Experimental Investigations on Electric-Field-Induced Crystallization in Erythritol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Thermodynamic Characterization of Erythritol
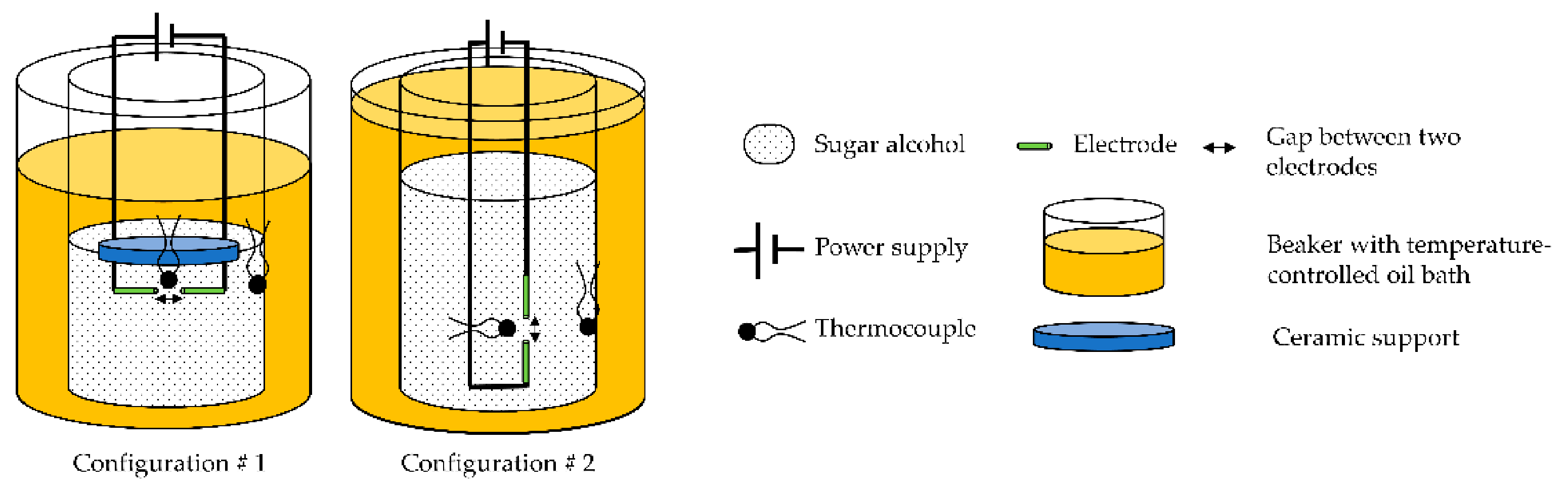
2.2.2. E-Field-Induced Crystallization
2.2.3. Configuration 1
- First, 5 g of powdered erythritol was placed in a 30 mL beaker. A unique sample was used for all tests.
- The electrical solicitation system consisted of an assembly of 2 silver electrodes (diameter 375 µm) fixed onto a ceramic support (Macor® glass ceramic, diameter 10 mm, thickness 1 mm). The ends of these electrodes were cut using wire cutters. The space between them was ca. 400 µm.
- The first thermocouple (TC1) was fixed on the ceramic support. Its end was located approximately 1 mm next to the electrodes.
- A second thermocouple (TC2) was also placed inside the beaker in contact with the glass at the same height as the electrodes.
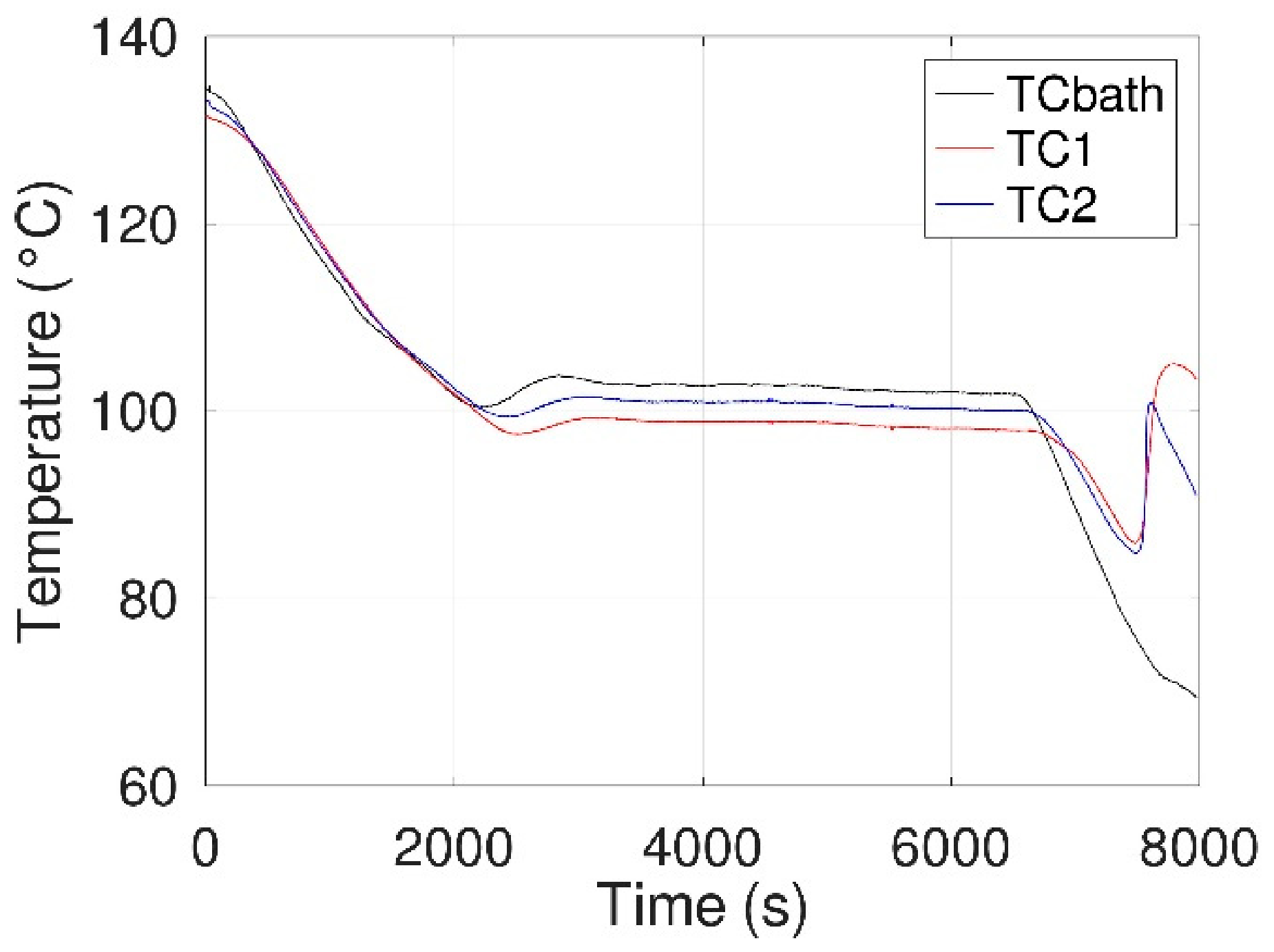
- The beaker was then heated using an oil bath (in a 500 mL beaker) and a hot plate. Homogeneity of the oil temperature (at approximately 140 °C) was ensured by magnetic stirring and controlled using a third thermocouple, called TCbath until the erythritol reached a temperature of about 130 °C. After complete melting of the erythritol, the electrode/thermocouple/ceramic assembly was immersed in the latter, maintaining approximately a 2 mm distance between the electrodes and the bottom of the beaker.
- A power supply was connected to the electrodes. The manual switch was used to stop the electrical solicitation (“floating electrode”).
- Before any electrical solicitation, the erythritol was freely cooled down by removing the beaker from the oil bath. For this purpose, a low-speed stepper motor (−3 mm/s) was added to the original device to extract the beaker, ensuring the repeatability of the experiments, while limiting vibrations during this step.
2.2.4. Configuration 2
- First, 15 g of powdered erythritol was placed in a 30 mL beaker, and a unique sample was used for all tests.
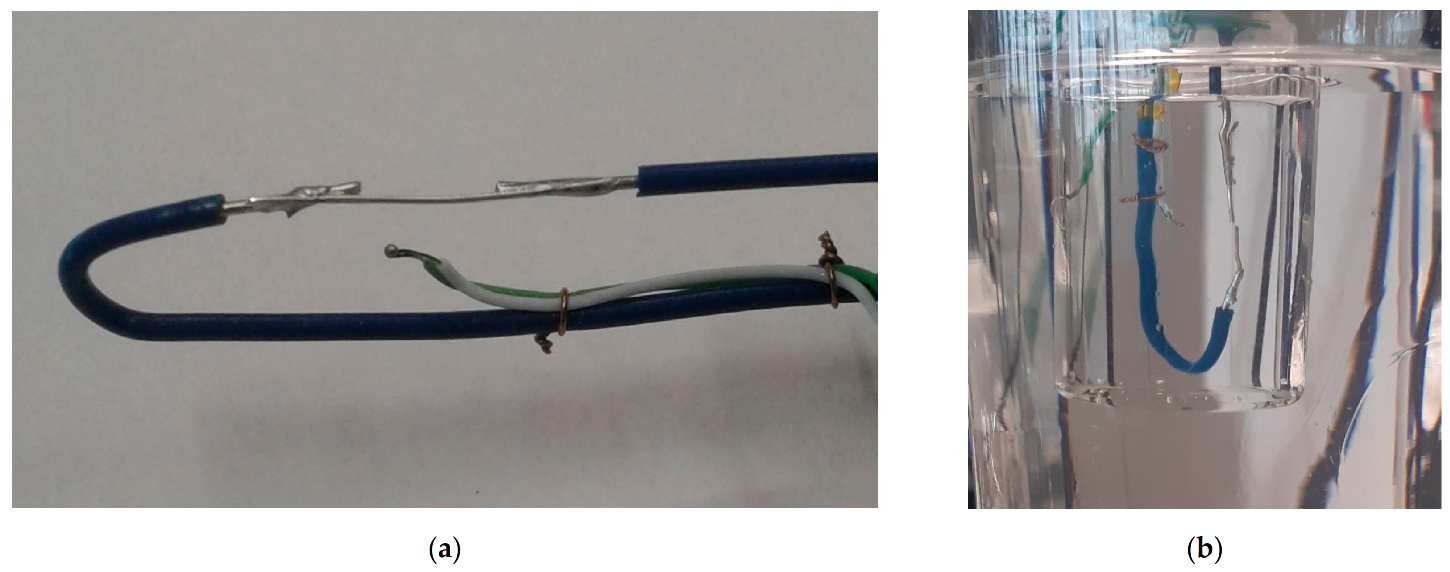
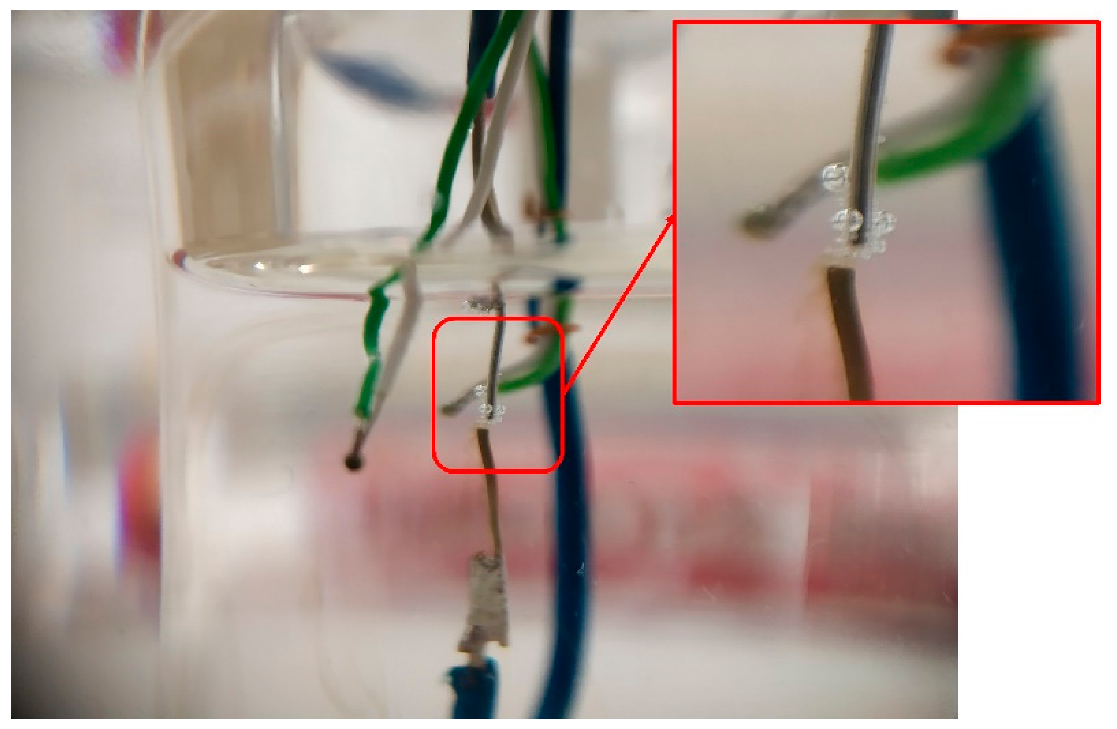
- The electrical solicitation system consisted of an assembly of two electrodes soldered on two rigid wires (in Figure 3). These electrodes were made with silver or copper wires (diameters 375 and 200 µm, respectively). The ends of the electrodes, with a gap of 0.15 mm, were cut using wire cutters.
- TC1 was also fixed on the rigid wires. Its extremity was located at approximately 1 mm from the electrodes. TC2 was placed inside the beaker in contact with the glass at the same height as the first thermocouple (in Figure 1).
- As in configuration 1, the beaker was then heated using the oil bath and the hot plate. The temperature of the oil was maintained at ca. 140 °C (TCbath) until the erythritol had completely melted. Then, the electrode/thermocouple assembly was immersed in the erythritol and connected to a power supply with a manual switch (“floating electrode”). One can note that a configuration without a manual switch, to allow discharge to the ground of the power supply, was also tested during our experiments.
- In this configuration, and before any electrical solicitation, the cooling of the erythritol to a given temperature was ensured by regulating the temperature of the oil bath.
3. Results and Discussion
3.1. Preliminary Tests
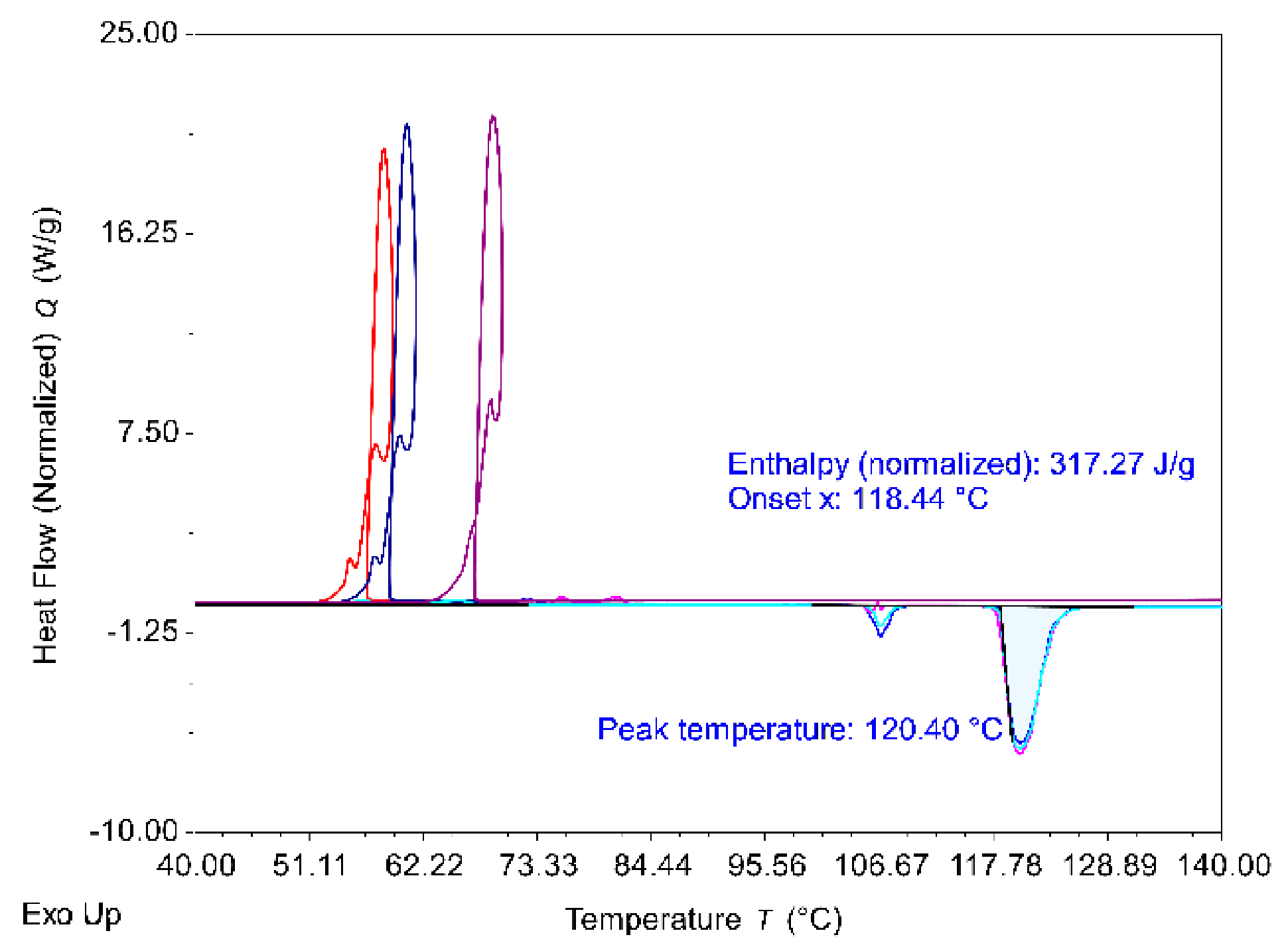
3.1.1. Thermodynamic Characterization of Erythritol
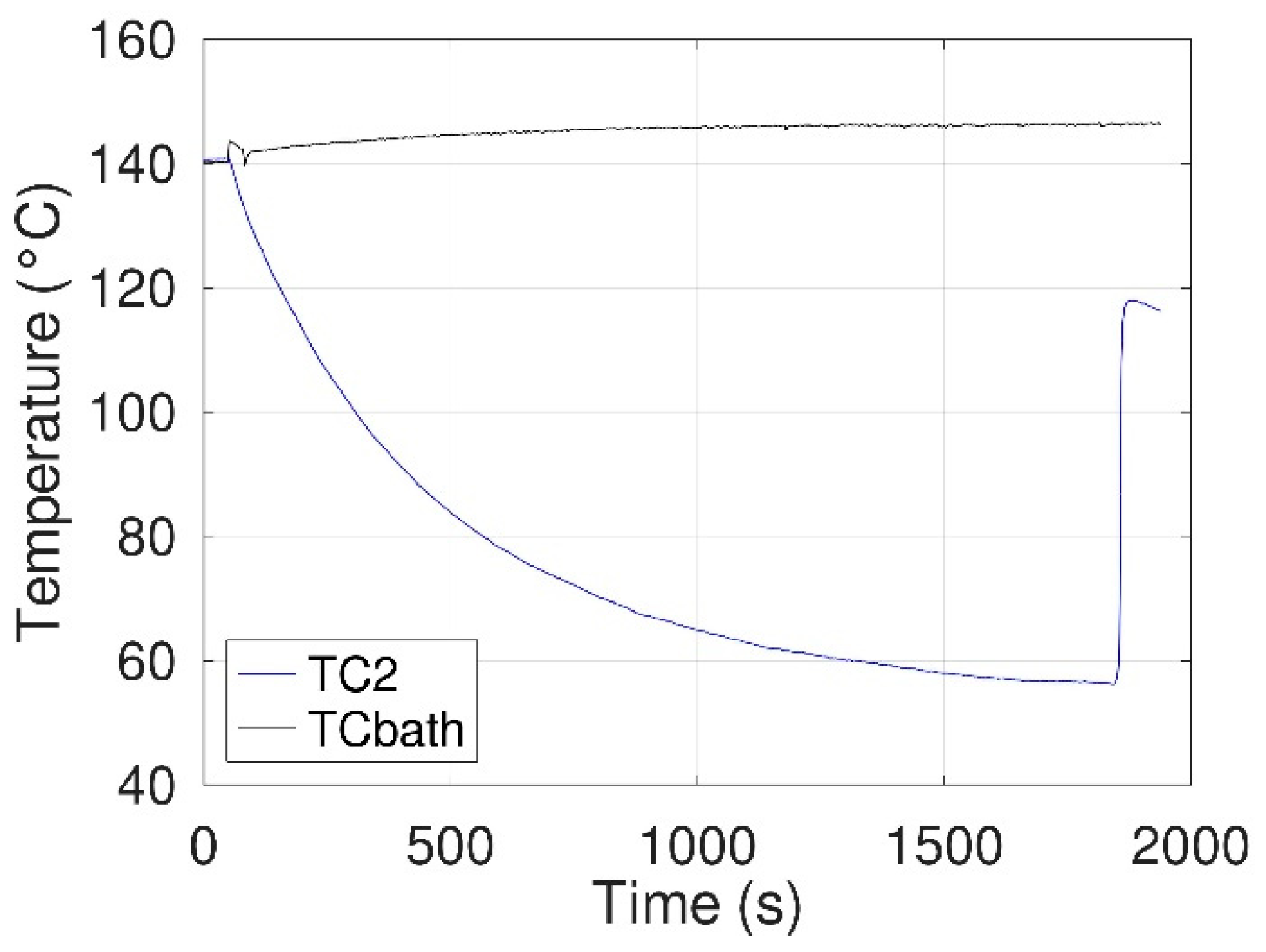
3.1.2. Configuration 1: Without an E-Field
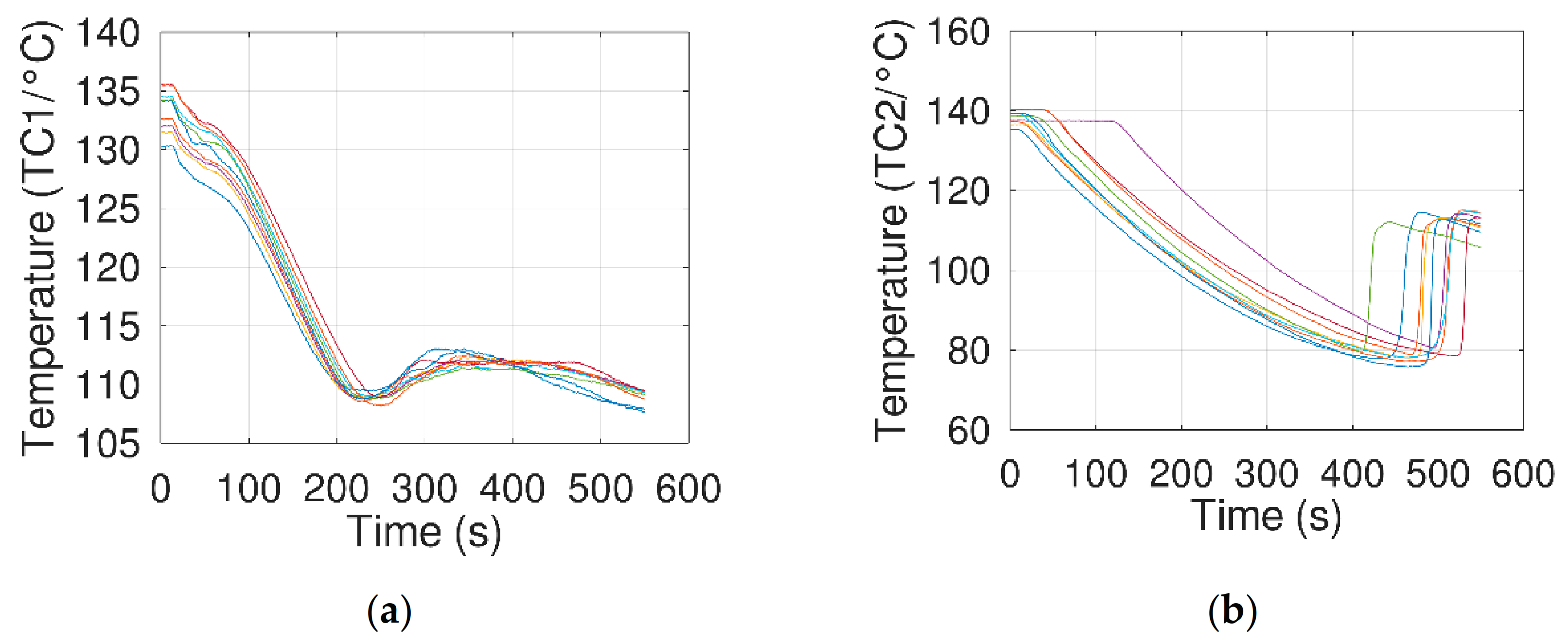
- A 30 mL beaker containing the sample was heated in an oil bath until erythritol melted and thermocouple TC1 indicated a temperature of about 130 °C (~12 °C above the melting temperature of erythritol).
- The beaker was then removed from the bath, using the low-speed stepper motor, to cool it down by natural convection.
- Temperatures were measured until crystallization of the erythritol.
3.1.3. Configuration 2: Without an E-Field
3.2. E-Field Impact on Erythritol Crystallization
3.2.1. Configuration 1
- A 30 mL beaker containing the sample was heated in an oil bath until erythritol melted and thermocouple TC1 indicated a temperature of about 130 °C (~12 °C above the melting temperature of erythritol).
- The sample was then carefully removed from the bath and cool down by natural convection.
- When TC1 indicated a temperature of 115 °C, a potential difference, from 25 to 200 V, was imposed for 3 s between the electrodes. This electrical stress was then interrupted using the manual switch.
- Temperatures from thermocouples TC1, TC2, and TCbath were recorded from step 2 until complete crystallization of the erythritol. During this time, the beaker was visually inspected to identify the initial crystallization location.
- Steps 1 to 4 were repeated for each experiment.
- During the tests with an E-field, the ammeter allowed us to measure electric currents from ca. 60 µA at 25 V to more than 1000 µA at 200 V. These amplitudes are consistent with the measurements of Jankowski and McCluskey. Moreover, data recorded via thermocouple TC1 presented a small peak when a potential difference was applied (Figure 8, at ca. 175 s), showing an interference with the potential difference measured between the thermocouple ends. These observations ensured that an electric current was flowing between the two electrodes.
- At 150 V, erythritol started to caramelize between the electrodes.
- At 200 V, a “dark cloud” linked to the degradation of erythritol was observed between the electrodes, an observation also reported by Jankowski and McCluskey but at 300 V [18]. This degradation at lower voltage can be explained by a small difference in the inter-electrode gaps of the two setups.
3.2.2. Configuration 2
- A 30 mL beaker containing the electrode assembly and the sugar alcohol was heated in an oil bath until erythritol melted and thermocouple TC1 indicated a temperature of about 130 °C (~12 °C above the melting temperature of erythritol).
- Using the thermal regulation of the hot plate, the oil bath was cooled down until TC1 indicated the desired temperature: 100, 95, or 90 °C in our tests. This temperature range was chosen in accordance with the results obtained by Duquesne et al. [26]. Indeed, at these temperatures, the crystal growth velocity of erythritol is maximum, which will facilitate its observation. One of the advantages of this procedure compared to that of configuration 1 is to avoid parasitic vibrations generated by the extraction of the beaker from the oil bath.
- When the temperature according to TC1 stabilized (±0.5 °C), a potential difference was imposed to the electrodes. Different electrical solicitations were tested:
- Solicitations from 5 to 60 s in DC at 10, 50, 100, 150, and 200 V
- Solicitations of 5 to 60 s in AC at 150 V with a frequency of 15, 30, 60, 200, 500, and 1000 Hz
- Pulse-type solicitations of ca. 15 kV using a piezoelectric element
- The electrical stress was then interrupted using the manual switch or the power supply switch (“floating electrode” or not, respectively).
- After an observation period of ca. 15 min, the hot plate was switched off to allow the free cooling of the setup.
- Temperatures from thermocouples TC1, TC2, and TCbath were recorded during steps 3 to 5 until complete crystallization of the erythritol. During this time, the beaker was visually inspected to identify the initial crystallization location.
- Steps 1 to 6 were repeated for each experiment.
4. Conclusions
- The electrodes/thermocouples act as thermal bridges, decreasing locally the temperature of erythritol to its crystallization temperature, leading to an apparent reduction in the degree of supercooling. It is possible that a similar effect was observed in the previous work.
- The purity of erythritol may have a significant impact on the observed results (99% in this work, food grade in the previous one)
- The complexity of the phenomena involved during E-field-induced crystallization experiments and the multiple possibilities for introducing bias, already highlighted in different papers, can explain the difficulty of reproducing and interpreting the authors’ results.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Forman, C.; Muritala, I.K.; Pardemann, R.; Meyer, B. Estimating the global waste heat potential. Renew. Sustain. Energy Rev. 2016, 57, 1568–1579. [Google Scholar] [CrossRef]
- Johnson, I.; Choate, W.T.; Davidson, A. Waste Heat Recovery. Technology and Opportunities in US Industry; BCS, Inc.: Laurel, MD, USA, 2008. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Pan, R.; Chiu, J.N.; Martin, V. Polyols as phase change materials for surplus thermal energy storage. Appl. Energy 2016, 162, 1439–1452. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.S.C.; Sandaran, S.C. Developments in organic solid–liquid phase change materials and their applications in thermal energy storage. Energy Convers. Manag. 2015, 95, 193–228. [Google Scholar] [CrossRef] [Green Version]
- Duquesne, M.; Del Barrio, E.P.; Godin, A. Nucleation triggering of highly undercooled Xylitol using an air lift reactor for seasonal thermal energy storage. Appl. Sci. 2019, 9, 267. [Google Scholar] [CrossRef] [Green Version]
- Kakiuchi, H.; Chihara, S.; Yamazaki, M.; Isaki, T. Heat Storage Material Composition. U.S. Patent 5785885, 28 July 1998. [Google Scholar]
- Godin, A.; Duquesne, M.; del Barrio, E.P.; Achchaq, F.; Monneyron, P. Bubble agitation as a new low-intrusive method to crystallize glass-forming materials. Energy Procedia 2017, 139, 352–357. [Google Scholar] [CrossRef]
- Beaupere, N.; Soupremanien, U.; Zalewski, L. Nucleation triggering methods in supercooled phase change materials (PCM), a review. Thermochim. Acta 2018, 670, 184–201. [Google Scholar] [CrossRef]
- Hammadi, Z.; Veesler, S. New approaches on crystallization under electric fields. Prog. Biophys. Mol. Biol. 2009, 101, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; He, H.-M.; Li, Y.; Li, Z.-R.; Zhou, Z.-J.; Wang, J.-J.; Wu, D.; Chen, W.; Gu, F.-L.; Sumpter, B.G. Electric field effects on the intermolecular interactions in water whiskers: Insight from structures, energetics, and properties. J. Phys. Chem. A 2015, 119, 2083–2090. [Google Scholar] [CrossRef]
- Sun, W.; Xu, X.; Zhang, H.; Xu, C. Effects of dipole polarization of water molecules on ice formation under an electrostatic field. Cryobiology 2008, 56, 93–99. [Google Scholar] [CrossRef]
- Kahk, J.M.; Tan, B.H.; Ohl, C.-D.; Loh, N.D. Viscous field-aligned water exhibits cubic-ice-like structural motifs. Phys. Chem. Chem. Phys. 2018, 20, 19877–19884. [Google Scholar] [CrossRef]
- Taleb, M.; Didierjean, C.; Jelsch, C.; Mangeot, J.P.; Capelle, B.; Aubry, A. Crystallization of proteins under an external electric field. J. Cryst. Growth 1999, 200, 575–582. [Google Scholar] [CrossRef]
- Koizumi, H.; Fujiwara, K.; Uda, S. Control of nucleation rate for tetragonal hen-egg white lysozyme crystals by application of an electric field with variable frequencies. Cryst. Growth Des. 2009, 9, 2420–2424. [Google Scholar] [CrossRef]
- Koizumi, H.; Uda, S.; Fujiwara, K.; Nozawa, J. Control of effect on the nucleation rate for hen egg white lysozyme crystals under application of an external ac electric field. Langmuir 2011, 27, 8333–8338. [Google Scholar] [CrossRef]
- Wursten, N. Cooling Crystallization under Influence of a Strong DC Electric Field. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2012. [Google Scholar]
- Jankowski, N.R.; McCluskey, F.P. Electrical supercooling mitigation in erythritol. In Proceedings of the International Heat Transfer Conference, Washington, DC, USA, 8–13 August 2010; IHTC14-22306. pp. 409–416. [Google Scholar] [CrossRef]
- Höhlein, S.; König-Haagen, A.; Brüggemann, D. Thermophysical characterization of MgCl2·6H2O, xylitol and erythritol as phase change materials (PCM) for latent heat thermal energy storage (LHTES). Materials 2017, 10, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ona, E.P.; Zhang, X.; Kyaw, K.; Watanabe, F.; Matsuda, H.; Kakiuchi, H.; Yabe, M.; Chihara, S. Relaxation of supercooling of erythritol for latent heat storage. J. Chem. Eng. Japan 2001, 34, 376–382. [Google Scholar] [CrossRef]
- GitHub-adafruit/Adafruit_MAX31856: Arduino Library for Adafruit MAX31856. Available online: https://github.com/adafruit/Adafruit_MAX31856 (accessed on 23 February 2021).
- Hozumi, T.; Saito, A.; Okawa, S.; Watanabe, K. Effects of electrode materials on freezing of supercooled water in electric freeze control. Int. J. Refrig. 2003, 26, 537–542. [Google Scholar] [CrossRef]
- Hozumi, T.; Saito, A.; Okawa, S.; Eshita, Y. Effects of shapes of electrodes on freezing of supercooled water in electric freeze control. Int. J. Refrig. 2005, 28, 389–395. [Google Scholar] [CrossRef]
- Kumano, H.; Hirata, T.; Mitsuishi, K.; Ueno, K. Experimental study on effect of electric field on hydrate nucleation in supercooled tetra-n-butyl ammonium bromide aqueous solution. Int. J. Refrig. 2012, 35, 1266–1274. [Google Scholar] [CrossRef]
- Kumano, H.; Goto, H.; Toyama, Y.; Kawakita, M. Study on TBAB hydrate nucleating activity of electrode products due to DC voltage application. Int. J. Refrig. 2018, 93, 10–17. [Google Scholar] [CrossRef]
- Duquesne, M.; Godin, A.; del Barrio, E.P.; Achchaq, F. Crystal growth kinetics of sugar alcohols as phase change materials for thermal energy storage. Energy Procedia 2017, 139, 315–321. [Google Scholar] [CrossRef]
- Shichiri, T.; Araki, Y. Nucleation mechanism of ice crystals under electrical effect. J. Cryst. Growth 1986, 78, 502–508. [Google Scholar] [CrossRef]




| Material | CAS Number | Formula | Phase Change Temperature (°C) | Mass-Specific Enthalpy (kJ/kg) | Degree of Supercooling (°C) |
|---|---|---|---|---|---|
| Erythritol | 149-32-6 | C4H10O4 | 117–120 | 315–379 | Up to 65 |
| Configuration | Material | Type | Dimensions (mm) | Gap (mm) |
|---|---|---|---|---|
| 1 | Silver | Wire | 0.4 Ø | 0.35 |
| 2 | Silver Copper | Wire | 0.4 Ø 0.2 Ø | 1.5 |
| Cycle | Mass-Specific Enthalpy (kJ/kg) | Melting Temperature (°C) | Temperature of Crystallization (°C) | Degree of Supercooling (°C) |
|---|---|---|---|---|
| 1 | 317.0 | 118.4 | 58.5 | 60.0 |
| 2 | 319.7 | 118.2 | 53.4 | 64.8 |
| 3 | 319.0 | 118.4 | 53.2 | 65.2 |
| Average | 318.6 | 118.3 | 55.0 | 63.3 |
| Standard deviation | 1.4 | 0.1 | 3.0 | 2.9 |
| Trial | Initial Nucleation Location | Local Temperature (°C) |
|---|---|---|
| PRE01 | Electrode assembly | 107.9 |
| PRE02 | Electrode assembly | 108.6 |
| PRE03 | Electrode assembly | 108.9 |
| PRE04 | Electrode assembly | 108.8 |
| PRE05 | Electrode assembly | 108.8 |
| PRE06 | Electrode assembly | 109.0 |
| PRE07 | Electrode assembly | 108.9 |
| PRE08 | Electrode assembly | 107.6 |
| PRE09 | Electrode assembly | 108.2 |
| Trial | Initial Nucleation Location | Local Temperature (°C) |
|---|---|---|
| PRE01 | Bottom of the electrode assembly | 68.6 |
| PRE02 | Top of the electrode assembly | 65.7 |
| PRE03 | Center of the electrode assembly | 75.5 |
| Trial | Voltage (V) | Initial Nucleation Location | Local Temperature (°C) |
|---|---|---|---|
| V01 | 25 | Electrode assembly | 108.1 |
| V02 | 50 | Electrode assembly | 109.8 |
| V03 | 100 | Electrode assembly | 108.2 |
| V04 | 150 | Electrode assembly | 108.9 |
| V05 | 200 | Electrode assembly | 108.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dauvergne, J.-L.; Nikulin, A.; Doppiu, S.; Palomo del Barrio, E. Experimental Investigations on Electric-Field-Induced Crystallization in Erythritol. Materials 2021, 14, 5110. https://doi.org/10.3390/ma14175110
Dauvergne J-L, Nikulin A, Doppiu S, Palomo del Barrio E. Experimental Investigations on Electric-Field-Induced Crystallization in Erythritol. Materials. 2021; 14(17):5110. https://doi.org/10.3390/ma14175110
Chicago/Turabian StyleDauvergne, Jean-Luc, Artem Nikulin, Stefania Doppiu, and Elena Palomo del Barrio. 2021. "Experimental Investigations on Electric-Field-Induced Crystallization in Erythritol" Materials 14, no. 17: 5110. https://doi.org/10.3390/ma14175110
APA StyleDauvergne, J.-L., Nikulin, A., Doppiu, S., & Palomo del Barrio, E. (2021). Experimental Investigations on Electric-Field-Induced Crystallization in Erythritol. Materials, 14(17), 5110. https://doi.org/10.3390/ma14175110






