Ni(II) concentration of 50 mg L–1.

Table 7.
The values of thermodynamic parameters ΔG0, ΔH0, and ΔS0.
The enthalpy ΔH0 is defined as a measure of the energy barrier that needs to be overcome by the reaction of molecules [48]. The ΔH0 values (Table 7) are positive, indicating that a large amount of heat was consumed in transferring Ni(II) from the liquid to the solid phase. The positive values of ΔH0 suggest that Ni(II) adsorption is endothermic, as reported elsewhere [27].
The changes in entropy (ΔS0) were 222.7, 203.8, 49.1, and 26.4 J·mol−1·K−1for the JP, SK, L, and S bentonites, respectively. The positive values of ΔS0 show that the adsorption process is irreversible. The change in entropy ΔS0 higher than −10−2 kJ·mol−1·K−1 indicated that the adsorption process takes place via a dissociation mechanism [16].
The degree of spontaneity and feasibility of an adsorption process is described by the Gibbs free energy (ΔG0). The values of ΔG0 were negative, confirming that the adsorption of Ni(II) onto the bentonites used in this work is a spontaneous and feasible process. More negative ΔG0 values imply a greater driving force for the adsorption process.
The values of ΔG0 decreased with the increasing temperature (as shown in Table 4). This indicated that the reaction is more favorable and spontaneous at higher temperatures, similar to the behavior reported previously [27].
4. Conclusions
In this study, the removal of nickel via adsorption onto natural bentonites from four different Slovak deposits was investigated in detail. Batch adsorption experiments were conducted at various initial concentrations of Ni(II), times, and temperatures.
The adsorption isotherms were described by the Langmuir isotherm model and by the Freundlich isotherm model. The maximum adsorption capacity for the bentonites used decreased following the order of SK > JP > L > S. The data obtained from the kinetic study best fitted the second-order kinetic model. The thermodynamic data indicated that the adsorption process was endothermic and spontaneous.
Based on the results obtained from this study, it can be concluded that of all used bentonites, the bentonites from Central Slovakia (Jelšový Potok and Stará Kremnička) are most suitable for the removal of nickel from aqueous solutions.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1944/14/2/282/s1, Figure S1: IR spectra of used bentonites after adsorption of Ni(II) ions.
Author Contributions
Conceptualization, Z.M., S.N. and L.K.; data curation, Z.M.; formal analysis, M.Š., Z.M. and V.K.; investigation, M.Š., Z.M. and V.K.; supervision, M.Š. and Z.M.; visualization, M.Š.; writing of the original draft, M.Š., Z.M. and V.K.; writing of review and editing, M.Š., Z.M., V.K., L.K. and S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific Grant Agency of the Slovak Republic under the grants 1/0291/19 and 1/0110/19.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and supplementary material.
Acknowledgments
The authors would like to thank the following Slovak companies—Envigeo Inc., REGOS Ltd. and GE.NE.S Inc.—for providing bentonites used in this work. In addition, we would like to thanks the Ministry of Education, Science and Technological Development of Republic of Serbia (contract No. 451-03-68/2020-14/200017).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Mladenović, N.; Kljajević, L.; Nenadović, S.; Ivanović, M.; Čalija, B.; Gulicovski, J.; Trivunac, K. The Applications of New Inorganic Polymer for Adsorption Cadmium from Waste Water. J. Inorg. Organomet. Polym. 2020, 30, 554–563. [Google Scholar] [CrossRef]
- Chen, C.; Huang, D.; Liu, J. Functions and Toxicity of Nickel in Plants: Recent Advances and Future Prospects. CLEAN Soil Air Water 2009, 37, 304–313. [Google Scholar] [CrossRef]
- Wang, R.; Ng, D.H.L.; Liu, S. Recovery of nickel ions from wastewater by precipitation approach using silica xerogel. J. Hazard. Mater. 2019, 380, 120826. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.D.; Błaszczyk, U. The impact of nickel on human health. J. Elem. 2008, 13, 685–696. [Google Scholar]
- Shang, W.; Yu, W.; Xiao, X.; Ma, Y.; Tan, P.; Ni, M. Unravel the influences of Ni substitution on Co-based electrodes for rechargeable alkaline Zn–Co batteries. J. Power Sources 2021, 483, 229192. [Google Scholar] [CrossRef]
- El-Maghrabi, H.H.; Nada, A.A.; Bekheet, M.F.; Roualdes, S.; Riedel, W.; Iatsunskyi, I.; Coy, E.; Gurlo, A.; Bechelany, M. Coaxial nanofibers of nickel/gadolinium oxide/nickel oxide as highly effective electrocatalysts for hydrogen evolution reaction. J. Colloid Interface Sci. 2020. [Google Scholar] [CrossRef]
- Ma, A.; Abushaikha, A.; Allen, S.J.; McKay, G. Ion exchange homogeneous surface diffusion modelling by binary site resin for the removal of nickel ions from wastewater in fixed beds. Chem. Eng. J. 2019, 358, 1–10. [Google Scholar] [CrossRef]
- Abubakar, M.; Binti Mohd Noor, S.F.; Ahmad, N. Effect of milling time on the performance of ceramic membrane from ball clay for the treatment of nickel plating wastewater. J. Aust. Ceram. Soc. 2019, 55, 667–679. [Google Scholar] [CrossRef]
- Rodrigues Pires da Silva, J.; Merçon, F.; Guimarães Costa, C.M.; Radoman Benjo, D. Application of reverse osmosis process associated with EDTA complexation for nickel and copper removal from wastewater. Desalination Water Treat. 2016, 57, 19466–19474. [Google Scholar] [CrossRef]
- Li, L.; Zhong, H.; Cao, Z.; Yuan, L. Recovery of Copper(II) and Nickel(II) from Plating Wastewater by Solvent Extraction. Chin. J. Chem. Eng. 2011, 19, 926–930. [Google Scholar] [CrossRef]
- Tran, T.K.; Chiu, K.F.; Lin, C.Y.; Leu, H.J. Electrochemical treatment of wastewater: Selectivity of the heavy metals removal process. Int. J. Hydrogen Energy 2017, 42, 27741–27748. [Google Scholar] [CrossRef]
- Wołowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepa, G.; Bajda, T. Removal of Heavy Metals and Metalloids from Water Using Drinking Water Treatment Residuals as Adsorbents: A Review. Minerals 2019, 9, 487. [Google Scholar] [CrossRef]
- Bisht, R.; Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalination 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Frišták, V.; Friesl-Hanl, W.; Pipíška, M.; Micháleková Richveisová, B.; Soja, G. The response of artificial aging to sorption properties of biochar for potentially toxic heavy metals. Nova Biotechnol. Chim. 2014, 13, 137–147. [Google Scholar] [CrossRef]
- Vakili, M. Nickel ion removal from aqueous solutions through the adsorption process: A review. Rev. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Melichová, Z.; Hromada, L. Adsorption of Pb2+ and Cu2+ ions from aqueous solutions on natural bentonite. Pol. J. Environ. Stud. 2013, 22, 457–464. [Google Scholar]
- Hoyer, M.; Kummer, N.A.; Merkel, B. Sorption of Lithium on Bentonite, Kaolin and Zeolite. Geosciences 2015, 5, 127–140. [Google Scholar] [CrossRef]
- Belousov, P.; Semenkova, A.; Egorova, T.; Romanchuk, A.; Zakusin, S.; Dorzhieva, O.; Tyupina, E.; Izosimova, Y.; Tolpeshta, I.; Chernov, M.; et al. Cesium Sorption and Desorption on Glauconite, Bentonite, Zeolite, and Diatomite. Minerals 2019, 9, 625. [Google Scholar] [CrossRef]
- Bakalár, T.; Kaňuchová, M.; Girová, A.; Pavolová, H.; Hromada, R.; Hajduová, Z. Characterization of Fe(III) Adsorption onto Zeolite and Bentonite. Int. J. Environ. Res. Public Health 2020, 17, 5718. [Google Scholar] [CrossRef] [PubMed]
- Burham, N.; Sayed, M. Adsorption Behavior of Cd2+ and Zn2+ onto Natural Egyptian Bentonitic Clay. Minerals 2016, 6, 129. [Google Scholar] [CrossRef]
- Bourliva, A.; Michailidis, K.; Sikalidis, C.; Filippidis, A.; Betsiou, M. Adsorption of Cd(II), Cu(II), Ni(II) and Pb(II) onto natural bentonite: Study in mono- and multi-metal systems. Environ. Earth Sci. 2015, 73, 5435–5444. [Google Scholar] [CrossRef]
- Krajňák, A.; Pivarčiová, L.; Rosskopfová, O.; Galamboš, M.; Rajec, P. Adsorption of nickel on rhyolitic Slovak bentonites. J. Radioanal. Nucl. Chem. 2015, 304, 587–593. [Google Scholar] [CrossRef]
- Pivarčiová, L.; Krajňák, A.; Rosskopfová, O.; Galamboš, M.; Rajec, P. Adsorption of nickel on andesitic bentonite Lieskovec. J. Radioanal. Nucl. Chem. 2015, 304, 851–858. [Google Scholar] [CrossRef]
- Sadeghalvad, B.; Azadmehr, A.R.; Motevalian, H. Statistical design and kinetic and thermodynamic studies of Ni(II) adsorption on bentonite. J. Cent. S. Univ. 2017, 24, 1529–1536. [Google Scholar] [CrossRef]
- Taha, A.A.; Ahmed, A.M.; Abdel Rahman, H.H.; Abouzeid, F.M.; Abdel Maksoud, M.O. Removal of nickel ions by adsorption on nano-bentonite: Equilibrium, kinetics, and thermodynamics. J. Dispers. Sci. Technol. 2017, 38, 757–767. [Google Scholar] [CrossRef]
- Rashid, A.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Fungal biomass composite with bentonite efficiency for nickel and zinc adsorption: A mechanistic study. Ecol. Eng. 2016, 91, 459–471. [Google Scholar] [CrossRef]
- Chang, Y.S. Adsorption of Cu(II) and Ni(II) ions from wastewater onto bentonite and bentonite/GO composite. Environ. Sci. Pollut. Res. 2020, 27, 33270–33296. [Google Scholar] [CrossRef]
- Górniak, K. Bentonite from the Central Slovakia Volcanic Field—A Prospective Raw Material for Polish Industry. Mineralogia 2017, 48, 23–38. [Google Scholar] [CrossRef]
- Osacký, M.; Binčík, T.; Paľo, T.; Uhlík, P.; Madejová, J.; Czímerová, A. Mineralogical and physico–chemical properties of bentonites from the Jastrabá Formation (Kremnické vrchy Mts., Western Carpathians). Geol. Carpathica 2019, 70, 433–445. [Google Scholar] [CrossRef]
- Uhlik, P.; Janosik, M.; Kraus, I.; Pentrak, M.; Caplovicova, M. Characterisation of bentonite from Hliník nad Hronom deposit (Jastrabá Formation of the Štiavnica stratovolcano, Western Carpathians). Acta Geol. Slovaca 2012, 4, 125–137. [Google Scholar]
- Madejová, J.; Gates, W.P.; Petit, S. IR spectra of clay minerals: Chapter 5. In Infrared and Raman Spectroscopies of Clay Minerals; Gates, W.P., Kloprogge, J.T., Madejová, J., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 107–149. ISBN 978-0-08-100355-8. ISSN 1572-4352. [Google Scholar]
- Bukka, K.; Miller, J.D.; Shabtai, J. FTIR Study of Deuterated Montmorillonites: Structural Features Relevant to Pillared Clay Stability. Clays Clay Miner. 1992, 40, 92–102. [Google Scholar] [CrossRef]
- Nenadović, S.; Kljajević, L.; Nenadović, M.; Mirković, M.; Marković, S.; Rakočević, Z. Mechanochemical treatment and structural properties of lead adsorption on kaolinite (Rudovci, Serbia). Environ. Earth Sci. 2015, 73, 7669–7677. [Google Scholar] [CrossRef]
- Kljajević, L.; Melichova, Z.; Kisić, D.; Nenadović, M.; Todorović, B.; Pavlović, V.; Nenadović, S. The influence of alumino-silicate matrix composition on surface hydrophobic properties. Sci. Sinter. 2018, 51, 163–173. [Google Scholar] [CrossRef]
- Kljajevic, L.M.; Melichova, Z.; Stojmenovic, M.; Todorovic, B.Z.; Pavlovic, V.B.; Citakovic, N.; Nenadovic, S.S. Structural and electrical properties of geopolymer materials based on different precursors (kaolin, bentonite and diatomite). Maced. J. Chem. Chem. Eng. 2019, 38, 283–292. [Google Scholar] [CrossRef]
- Shroff, K.A.; Vaidya, V.K. Effect of pre-treatments on biosorption of Ni (II) by dead biomass of Mucor hiemalis. Eng. Life Sci. 2011, 11, 588–597. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X. Adsorption and Desorption of Nickel(II) Ions from Aqueous Solution by a Lignocellulose/Montmorillonite Nanocomposite. PLoS ONE 2015, 10, e0117077. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M.; Citak, D.; Soylak, M. Equilibrium, kinetic and thermodynamic studies of adsorption of Pb(II) from aqueous solution onto Turkish kaolinite clay. J. Hazard. Mater. 2007, 149, 283–291. [Google Scholar] [CrossRef]
- El-Enein, S.A.; Okbah, M.A.; Hussain, S.G.; Soliman, N.F.; Ghounam, H.H. Adsorption of Selected Metals Ions in Solution Using Nano-Bentonite Particles: Isotherms and Kinetics. Environ. Process. 2020, 7, 463–477. [Google Scholar] [CrossRef]
- Vijaya, Y.; Popuri, S.R.; Boddu, V.M.; Krishnaiah, A. Modified chitosan and calcium alginate biopolymer sorbents for removal of nickel (II) through adsorption. Carbohydr. Polym. 2008, 72, 261–271. [Google Scholar] [CrossRef]
- Mu’azu, N.D.; Bukhari, A.; Munef, K. Effect of montmorillonite content in natural Saudi Arabian clay on its adsorptive performance for single aqueous uptake of Cu(II) and Ni(II). J. King Saud Univ. 2020, 32, 412–422. [Google Scholar] [CrossRef]
- Mohammed-Azizi, F.; Dib, S.; Boufatit, M. Removal of heavy metals from aqueous solutions by Algerian bentonite. Desalination Water Treat. 2013, 51, 4447–4458. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; Neto, A.F.; Gimenes, A.M.L.; da Silva, M.G. Sorption kinetics and equilibrium for the removal of nickel ions from aqueous phase on calcined Bofe bentonite clay. J. Hazard. Mater. 2010, 177, 362–371. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Milonjić, S.K. A consideration of the correst calculation of thermodynamic parameters of adsorption. J. Serb. Chem. Soc. 2007, 72, 1363–1367. [Google Scholar] [CrossRef]
- Shi, T.; Jia, S.; Chen, Y.; Wen, Y.; Du, C.; Guo, H.; Wang, Z. Adsorption of Pb(II), Cr(III), Cu(II), Cd(II) and Ni(II) onto a vanadium mine tailing from aqueous solution. J. Hazard. Mater. 2009, 169, 838–846. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).