Abstract
This study aimed to evaluate the possibility of using Fourier Transform Infrared (FTIR) spectroscopy to track binders produced by three different plants: plants A, B, and C. The work included the quality assessment of 80 bituminous materials graded as BND 70/100 and 100/130 according to GOST 33133 (Russian interstate standard) and chemical analyses using FTIR spectroscopy. FTIR analyses were conducted before and after short-term ageing in a Rolling Thin Film Oven Test (RTFOT). Thus, the number of binder samples was multiplied by two (2) for a final total of 160 infrared (IR) spectra. All infrared spectra were normalised to ensure the reliability of results, and the standard deviation and variance coefficient were included. The principal purpose of the present work was to track the origin and the ageing extent of the bituminous binders under study.
1. Introduction
The traditional type of organic binder is petroleum bitumen or petroleum bituminous materials []. Bituminous materials are one of the most important tonnages produced by the petroleum refining industries, as bituminous materials are also one of the most widely used materials in the world [,,]. The widespread use of bituminous materials in many fields of human activity, such as insulating and binding materials for road construction, airfield repair, pavements, roofs, etc., is mainly due to their physicochemical characteristics []. Improving or optimising the physicochemical characteristics of bituminous materials is a challenge for the scientific community today in order to meet the modern requirements of our society or our civilization. However, bitumen production technology differs from region to region depending on ecological, climatic, and sanitary conditions or requirements []. As a result, the technical specifications for the quality of bituminous materials produced in leading countries such as the United States of America, China, Canada, Spain, the Russian Federation, and France differ. Climatic conditions, road traffic density, and the technology used to produce bituminous materials contribute to these differences [,,].
In the Russian Federation, oxidised bitumen is used as road bitumen as prescribed by the GOST 33133 standard. The most common binders’ grades of GOST 33133 are BND 100/130, 70/100, and 50/70 []. The road bitumen in this region of the world is subject to higher requirements in terms of frost resistance (Fraass breaking point) and high-temperature resistance (softening temperature), as well as in terms of adhesive properties [].
The process of bitumen manufacture from crude oil is rather complex [,]. Oxidation, cracking (vacuum distillation with rectification), and compounding are fundamental techniques used to produce petroleum bituminous materials [,,,]. The primary raw material used for the production of petroleum bitumen is tar or vacuum residue. The technology of manufacturing bitumen involves several stages, regardless of the production technique, starting with the desalination of the crude oil, then atmospheric and vacuum distillation [,,]. The most widely used bitumen manufacturing process in the Russian Federation is the air blowing process due to its simplicity, reliability, and cost-effectiveness. Furthermore, Russian legislation allows air-blown or oxidised bitumen as a binder for pavement or roadways, which is not the case in many Western countries due to their laws and technical requirements []. However, other processes are also used in Russia, such as compounding to manufacture polymer-modified bitumen [,].
Most specifications and standards are based on the physicochemical properties of bituminous binders, measured empirically [,,]. Thus, for many manufacturers and even dealers, one of the crucial objectives to ensure a good reputation with these customers is to guarantee the physicochemical properties of the binders. This comprehensive guarantee implies the possibility of identifying and tracking its products among other products in the same product categories. Identification is therefore one of the important goals of mass production. Gazpromneft-Bituminous Materials, in this approach, undertakes to develop traceability methods for its manufactured products. One of the most suitable identification methods is to study the product’s chemical structure to determine its distinctions and thus solve the traceability challenges.
Moreover, economically, the identification or tracking method chosen or developed should not be expensive, but fast and efficient. Here, FTIR analysis was positioned as the method of the first choice for the study of road bitumen or asphalt pavements [,]. The choice was based on FTIR analyses’ reputation in the field of bituminous materials, as evidenced via numerous published studies, such as those of the Western Research Institute and Petersen []. As already mentioned, this method of experimental analysis is frequently used because it provides reliable and precise information on the chemical structure of the samples studied [,].
The chemical structure of bitumen or bituminous materials is complex. Four chemical fractions are obtained using the bitumen separation method ASTM D 4124-01 or ASTM 2007 [,,]: asphaltenes, resins, aromatic compounds, and saturated compounds. Bitumen, in its structure, is a colloidal system in which asphaltenes or asphaltene micelles are dispersed in maltenes. The middle of the dispersion of maltenes includes resins and oils (saturates and aromatics) []. Resins, i.e., the polar components of maltenes, stabilise the micelles of asphaltenes.
Consequently, the oxidation or ageing process includes many oxidative reactions that cause the generation of transitions from one polarity-based fraction to another [,,]. The ageing of bituminous materials is an important problem because sulphoxides and carbonyl groups are formed during the oxidative ageing process [,,]. Carbonyl groups in bitumen include carboxylic acid, ester, ketone, aldehyde, anhydride, and amide [,]. Therefore, many studies have focused on anti-ageing to find lasting solutions for thermochemical stability [,]. One of the modern solutions is the introduction of epoxy-based compounds as anti-ageing technologies for bituminous materials [,,].
Following the literature, FTIR analysis is one of the most effective analytical methods for studying the chemical composition of oil, vacuum residues, and bitumen []. The range from 4000 cm−1 to 400 cm−1 is the interval where the fundamental molecular vibrations of functional groups are displayed in the spectrum as absorption bands [,,,]. According to field studies, bands at 2920 and 2850 cm−1 are assigned, respectively, C–H aliphatic asymmetric and symmetric stretching vibrations [,,]. The absorption region of 1740–1710 cm−1 corresponds to the C=O stretching of carbonyl groups. It is good to report that the band at 1710 cm−1 often determines the oxidation degree of oils and bitumen. The band at 1600 cm−1 is associated with C=C due to its aromatic compounds, and 1030 cm−1 the bands’ sulphoxide groups. The bands at 1375 and 1455 cm−1 are assigned to the CH2 groups > 4, CH and CH2CH3 groups. The paraffin structures are especially at the band at 720 cm−1 [,,]. Under 1500 cm−1, known as the fingerprinting region [,], is a complex and informative part of the IR spectrum that typifies the molecule under study. Especially, in our particular case, this is the bitumen.
The binder tracking is topical because oxidised bitumen is mainly used for roofs, floor coverings, waterproofing complexes, adhesives, and anticorrosion products according to standard EN 13304 [,]. As mentioned above, in the Russian Federation, oxidised bitumen is used as road bitumen and as a feedstock for modified bitumen and other bituminous materials []. Road projects are frequently supplied with various bituminous materials from various factories, manufacturers, and dealers [,]. During road project operations, bituminous material passports (origin) can get lost. In this regard, there are cases where customers (road projects) are not satisfied with the quality of the products supplied []. Thus, such cases can become a source of conflict between dealers, manufacturers, and customers. Maintaining good business relationships with customers or dealers is essential for any manufacturer. One solution is to identify or track bituminous materials by manufacturer, dealer, or customer, which helps maintain the partnership. Furthermore, many oil plants around the world use almost identical sources of oil for their production. For example, in Russia, crude oil supplied to refineries is usually delivered via pipelines [,], thereby having the same crude oil.
The investigation aimed to discover or find the differences between bituminous binders from different plants using FTIR in order to recognise or track their origin. FTIR analyses were performed before and after short-term ageing in the Rolling Thin Film Oven Test (RTFOT), as shown in Figure 1. The study also included the physicochemical properties assessment of bituminous binders graded as BND 70/100 and 100/130 according to GOST 33133.
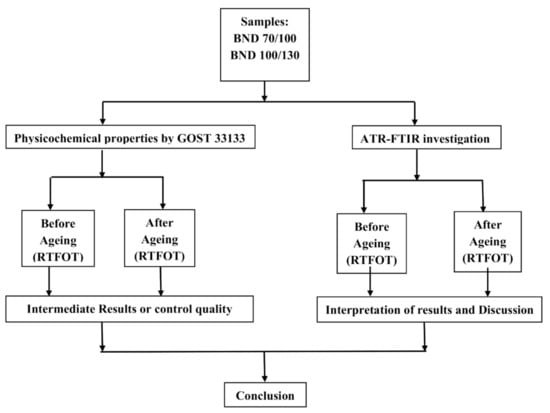
Figure 1.
Flowchart of investigation design.
2. Materials and Methods
2.1. Materials
The materials in this study were 80 samples of road bitumen (BND) graded as BND 70/100 and 100/130, according to GOST 33133, collected directly under GOST 32268-2013 (standard for sampling bituminous materials) from three Gazpromneft-Bituminous Materials plants (plants installations) over six months. Binders were sampled at 40 specimens BND 70/100 and 40 specimens BND 100/130, as shown in Figure 2. Note that the survey was carried out before and after short-term ageing (RTFOT under GOST 33140) and that all the samples studied were formulated by the air-blowing process from different batches, that is, different production periods.
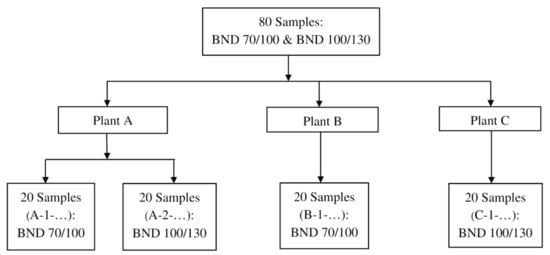
Figure 2.
Flowchart of samples distribution in investigation.
2.2. Methods
The advancement of technologies and methods offers a multitude of possibilities for studying bitumen and, in particular, its chemical structure. In Table 1, the Russian standard GOST 33133-2014 was used in addition to the FTIR []. As well, the variance coefficient (CV) was applied due to the size of the data, and it was defined as the standard deviation divided by the mean. The algorithm for the variance coefficient and standard deviation, see Equations (1) and (2) below, makes it possible to measure the dispersion of the data obtained and facilitate their analysis [].

Table 1.
GOST 33133–2014, physicochemical properties of bitumen BND 70/100 and 100/130.
SD is the standard deviation, while M is the mean.
where N is the number of binder samples by plants, X is each value of variables, and M is the mean.
It was deliberately decided that the variance coefficient be set at 30% due to certain factors, such as the production batch and the origin of the binders.
2.2.1. Russian Interstate Standards for Road Bitumen: GOST 33133–2014
2.2.2. Infrared Spectroscopy Analysis: ATR-FTIR Spectroscopy
The study was conducted before and after short-term ageing (RTFOT under GOST 33140). IR spectra of samples in the range of 4000–400 cm−1 (fundamental region) were obtained using the IR Affinity-1S spectrophotometer (Shimadzu, Kyoto, Japan) with the console Quest ATR Diamond (Single-Reflection) at room temperature between 22 and 27 °C. The binder samples were placed on a diamond surface, and interferograms were recorded with a 4 cm−1 resolution and averaged over 30 scans. After subtracting the baseline, the obtained IR spectra were normalised to the absorption band of 2920 (±3) cm−1 according to Equation (3):
where Absnorm (wn) is the absorbance spectrum normalised as a function of wavenumber; Abs (wn) is the original absorbance spectrum; Abs (2920 (±3) cm−1) are original absorbance values at 2920 (±3) cm−1. The normalisation of this band resulted in an absorbance value set at 1.0, and the full spectrum was multiplied by a ratio factor, as shown in Figure 3 []. The IR spectra’ normalisation, as fixed in Hofko, B., Alavi, M.Z. et al., was based on the idea to eliminate any changes in the absorbance spectra due to the variation of the infrared beam penetration between samples, which would bias the interpretation of results []. The binder samples’ chemical structures via IR were based on the calculation of spectrometry indices defined as the ratio of the absorbance (a.u.) values in the maxima of the corresponding absorption bands in Equations (4)–(9) [,].
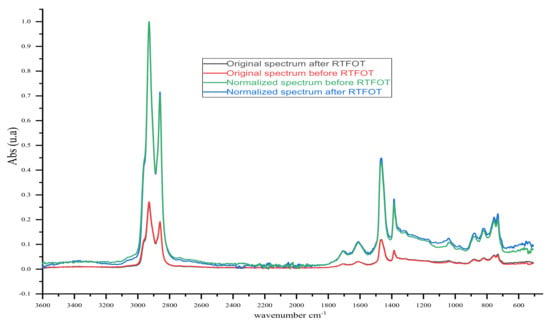
Figure 3.
Example of original and normalised IR spectra of bitumen samples.
Aromaticity indices are aromatic rings that make up the structure of bitumen to the relative content of aliphatic fragments.
Oxidation or carbonyl indices (oxidation rate) are parameters that characterise the oxidation degree. An increase in the oxidation degree accompanies the oxidative ageing process. In other words, this is the increase in oxidised compounds such as carbonyl groups in the bitumen.
Isomerisation indices describe the branched-chain compound hydrocarbons in the chemical structure of bitumen.
Aliphaticity indices are aliphatic fragments to the relative content of aromatic compounds present in bitumen samples.
Sulphurisation indices represent the sulphurised compounds in the chemical structure of bitumen.
Asphaltenes indices are the polycondensed aromatic compounds in the chemical and colloidal structure of bitumen.
Based on the set goals and origin of bituminous binders, the variance coefficient of chemical structures was set at 30%.
3. Results and Discussions
3.1. Physicochemical Properties of Binder Samples by Plants
The samples’ physicochemical qualities under GOST 33133 for grades BND 70/100 and 100/130 are the focus of the first portion of the inquiry. The physicochemical properties of binder samples from manufacturers or plants are presented in Table 2, Table 3, Table 4 and Table 5.

Table 2.
The physicochemical properties of BND 70/100 from plant A.

Table 3.
The physicochemical properties of BND 100/130 from plant A.

Table 4.
The physicochemical properties of BND 70/100 from plant B.

Table 5.
The physicochemical properties of BND 100/130 from plant C.
During the evaluation of the samples, it was discovered that nearly half of the BND 70/100 binder samples from plant A exhibited insufficient “ductility at 0 °C,” i.e., nine (9) of the twenty (20) samples. Of the nine (9) samples that did not meet the “ductility at 0 °C” criteria, five (5) developed a rupture (crack) since the start of the tests. In brief, 45% of BND 70/100 from plant A were not BND 70/100 after being evaluated according to GOST 33133 standards.
During the study, the BPA 5 breakpoint analyser was damaged. Some samples were not tested. As a result, when compared with other indicators, the statistical data for the Fraass breaking point can be regarded as insufficient.
In the first part of our research, we decided to establish the means of physicochemical properties to find some differences between the BND presented by plants and to evaluate the physicochemical properties of bituminous binders under the standard GOST 33133. The variance coefficient must be less than or equal to 30% for a statistic to be valid. This value of 30% is motivated by the fact that there are three (3) fundamental factors in the production of bitumen by air blowing: temperature, pressure, and airflow rate/raw material. We considered 10% as the variance coefficient due to corrections based on technical problems during the production process for each factor. Thus, the sum of the variance coefficient of each factor is equal to 30%, which is the variance coefficient of the production process on the physicochemical properties and chemical structures by the bituminous binders’ species. Moreover, the four bituminous binders or four bituminous binders species studied were manufactured somewhat differently, and each binder sample came from different production batches. The bituminous binders are graded into pairs as BND 70/100 and 100/130 according to GOST 33133. Therefore, they must have very close physicochemical properties in pairs. The intermediate results with the remarks are presented in Table 6 below:

Table 6.
Summary table of mean physicochemical properties BND 70/100 and 100/130.
- Most of the studied samples had physicochemical properties under GOST 33133 requirements for BNDs 70/100 and 100/130. However, it should be noted that the samples studied here are far from being superior bituminous binders due to the low margin recorded.
- The test results further showed that 45% of the BND 70/100 samples and 60% of the BND 100/130 samples from Plant A did not meet GOST requirements, including “ductility at 0 °C” for BND 70/100 and “mass loss (%) after RTFOT ageing” for BND 100/130. Regarding “ductility at 0 °C” for BND 70/100 from Plant A, five (5) showed failure (crack) since the testing began. Thus, these five (5) measurements were not included in the statistical mean. On the other hand, concerning the property “mass loss (%) after RTFOT ageing” of BND100/130 from plant A, the parameters of the standard deviation/variance coefficient confirmed the remark. However, the arithmetic means of “mass loss (%) after ageing RTFOT” ET 100/130 from plant A was following GOST 33133, as for BAND 70/100 from plant B.
- Regarding the identification or tracking of bituminous binders, it is noted that the “rotational viscosity at 1.5 s−1 and 60 °C, Pa∙s before RTFOT” of the bituminous binder samples from plants A and C was generally less than 200 Pa∙s before RTFOT, and after short-term ageing, less than 500 Pa∙s. On the other hand, the rotational viscosity values of bituminous binders from plant B were close to 400 Pa∙s before RTFOT and 900 Pa∙s after RTFOT.
3.2. Results of Fourier-Transform Infrared Spectroscopy Investigation
Studies using IR spectroscopy are divided into two parts: visual analysis of IR spectra and calculation of spectrometry indices with the interpretation of the data obtained. Visual analysis of IR spectra was performed to find and determine functional groups. A visual analysis should establish whether or not there are differences between one sample and another for chemical compounds.
Each binder sample in the infrared spectroscopy investigation was studied before and after short-term ageing (RTFOT). Thus, the initial number of binder samples studied was multiplied by two (2) to study the samples after short-term ageing. Therefore, 160 samples were studied, which is equivalent to 160 infrared spectra with their spectral data. 160 IR spectra (samples) were shared between four species of binders or types of binder. For each type of bituminous binder, there were 40 IR spectra distributed as follows: 20 IR spectra before and after short-term ageing.
In order to measure and ensure the reliability of the results obtained, we also included the standard deviation and the variance coefficient in this study, while maintaining the same standard of statistical validity at 30%. FTIR analyses of bituminous binders or binder samples by plants yielded the following IR spectra shown in Figure 4, Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9. Figure 4 and Figure 5 show 80 IR spectra of BND 70/100 from plants A and B, distributed as follows: 40 IR spectra before and 40 IR spectra after short-term ageing (RTFOT). The same distribution of IR spectra for BND 100/130 from plants A and C apply to Figure 6 and Figure 7.
Figure 4.
IR spectra of BND 70/100 from plants A and B before short-term ageing RTFOT.
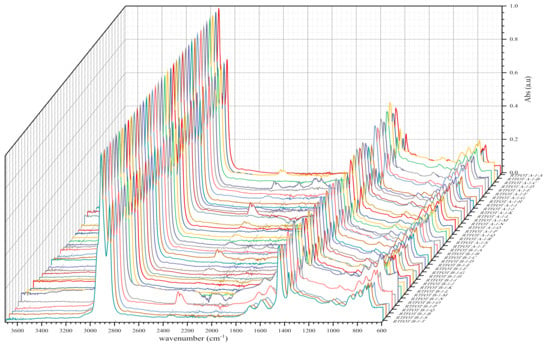
Figure 5.
IR spectra of BND 70/100 from plants A and B after short-term ageing RTFOT.
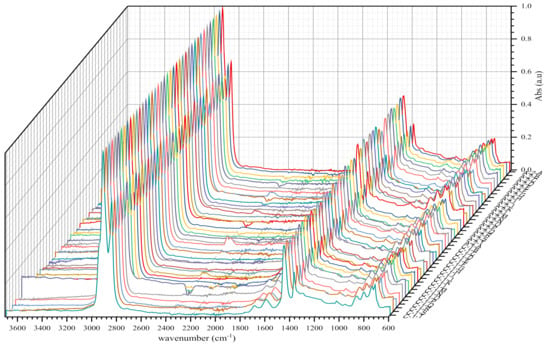
Figure 6.
IR spectra of BND 100/130 from plants A and C before short-term ageing RTFOT.
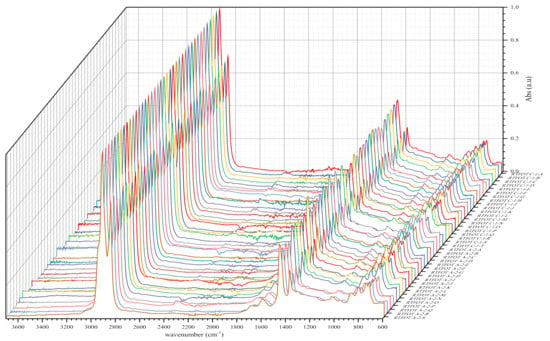
Figure 7.
IR spectra of BND 100/130 from plants A and C after short-term ageing RTFOT.
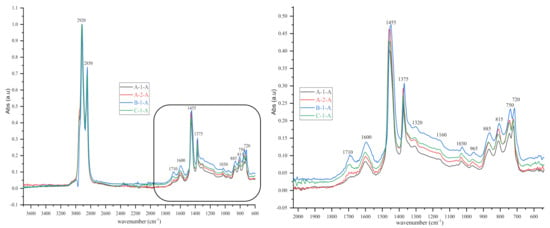
Figure 8.
IR spectra (3700–600 cm‒1 and 2000–600 cm‒1) of some bituminous binders before short-term ageing (RTFOT).
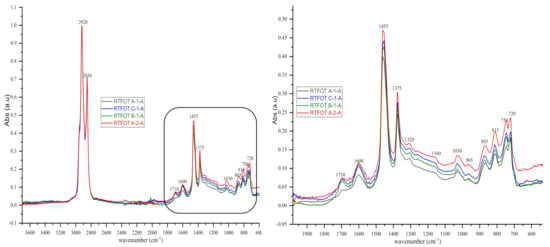
Figure 9.
IR spectra (3700–600 cm‒1 and 2000–600 cm‒1) of some bituminous binders after short-term ageing RTFOT.
The enumeration and detection of each band or number of waves of chemical compounds of the binder samples studied are shown in Table 7. Likewise, the results of spectrometry indices data are shown in Table 8, Table 9, Table 10 and Table 11. Using spectrometry indices to analyse the chemical structure of bituminous binders can help detect, track, and understand the stability of their chemical composition.

Table 7.
ATR-FTIR spectra’s data of bituminous binders.

Table 8.
Calculation of spectrometry indices of BND 70/100 from plant A before and after RTFOT.

Table 9.
Calculation of spectrometry indices of BND 100/130 from plant A before and after RTFOT.

Table 10.
Calculation of spectrometry indices of BND 70/100 from plant B before and after RTFOT.

Table 11.
Calculation of spectrometry indices of BND 100/130 from plant C before and after RTFOT.
As shown in the figures above, visual inspection of the IR spectra did not yield the expected results. Indeed, all the samples of binder studied had almost identical chemical functions, as can be seen in Table 7. Except for BND 70/100 binders from Plant A, we found two peaks at 1710 cm−1 and 1600 cm−1 in virtually all other IR spectra of binder samples, precisely in the range 1590–1740 cm−1. The peculiarity of the BND 70/100 from plant A compared with the others studied was that they only had the band at 1600 cm−1. This observation is supported by the IR spectra of BND 70/100 from plant A before and after RTFOT shown in Figure 4, Figure 5, Figure 8 and Figure 9. All BND 70/100 and 100/130 IR spectra from plants B and C had bands at 1710 and 1600 cm−1, as well as BND 100/130 from plant A.
Briefly, the only visual difference was seen in the range of 1740–1690 cm−1 (1710 cm−1), while the carbonyl, hydroxyl, and ether (C=O) groups were among the chemical classes represented by the 1710 cm−1 band. According to the spectral inspection results of BND 70/100 from plant A, these BND 70/100 are produced without oxidation or with minimal oxidation, that is, under mild oxidation conditions. This interpretation of the BND 70/100 binder samples is due to their IR spectra, in which there is virtually no peak at 1710 cm−1.
Further analysis was performed to identify or track, including quality control of the binder samples, for the reasons stated above. This in-depth or further analysis was based on the oxidation degree via oxidation indices (spectrometry indices). The spectrometry indices were defined as the ratio of the absorbance values (a.u) to the maxima of the corresponding absorption bands. Before calculating the spectrometry indices, the baselines of the IR spectra were subtracted and the IR spectra were normalised.
The chemical structures of the analysed binder samples are or should be reflected by the spectrometry indices. The algorithm for calculating spectrometry indices includes the normalisation. The normalisation was carried out to eliminate any modification of the absorbance spectra, due to a variation in the penetration of the infrared beam between the samples which would distort the interpretation of the results. Among the spectrometry indices, one of the most important is the oxidation indices due to the visual differences observed in the IR spectra of the binder samples. The oxidation indices, or oxidation rate, are the parameters which characterise the degree of oxidation. The increase in carbonyl compounds characterises the process of chemical oxidation of oils and bitumen, as already mentioned in the introduction. The sulphurisation and aromatisation processes also accompany the chemical oxidation process. Therefore, other indices, such as aromaticity and sulphurisation, were included in our studies to confirm the results on the degree of oxidation.
The BND 70/100 spectrometry indices of plant A corresponded to the observations mentioned above. In this sense, the oxidation indices of BND 70/100 from plant A were the lowest because there are no bands of carbonyl compounds in their IR spectra. Furthermore, during our visual inspection of the infrared spectra samples, another phenomenon caught our attention. We observed that the IR spectra of the 100/130 BAND of plant A had two bands at 1710 and 1600 cm−1 in the range of 1740–1590 cm−1. Visual evaluation of the IR BND 100/130 spectra of plant A was confirmed by spectrometry indices (see Figure 6, Figure 7, Figure 8 and Figure 9). The degree of oxidation of BND 100/130 from plant A, on the other hand, was significantly higher than the BND 70/100 of the same plant, according to spectrometry indices. This fact, from our point of view, is somewhat peculiar. BND 70/100 and 100/130 are derived from the same plant A. If BND 70/100 and 100/130 are made with the same technology, BND 100/130 should have an oxidation grade of less than 70/100, not the other way around, in the sense that the 70/100 performance band is harder than BND 100/130. Based on our observations, there is every reason to believe that the production technology used for BND 70/100 differs from the technology used for BND 100/130. In this case, this assumption is the only rational one. It is perhaps conceivable that the raw materials for BND 70/100 and 100/130 are, though unlikely, implausible because Plant A is supplied via pipeline. Nevertheless, Table 8 and Table 9 show that the BND 70/100 and 100/130 binder samples follow the ageing law, because the coefficient of variance after short-term ageing (RTFOT) was within the norm (standard); see Summary Table 12.

Table 12.
The mean of spectrometry indices per plant with their standard deviation (SD) and their variance coefficient (CV).
Looking at the IR spectra (Figure 4, Figure 5, Figure 8, and Figure 9) and spectrometry indices (Table 10) of BND 70/100 from plant B, we observed a significant (high) oxidation degree before and after short-term ageing. According to Table 10 and Table 12, the degree of oxidation before and after short-term ageing (RFTOT) is plus or minus 0.160, which shows that the chemical structure of these binders is generally stable, as shown in Figure 10. The regular variance coefficient and the standard deviations confirm these observations. The values of the other spectrometry indices in Table 12 confirm this assumption.
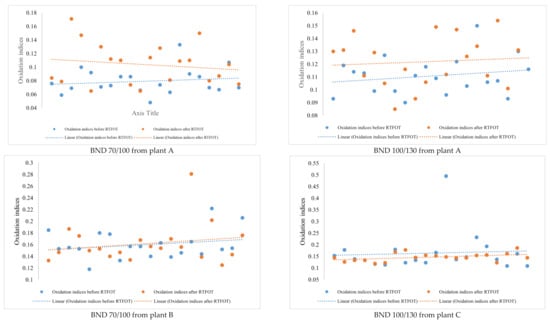
Figure 10.
Comparison between the oxidation indices before and after RTFOT by plant and binder samples’ grades.
The analysis of BND 100/130 from Plant C seemed to be the most curious of all the results of the present study. Curiosity was found in the results obtained. The variance coefficient before ageing (RTFOT) for the oxidation indices was 51.2%, as shown in Table 12, and therefore outside the standards set. This allows us to conclude, at this stage, by the dispersion of the data, that the BNDs from plant C are not very stable. In addition, post-ageing studies (RTFOT) showed that the values of the spectrometry indices change in the opposite direction of ageing. Usually, sulphoxides, carbonyl groups and aromatic compounds increase upon oxidation of the binder, as shown earlier in the introduction. Therefore, after oxidation, an increase in the content of sulphoxides, carbonyl groups, and aromatic compounds is to be expected. However, for the BND from plant C, the mean values of the spectrometry indices showed the opposite of the results expected after short-term ageing (RTFOT). The oxidation indices of BND 100/130 binder samples before RTFOT were higher than after short-term ageing (RTFOT). On the other hand, the proportion of sulphoxides decreased, as did carbonyl groups and aromatics. Rejuvenation of the binder samples (chemical structure) was logically observed. The rejuvenation phenomenon is a positive process because it guarantees the quality of the binders during operation life. Unfortunately, as shown in Table 11, all BND 100/130 binder samples after short-term ageing (RTFOT) did not follow the rejuvenation phenomenon because some samples showed a high degree of oxidation. Therefore, rejuvenation may be an identifying or tracking factor for the BND 100/130 binder samples from Plant C.
Over the study of bituminous materials, it was noted that there are certain correlations between the FTIR spectroscopy data and the physicochemical properties in Table 2, Table 3, Table 4, Table 5 and Table 6. More precisely, the correlation is significant between the chemical structures and rheological data. For example, according to FTIR analyses, the bituminous binders from plant C exhibited the relatively highest and most stable degree of oxidation, accompanied by fairly high asphaltene, aromaticity, and sulphurisation indices, which are reflected in the highest values for “rotational viscosity at 1.5 s−1 and 60 °C” before and after short-term ageing (RTFOT). In addition, the bituminous binders from plant C with low aliphaticity indices, probably low in n-paraffin, had good physicochemical properties at zero and negative temperatures. On the other side, t BND 70/100 bituminous binders from plant A had poor physicochemical properties at zero or low temperatures (see ductility at 0 °C) with very high aliphaticity indices. At the same time, the BND 70/100 bituminous binders from plant A also showed relatively low oxidation indices before and after short-term ageing (RTFOT) according to FTIR results, which is visible in the values of “rotational viscosity at 1.5 s−1 and 60 °C.
4. Conclusions
The binder tracking is topical as road projects are often supplied with various bituminous materials from different manufacturers and dealers. During road project operations, bituminous material passports (origin) can get lost. In this regard, there are cases when customers (road projects) are not satisfied with the quality of the product supplied. Many oil plants around the world have almost the same sources of oil. For example, in Russia, crude oil supplied to refineries is usually carried out via pipeline. Therefore, this investigation was conducted to find the differences between bituminous binders from different plant facilities using FTIR analyses to track their origin. The work also included the assessment of the quality of bituminous binders graded as BND 70/100 and 100/130 according to GOST 33133.
As a result of the first part of the work, the road bitumen was tested according to GOST 33133, as shown in Table 13. Most of the bituminous binders studied have physicochemical properties under the requirements of GOST 33133 for TAPES 70/100 and 100/130, which are consistent with the standard deviation/variance coefficient. However, the test results obtained showed that 45% of the BND 70/100 samples and 60% of the BND 100/130 samples from Plant A did not meet GOST requirements, including “ductility at 0 °C” for BND 70/100 and “mass loss (%) after RTFOT ageing” for BND 100/130. Regarding the identification or tracking of bituminous binders, it is noted that the “rotational viscosity at 1.5 s−1 and 60 °C, Pa∙s before RTFOT” of the bituminous binder samples from plants A and C was generally less than 200 Pa∙s before RTFOT and less than 500 Pa∙s after short-term ageing. On the other hand, the rotational viscosity of the bituminous binders from plant B was close to 400 Pa∙s before RTFOT and 900 Pa∙s after RTFOT.

Table 13.
Summarising the mean physicochemical properties of the bituminous binders studied.
According to the results of the second part of the investigation (FTIR analyses), it can be concluded that the chemical structures of the binder samples before and after short-term ageing (RTFOT) were mostly stable. Most binder samples obeyed the law of ageing, except for BND 100/130 from Plant C, which was unpredictable, i.e., complicated statistical series. Therefore, the spectral data and spectrometry indices of the binder samples provided information about their differences; see summary Table 14 of experimental studies on FTIR spectroscopy.

Table 14.
Summary of Fourier-transform infrared spectroscopy investigation.
In summary of the investigation, there were some differences between NDBs from different plants. The significant differences in Table 14 can be used to partially identify the bitumen, i.e., recognise or track its place of manufacture (origin). However, these differences can only be used in Gazpromneft-Bituminous Materials. The explanation lies in the fact that the chemical structures of BND 70/100 and 100/130 from competitors (other companies or plants) are unknown. Therefore, this point shows the limitations of this tracking method to identify or recognise products made by Gazpromneft-bituminous materials. Consequently, it is recommended to develop and introduce unique chemical markers (tracers) to achieve effective traceability and identify Gazpromneft-Bituminous Materials products.
On the other hand, this work can be considered as a start for new research using FTIR spectroscopy as an internal traceability tool for large companies such as Gazpromneft-Bituminous Materials or to explore other experimental methods more suited to effective traceability of bituminous binders.
Author Contributions
S.-B.A.: conceptualization, methodology, investigation, formal analysis, writing—original draft; A.A.G.: data curation; O.N.V.: conceptualization, methodology; A.V.K.: supervision, validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Institutional Review Board (IRB) of Scientifical and Educational Centre Bituminous Materials, National University of Oil and Gas “Gubkin University”, and Research and Development of Gazpromneft-Bituminous Materials.
Acknowledgments
Thanks to Gazpromneft-Bituminous Materials for agreeing to publish this investigation.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclaimer
The trade name or company name has been used solely for informational purposes and not for the approval of its products, advertisements, promotions or certifications.
Abbreviations
| ATR-FTIR Spectroscopy | Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy |
| RTFOT | Rolling Thin Film Oven Test |
| GOST | Russian interstate standards |
| BND | Bituminous oil road |
References
- Rudenskaya, I.M.; Rudensky, A.V. Organic Binders for Road Construction; Infra-M: Moscow, Russia, 2010; p. 257. [Google Scholar]
- Kemalov, F.A.; Kemalov, R.A.; Ganieva, T.F. Production of Oxidised Bitumen. Kazan State Technological University: Kazan, Russia, 2010. [Google Scholar]
- Gureev, A.A. Petroleum Binders; Publishing House Nedra, LLC: Moscow, Russia, 2018. [Google Scholar]
- Motamedi, M.; Attar, M.M.; Rostami, M. Performance enhancement of the oxidized bitumen binder using epoxy resin. Prog. Org. Coat. 2017, 102, 178–185. [Google Scholar] [CrossRef]
- Adiko, S.-B.; Gureev, A.A.; Khasanova, N.M.; Sakharov, B.V. Processing of High-Paraffinic vacuum residues by thermocatalytic methods to obtain bitumen. Constr. Build. Mater. 2021, 285, 122880. [Google Scholar] [CrossRef]
- EN. 12591. Bitumen and Bituminous Binders. Specifications for Paving Grade Bitumens; CEN: Brussels, Belgium, 2009; p. 28. [Google Scholar]
- Asi, I.M. Performance evaluation of SUPERPAVE and Marshall asphalt mix designs to suite Jordan climatic and traffic conditions. Constr. Build. Mater. 2007, 21, 1732–1740. [Google Scholar] [CrossRef]
- Djimasbe, R.; Galiullin, E.A.; Varfolomeev, M.A.; Fakhrutdinov, R.Z.; Al-Muntaser, A.A.; Farhadian, A. Experimental study of non-oxidized and oxidized bitumen obtained from heavy oil. Sci. Rep. 2021, 11, 8107. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.J. Introduction to Crude Oil and Petroleum Processing. In Handbook of Petroleum Processing; Kokayeff, P., Zink, S., Roxas, P., Eds.; Springer: Berlin, Germany, 2015; pp. 1–39. [Google Scholar]
- Lesueur, D. The colloidal structure of bitumen: Consequences on the rheology and on the mechanisms of bitumen modification. Adv. Colloid Interface Sci. 2009, 145, 42–82. [Google Scholar] [CrossRef] [PubMed]
- Speight, J.G. The Chemistry and Technology of Petroleum; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Hunter, R.N.; Self, A.; Read, J. The Shell Bitumen Handbook, 6nd ed.; ICE Publishing: London, UK, 2015; p. 788. [Google Scholar]
- BSI, BS EN 13304. Bitumen and ituminous binders. In Framework for Specification of Oxidized Bitumen; NSAI: Dublin, Ireland, 2009; p. 8. [Google Scholar]
- Yao, H.; Dai, Q.; You, Z. Fourier Transform Infrared Spectroscopy characterization of aging-related properties of original and nano-modified asphalt binders. Constr. Build. Mater. 2015, 101, 1078–1087. [Google Scholar] [CrossRef]
- MNivitha, R.; Prasad, E.; Krishnan, J.M. Ageing in modified bitumen using FTIR spectroscopy. Int. J. Pavement Eng. 2016, 17, 565–577. [Google Scholar] [CrossRef]
- Petersen, J.C. A review of the fundamentals of asphalt oxidation: Chemical, physicochemical, physical property, and durability relationships. Transp. Res. Circ. 2009, 10, 78. [Google Scholar]
- Tauste, R.; Moreno-Navarro, F.; Sol-Sánchez, M.; Rubio-Gámez, M.C. Understanding the bitumen ageing phenomenon: A review. Constr. Build. Mater. 2018, 192, 593–609. [Google Scholar] [CrossRef]
- Cong, P.; Guo, X.; Mei, L. Investigation on rejuvenation methods of aged SBS modified asphalt binder. Fuel 2020, 279, 118556. [Google Scholar] [CrossRef]
- Camargo, I.G.d.N.; Dhia, T.B.; Loulizi, A.; Hofko, B.; Mirwald, J. Anti-aging additives: Proposed evaluation process based on literature review. Road Mater. Pavement Des. 2021, 22, S134–S153. [Google Scholar] [CrossRef]
- Apostolidis, P.; Liu, X.; Erkens, S.; Scarpas, T. Oxidative aging of epoxy asphalt. Int. J. Pavement Eng. 2020, 1–11. [Google Scholar] [CrossRef]
- Weigel, S.; Stephan, D. The prediction of bitumen properties based on FTIR and multivariate analysis methods. Fuel 2017, 208, 655–661. [Google Scholar] [CrossRef]
- Pipintakos, G.; Ching, H.V.; Soenen, H.; Sjövall, P.; Mühlich, U.; van Doorslaer, S.; Varveri, A.; Lu, X. Experimental investigation of the oxidative ageing mechanisms in bitumen. Constr. Build. Mater. 2020, 260, 119702. [Google Scholar]
- Omar, M.A.; Nara, K.R.; Obieglo, A.; Belk, N.; Schalausky, R. Analysis of non-load bearing, 2 component epoxy-adhesive to the hemming process variations; Thermo-Gravimetric, Differential Scanning Calorimetry, and Fourier Transform Infrared Spectroscopy FT-IR analyses. Prog. Org. Coat. 2009, 65, 104–108. [Google Scholar] [CrossRef]
- Tarasevich, B.N. IR Spectra of Basic Classes of Organic Compounds; MSU: Moscow, Russia, 2012; p. 6. [Google Scholar]
- Tarasevich, B.N. Fundamentals of IR Spectroscopy with Fourier Transform. Sample Preparation in IR Spectroscopy; MSU: Moscow, Russia, 2012. [Google Scholar]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 10815–10837. [Google Scholar]
- Moretti, L.; Mandrone, V.; D’Andrea, A.; Caro, S. Comparative “from cradle to gate” life cycle assessments of Hot Mix Asphalt (HMA) materials. Sustainability 2017, 9, 400. [Google Scholar] [CrossRef] [Green Version]
- Hunter, R.N. Bituminous Mixtures in Road Construction, 2nd ed.; Thomas Telford: Telford, UK, 1994; p. 441. [Google Scholar]
- Isenring, T.; Koster, H.; Scazziga, I. Experiences with Porous Asphalt in Switzerland. Transp. Res. Rec 1990, 1265, 41–53. [Google Scholar]
- Gulen, S.G. Regionalization in the world crude oil market. Energy J. 1999, 20, 125–139. [Google Scholar] [CrossRef]
- Nakanishi, T.; Komiyama, R. Supply and Demand Analysis on Petroleum Products and Crude Oils for Asia and the World. IEEJ august 2006, 80. [Google Scholar]
- Abdi, H. Coefficient of variation. Encycl. Res. Des. 2010, 1, 169–171. [Google Scholar]
- Hofko, B.; Alavi, M.Z.; Grothe, H.; Jones, D.; Harvey, J. Repeatability and sensitivity of FTIR ATR spectral analysis methods for bituminous binders. Mater. Struct. 2017, 50, 187. [Google Scholar] [CrossRef] [Green Version]
- Kemalov, R.A.; Kemalov, A.F. Scientific and practical aspects of obtaining bitumen-emulsion mastics. Oil Gas. Technol. 2012, 6, 31–39. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).