Position Control and Force Estimation Method for Surgical Forceps Using SMA Actuators and Sensors
Abstract
:1. Introduction
1.1. State of the Art
1.2. Load Sensing and Haptic Feedback
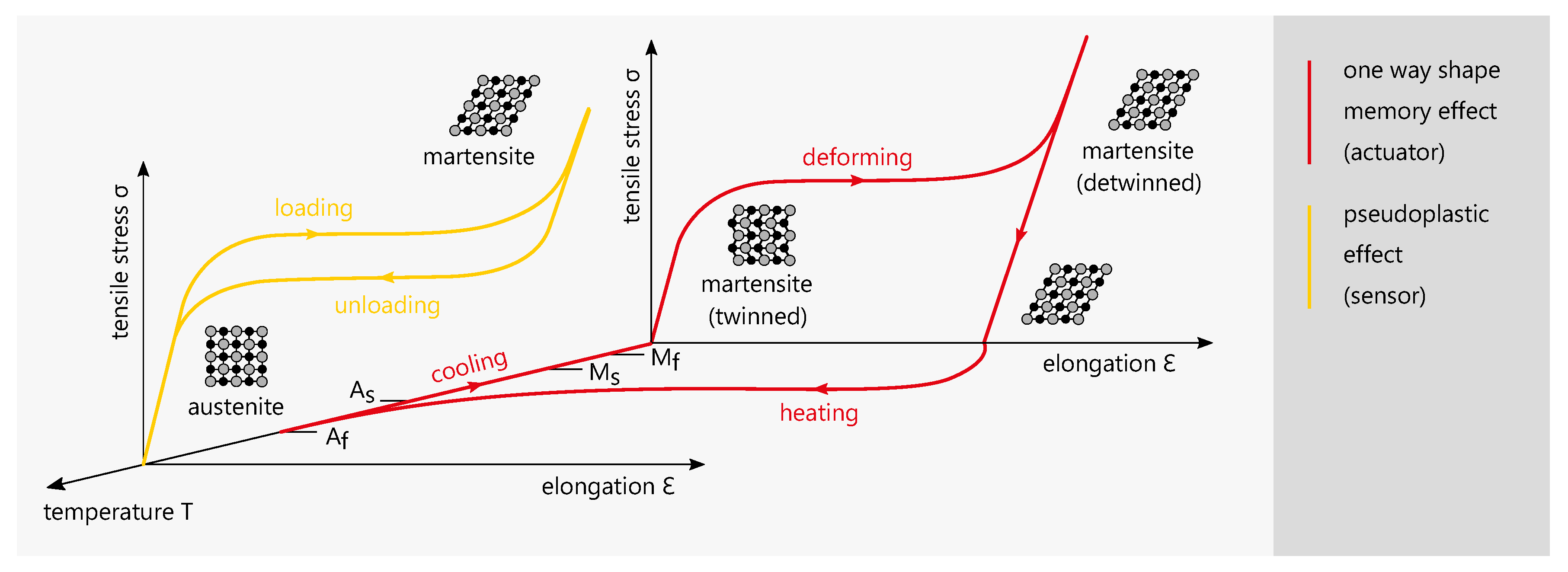
1.3. Shape Memory Alloys
2. Materials and Methods
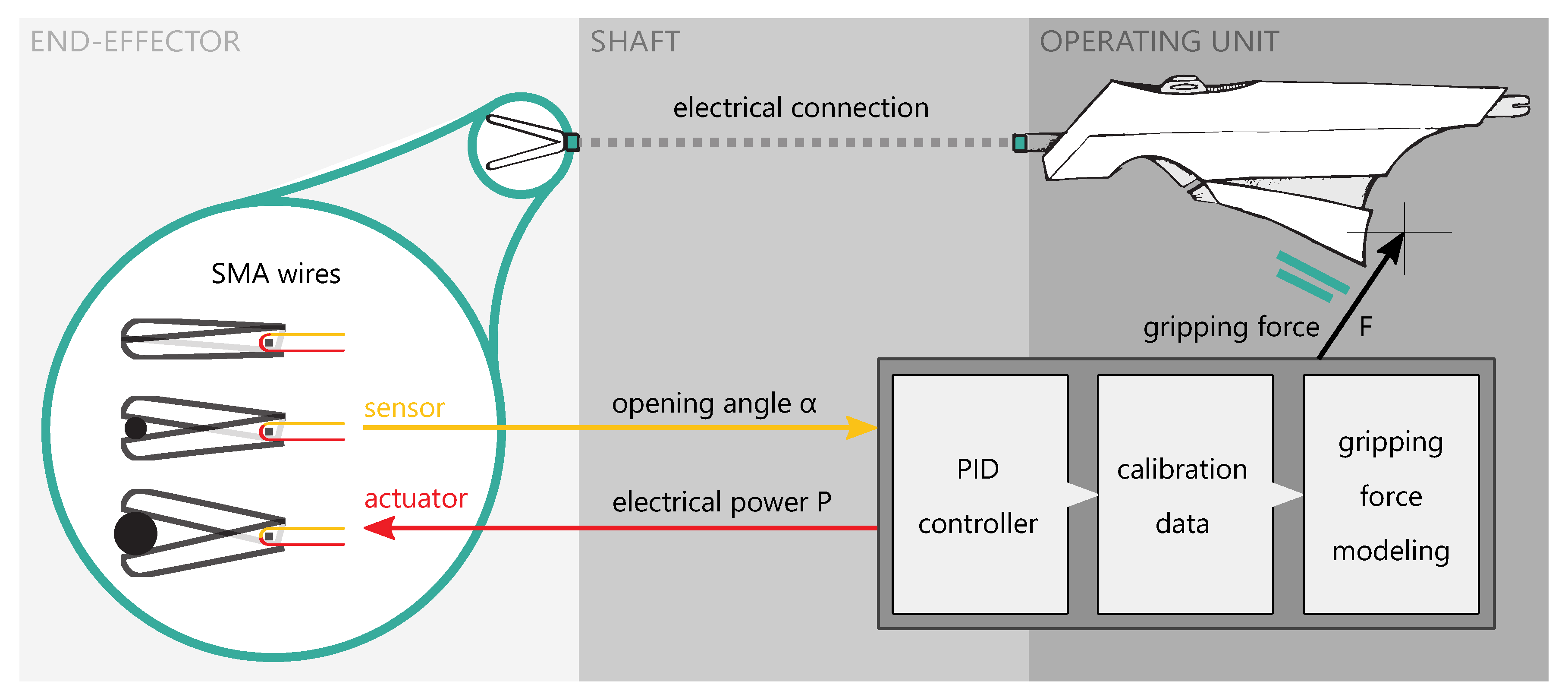
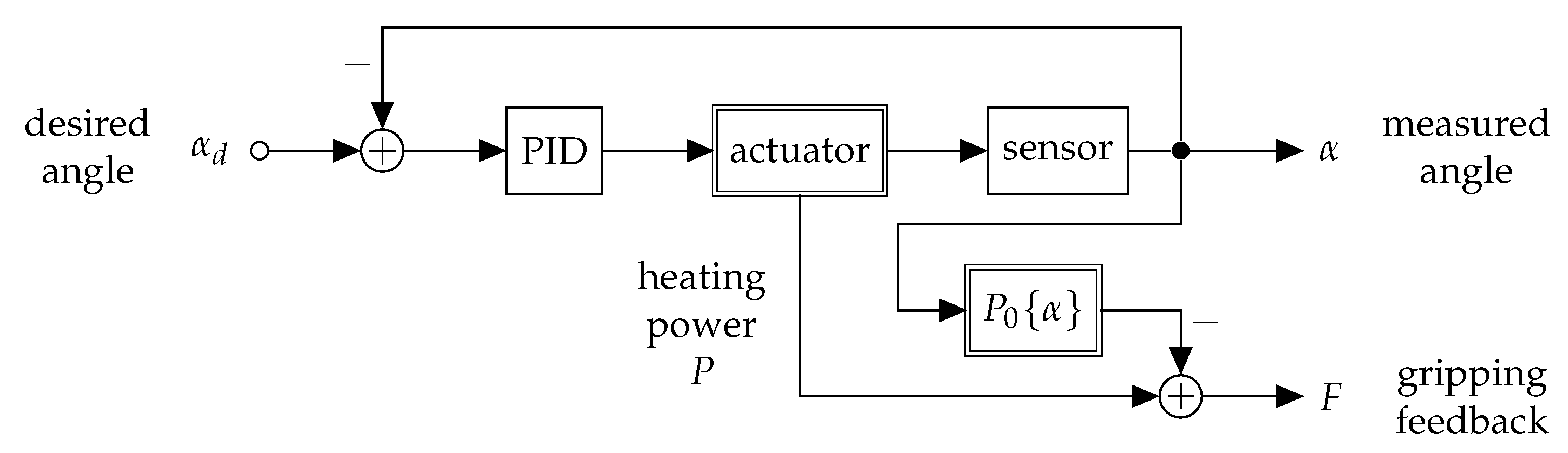
2.1. Concept and Approach
2.2. NiTi as Actuator and Sensor
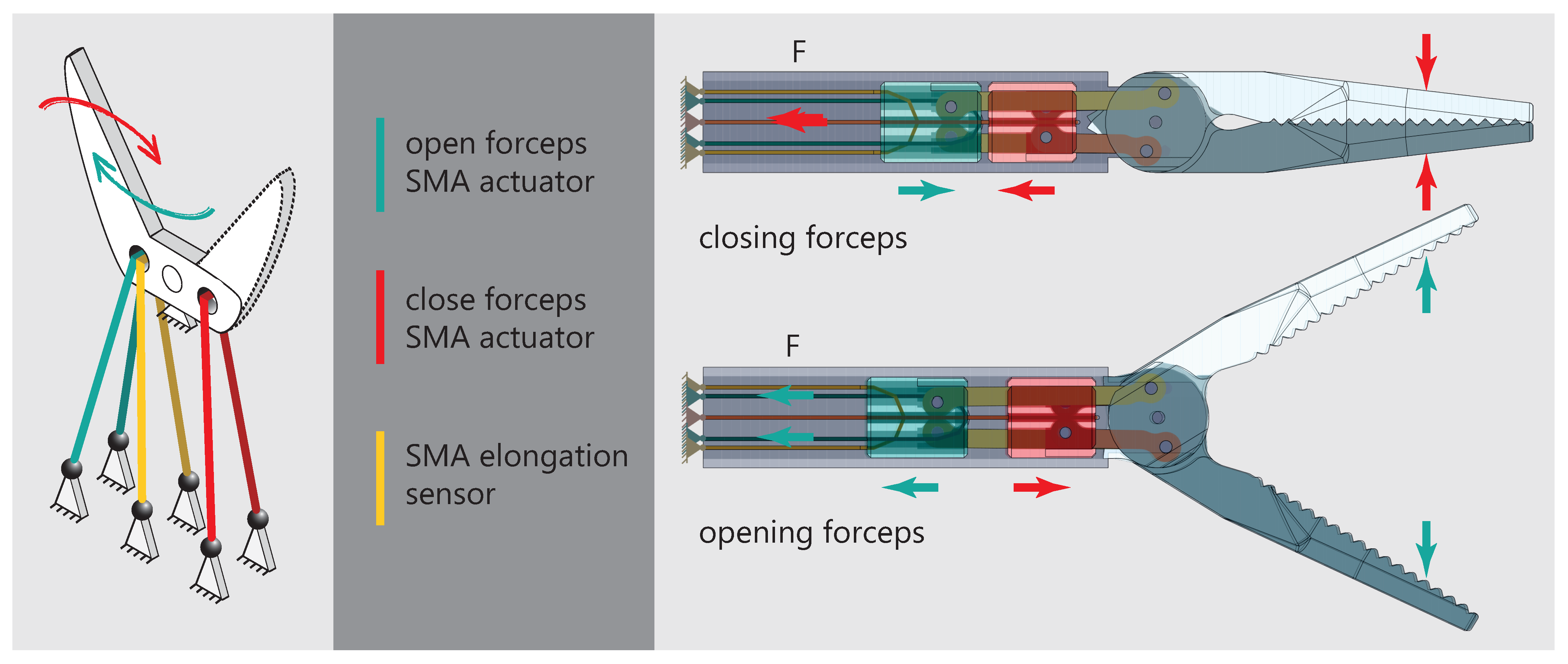
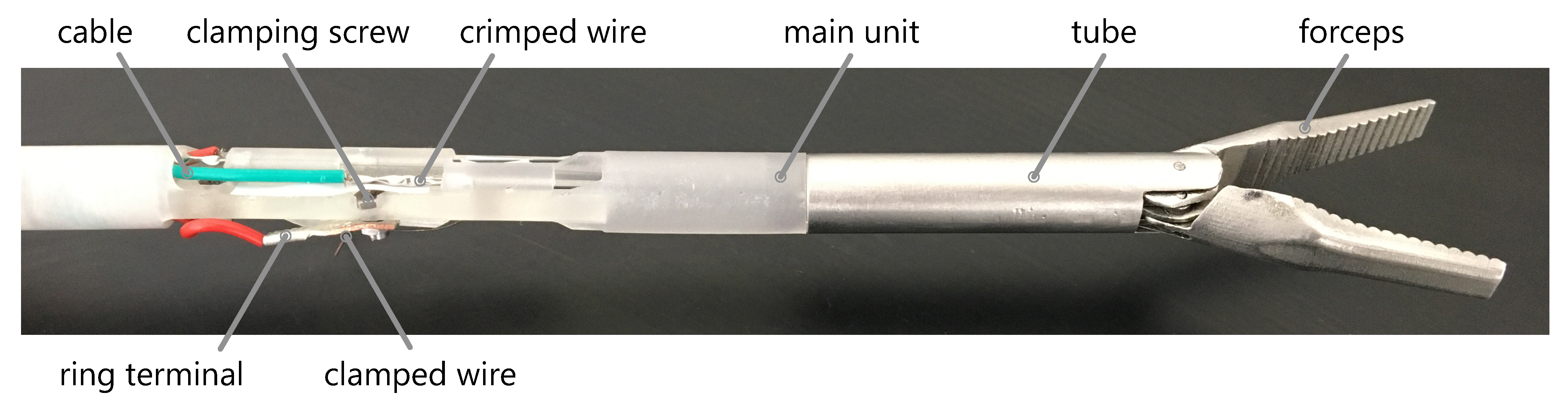
2.3. Effector Mechanics
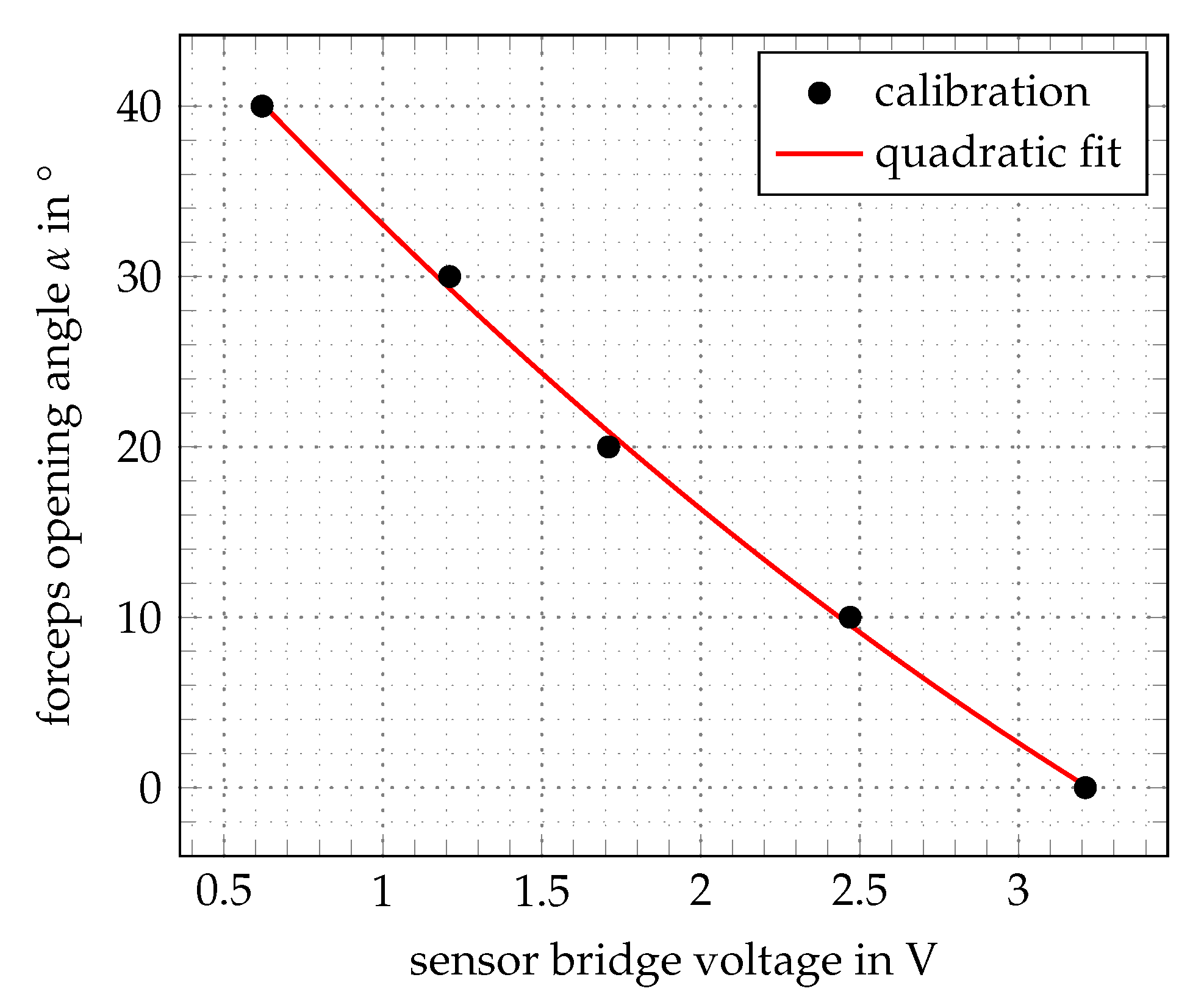
2.4. Control and Electronics
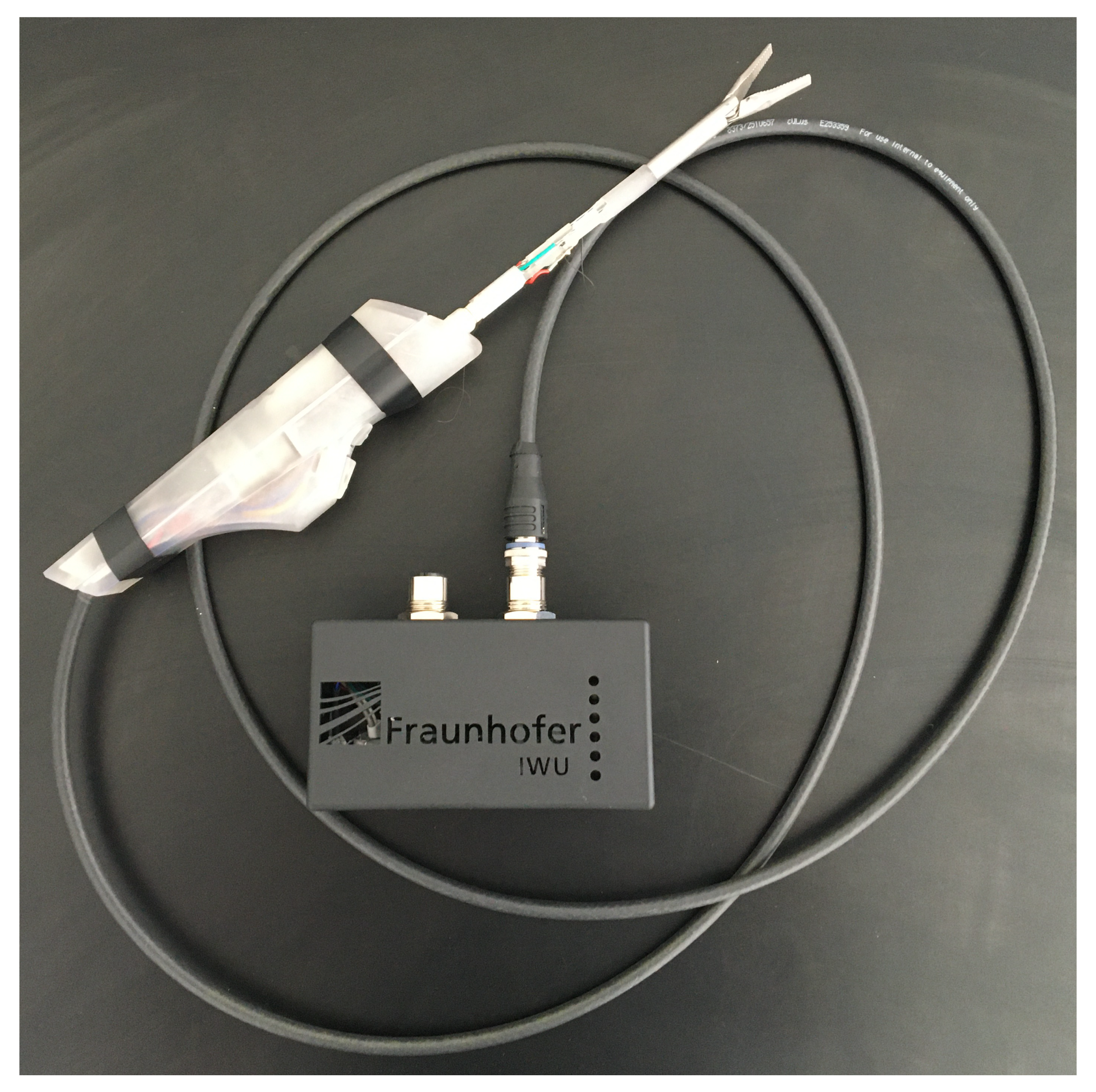
2.5. Functional Model Manufacturing
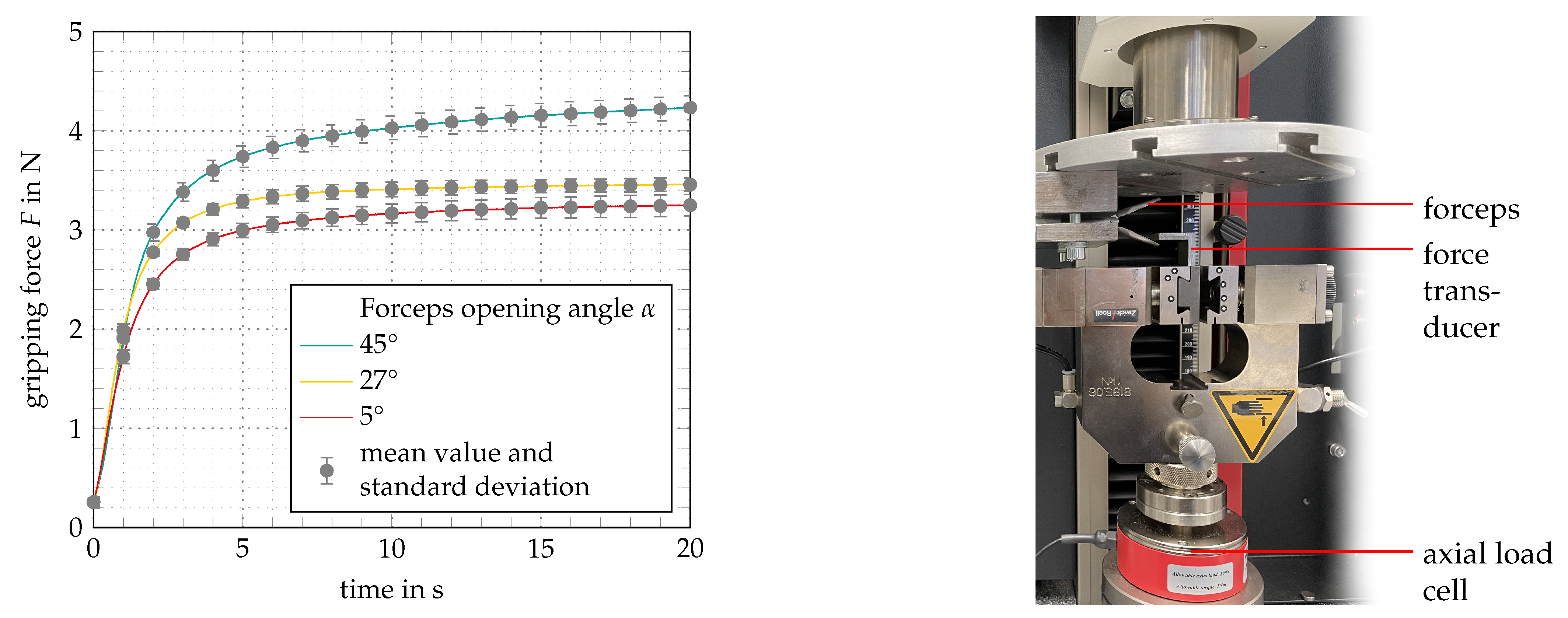
3. Results and Discussion
4. Conclusions
- The approach to realize a software control model to use position control for gripping force estimation instead of using external load sensors is a novelty. The developed control model is not based on a physical-oriented model but rather a relative-model on calibrating parameters as ground truth within a specific assembly.
- A new forceps mechanism was designed to meet the special requirements of the SMA actuators and sensor. By using two deflected actuators in an antagonistic arrangement, the cooling performance and force output of the forceps could be increased. Another SMA wire acts as an elongation sensor to detect the opening angle of the forceps.
- By mainly using rapid prototyping technologies, a functional model was manufactured. In an evaluation setting, the working principle could be proven. Gripping forces of up to ± could be measured.
- Compared to conventional laparoscopic instruments, no mechanical coupling is used. The force transmission between the operating unit and the forceps is adjustable and there is a great freedom in designing the control elements, because the connection is only via an electric signal.
- Compared to robotic systems, it is less complex and therefore more cost-effective.
- By not using an external load sensor, the system can be capsulated, which is important for the cleaning process of the instrument and the prevention of cross-contamination.
- Slow dynamics of the functioning model can be improved by optimizing the cooling performance and tuning of the PID coefficients.
- The generation of the feedback force is realized via a vibration motor in the functional model. This can be improved to increase usability.
- Thermal management and electric isolation has not yet been addressed, but is planned for future works.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Runciman, M.; Darzi, A.; Mylonas, G.P. Soft Robotics in Minimally Invasive Surgery. Soft Robot. 2019, 6, 423–443. [Google Scholar] [CrossRef] [Green Version]
- Antoniou, S.A.; Antoniou, G.A.; Antoniou, A.I.; Granderath, F.A. Past, Present, and Future of Minimally Invasive Abdominal Surgery. JSLS J. Soc. Laparoendosc. Surg. 2015, 19. [Google Scholar] [CrossRef] [Green Version]
- Tsui, C.; Klein, R.; Garabrant, M. Minimally invasive surgery: National trends in adoption and future directions for hospital strategy. Surg. Endosc. 2013, 27, 2253–2257. [Google Scholar] [CrossRef]
- Donner, S. Entwicklung von Richtlinien für die Prospektiv-Nutzergerechte Gestaltung Minimal-Invasiver Chirurgieinstrumente. Ph.D. Thesis, Technical University of Berlin, Berlin, Germany, 2014. [Google Scholar] [CrossRef]
- Spruce, L. Back to Basics: Minimally Invasive Surgery. AORN J. 2019, 109, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M. Finanzielle Auswirkungen Innovativer Medizintechnik mit Einspareffekten im Gesundheitswesen; Eine Gemeinschaftsstudie der Technischen Universität, der Unternehmerberatung Droege & Comp und des Industrieverbandes SPECTARIS eV: Berlin, Germany, 2006. [Google Scholar]
- Breedveld, P.; Stassen, H.G.; Meijer, D.W.; Jakimowicz, J.J. Manipulation in laparoscopic surgery: Overview of impeding effects and supporting aids. J. Laparoendosc. Adv. Surg. Tech. Part 1999, 9, 469–480. [Google Scholar] [CrossRef]
- Heijnsdijk, E.A.M.; Pasdeloup, A.; Dankelman, J.; Gouma, D.J. The optimal mechanical efficiency of laparoscopic forceps. Surg. Endosc. 2004, 18, 1766–1770. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Wang, X.; He, C.; Zhang, L. Development of a novel mechanism for minimally invasive surgery. In Proceedings of the 2010 IEEE International Conference on Robotics and Biomimetics, Tianjin, China, 14–18 December 2010; pp. 1370–1375. [Google Scholar] [CrossRef]
- Park, A.; Lee, G.; Seagull, F.J.; Meenaghan, N.; Dexter, D. Patients benefit while surgeons suffer: An impending epidemic. J. Am. Coll. Surg. 2010, 210, 306–313. [Google Scholar] [CrossRef]
- Alleblas, C.C.J.; Vleugels, M.P.H.; Stommel, M.W.J.; Nieboer, T.E. Performance of a Haptic Feedback Grasper in Laparoscopic Surgery: A Randomized Pilot Comparison With Conventional Graspers in a Porcine Model. Surg. Innov. 2019, 26, 573–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meccariello, G.; Faedi, F.; AlGhamdi, S.; Montevecchi, F.; Firinu, E.; Zanotti, C.; Cavaliere, D.; Gunelli, R.; Taurchini, M.; Amadori, A.; et al. An experimental study about haptic feedback in robotic surgery: May visual feedback substitute tactile feedback? J. Robot. Surg. 2016, 10, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.M.; Frelich, M.J.; Bosler, M.E.; Gould, J.C. Cost analysis of robotic versus laparoscopic general surgery procedures. Surg. Endosc. 2017, 31, 185–192. [Google Scholar] [CrossRef]
- Okamura, A.M. Haptic feedback in robot-assisted minimally invasive surgery. Curr. Opin. Urol. 2009, 19, 102–107. [Google Scholar] [CrossRef]
- Hagen, M.E.; Meehan, J.J.; Inan, I.; Morel, P. Visual clues act as a substitute for haptic feedback in robotic surgery. Surg. Endosc. 2008, 22, 1505–1508. [Google Scholar] [CrossRef] [PubMed]
- Koehn, J.K.; Kuchenbecker, K.J. Surgeons and non-surgeons prefer haptic feedback of instrument vibrations during robotic surgery. Surg. Endosc. 2015, 29, 2970–2983. [Google Scholar] [CrossRef]
- van der Meijden, O.A.J.; Schijven, M.P. The value of haptic feedback in conventional and robot-assisted minimal invasive surgery and virtual reality training: A current review. Surg. Endosc. 2009, 23, 1180–1190. [Google Scholar] [CrossRef] [Green Version]
- Braun, E.U.; Mayer, H.; Knoll, A.; Lange, R.; Bauernschmitt, R. The Must-Have in Robotic Heart Surgery: Haptic Feedback. In Medical Robotics; Bozovic, V., Ed.; I-Tech Education and Publishing: Vienna, Austria, 2008. [Google Scholar] [CrossRef] [Green Version]
- Reiley, C.E.; Akinbiyi, T.; Burschka, D.; Chang, D.C.; Okamura, A.M.; Yuh, D.D. Effects of visual force feedback on robot-assisted surgical task performance. J. Thorac. Cardiovasc. Surg. 2008, 135, 196–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saracino, A.; Deguet, A.; Staderini, F.; Boushaki, M.N.; Cianchi, F.; Menciassi, A.; Sinibaldi, E. Haptic feedback in the da Vinci Research Kit (dVRK): A user study based on grasping, palpation, and incision tasks. Int. J. Med. Robot. Comput. Assist. Surg. MRCAS 2019, 15, e1999. [Google Scholar] [CrossRef] [PubMed]
- Westebring-van der Putten, E.P.; Berben, M.C.J.; Goossens, R.H.M.; Jakimowicz, J.J.; Dankelman, J. The opinion and experience of surgeons with laparoscopic bowel grasper haptics. J. Biomed. Sci. Eng. 2010, 3, 422–429. [Google Scholar] [CrossRef]
- Gotthardt, R.; Scherrer, P.; Stalmans, R. Smart Materials Based on Shape Memory Alloys: Examples from Europe. Mater. Sci. Forum 2000, 327–328, 83–90. [Google Scholar] [CrossRef]
- Duerig, T.; Pelton, A.; Stöckel, D. An overview of nitinol medical applications. Mater. Sci. Eng. 1999, 273–275, 149–160. [Google Scholar] [CrossRef]
- Gerhard, C. Aktive Beeinflussung von Mechanismen Mittels Integrierter Formgedächtnisaktoren; Dissertation; Technische Universität Dresden: Chemnitz, Germany, 2014. [Google Scholar]
- Lagoudas, D.C. Shape Memory Alloys: Modeling and Engineering Applications; Springer: New York, NY, USA, 2008. [Google Scholar]
- Guo, Y.; Klink, A.; Fu, C.; Snyder, J. Machinability and surface integrity of Nitinol shape memory alloy. CIRP Ann. 2013, 62, 83–86. [Google Scholar] [CrossRef]
- Kaack, M. Elastische Eigenschaften von NiTi-Formgedächtnis-Legierungen; Univ., Diss.: Bochum, Germany, 2002. [Google Scholar]
- Kianzad, S.; Amini, A.; Karkouti, S.O. Force control of laparoscopy grasper using antagonistic shape memory alloy. In Proceedings of the 2011 1st Middle East Conference on Biomedical Engineering, Sharjah, United Arab Emirates, 21–24 February 2011; pp. 335–338. [Google Scholar] [CrossRef]
- Kode, V.; Cavusoglu, M.C. Design and Characterization of a Novel Hybrid Actuator Using Shape Memory Alloy and DC Micromotor for Minimally Invasive Surgery Applications. IEEE/ASME Trans. Mechatronics 2007, 12, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Quintanar-Guzmán, S.; Kannan, S.; Aguilera-González, A.; Olivares-Mendez, M.A.; Voos, H. Operational space control of a lightweight robotic arm actuated by shape memory alloy wires: A comparative study. J. Intell. Mater. Syst. Struct. 2019, 30, 1368–1384. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.M.; Shi, Z.Y.; Liu, D.; Ma, C.; Zhang, Z.H. An accurately controlled antagonistic shape memory alloy actuator with self-sensing. Sensors 2012, 12, 7682–7700. [Google Scholar] [CrossRef] [Green Version]
- Langbein, S.; Czechowicz, A. (Eds.) Konstruktionspraxis Formgedächtnistechnik; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2013. [Google Scholar] [CrossRef]
- Tonutti, M.; Elson, D.S.; Yang, G.Z.; Darzi, A.W.; Sodergren, M.H. The role of technology in minimally invasive surgery: State of the art, recent developments and future directions. Postgrad. Med. J. 2017, 93, 159–167. [Google Scholar] [CrossRef]
- Okuda, Y.; Nakai, A.; Sato, T.; Kurata, M.; Shimoyama, I.; Oda, T.; Ohkohci, N. New device with force sensors for laparoscopic liver resection-investigation of grip force and histological damage. Minim. Invasive Ther. Allied Technol. 2020, 1–6. [Google Scholar] [CrossRef]
- Wottawa, C.R.; Genovese, B.; Nowroozi, B.N.; Hart, S.D.; Bisley, J.W.; Grundfest, W.S.; Dutson, E.P. Evaluating tactile feedback in robotic surgery for potential clinical application using an animal model. Surg. Endosc. 2016, 30, 3198–3209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, C.R.; Stylopoulos, N.; Howe, R.D. The Role of Force Feedback in Surgery: Analysis of Blunt Dissection. In Proceedings of the 10th Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systems, HAPTICS 2002, Orlando, FL, USA, 24–25 March 2002; pp. 68–74. [Google Scholar] [CrossRef]
- Ashrafiuon, H.; Eshraghi, M.; Elahinia, M.H. Position Control of a Three-link Shape Memory Alloy Actuated Robot. J. Intell. Mater. Syst. Struct. 2006, 17, 381–392. [Google Scholar] [CrossRef]
- Price, A.D.; Jnifene, A.; Naguib, H.E. Design and control of a shape memory alloy based dexterous robot hand. Smart Mater. Struct. 2007, 16, 1401–1414. [Google Scholar] [CrossRef]
- Ma, N.; Song, G. Control of shape memory alloy actuator using pulse width modulation. Smart Mater. Struct. 2003, 12, 712–719. [Google Scholar] [CrossRef] [Green Version]
- Chernyshov, G.; Tag, B.; Caremel, C.; Cao, F.; Liu, G.; Kunze, K. Shape memory alloy wire actuators for soft, wearable haptic devices. In Proceedings of the 2018 ACM International Symposium on Wearable Computers; Kunze, K., Balan, R.K., Lee, Y., Beigl, M., Peiris, R., Eds.; ACM: New York, NY, USA, 2018; pp. 112–119. [Google Scholar] [CrossRef]
- Kunze, K.; Balan, R.K.; Lee, Y.; Beigl, M.; Peiris, R. (Eds.) On attention models for human activity recognition. In Proceedings of the 2018 ACM International Symposium on Wearable Computers; ACM: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Yarosh, S.; Mejia, K.; Unver, B.; Wang, X.; Yao, Y.; Campbell, A.; Holschuh, B. SqueezeBands. Proc. ACM -Hum.-Comput. Interact. 2017, 1, 1–18. [Google Scholar] [CrossRef]
- Cooke, I.; DeClerck, B.; Hallett, J.; Miller, T.; Mitchell, A.; Rashidi, R. A Magnetic and Shape Memory Alloy Actuated Gripper for Surgical Applications. Volume 4: Dynamics, Vibration, and Control. Am. Soc. Mech. Eng. 2019, 59414, V004T05A035. [Google Scholar] [CrossRef]
- Kode, V.; Cavusoglu, M.C.; Azar, M.T. Design and characterization of a novel hybrid actuator using shape memory alloy and DC motor for minimally invasive surgery applications. In Proceedings of the IEEE International Conference Mechatronics and Automation, Niagara Falls, ON, Canada, 29 July–1 August 2005; pp. 416–420. [Google Scholar] [CrossRef] [Green Version]
| Description | Symbol | Actuator Wire | Sensor Wire |
|---|---|---|---|
| Material | NiTi (Memry Alloy H) | NiTi (Memry Alloy S) | |
| Wire Diameter | d | 0.3 mm | 0.05 mm |
| Martensite start transformation temperature at zero stress level | 61.5 °C | 14.8 °C | |
| Martensite finish transformation temperature at zero stress level | 52.8 °C | −12.7 °C | |
| Austenite start transformation temperature at zero stress level | 72.4 °C | −13.0 °C | |
| Austenite finish transformation temperature at zero stress level | 85.5 °C | 18.3 °C | |
| Specified life cycles of actuator wires | n | 1000 | 1000 |
| Maximum tensile stress for n | 500 MPa | 800 MPa | |
| Maximum reversal elongation at | 4.5% | 5% |
| Support Structure | Contour | Area | |
|---|---|---|---|
| Laser power | 100 | 75 | 113 |
| Velocity | / | / | / |
| Spot size | 55 μm | 55 μm | 55 μm |
| Layer thickness | 20 μm | 20 μm | 20 μm |
| Description | Symbol | Value |
|---|---|---|
| Instrument diameter | d | 8 |
| Maximum forceps opening angle | 60° | |
| Maximum gripping force at 5° | F | ± |
| Maximum gripping force at 27° | F | ± |
| Maximum gripping force at 45° | F | ± |
| Duration for complete opening/ closing | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braun, D.; Weik, D.; Elsner, S.; Hunger, S.; Werner, M.; Drossel, W.-G. Position Control and Force Estimation Method for Surgical Forceps Using SMA Actuators and Sensors. Materials 2021, 14, 5111. https://doi.org/10.3390/ma14175111
Braun D, Weik D, Elsner S, Hunger S, Werner M, Drossel W-G. Position Control and Force Estimation Method for Surgical Forceps Using SMA Actuators and Sensors. Materials. 2021; 14(17):5111. https://doi.org/10.3390/ma14175111
Chicago/Turabian StyleBraun, Dennis, David Weik, Sophia Elsner, Sandra Hunger, Michael Werner, and Welf-Guntram Drossel. 2021. "Position Control and Force Estimation Method for Surgical Forceps Using SMA Actuators and Sensors" Materials 14, no. 17: 5111. https://doi.org/10.3390/ma14175111
APA StyleBraun, D., Weik, D., Elsner, S., Hunger, S., Werner, M., & Drossel, W.-G. (2021). Position Control and Force Estimation Method for Surgical Forceps Using SMA Actuators and Sensors. Materials, 14(17), 5111. https://doi.org/10.3390/ma14175111






