Synthesis and Properties of Inositol Nanocapsules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Inositol Nanocapsules
2.3. Characterization of the Nanocapsules
3. Results and Discussion
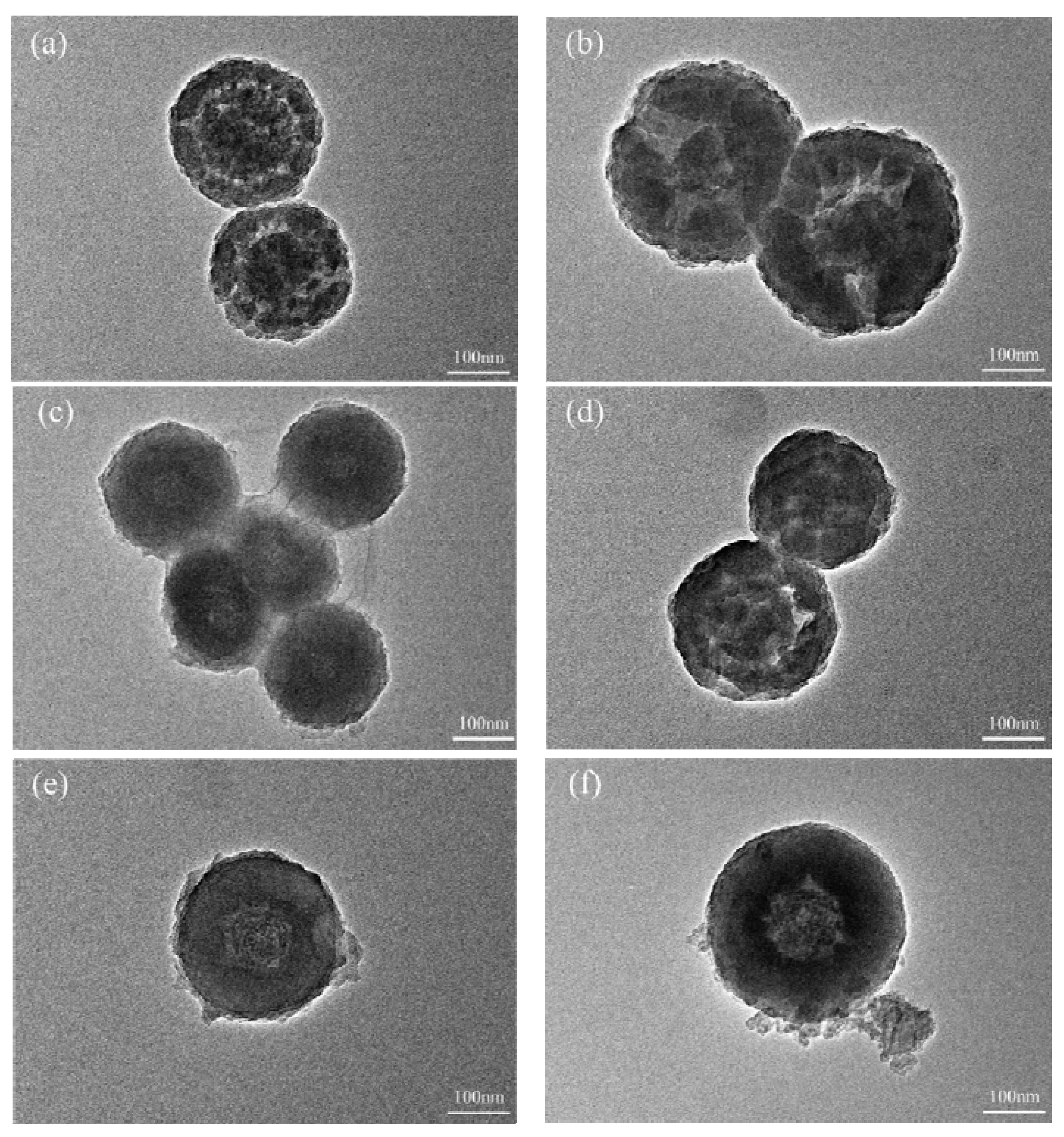
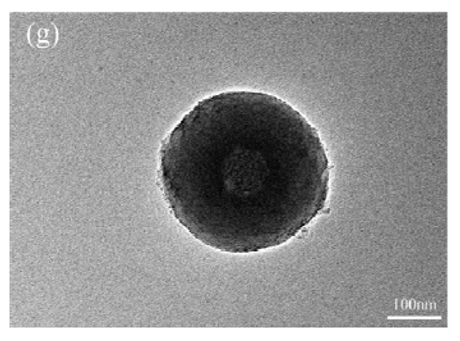
3.1. Morphology of the Nanocapsules
3.2. Chemical Characterization of the Nanocapsules
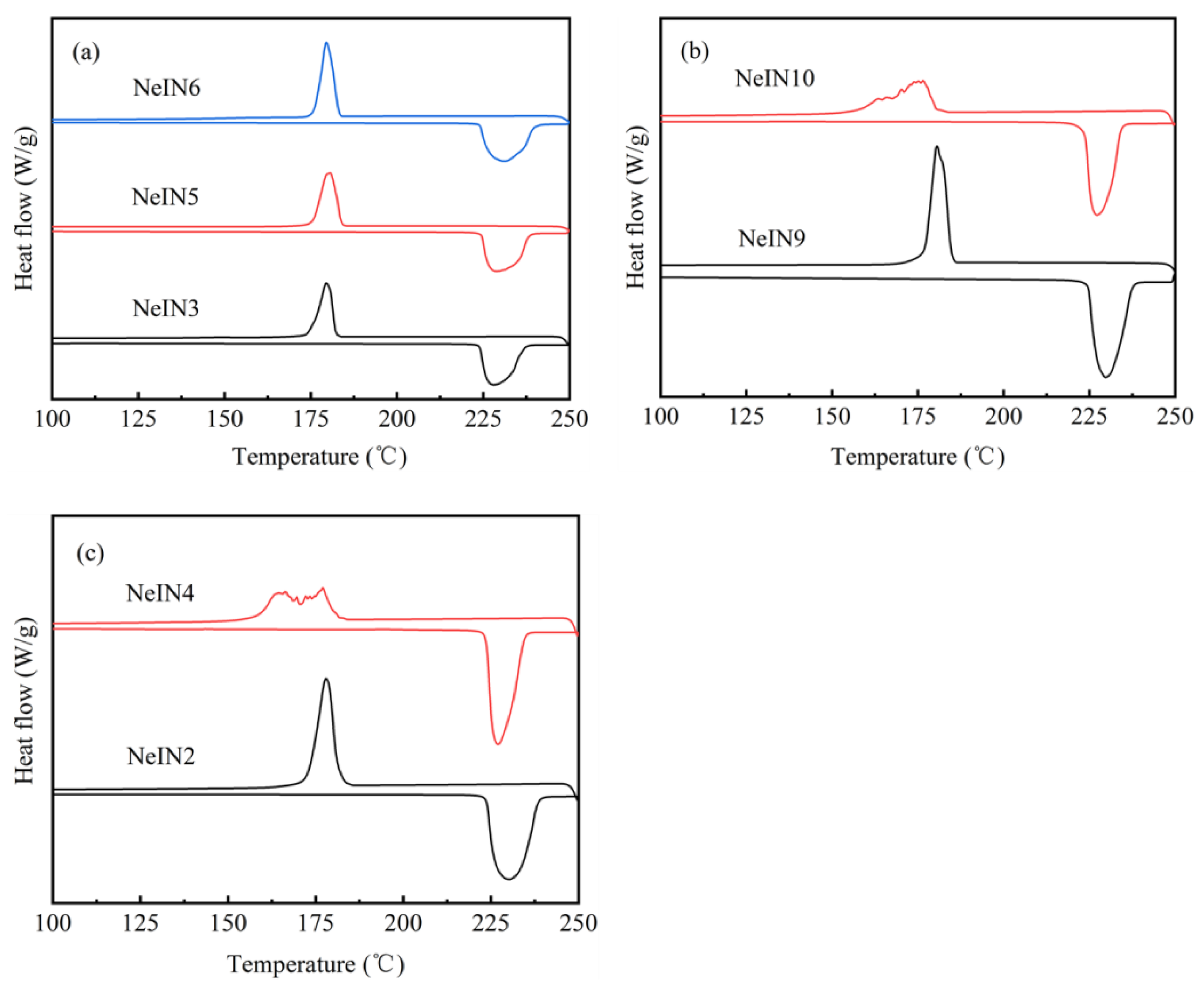
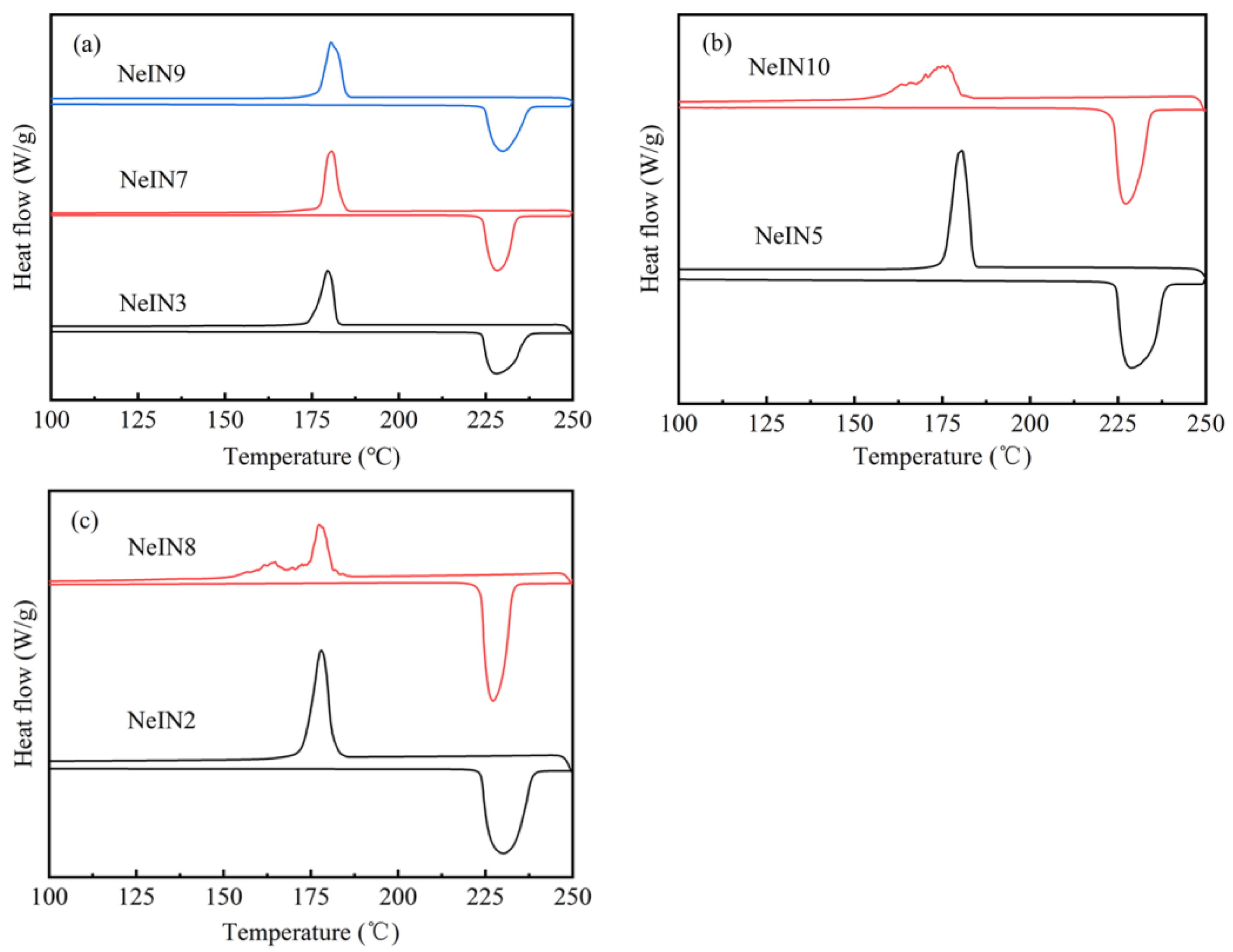
3.3. Phase-Change Properties of the Nanocapsules
3.4. Effect of Time for Adding the Precursors
3.5. Effect of TEOS Amount
3.6. Effect of TES Amount
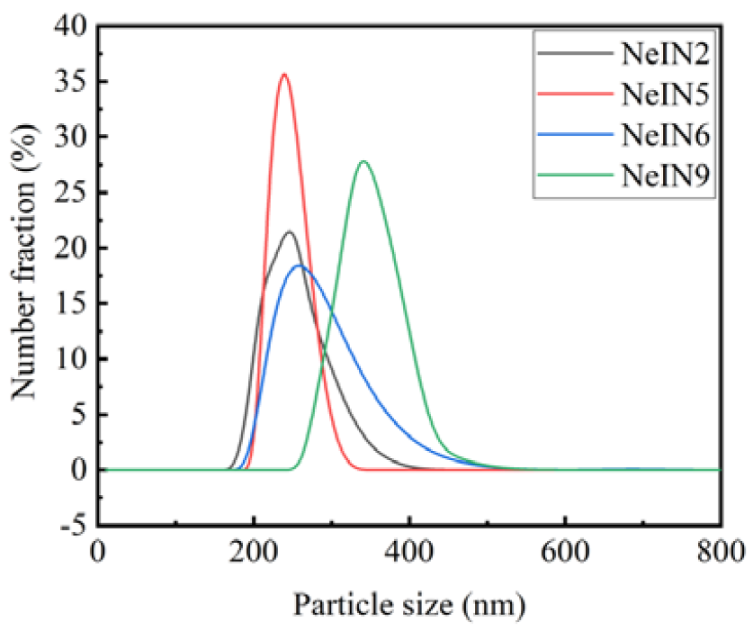
3.7. Particle Size Distribution of the Nanocapsules
4. Conclusions
- (1)
- Inositol was successfully encapsulated by SiO2 shell and no chemical reaction between the inositol core and SiO2 shell was observed.
- (2)
- The shell thickness of the nanocapsules increased while the supercooling degrees decreased with increasing time for adding the precursors. The results suggest that the SiO2 shell could act as a nucleating agent which promotes the nucleation of the inositol core.
- (3)
- The nanocapsules that were well-dispersed, spherically-shaped with uniform size also exhibited good phase-change properties. In order to obtain good morphology and phase-change properties, the time for adding the precursors should increase with the amount of the precursors. Excess or insufficient time for adding the precursors, TEOS amount or TES amount resulted in reduced encapsulation performance, including poor size uniformity, multiple nucleation, decreased encapsulation ratio and energy storage efficiency. NeIN6 exhibited the best performance with melting enthalpy, encapsulation ratio and energy storage efficiency of 216.0 kJ/kg, 83.1 % and 82.1 %, respectively.
- (4)
- The TES amount remarkably affected the size of the nanocapsules, while the effects of the TEOS amount and the time for adding the precursors were neglectable.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pasupathy, A.; Velraj, R.; Seeniraj, R.V. Phase change material-based building architecture for thermal management in residential and commercial establishments. Renew. Sust. Energy Rev. 2008, 12, 39–64. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- del Barrio, E.P.; Godin, A.; Duquesne, M.; Daranlot, J.; Jolly, J.; Alshaer, W.; Kouadio, T.; Sommier, A. Characterization of different sugar alcohols as phase thermal energy storage applications. Sol. Energy Mat. Sol. C 2017, 159, 560–569. [Google Scholar] [CrossRef]
- Bayes-Garcia, L.; Ventola, L.; Cordobilla, R.; Benages, R.; Calvet, T.; Cuevas-Diarte, M.A. Phase Change Materials (PCM) microcapsules with different shell compositions: Preparation, characterization and thermal stability. Sol. Energy Mat. Sol. C 2010, 94, 1235–1240. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sust. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Sari, A.; Alkan, C.; Karaipekli, A. Preparation, characterization and thermal properties of PMMA/n-heptadecane microcapsules as novel solid-liquid microPCM for thermal energy storage. Appl. Energy 2010, 87, 1529–1534. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Shi, H.; Yang, W.T. Fabrication and performances of new kind microencapsulated phase change material based on stearic acid core and polycarbonate shell. Energy Convers. Manag. 2012, 64, 1–7. [Google Scholar] [CrossRef]
- Kumar, G.N.; Al-Aifan, B.; Parameshwaran, R.; Ram, V.V. Facile synthesis of microencapsulated 1-dodecanol/melamine-formaldehyde phase change material using in-situ polymerization for thermal energy storage. Colloid. Surface A 2021, 610, 125698. [Google Scholar] [CrossRef]
- Fang, G.Y.; Chen, Z.; Li, H. Synthesis and properties of microencapsulated paraffin composites with SiO2 shell as thermal energy storage materials. Chem. Eng. J. 2010, 163, 154–159. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Wang, X.D.; Wu, D.Z. Silica encapsulation of n-octadecane via sol-gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interf. Sci. 2010, 343, 246–255. [Google Scholar]
- Zhang, H.Z.; Sun, S.Y.; Wang, X.D.; Wu, D.Z. Fabrication of microencapsulated phase change materials based on n-octadecane core and silica shell through interfacial polycondensation. Colloid. Surface A 2011, 389, 104–117. [Google Scholar] [CrossRef]
- Gui, H.; Li, Y.; Du, D.; Liang, F.; Yang, Z. High-performance phase change material capsule by Janus particle. Mater Today Energy 2021, 20, 100107. [Google Scholar]
- Sari, A.; Saleh, T.A.; Hekimoglu, G.; Tyagi, V.V.; Sharma, R.K. Microencapsulated heptadecane with calcium carbonate as thermal conductivity-enhanced phase change material for thermal energy storage. J. Mol. Liq. 2021, 328, 115508. [Google Scholar] [CrossRef]
- Fredi, G.; Dire, S.; Callone, E.; Ceccato, R.; Mondadori, F.; Pegoretti, A. Docosane-Organosilica Microcapsules for Structural Composites with Thermal Energy Storage/Release Capability. Materials 2019, 12, 1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Wang, X.D.; Wu, D.Z. New approach for sol-gel synthesis of microencapsulated n-octadecane phase change material with silica wall using sodium silicate precursor. Energy 2014, 67, 223–233. [Google Scholar] [CrossRef]
- He, L.J.; Mo, S.P.; Lin, P.C.; Jia, L.S.; Chen, Y.; Cheng, Z.D. Synthesis and properties of nanoencapsulated D-mannitol for medium temperature thermal energy storage. Sol. Energy Mat. Sol. C 2020, 209, 110473. [Google Scholar] [CrossRef]
- Salaun, F.; Bedek, G.; Devaux, E.; Dupont, D.; Gengembre, L. Microencapsulation of a cooling agent by interfacial polymerization: Influence of the parameters of encapsulation on poly(urethane-urea) microparticles characteristics. J. Membrane Sci. 2011, 370, 23–33. [Google Scholar] [CrossRef]
- Makuta, T.; Kadoya, K.; Izumi, H.; Miyatake, M. Synthesis of cyanoacrylate-covered xylitol microcapsules for thermal storage. Chem. Eng. J. 2015, 273, 192–196. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Zhang, T.; Zhang, D.Y.; Ji, H. Supercooling suppression and thermal behavior improvement of erythritol as phase change material for thermal energy storage. Sol. Energy Mat. Sol. C 2017, 171, 60–71. [Google Scholar] [CrossRef]
- Pethurajan, V.; Sivan, S.; Konatt, A.J.; Reddy, A.S. Facile approach to improve solar thermal energy storage efficiency using encapsulated sugar alcohol based phase change material. Sol. Energy Mat. Sol. C 2018, 185, 524–535. [Google Scholar] [CrossRef]
- Shao, X.F.; Wang, C.; Yang, Y.J.; Feng, B.; Zhu, Z.Q.; Wang, W.J.; Zeng, Y.; Fan, L.W. Screening of sugar alcohols and their binary eutectic mixtures as phase change materials for low-to-medium temperature latent heat storage. (I): Non-isothermal melting and crystallization behaviors. Energy 2018, 160, 1078–1090. [Google Scholar] [CrossRef]
- Cao, F.Y.; Yang, B. Supercooling suppression of microencapsulated phase change materials by optimizing shell composition and structure. Appl. Energy 2014, 113, 1512–1518. [Google Scholar] [CrossRef]
- Yang, J.M.; Kim, J.S. The microencapsulation of calcium chloride hexahydrate as a phase-change material by using the hybrid coupler of organoalkoxysilanes. J. Appl. Polym. Sci. 2018, 135, 45821. [Google Scholar] [CrossRef]
- Jebasingh, B.E.; Arasu, A.V. A detailed review on heat transfer rate, supercooling, thermal stability and reliability of nanoparticle dispersed organic phase change material for low-temperature applications. Mater. Today Energy 2020, 16, 100408. [Google Scholar] [CrossRef]
- Gunther, E.; Huang, L.; Mehling, H.; Dotsch, C. Subcooling in PCM emulsions—Part 2: Interpretation in terms of nucleation theory. Thermochim. Acta 2011, 522, 199–204. [Google Scholar] [CrossRef]

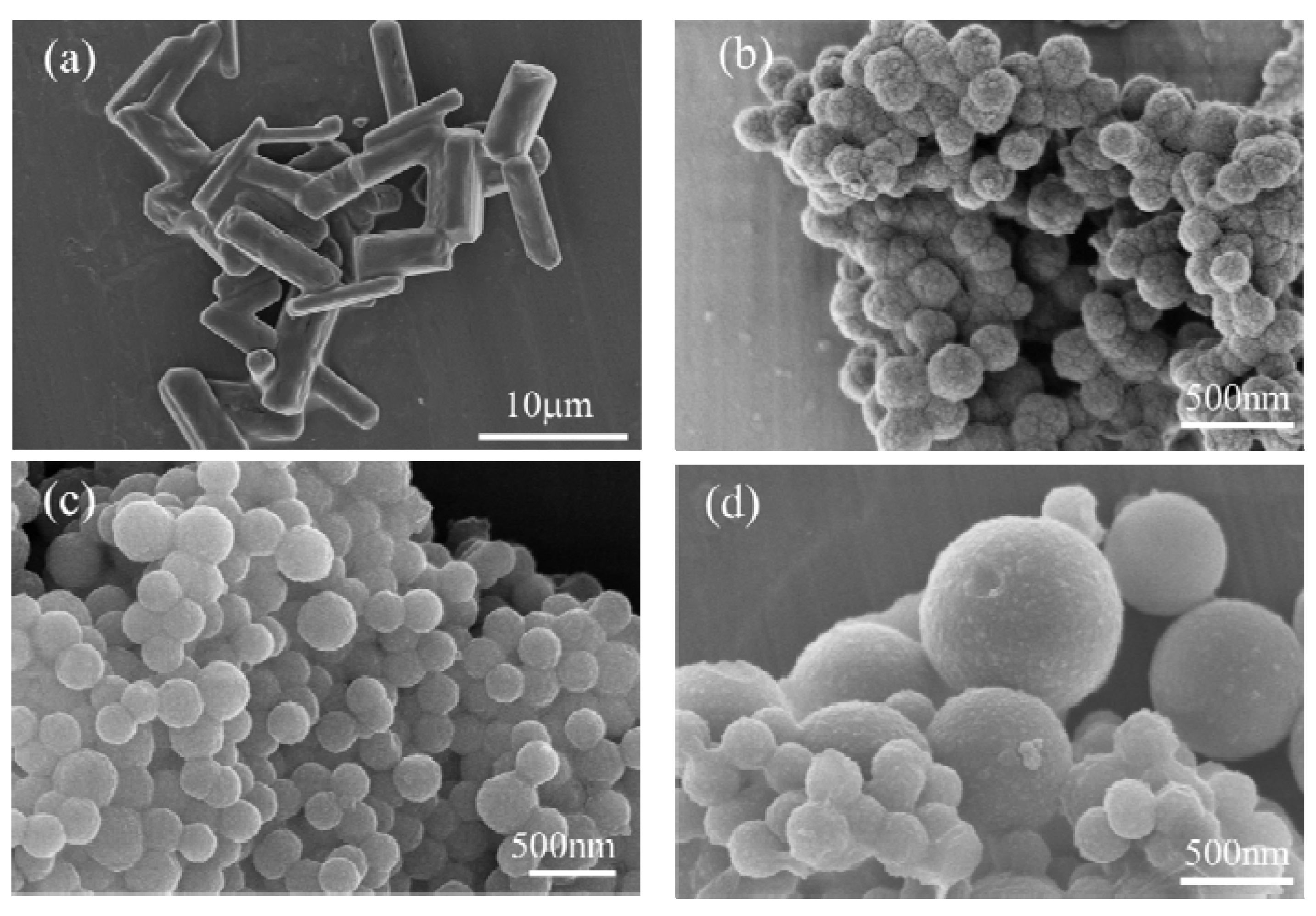

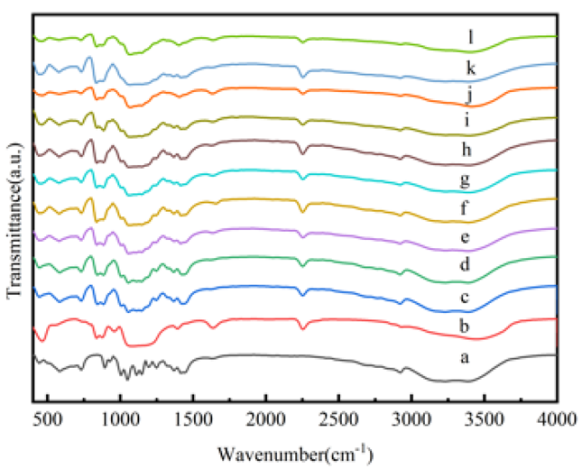
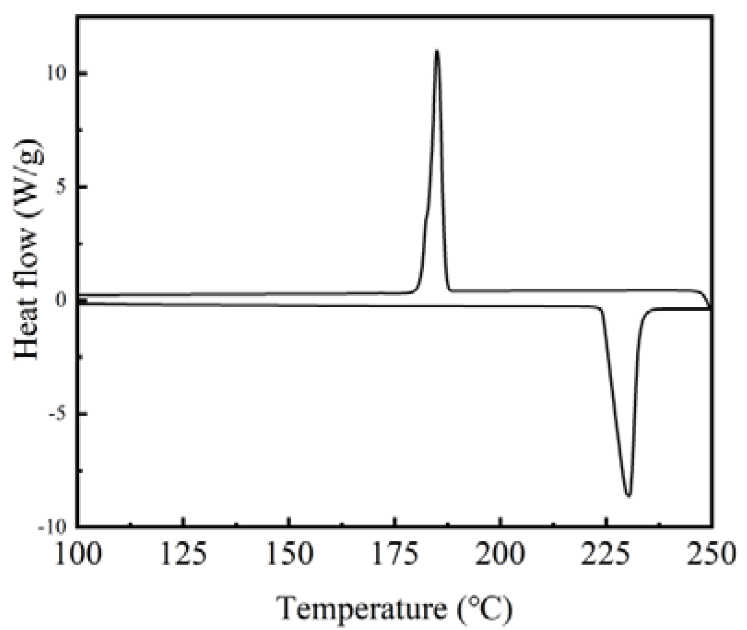
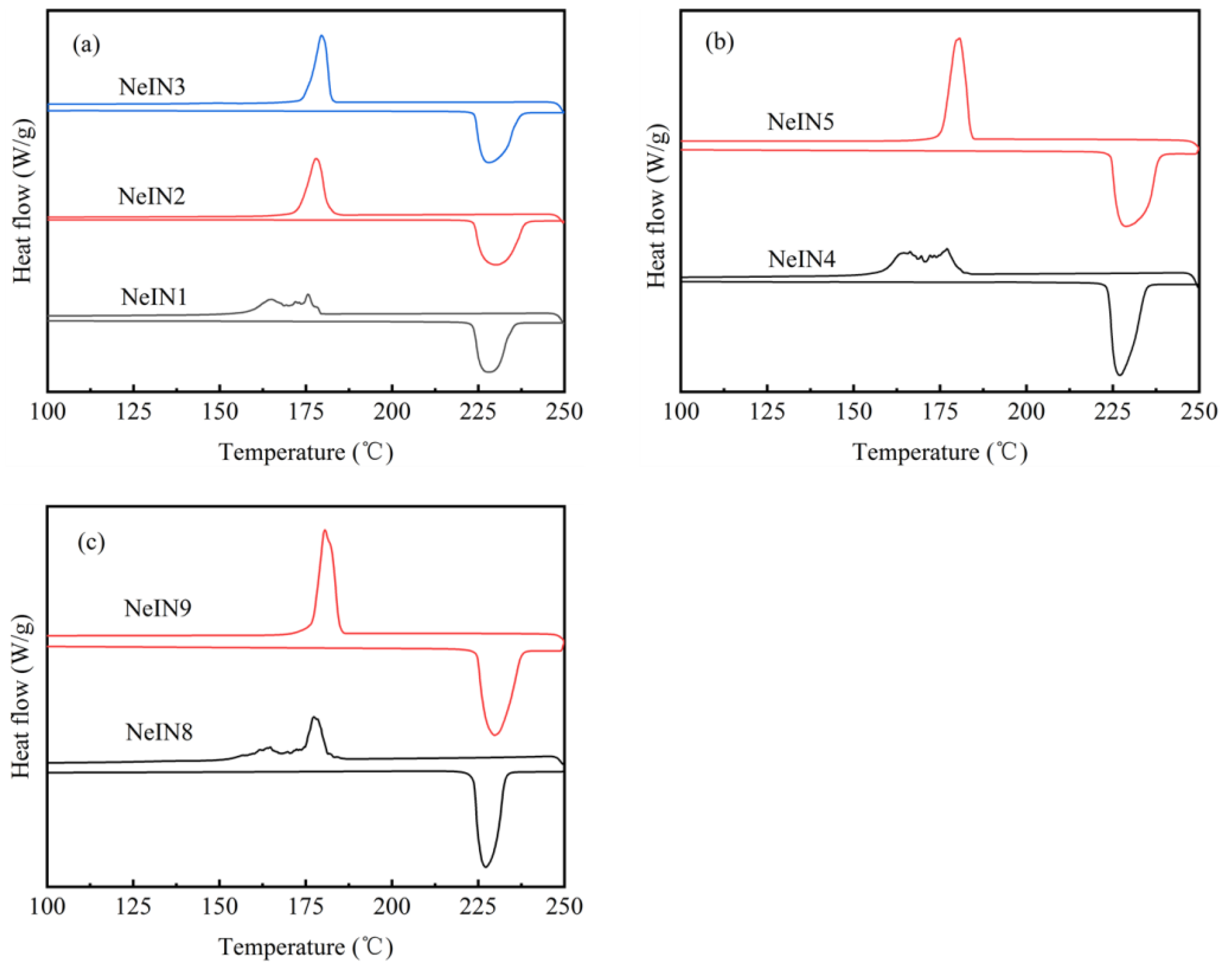
| Sample | TES (mL) | TEOS (mL) | Time for Adding Precursors (min) |
|---|---|---|---|
| NeIN1 | 5 | 9 | 20 |
| NeIN2 | 5 | 9 | 30 |
| NeIN3 | 5 | 9 | 40 |
| NeIN4 | 5 | 10 | 30 |
| NeIN5 | 5 | 10 | 40 |
| NeIN6 | 5 | 11 | 40 |
| NeIN7 | 6 | 9 | 40 |
| NeIN8 | 7 | 9 | 30 |
| NeIN9 | 7 | 9 | 40 |
| NeIN10 | 7 | 10 | 40 |
| Sample | Tm (°C) | Hm (kJ/kg) | Ts (°C) | Hs (kJ/kg) | ΔT (°C) |
|---|---|---|---|---|---|
| Present | 224.9 | 260.9 | 191.4 | 198.0 | 33.5 |
| Ref. [21] | 224.5 ± 0.2 | 261.8 ± 0.1 | 181.4 ± 0.5 | 198.6 ± 0.2 | 43.1 ± 0.7 |
| Sample | Tm (°C) | Hm (kJ/kg) | Ts (°C) | Hs (kJ/kg) | R (%) | E (%) | ΔT (°C) |
|---|---|---|---|---|---|---|---|
| NeIN1 | 227.1 | 167.2 | 175.9 | 96.0 | 64.1 | 57.4 | 51.2 |
| NeIN2 | 228.6 | 192.6 | 179.6 | 131.4 | 73.8 | 70.6 | 48.9 |
| NeIN3 | 226.5 | 188.6 | 181.2 | 126.6 | 72.3 | 68.7 | 45.3 |
| NeIN4 | 226.2 | 176.2 | 177.3 | 100.6 | 67.5 | 60.3 | 48.9 |
| NeIN5 | 227.4 | 210.4 | 182.4 | 144.7 | 77.2 | 75.4 | 45.0 |
| NeIN6 | 228.9 | 216.0 | 183.1 | 160.8 | 83.1 | 82.1 | 45.8 |
| NeIN7 | 227.1 | 191.0 | 182.2 | 126.5 | 73.3 | 69.2 | 44.9 |
| NeIN8 | 226.4 | 121.4 | 177.9 | 67.3 | 46.5 | 41.1 | 48.5 |
| NeIN9 | 227.2 | 196.6 | 182.3 | 143.3 | 75.3 | 74.0 | 44.9 |
| NeIN10 | 226.3 | 165.1 | 176.8 | 85.5 | 63.3 | 54.6 | 49.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, S.; Li, Y.; Shan, S.; Jia, L.; Chen, Y. Synthesis and Properties of Inositol Nanocapsules. Materials 2021, 14, 5481. https://doi.org/10.3390/ma14195481
Mo S, Li Y, Shan S, Jia L, Chen Y. Synthesis and Properties of Inositol Nanocapsules. Materials. 2021; 14(19):5481. https://doi.org/10.3390/ma14195481
Chicago/Turabian StyleMo, Songping, Yuanhong Li, Shaofei Shan, Lisi Jia, and Ying Chen. 2021. "Synthesis and Properties of Inositol Nanocapsules" Materials 14, no. 19: 5481. https://doi.org/10.3390/ma14195481
APA StyleMo, S., Li, Y., Shan, S., Jia, L., & Chen, Y. (2021). Synthesis and Properties of Inositol Nanocapsules. Materials, 14(19), 5481. https://doi.org/10.3390/ma14195481






