Hyaluronic-Acid-Based Organic-Inorganic Composites for Biomedical Applications
Abstract
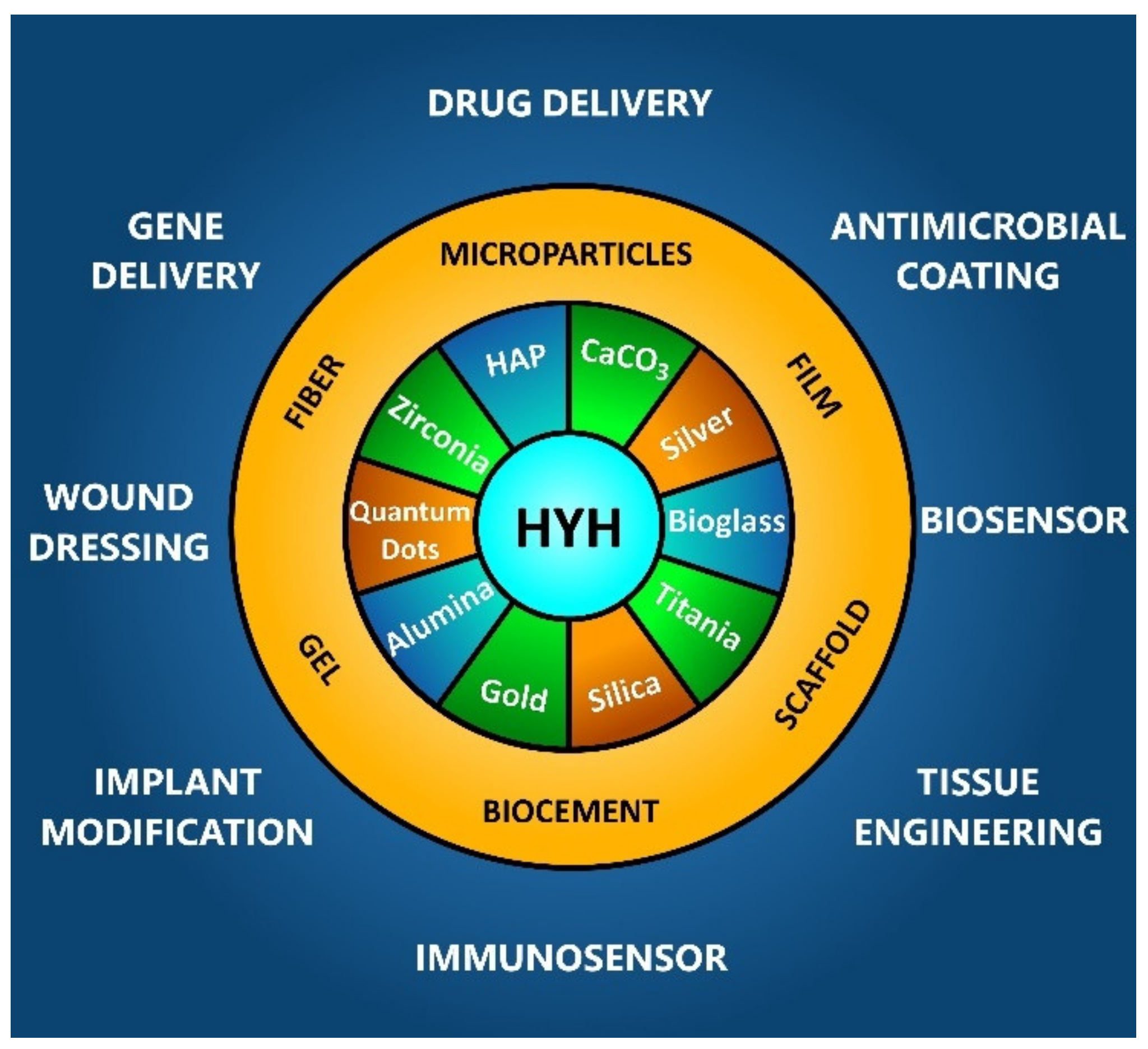
1. Introduction
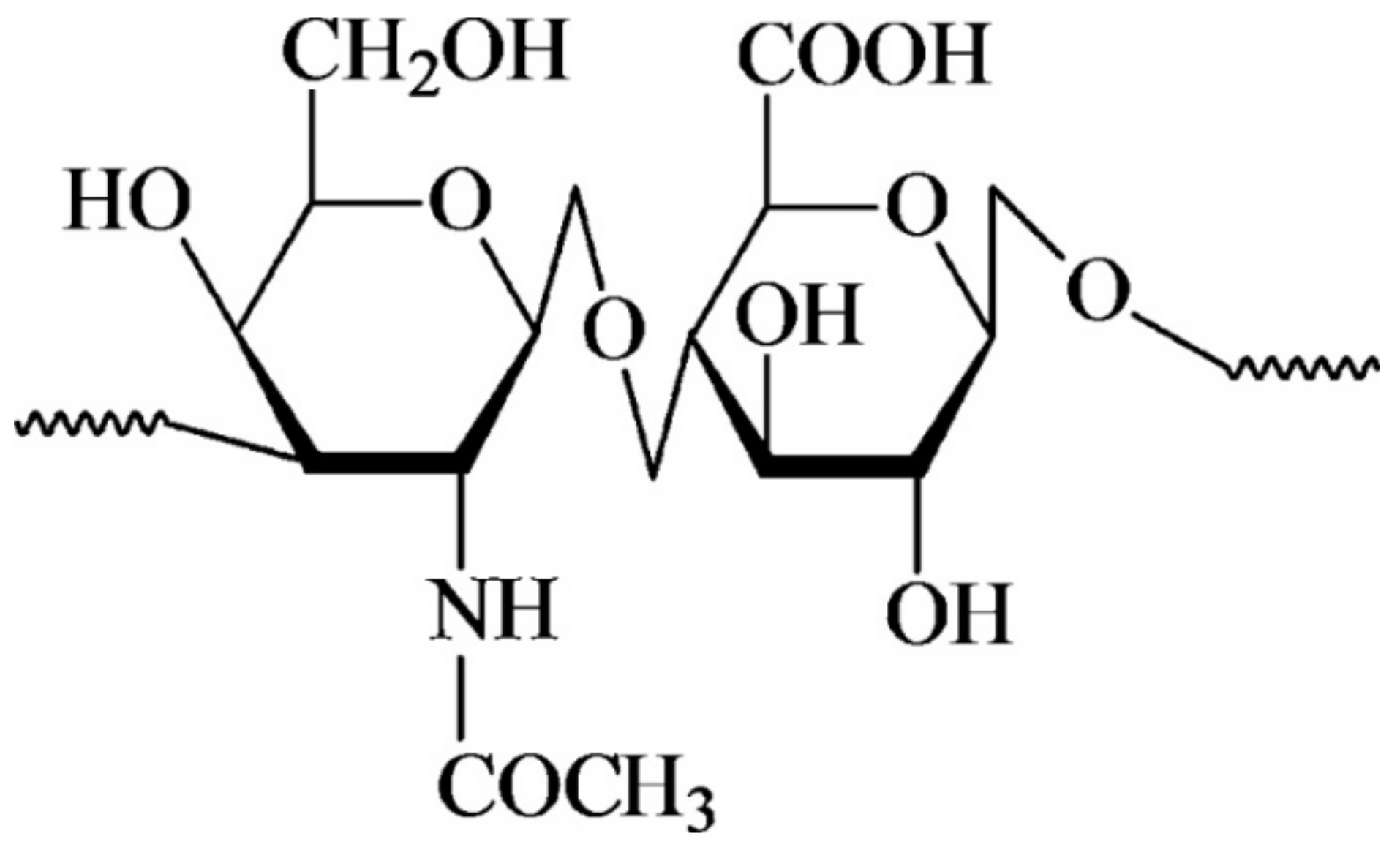
2. Structure and Functional Properties of HYH
3. HYH–HAP and HYH–CaP Composites
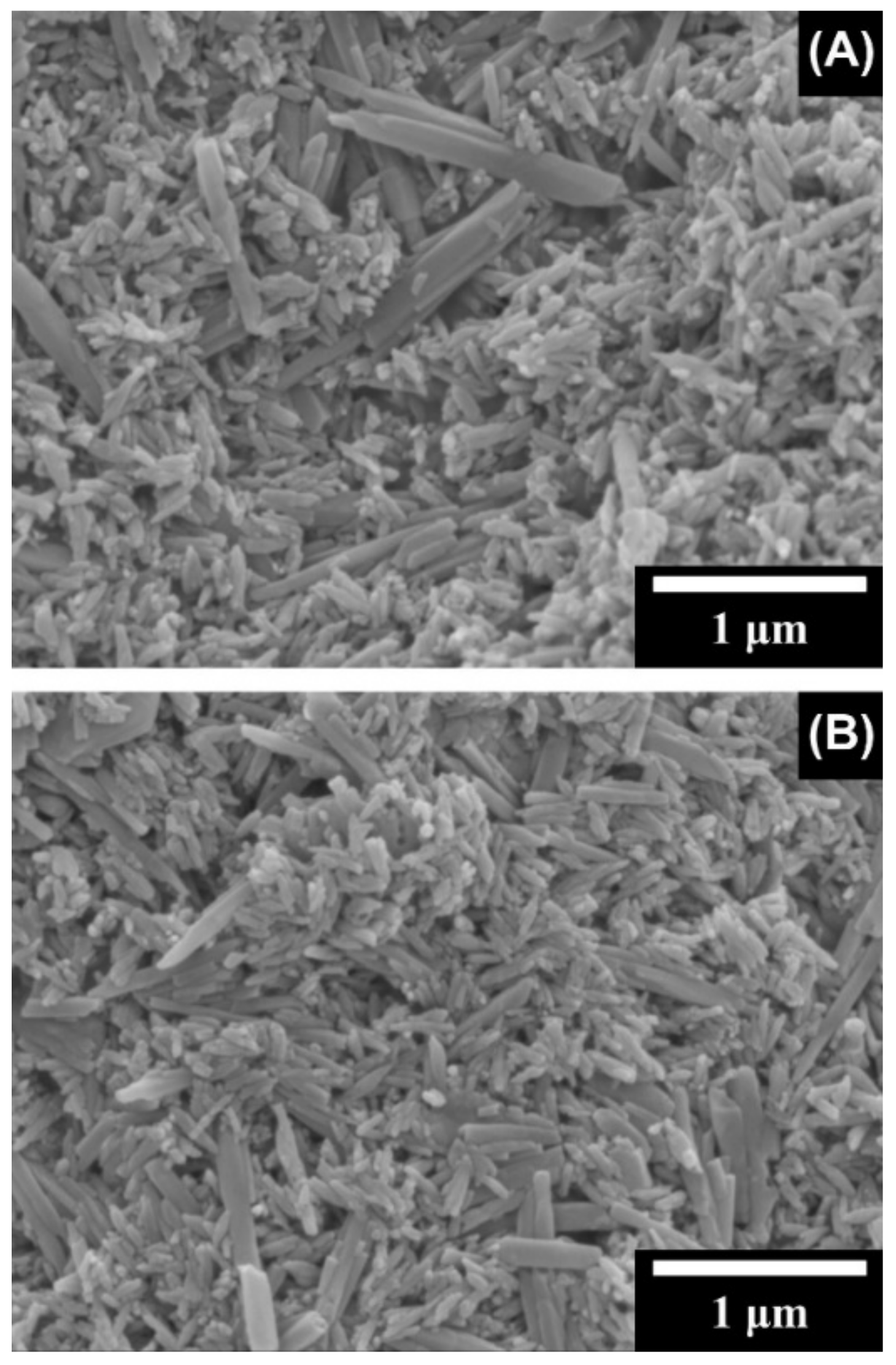
3.1. Composite Gels
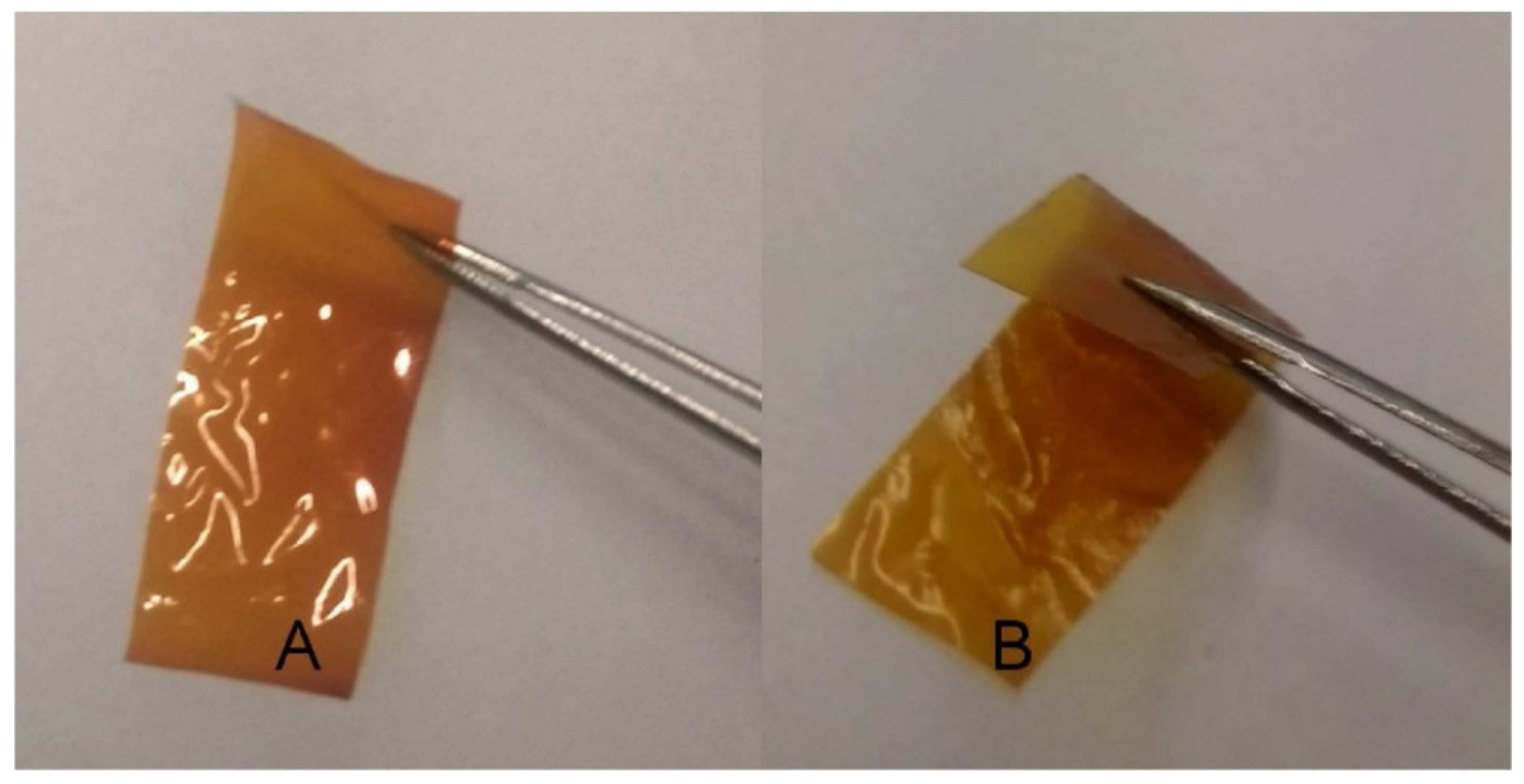
3.2. Composite Films and Coatings
3.2.1. Electrophoretic Deposition
3.2.2. Other Deposition Techniques
3.3. Scaffolds
3.4. Biocements
3.5. Composite Particles
4. HYH–Bioceramic and HYH–Bioglass Composites
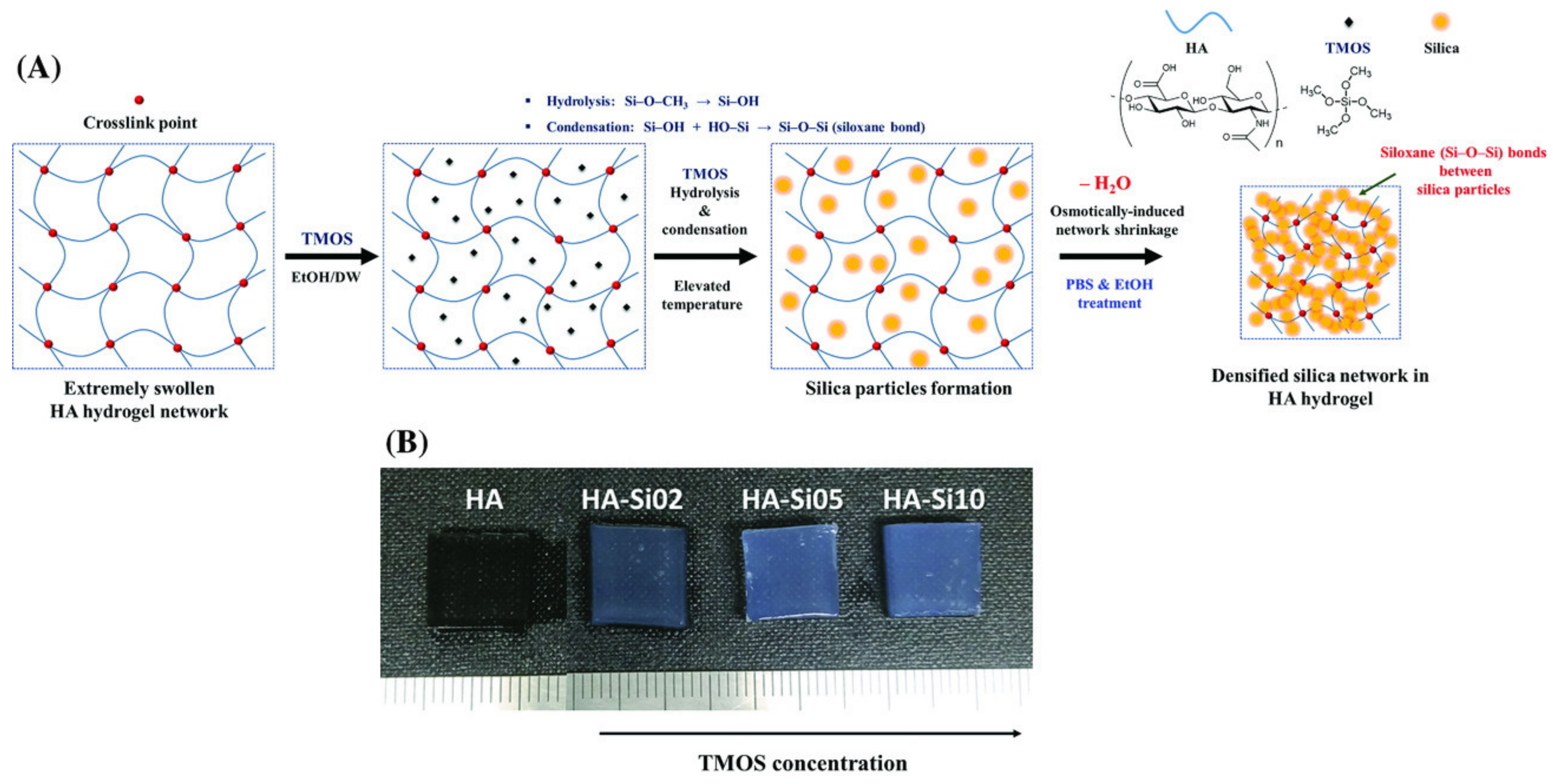
4.1. Silica
4.2. Titania
4.3. Calcium Carbonate, Alumina, and Zirconia
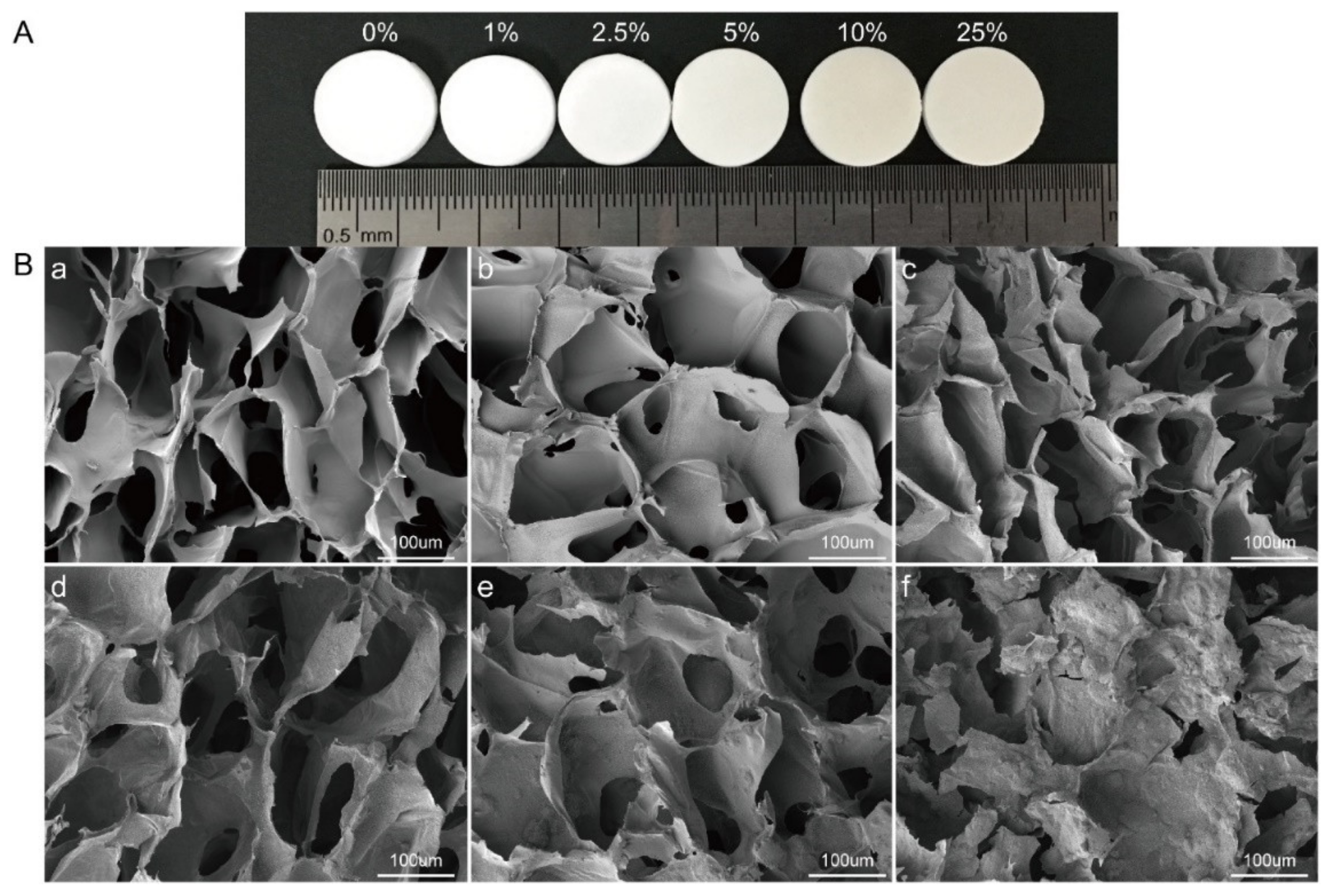
4.4. Bioglass
5. HYH Composites Containing Silver, Gold Nanoparticles, and Quantum Dots
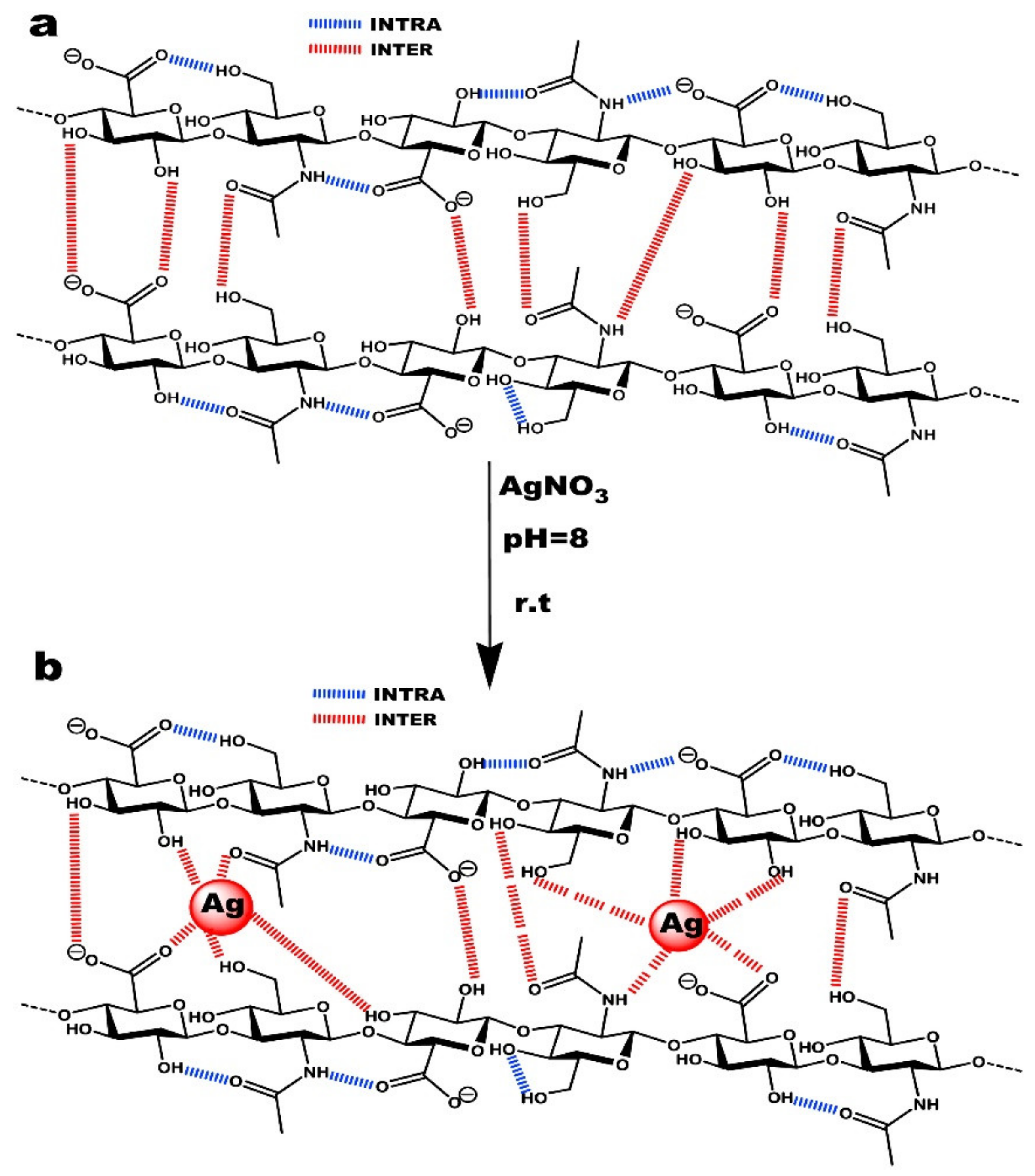
5.1. Silver
5.2. Gold
5.3. Quantum Dots
6. Conclusions and Future Trends
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Blackburn, M.R.; Hubbard, C.; Kiessling, V.; Bi, Y.; Kloss, B.; Tamm, L.K.; Zimmer, J. Distinct reaction mechanisms for hyaluronan biosynthesis in different kingdoms of life. Glycobiology 2018, 28, 108–121. [Google Scholar] [CrossRef]
- Baggenstoss, B.A.; Harris, E.N.; Washburn, J.L.; Medina, A.P.; Nguyen, L.; Weigel, P.H. Hyaluronan synthase control of synthesis rate and hyaluronan product size are independent functions differentially affected by mutations in a conserved tandem B-X7-B motif. Glycobiology 2017, 27, 154–164. [Google Scholar] [CrossRef]
- Gupta, A.; El-Amin, S.F.; Levy, H.J.; Sze-Tu, R.; Ibim, S.E.; Maffulli, N. Umbilical cord-derived Wharton’s jelly for regenerative medicine applications. J. Orthop. Surg. Res. 2020, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Törrönen, K.; Nikunen, K.; Kärnä, R.; Tammi, M.; Tammi, R.; Rilla, K. Tissue distribution and subcellular localization of hyaluronan synthase isoenzymes. Histochem. Cell Biol. 2014, 141, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Vasi, A.-M.; Popa, M.I.; Butnaru, M.; Dodi, G.; Verestiuc, L. Chemical functionalization of hyaluronic acid for drug delivery applications. Mater. Sci. Eng. C 2014, 38, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Palmer, J.W. The polysaccharide of the vitreous humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar] [CrossRef]
- Levene, P.; Lopez-Suarez, J. Mucins and mucoids. J. Biol. Chem. 1918, 36, 105–126. [Google Scholar] [CrossRef]
- Bayer, I.S. Hyaluronic acid and controlled release: A review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Chen, L.H.; Xue, J.F.; Zheng, Z.Y.; Shuhaidi, M.; Thu, H.E.; Hussain, Z. Hyaluronic acid, an efficient biomacromolecule for treatment of inflammatory skin and joint diseases: A review of recent developments and critical appraisal of preclinical and clinical investigations. Int. J. Biol. Macromol. 2018, 116, 572–584. [Google Scholar] [CrossRef]
- Graça, M.F.; Miguel, S.P.; Cabral, C.S.; Correia, I.J. Hyaluronic acid-based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Salehi Dashtebayaz, M.S.; Nourbakhsh, M.S. Interpenetrating networks hydrogels based on hyaluronic acid for drug delivery and tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 442–451. [Google Scholar] [CrossRef]
- Schuurmans, C.C.; Mihajlovic, M.; Hiemstra, C.; Ito, K.; Hennink, W.E.; Vermonden, T. Hyaluronic acid and chondroitin sulfate (meth) acrylate-based hydrogels for tissue engineering: Synthesis, characteristics and pre-clinical evaluation. Biomaterials 2021, 268, 120602. [Google Scholar] [CrossRef] [PubMed]
- Salwowska, N.M.; Bebenek, K.A.; Żądło, D.A.; Wcisło-Dziadecka, D.L. Physiochemical properties and application of hyaluronic acid: A systematic review. J. Cosmet. Dermatol. 2016, 15, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, R.; Baker, K.; Zhitomirsky, I. Electrophoretic deposition of polymers and proteins for biomedical applications. Adv. Colloid Interface Sci. 2020, 284, 102272. [Google Scholar] [CrossRef]
- Petta, D.; D’Amora, U.; Ambrosio, L.; Grijpma, D.; Eglin, D.; D’Este, M. Hyaluronic acid as a bioink for extrusion-based 3D printing. Biofabrication 2020, 12, 032001. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.-Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Ma, M.; Chen, Y.; Zhang, K.; Wang, X.; Cui, X.; Shi, J. Hyaluronic acid-conjugated mesoporous silica nanoparticles: Excellent colloidal dispersity in physiological fluids and targeting efficacy. J. Mater. Chem. 2012, 22, 5615–5621. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Lin, Y.; Yin, M.; Ren, J.; Qu, X. Bioresponsive hyaluronic acid-capped mesoporous silica nanoparticles for targeted drug delivery. Chem. A Eur. J. 2013, 19, 1778–1783. [Google Scholar] [CrossRef]
- Hien, N.Q.; van Phu, D.; Duy, N.N.; Quoc, L.A. Radiation synthesis and characterization of hyaluronan capped gold nanoparticles. Carbohydr. Polym. 2012, 89, 537–541. [Google Scholar] [CrossRef]
- Ishii, S.; Sugiyama, T.; Cross, J.S.; Ikoma, T. Effects of Particle Sizes and Natural Polymers on Mechanical Properties of Alpha Tricalcium Phosphate Cements. MRS Adv. 2016, 1, 1277–1282. [Google Scholar] [CrossRef]
- Berts, I.; Gerelli, Y.; Hilborn, J.; Rennie, A.R. Structure of polymer and particle aggregates in hydrogel composites. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 421–429. [Google Scholar] [CrossRef]
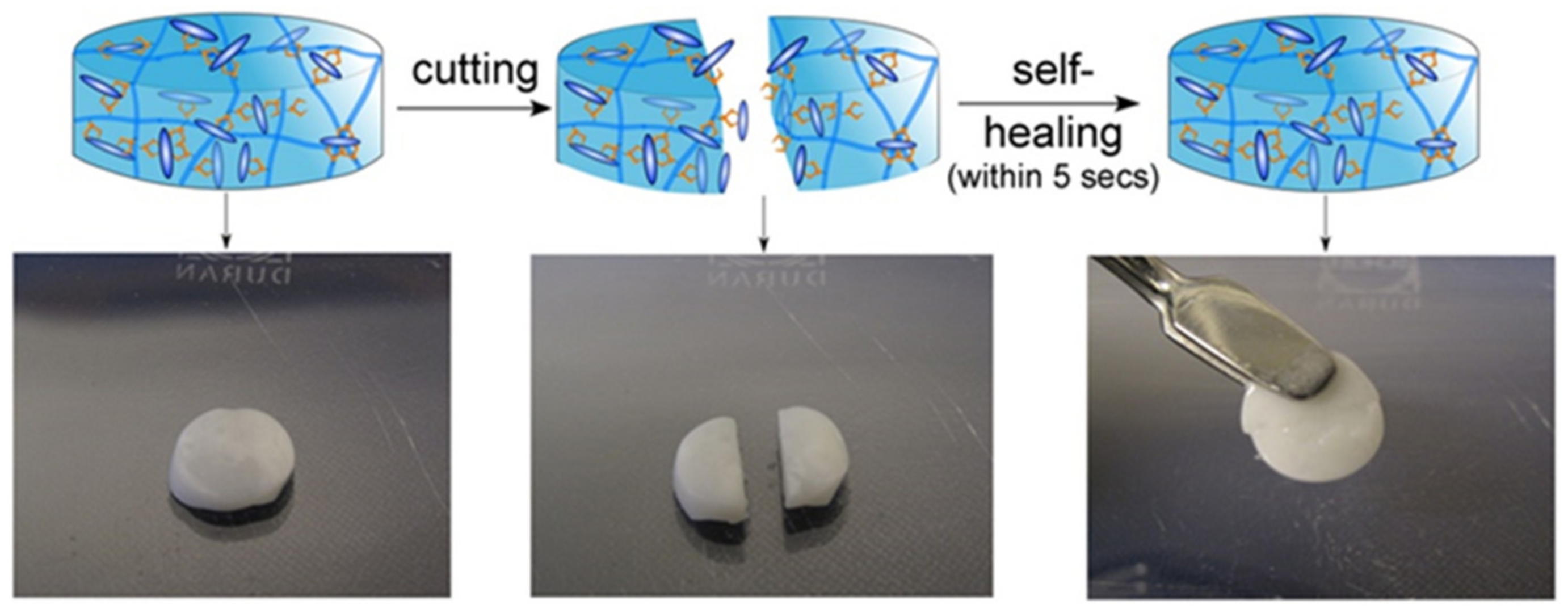
- Nejadnik, M.R.; Yang, X.; Bongio, M.; Alghamdi, H.S.; van den Beucken, J.J.; Huysmans, M.C.; Jansen, J.A.; Hilborn, J.; Ossipov, D.; Leeuwenburgh, S.C. Self-healing hybrid nanocomposites consisting of bisphosphonated hyaluronan and calcium phosphate nanoparticles. Biomaterials 2014, 35, 6918–6929. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhitomirsky, I. Electrodeposition of hyaluronic acid and composite films. Surf. Eng. 2009, 25, 621–627. [Google Scholar] [CrossRef]
- Gatej, I.; Popa, M.; Rinaudo, M. Role of the pH on hyaluronan behavior in aqueous solution. Biomacromolecules 2005, 6, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Abou-Okeil, A.; Fahmy, H.; El-Bisi, M.; Ahmed-Farid, O. Hyaluronic acid/Na-alginate films as topical bioactive wound dressings. Eur. Polym. J. 2018, 109, 101–109. [Google Scholar] [CrossRef]
- Li, J.; Qiao, M.; Ji, Y.; Lin, L.; Zhang, X.; Linhardt, R.J. Chemical, enzymatic and biological synthesis of hyaluronic acids. Int. J. Biol. Macromol. 2020, 152, 199–206. [Google Scholar] [CrossRef]
- Singh, A.; Li, P.; Beachley, V.; McDonnell, P.; Elisseeff, J.H. A hyaluronic acid-binding contact lens with enhanced water retention. Contact Lens Anterior Eye 2015, 38, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Korogiannaki, M.; Rastegari, B.; Zhang, J.; Chen, M.; Fu, Q.; Sheardown, H.; Filipe, C.D.; Hoare, T. “Click” chemistry-tethered hyaluronic acid-based contact lens coatings improve lens wettability and lower protein adsorption. ACS Appl. Mater. Interfaces 2016, 8, 22064–22073. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, S.-O.; Linardy, E.; Dreaden, E.C.; Zhdanov, V.P.; Hammond, P.T.; Cho, N.-J. Adsorption of hyaluronic acid on solid supports: Role of pH and surface chemistry in thin film self-assembly. J. Colloid Interface Sci. 2015, 448, 197–207. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, J.; Wu, B.; Shen, Z. pH-responsive hyaluronic acid nanoparticles coloaded with sorafenib and cisplatin for treatment of hepatocellular carcinoma. J. Biomater. Appl. 2019, 34, 219–228. [Google Scholar] [CrossRef]
- Wise, E.R.; Maltsev, S.; Davies, M.E.; Duer, M.J.; Jaeger, C.; Loveridge, N.; Murray, R.C.; Reid, D.G. The organic–mineral interface in bone is predominantly polysaccharide. Chem. Mater. 2007, 19, 5055–5057. [Google Scholar] [CrossRef]
- Li, Q.; Li, M.; Zhu, P.; Wei, S. In vitro synthesis of bioactive hydroxyapatite using sodium hyaluronate as a template. J. Mater. Chem. 2012, 22, 20257–20265. [Google Scholar] [CrossRef]
- Milošev, I.; Hmeljak, J.; Cör, A. Hyaluronic acid stimulates the formation of calcium phosphate on CoCrMo alloy in simulated physiological solution. J. Mater. Sci. Mater. Med. 2013, 24, 555–571. [Google Scholar] [CrossRef]
- Ohba, S.; Wang, W.; Itoh, S.; Takagi, Y.; Nagai, A.; Yamashita, K. Efficacy of platelet-rich plasma gel and hyaluronan hydrogel as carriers of electrically polarized hydroxyapatite microgranules for accelerating bone formation. J. Biomed. Mater. Res. Part A 2012, 100, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- Wenz, A.; Borchers, K.; Tovar, G.E.; Kluger, P.J. Bone matrix production in hydroxyapatite-modified hydrogels suitable for bone bioprinting. Biofabrication 2017, 9, 044103. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Zamanian, A.; Behnamghader, A.; Joupari, M.D. Bioactive and biostable hyaluronic acid-pullulan dermal hydrogels incorporated with biomimetic hydroxyapatite spheres. Mater. Sci. Eng. C 2020, 112, 110906. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-H.; Koh, Y.-H.; Kim, S.-W.; Park, J.-U.; Kim, H.-E.; Song, J. Strong and biostable hyaluronic acid–calcium phosphate nanocomposite hydrogel via in situ precipitation process. Biomacromolecules 2016, 17, 841–851. [Google Scholar] [CrossRef]
- Arjama, M.; Mehnath, S.; Rajan, M.; Jeyaraj, M. Injectable cuttlefish HAP and macromolecular fibroin protein hydrogel for natural bone mimicking matrix for enhancement of osteoinduction progression. React. Funct. Polym. 2021, 160, 104841. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Fan, Y.-F.; Baek, J.-U.; Song, J.; Choi, T.-H.; Kim, S.-W.; Kim, H.-E. Long-lasting and bioactive hyaluronic acid-hydroxyapatite composite hydrogels for injectable dermal fillers: Physical properties and in vivo durability. J. Biomater. Appl. 2016, 31, 464–474. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Wu, A.; Xu, H. An injectable nano-hydroxyapatite (n-HA)/glycol chitosan (G-CS)/hyaluronic acid (HyA) composite hydrogel for bone tissue engineering. RSC Adv. 2016, 6, 33529–33536. [Google Scholar] [CrossRef]
- Hong, S.Y.; Tran, T.V.T.; Kang, H.J.; Tripathi, G.; Lee, B.T.; Bae, S.H. Synthesis and characterization of biphasic calcium phosphate laden thiolated hyaluronic acid hydrogel based scaffold: Physical and in-vitro biocompatibility evaluations. J. Biomater. Sci. Polym. Ed. 2020, 32, 337–354. [Google Scholar] [CrossRef]
- Dennis, S.C.; Whitlow, J.; Detamore, M.S.; Kieweg, S.L.; Berkland, C.J. Hyaluronic-acid–hydroxyapatite colloidal gels combined with micronized native ECM as potential bone defect fillers. Langmuir 2017, 33, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Fan, Y.; Cheon, K.H.; Baek, J.; Kim, S.; Kim, H.E. Hyaluronic acid-hydroxyapatite nanocomposite hydrogels for enhanced biophysical and biological performance in a dermal matrix. J. Biomed. Mater. Res. Part A 2017, 105, 3315–3325. [Google Scholar] [CrossRef] [PubMed]
- Kettenberger, U.; Luginbuehl, V.; Procter, P.; Pioletti, D.P. In vitro and in vivo investigation of bisphosphonate-loaded hydroxyapatite particles for peri-implant bone augmentation. J. Tissue Eng. Regen. Med. 2017, 11, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Timpu, D.; Sacarescu, L.; Vasiliu, T.; Dinu, M.V.; David, G. Surface cationic functionalized nano-hydroxyapatite—Preparation, characterization, effect of coverage on properties and related applications. Eur. Polym. J. 2020, 132, 109759. [Google Scholar] [CrossRef]
- Jeong, S.H.; Sun, J.Y.; Kim, H.E. Dual-Crosslinking of Hyaluronic Acid–Calcium Phosphate Nanocomposite Hydrogels for Enhanced Mechanical Properties and Biological Performance. Macromol. Mater. Eng. 2017, 302, 1700160. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, B.; Yin, P.; Zhao, L.; Wang, Y.; Fu, Z.; Dang, R.; Xu, J.; Zhang, J.; Wen, N. Integration of human umbilical cord mesenchymal stem cells-derived exosomes with hydroxyapatite-embedded hyaluronic acid-alginate hydrogel for bone regeneration. ACS Biomater. Sci. Eng. 2020, 6, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Taşdelen, B.; Erdoğan, S.; Bekar, B. Radiation synthesis and characterization of chitosan/hyraluronic acid/hydroxyapatite hydrogels: Drug uptake and drug delivery systems. Mater. Today Proc. 2018, 5, 15990–15997. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Hsieh, C.-Y.; Yeh, C.-Y.; Lin, F.-H. The development of gelatin/hyaluronate copolymer mixed with calcium sulfate, hydroxyapatite, and stromal-cell-derived factor-1 for bone regeneration enhancement. Polymers 2019, 11, 1454. [Google Scholar] [CrossRef]
- Choi, S.; Lee, J.S.; Shin, J.; Lee, M.S.; Kang, D.; Hwang, N.S.; Lee, H.; Yang, H.S.; Cho, S.-W. Osteoconductive hybrid hyaluronic acid hydrogel patch for effective bone formation. J. Control. Release 2020, 327, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhitomirsky, I. Cathodic electrophoretic deposition of manganese dioxide films. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 248–253. [Google Scholar] [CrossRef]
- Zhitomirsky, I.; Gal-Or, L. Formation of hollow fibers by electrophoretic deposition. Mater. Lett. 1999, 38, 10–17. [Google Scholar] [CrossRef]
- Deen, I.; Pang, X.; Zhitomirsky, I. Electrophoretic deposition of composite chitosan–halloysite nanotube–hydroxyapatite films. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 38–44. [Google Scholar] [CrossRef]
- Ma, R.; Zhitomirsky, I. Electrophoretic Deposition of Organic-Inorganic Composites for Biomedical Applications. Mater. Sci. Forum 2012, 706, 617–622. [Google Scholar] [CrossRef]
- Ma, R.; Li, Y.; Zhitomirsky, I. Electrophoretic deposition of hyaluronic acid and composite films for biomedical applications. JOM 2010, 62, 72–75. [Google Scholar] [CrossRef]
- Deen, I.; Zhitomirsky, I. Electrophoretic deposition of composite halloysite nanotube–hydroxyapatite–hyaluronic acid films. J. Alloy. Compd. 2014, 586, S531–S534. [Google Scholar] [CrossRef]
- Grandfield, K.; Sun, F.; FitzPatrick, M.; Cheong, M.; Zhitomirsky, I. Electrophoretic deposition of polymer-carbon nanotube–hydroxyapatite composites. Surf. Coat. Technol. 2009, 203, 1481–1487. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Shi, Y.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. Electrophoretic deposition and electrochemical behavior of novel graphene oxide-hyaluronic acid-hydroxyapatite nanocomposite coatings. Appl. Surf. Sci. 2013, 284, 804–810. [Google Scholar] [CrossRef]
- Huang, C.; Fang, G.; Zhao, Y.; Bhagia, S.; Meng, X.; Yong, Q.; Ragauskas, A.J. Bio-inspired nanocomposite by layer-by-layer coating of chitosan/hyaluronic acid multilayers on a hard nanocellulose-hydroxyapatite matrix. Carbohydr. Polym. 2019, 222, 115036. [Google Scholar] [CrossRef]
- Aebli, N.; Stich, H.; Schawalder, P.; Theis, J.C.; Krebs, J. Effects of bone morphogenetic protein-2 and hyaluronic acid on the osseointegration of hydroxyapatite-coated implants: An experimental study in sheep. J. Biomed. Mater. Res. A 2005, 73, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Myers, C.; Zaidel, L.; Nalam, P.C.; Caporizzo, M.A.; Daep, C.A.; Eckmann, D.M.; Masters, J.G.; Composto, R.J. Competitive adsorption of polyelectrolytes onto and into pellicle-coated hydroxyapatite investigated by QCM-D and force spectroscopy. ACS Appl. Mater. Interfaces 2017, 9, 13079–13091. [Google Scholar] [CrossRef]
- Lei, P.; Bian, W.-G.; Liang, F.-H.; Xu, H.-Z. Implanting hydroxyapatite-coated porous titanium with bone morphogenetic protein-2 and hyaluronic acid into distal femoral metaphysis of rabbits. Chin. J. Traumatol. (Engl. Ed.) 2008, 11, 179–185. [Google Scholar]
- Hakobyan, S.; Roohpour, N.; Gautrot, J.E. Modes of adsorption of polyelectrolytes to model substrates of hydroxyapatite. J. Colloid Interface Sci. 2019, 543, 237–246. [Google Scholar] [CrossRef]
- Dubus, M.; Varin-Simon, J.; Prada, P.; Scomazzon, L.; Reffuveille, F.; Alem, H.; Boulmedais, F.; Mauprivez, C.; Rammal, H.; Kerdjoudj, H. Biopolymers-calcium phosphate antibacterial coating reduces the pathogenicity of internalized bacteria by mesenchymal stromal cells. Biomater. Sci. 2020, 8, 5763–5773. [Google Scholar] [CrossRef] [PubMed]
- Faruq, O.; Kim, B.; Padalhin, A.R.; Lee, G.H.; Lee, B.-T. A hybrid composite system of biphasic calcium phosphate granules loaded with hyaluronic acid–gelatin hydrogel for bone regeneration. J. Biomater. Appl. 2017, 32, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Sionkowska, A.; Kozlowska, J.; Osyczka, A. New composite materials prepared by calcium phosphate precipitation in chitosan/collagen/hyaluronic acid sponge cross-linked by EDC/NHS. Int. J. Biol. Macromol. 2018, 107, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jang, T.-S.; Pan, H.M.; Jung, H.-D.; Sia, M.W.; Xie, S.; Hang, Y.; Chong, S.K.M.; Wang, D.; Song, J. 3D freeform printing of nanocomposite hydrogels through in situ precipitation in reactive viscous fluid. Int. J. Bioprint. 2020, 6, 258. [Google Scholar]
- Kaczmarek, B.; Sionkowska, A.; Osyczka, A. The application of chitosan/collagen/hyaluronic acid sponge cross-linked by dialdehyde starch addition as a matrix for calcium phosphate in situ precipitation. Int. J. Biol. Macromol. 2018, 107, 470–477. [Google Scholar] [CrossRef]
- Li, M.; Jia, W.; Zhang, X.; Weng, H.; Gu, G.; Chen, Z. Hyaluronic acid oligosaccharides modified mineralized collagen and chitosan with enhanced osteoinductive properties for bone tissue engineering. Carbohydr. Polym. 2021, 260, 117780. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Dvorakova, J.; Kolaja Dobra, J.; Babuska, V. Biological Evaluation of Polyvinyl Alcohol Hydrogels Enriched by Hyaluronic Acid and Hydroxyapatite. Int. J. Mol. Sci. 2020, 21, 5719. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Hsieh, M.-F.; Fang, C.-H.; Jiang, C.-P.; Lin, B.; Lee, H.-M. Osteochondral regeneration induced by TGF-β loaded photo cross-linked hyaluronic acid hydrogel infiltrated in fused deposition-manufactured composite scaffold of hydroxyapatite and poly (ethylene glycol)-block-poly (ε-caprolactone). Polymers 2017, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.L.; Lee, B.-T. A combination of biphasic calcium phosphate scaffold with hyaluronic acid-gelatin hydrogel as a new tool for bone regeneration. Tissue Eng. Part A 2014, 20, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, P.; Liu, X.; Wang, P.; Xue, W.; Ren, Y.; Yang, R.; Chi, B.; Ye, Z. Bioinspired mineral-polymeric hybrid hyaluronic acid/poly (γ-glutamic acid) hydrogels as tunable scaffolds for stem cells differentiation. Carbohydr. Polym. 2021, 264, 118048. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Fan, T.; Zhang, Y.; Zhao, Y.; Shi, X.; Zhang, Q. Biomimetic mineralized hierarchical hybrid scaffolds based on in situ synthesis of nano-hydroxyapatite/chitosan/chondroitin sulfate/hyaluronic acid for bone tissue engineering. Colloids Surf. B Biointerfaces 2017, 157, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Sionkowska, A.; Gołyńska, M.; Polkowska, I.; Szponder, T.; Nehrbass, D.; Osyczka, A. In vivo study on scaffolds based on chitosan, collagen, and hyaluronic acid with hydroxyapatite. Int. J. Biol. Macromol. 2018, 118, 938–944. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Q.; Zhang, G.; Yang, S.; Wu, J.; Zhang, Q. Preparation and biocompatibility of nanohybrid scaffolds by in situ homogeneous formation of nano hydroxyapatite from biopolymer polyelectrolyte complex for bone repair applications. Colloids Surf. B: Biointerfaces 2012, 93, 100–107. [Google Scholar] [CrossRef]
- Gun-II, I.; Ji-Hyun, A.; So-Young, K.; Baek-Sun, C.; Shi-Woo, L. A hyaluronate–atelocollagen/B-tricalcium phosphate-hydroxyapatite biphasic scaffold for the repair of osteochondral defects: A porcine study. Tissue Eng. 2010, 16, 1189. [Google Scholar]
- Kaczmarek, B.; Sionkowska, A.; Osyczka, A. The comparison of physic-chemical properties of chitosan/collagen/hyaluronic acid composites with nano-hydroxyapatite cross-linked by dialdehyde starch and tannic acid. Polym. Test. 2017, 62, 171–176. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Otrocka-Domagała, I.; Polkowska, I. In vivo studies of novel scaffolds with tannic acid addition. Polym. Degrad. Stab. 2018, 158, 26–30. [Google Scholar] [CrossRef]
- Kang, H.-J.; Park, S.-S.; Saleh, T.; Ahn, K.-M.; Lee, B.-T. In vitro and in vivo evaluation of Ca/P-hyaluronic acid/gelatin based novel dental plugs for one-step socket preservation. Mater. Des. 2020, 194, 108891. [Google Scholar] [CrossRef]
- Nath, S.D.; Abueva, C.; Sarkar, S.K.; Lee, B.T. BMP-2 Immoblized in BCP-Chitosan-Hyaluronic Acid Hybrid Scaffold for Bone Tissue Engineering. Korean J. Mater. Res. 2014, 24, 704–709. [Google Scholar] [CrossRef][Green Version]
- Babo, P.S.; Cai, X.; Plachokova, A.S.; Reis, R.L.; Jansen, J.; Gomes, M.E.; Walboomers, X.F. Evaluation of a platelet lysate bilayered system for periodontal regeneration in a rat intrabony three-wall periodontal defect. J. Tissue Eng. Regen. Med. 2018, 12, e1277–e1288. [Google Scholar] [CrossRef]
- Hesaraki, S.; Nezafati, N. In vitro biocompatibility of chitosan/hyaluronic acid-containing calcium phosphate bone cements. Bioprocess Biosyst. Eng. 2014, 37, 1507–1516. [Google Scholar] [CrossRef]
- Luo, J.; Engqvist, H.; Persson, C. A ready-to-use acidic, brushite-forming calcium phosphate cement. Acta Biomater. 2018, 81, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Fang, Y.-H.; Sivasubramanian, S.; Lin, F.-H.; Lin, C.-P. Hydroxyapatite-calcium sulfate-hyaluronic acid composite encapsulated with collagenase as bone substitute for alveolar bone regeneration. Biomaterials 2016, 74, 99–108. [Google Scholar] [CrossRef]
- Babo, P.S.; Santo, V.E.; Gomes, M.E.; Reis, R.L. Development of an injectable calcium phosphate/hyaluronic acid microparticles system for platelet lysate sustained delivery aiming bone regeneration. Macromol. Biosci. 2016, 16, 1662–1677. [Google Scholar] [CrossRef]
- Alkhraisat, M.; Rueda, C.; Marino, F.; Torres, J.; Jerez, L.; Gbureck, U.; Cabarcos, E. The effect of hyaluronic acid on brushite cement cohesion. Acta Biomater. 2009, 5, 3150–3156. [Google Scholar] [CrossRef]
- Ahmadzadeh-Asl, S.; Hesaraki, S.; Zamanian, A. Preparation and characterisation of calcium phosphate–hyaluronic acid nanocomposite bone cement. Adv. Appl. Ceram. 2011, 110, 340–345. [Google Scholar] [CrossRef]
- Cui, X.; Huang, C.; Chen, Z.; Zhang, M.; Liu, C.; Su, K.; Wang, J.; Li, L.; Wang, R.; Li, B. Hyaluronic acid facilitates bone repair effects of calcium phosphate cement by accelerating osteogenic expression. Bioact. Mater. 2021, 6, 3801–3811. [Google Scholar] [CrossRef] [PubMed]
- Amirian, J.; Makkar, P.; Lee, G.H.; Paul, K.; Lee, B.T. Incorporation of alginate-hyaluronic acid microbeads in injectable calcium phosphate cement for improved bone regeneration. Mater. Lett. 2020, 272, 127830. [Google Scholar] [CrossRef]
- Kai, D.; Li, D.; Zhu, X.; Zhang, L.; Fan, H.; Zhang, X. Addition of sodium hyaluronate and the effect on performance of the injectable calcium phosphate cement. J. Mater. Sci. Mater. Med. 2009, 20, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Wolke, J.G.; Jansen, J.A.; Leeuwenburgh, S.C. Influence of polymeric additives on the cohesion and mechanical properties of calcium phosphate cements. J. Mater. Sci. Mater. Med. 2016, 27, 58. [Google Scholar] [CrossRef] [PubMed]
- Pek, Y.S.; Kurisawa, M.; Gao, S.; Chung, J.E.; Ying, J.Y. The development of a nanocrystalline apatite reinforced crosslinked hyaluronic acid–tyramine composite as an injectable bone cement. Biomaterials 2009, 30, 822–828. [Google Scholar] [PubMed]
- Zhou, Z.; Li, H.; Wang, K.; Guo, Q.; Li, C.; Jiang, H.; Hu, Y.; Oupicky, D.; Sun, M. Bioreducible cross-linked hyaluronic acid/calcium phosphate hybrid nanoparticles for specific delivery of siRNA in melanoma tumor therapy. ACS Appl. Mater. Interfaces 2017, 9, 14576–14589. [Google Scholar] [CrossRef]
- Kang, Y.; Sun, W.; Li, S.; Li, M.; Fan, J.; Du, J.; Liang, X.J.; Peng, X. Oligo Hyaluronan-Coated Silica/Hydroxyapatite Degradable Nanoparticles for Targeted Cancer Treatment. Adv. Sci. 2019, 6, 1900716. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-Y.; Han, H.S.; Lee, S.C.; Kang, Y.M.; Kim, I.-S.; Park, J.H. Mineralized hyaluronic acid nanoparticles as a robust drug carrier. J. Mater. Chem. 2011, 21, 7996–8001. [Google Scholar] [CrossRef]
- Kong, L.; Mu, Z.; Yu, Y.; Zhang, L.; Hu, J. Polyethyleneimine-stabilized hydroxyapatite nanoparticles modified with hyaluronic acid for targeted drug delivery. RSC Adv. 2016, 6, 101790–101799. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, J.E.; Byun, E.; Kim, N.W.; Lee, K.; Lee, H.; Sim, S.J.; Lee, D.S.; Jeong, J.H. Target-specific delivery of siRNA by stabilized calcium phosphate nanoparticles using dopa–hyaluronic acid conjugate. J. Control. Release 2014, 192, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Du, S.; Ni, J.; Zhou, J.; Yao, J. Mitochondria and nuclei dual-targeted heterogeneous hydroxyapatite nanoparticles for enhancing therapeutic efficacy of doxorubicin. Biomaterials 2016, 94, 70–83. [Google Scholar] [CrossRef]
- Zhou, R.; Li, Y.; Xiao, D.; Li, T.; Zhang, T.; Fu, W.; Lin, Y. Hyaluronan-directed fabrication of co-doped hydroxyapatite as a dual-modal probe for tumor-specific bioimaging. J. Mater. Chem. B 2020, 8, 2107–2114. [Google Scholar] [CrossRef]
- Qiu, C.; Wei, W.; Sun, J.; Zhang, H.-T.; Ding, J.-S.; Wang, J.-C.; Zhang, Q. Systemic delivery of siRNA by hyaluronan-functionalized calcium phosphate nanoparticles for tumor-targeted therapy. Nanoscale 2016, 8, 13033–13044. [Google Scholar] [CrossRef]
- Wei, J.; Shi, J.; Wu, Q.; Yang, L.; Cao, S. Hollow hydroxyapatite/polyelectrolyte hybrid microparticles with controllable size, wall thickness and drug delivery properties. J. Mater. Chem. B 2015, 3, 8162–8169. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shi, J.; Ma, H.; Chen, R.; Li, J.; Cao, S. Hierarchical hydroxyapatite/polyelectrolyte microcapsules capped with AuNRs for remotely triggered drug delivery. Mater. Sci. Eng. C 2019, 99, 1236–1245. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Wang, H.; Zhong, W.; Sun, C.; Sun, W.; Wu, H. Zoledronic Acid-Loaded Hybrid Hyaluronic Acid/Polyethylene Glycol/Nano-Hydroxyapatite Nanoparticle: Novel Fabrication and Safety Verification. Front. Bioeng. Biotechnol. 2021, 9, 629928. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, J.; Hwang, C.H.; Kim, H.E.; Jeong, S.H. Strategy for Preparing Mechanically Strong Hyaluronic Acid–Silica Nanohybrid Hydrogels via In Situ Sol–Gel Process. Macromol. Mater. Eng. 2018, 303, 1800213. [Google Scholar] [CrossRef]
- Sánchez-Téllez, D.; Rodríguez-Lorenzo, L.; Téllez-Jurado, L. Siloxane-inorganic chemical crosslinking of hyaluronic acid–based hybrid hydrogels: Structural characterization. Carbohydr. Polym. 2020, 230, 115590. [Google Scholar] [CrossRef] [PubMed]
- Shchipunov, Y.A.; Krekoten, A.V.; Postnova, I.V. Gelling of otherwise nongelable polysaccharides. J. Colloid Interface Sci. 2005, 287, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Vallés-Lluch, A.; Poveda-Reyes, S.; Amoros, P.; Beltran, D.; Monleón Pradas, M. Hyaluronic acid–silica nanohybrid gels. Biomacromolecules 2013, 14, 4217–4225. [Google Scholar] [CrossRef]
- Li, Z.; Chen, H.; Zhuo, Z.; Huang, D.; Luo, F.; Chen, L.; Wang, J.; Guo, L.; Qiu, B.; Lin, Z. Electrochemiluminescence biosensor for hyaluronidase activity detection and inhibitor assay based on the electrostatic interaction between hyaluronic acid and Ru (bpy) 32+. Sens. Actuators B Chem. 2018, 275, 409–414. [Google Scholar] [CrossRef]
- Franca, C.G.; Plaza, T.; Naveas, N.; Santana, M.H.A.; Manso-Silván, M.; Recio, G.; Hernandez-Montelongo, J. Nanoporous silicon microparticles embedded into oxidized hyaluronic acid/adipic acid dihydrazide hydrogel for enhanced controlled drug delivery. Microporous Mesoporous Mater. 2021, 310, 110634. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Han, Y.; Lai, J.; Chen, J. Multistage-targeted gold/mesoporous silica nanocomposite hydrogel as in situ injectable drug release system for chemophotothermal synergistic cancer therapy. ACS Appl. Bio Mater. 2019, 3, 421–431. [Google Scholar] [CrossRef]
- Piantanida, E.; Boškoski, I.; Quero, G.; Gallo, C.; Zhang, Y.; Fiorillo, C.; Arena, V.; Costamagna, G.; Perretta, S.; de Cola, L. Nanocomposite hyaluronic acid-based hydrogel for the treatment of esophageal fistulas. Mater. Today Bio 2021, 10, 100109. [Google Scholar] [CrossRef]
- Ma, R.; Zhitomirsky, I. Electrophoretic deposition of silica–hyaluronic acid and titania–hyaluronic acid nanocomposites. J. Alloys Compd. 2011, 509, S510–S513. [Google Scholar] [CrossRef]
- Hwang, C.; Min, Y.; Seong, Y.-J.; Kim, D.-E.; Kim, H.-E.; Jeong, S.-H. Enhanced biolubrication on biomedical devices using hyaluronic acid-silica nanohybrid hydrogels. Colloids Surf. B Biointerfaces 2019, 184, 110503. [Google Scholar] [CrossRef]
- Zouari, R.; Michel, M.; di Martino, J.; Ball, V. Production of free standing composite membranes or of patterned films after sol–gel reactions in an exponential layer-by-layer architecture. Mater. Sci. Eng. C 2010, 30, 1291–1297. [Google Scholar] [CrossRef]
- Nie, K.; An, Q.; Zink, J.I.; Yu, X.; Zhang, Y. Layer by Layer Mesoporous Silica-Hyaluronic Acid-Cyclodextrin Bifunctional “Lamination”: Study of the Application of Fluorescent Probe and Host–Guest Interactions in the Drug Delivery Field. Materials 2018, 11, 1745. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Yu, X.; Kumar, N.; Zhang, Y. Versatile layer-by-layer highly stable multilayer films: Study of the loading and release of FITC-labeled short peptide in the drug delivery field. Materials 2019, 12, 1206. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Long, T.; Yue, B. Immobilization of type I collagen/hyaluronic acid multilayer coating on enoxacin loaded titania nanotubes for improved osteogenesis and osseointegration in ovariectomized rats. Colloids Surf. B Biointerfaces 2019, 175, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, Y.; Gao, H.; Ge, P.; Ren, K.; Gao, J.; Cao, Y.; Han, D.; Zhang, J. One-step fabrication of functionalized poly (etheretherketone) surfaces with enhanced biocompatibility and osteogenic activity. Mater. Sci. Eng. C 2018, 88, 70–78. [Google Scholar] [CrossRef]
- Mu, C.; Hu, Y.; Huang, L.; Shen, X.; Li, M.; Li, L.; Gu, H.; Yu, Y.; Xia, Z.; Cai, K. Sustained raloxifene release from hyaluronan-alendronate-functionalized titanium nanotube arrays capable of enhancing osseointegration in osteoporotic rabbits. Mater. Sci. Eng. C 2018, 82, 345–353. [Google Scholar] [CrossRef]
- Yu, Y.; Ran, Q.; Shen, X.; Zheng, H.; Cai, K. Enzyme responsive titanium substrates with antibacterial property and osteo/angio-genic differentiation potentials. Colloids Surf. B Biointerfaces 2020, 185, 110592. [Google Scholar] [CrossRef]
- Yuan, Z.; Huang, S.; Lan, S.; Xiong, H.; Tao, B.; Ding, Y.; Liu, Y.; Liu, P.; Cai, K. Surface engineering of titanium implants with enzyme-triggered antibacterial properties and enhanced osseointegration in vivo. J. Mater. Chem. B 2018, 6, 8090–8104. [Google Scholar] [CrossRef]
- Safaei, M.; Taran, M. Fabrication, characterization, and antifungal activity of sodium hyaluronate-TiO2 bionanocomposite against Aspergillus niger. Mater. Lett. 2017, 207, 113–116. [Google Scholar] [CrossRef]
- Safaei, M.; Taran, M.; Imani, M.M.; Moradpoor, H.; Golshah, A.; Upadhyay, P. In vitro evaluation of anticancer activity of sodium hyaluronate-titanium dioxide bionanocomposite. Curr. Issues Pharm. Med. Sci. 2019, 32, 99–103. [Google Scholar] [CrossRef]
- Giang, N.N.; Won, H.J.; Lee, G.; Park, S.Y. Cancer cells targeted visible light and alkaline Phosphatase-Responsive TiO2/Cu2+ carbon Dots-Coated wireless electrochemical biosensor. Chem. Eng. J. 2021, 417, 129196. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Robinson, D.; Eisman, J.; Levy, A.; Zaslav, K.; Shani, J.; Altschuler, N. Osteochondral regeneration using a novel aragonite-hyaluronate bi-phasic scaffold in a goat model. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1452–1464. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Zhou, H.; Li, X. Biomimetic crystallization of toplike calcite single crystals with an extensive (00.1) face in the presence of sodium hyaluronate. Cryst. Growth Des. 2010, 10, 4722–4727. [Google Scholar] [CrossRef]
- Li, X.; Xu, P.; Cheng, Y.; Zhang, W.; Zheng, B.; Wang, Q. Nano-pearl powder/chitosan-hyaluronic acid porous composite scaffold and preliminary study of its osteogenesis mechanism. Mater. Sci. Eng. C 2020, 111, 110749. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Georgieva, R.; Steffen, A.; Smuda, K.; Bäumler, H. Structure and properties of hybrid biopolymer particles fabricated by co-precipitation cross-linking dissolution procedure. J. Colloid Interface Sci. 2018, 514, 156–164. [Google Scholar] [CrossRef]
- Ramalapa, B.; Crasson, O.; Vandevenne, M.; Gibaud, A.; Garcion, E.; Cordonnier, T.; Galleni, M.; Boury, F. Protein–polysaccharide complexes for enhanced protein delivery in hyaluronic acid templated calcium carbonate microparticles. J. Mater. Chem. B 2017, 5, 7360–7368. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, L.; Liu, Y.; Xu, X.; Wu, C. Targeted delivery of paclitaxel in liver cancer using hyaluronic acid functionalized mesoporous hollow alumina nanoparticles. BioMed Res. Int. 2019, 2019, 2928507. [Google Scholar] [CrossRef]
- Joung, C.-K.; Kim, H.-N.; Lim, M.-C.; Jeon, T.-J.; Kim, H.-Y.; Kim, Y.-R. A nanoporous membrane-based impedimetric immunosensor for label-free detection of pathogenic bacteria in whole milk. Biosens. Bioelectron. 2013, 44, 210–215. [Google Scholar] [CrossRef]
- Bae, M.S.; Kim, J.E.; Lee, J.B.; Heo, D.N.; Yang, D.H.; Kim, J.-H.; Kwon, K.-R.; Bang, J.B.; Bae, H.; Kwon, I.K. ZrO2 surface chemically coated with hyaluronic acid hydrogel loading GDF-5 for osteogenesis in dentistry. Carbohydr. Polym. 2013, 92, 167–175. [Google Scholar] [CrossRef]
- Zhou, Z.; He, S.; Ou, B.; Huang, T.; Zeng, W.; Liu, L.; Liu, Q.; Chen, J.; Zhao, Y.; Yang, Z. Influence of nano-bioactive glass (NBG) content on properties of gelatin-hyaluronic acid/NBG composite scaffolds. J. Macromol. Sci. Part B 2014, 53, 1145–1155. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Yu, X.; Chen, X.; Li, X.; Yang, X.; Li, S.; Zhang, X.; Xiang, A.P. Evaluation of human mesenchymal stem cells response to biomimetic bioglass-collagen-hyaluronic acid-phosphatidylserine composite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 88, 264–273. [Google Scholar] [CrossRef]
- Sohrabi, M.; Hesaraki, S.; Kazemzadeh, A. The influence of polymeric component of bioactive glass-based nanocomposite paste on its rheological behaviors and in vitro responses: Hyaluronic acid versus sodium alginate. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Vale, A.; Sousa, M.; Barbosa, A.; Torrado, E.; Mano, J.; Alves, N. Antibacterial bioadhesive layer-by-layer coatings for orthopedic applications. J. Mater. Chem. B 2016, 4, 5385–5393. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.C.; Vale, A.C.; Reis, R.L.; Alves, N.M. Bioactive and adhesive properties of multilayered coatings based on catechol-functionalized chitosan/hyaluronic acid and bioactive glass nanoparticles. Int. J. Biol. Macromol. 2020, 157, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Vale, A.C.; Pires, R.A.; Botelho, G.; Reis, R.L.; Alves, N.M. Spin-coated polysaccharide-based multilayered freestanding films with adhesive and bioactive moieties. Molecules 2020, 25, 840. [Google Scholar] [CrossRef]
- Vale, A.C.; Pereira, P.; Barbosa, A.M.; Torrado, E.; Mano, J.F.; Alves, N.M. Antibacterial free-standing polysaccharide composite films inspired by the sea. Int. J. Biol. Macromol. 2019, 133, 933–944. [Google Scholar] [CrossRef]
- Diba, M.; An, J.; Schmidt, S.; Hembury, M.; Ossipov, D.; Boccaccini, A.R.; Leeuwenburgh, S.C. Exploiting Bisphosphonate-Bioactive-Glass Interactions for the Development of Self-Healing and Bioactive Composite Hydrogels. Macromol. Rapid Commun. 2016, 37, 1952–1959. [Google Scholar] [CrossRef]
- Manferdini, C.; Guarino, V.; Zini, N.; Raucci, M.G.; Ferrari, A.; Grassi, F.; Gabusi, E.; Squarzoni, S.; Facchini, A.; Ambrosio, L. Mineralization behavior with mesenchymal stromal cells in a biomimetic hyaluronic acid-based scaffold. Biomaterials 2010, 31, 3986–3996. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, M.; Hesaraki, S.; Kazemzadeh, A.; Alizadeh, M. Development of injectable biocomposites from hyaluronic acid and bioactive glass nano-particles obtained from different sol–gel routes. Mater. Sci. Eng. C 2013, 33, 3730–3744. [Google Scholar] [CrossRef]
- Kummara, M.R.; Kumar, A.; Soo, H.S. Development of antibacterial paper coated with sodium hyaluronate stabilized curcumin-Ag nanohybrid and chitosan via polyelectrolyte complexation for medical applications. Mater. Res. Express 2017, 4, 115401. [Google Scholar] [CrossRef]
- Lu, B.; Lu, F.; Zou, Y.; Liu, J.; Rong, B.; Li, Z.; Dai, F.; Wu, D.; Lan, G. In situ reduction of silver nanoparticles by chitosan-l-glutamic acid/hyaluronic acid: Enhancing antimicrobial and wound-healing activity. Carbohydr. Polym. 2017, 173, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 223, 115023. [Google Scholar] [CrossRef]
- Yahyaei, B.; Peyvandi, N.; Akbari, H.; Arabzadeh, S.; Afsharnezhad, S.; Ajoudanifar, H.; Pourali, P. Production, assessment, and impregnation of hyaluronic acid with silver nanoparticles that were produced by Streptococcus pyogenes for tissue engineering applications. Appl. Biol. Chem. 2016, 59, 227–237. [Google Scholar] [CrossRef]
- Anisha, B.; Biswas, R.; Chennazhi, K.; Jayakumar, R. Chitosan–hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int. J. Biol. Macromol. 2013, 62, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Catanzano, O.; D’Esposito, V.; Pulcrano, G.; Maiolino, S.; Ambrosio, M.R.; Esposito, M.; Miro, A.; Ungaro, F.; Formisano, P.; Catania, M.R. Ultrasmall silver nanoparticles loaded in alginate–hyaluronic acid hybrid hydrogels for treating infected wounds. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 626–634. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, S.-H.; Shalumon, K.; Chen, J.-P. Dual functional core–sheath electrospun hyaluronic acid/polycaprolactone nanofibrous membranes embedded with silver nanoparticles for prevention of peritendinous adhesion. Acta Biomater. 2015, 26, 225–235. [Google Scholar] [CrossRef]
- Khachatryan, G.; Khachatryan, K.; Grzyb, J.; Fiedorowicz, M. Formation and properties of hyaluronan/nano Ag and hyaluronan-lecithin/nano Ag films. Carbohydr. Polym. 2016, 151, 452–457. [Google Scholar] [CrossRef]
- Khachatryan, G.; Khachatryan, K.; Krystyjan, M.; Krzan, M.; Khachatryan, L. Functional properties of composites containing silver nanoparticles embedded in hyaluronan and hyaluronan-lecithin matrix. Int. J. Biol. Macromol. 2020, 149, 417–423. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.; Hrdina, R.; Burgert, L.; Krylová, G.; Abdel-Rahman, R.M.; Krejčová, A.; Steinhart, M.; Beneš, L. Green synthesis of hyaluronan fibers with silver nanoparticles. Carbohydr. Polym. 2012, 89, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohsen, A.; Jancar, J.; Abdel-Rahman, R.; Vojtek, L.; Hyršl, P.; Dušková, M.; Nejezchlebová, H. A novel in situ silver/hyaluronan bio-nanocomposite fabrics for wound and chronic ulcer dressing: In vitro and in vivo evaluations. Int. J. Pharm. 2017, 520, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohsen, A.; Hrdina, R.; Burgert, L.; Abdel-Rahman, R.M.; Hašová, M.; Šmejkalová, D.; Kolář, M.; Pekar, M.; Aly, A. Antibacterial activity and cell viability of hyaluronan fiber with silver nanoparticles. Carbohydr. Polym. 2013, 92, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohsen, A.; Pavliňák, D.; Čileková, M.; Lepcio, P.; Abdel-Rahman, R.; Jančář, J. Electrospinning of hyaluronan/polyvinyl alcohol in presence of in-situ silver nanoparticles: Preparation and characterization. Int. J. Biol. Macromol. 2019, 139, 730–739. [Google Scholar] [CrossRef]
- Cui, X.; Li, C.M.; Bao, H.; Zheng, X.; Lu, Z. In situ fabrication of silver nanoarrays in hyaluronan/PDDA layer-by-layer assembled structure. J. Colloid Interface Sci. 2008, 327, 459–465. [Google Scholar] [CrossRef]
- Fahmy, H.; Aly, A.; Abou-Okeil, A. A non-woven fabric wound dressing containing layer–by–layer deposited hyaluronic acid and chitosan. Int. J. Biol. Macromol. 2018, 114, 929–934. [Google Scholar] [CrossRef]
- Liu, P.; Hao, Y.; Ding, Y.; Yuan, Z.; Liu, Y.; Cai, K. Fabrication of enzyme-responsive composite coating for the design of antibacterial surface. J. Mater. Sci. Mater. Med. 2018, 29, 160. [Google Scholar] [CrossRef] [PubMed]
- Taketa, T.B.; Bataglioli, R.A.; Neto, J.B.M.R.; Beppu, M.M. Probing axial metal distribution on biopolymer-based layer-by-layer films for antimicrobial use. Colloids Surf. B Biointerfaces 2021, 199, 111505. [Google Scholar] [CrossRef]
- Malcher, M.; Volodkin, D.; Heurtault, B.; André, P.; Schaaf, P.; Möhwald, H.; Voegel, J.-C.; Sokolowski, A.; Ball, V.; Boulmedais, F. Embedded silver ions-containing liposomes in polyelectrolyte multilayers: Cargos films for antibacterial agents. Langmuir 2008, 24, 10209–10215. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Jiang, Y.; Tang, Q.; Ji, J.; Wang, B.; Huang, Y.; Qian, Z. Silver nanoparticles reinforced (poly-(l-lysine)/hyaluronic acid) free-standing films: The mechanical strength and antibacterial activity. J. Biomed. Nanotechnol. 2017, 13, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Son, K.J.; Koh, W.-G. Metal-enhanced fluorescence using silver nanoparticles-embedded polyelectrolyte multilayer films for microarray-based immunoassays. Colloid Polym. Sci. 2014, 292, 1355–1364. [Google Scholar] [CrossRef]
- Francesko, A.; Ivanova, K.; Hoyo, J.; Pérez-Rafael, S.; Petkova, P.; Fernandes, M.M.; Heinze, T.; Mendoza, E.; Tzanov, T. Bottom-up Layer-by-Layer assembling of antibacterial freestanding nanobiocomposite films. Biomacromolecules 2018, 19, 3628–3636. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Han, X.; Deng, J.; Zhang, J.; Li, L.; Ni, J.; Huang, Y.; Xie, X.; Chen, S.; Ke, L. An injectable hydrogel based on phenylboronic acid hyperbranched macromer encapsulating gold nanorods and Astragaloside IV nanodrug for myocardial infarction. Chem. Eng. J. 2021, 413, 127423. [Google Scholar] [CrossRef]
- Fischer, R.L.; McCoy, M.G.; Grant, S.A. Electrospinning collagen and hyaluronic acid nanofiber meshes. J. Mater. Sci. Mater. Med. 2012, 23, 1645–1654. [Google Scholar] [CrossRef]
- Prokopović, V.Z.; Vikulina, A.S.; Sustr, D.; Duschl, C.; Volodkin, D. Biodegradation-resistant multilayers coated with gold nanoparticles. Toward a tailor-made artificial extracellular matrix. ACS Appl. Mater. Interfaces 2016, 8, 24345–24349. [Google Scholar] [CrossRef]
- Ratto, F.; Matteini, P.; Centi, S.; Rossi, F.; Pini, R. Gold nanorods as new nanochromophores for photothermal therapies. J. Biophotonics 2011, 4, 64–73. [Google Scholar] [CrossRef]
- Schmidt, S.; Madaboosi, N.; Uhlig, K.; Köhler, D.; Skirtach, A.; Duschl, C.; Möhwald, H.; Volodkin, D.V. Control of cell adhesion by mechanical reinforcement of soft polyelectrolyte films with nanoparticles. Langmuir 2012, 28, 7249–7257. [Google Scholar] [CrossRef]
- Volodkin, D.; Madaboosi, N.; Blacklock, J.; Skirtach, A.; Mohwald, H. Surface-supported multilayers decorated with bio-active material aimed at light-triggered drug delivery. Langmuir 2009, 25, 14037–14043. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Rapenne, L.; Chaudouet, P.; Ji, J.; Picart, C. In situ synthesis of gold nanoparticles in exponentially-growing layer-by-layer films. J. Colloid Interface Sci. 2012, 388, 56–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, H.-T.; Chuang, E.-Y.; Ma, H.; Tsai, C.-H.; Perng, C.-K. Fabrication of quantum dot-conjugated collagen/hyaluronic acid porous scaffold. Ann. Plast. Surg. 2012, 69, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Perng, C.-K.; Wang, Y.-J.; Tsi, C.-H.; Ma, H. In vivo angiogenesis effect of porous collagen scaffold with hyaluronic acid oligosaccharides. J. Surg. Res. 2011, 168, 9–15. [Google Scholar] [CrossRef]
- Jaffar, S.; Nam, K.T.; Khademhosseini, A.; Xing, J.; Langer, R.S.; Belcher, A.M. Layer-by-layer surface modification and patterned electrostatic deposition of quantum dots. Nano Lett. 2004, 4, 1421–1425. [Google Scholar] [CrossRef]
- Khachatryan, G.; Khachatryan, K.; Stobinski, L.; Tomasik, P.; Fiedorowicz, M.; Lin, H. CdS and ZnS quantum dots embedded in hyaluronic acid films. J. Alloys Compd. 2009, 481, 402–406. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikkema, R.; Keohan, B.; Zhitomirsky, I. Hyaluronic-Acid-Based Organic-Inorganic Composites for Biomedical Applications. Materials 2021, 14, 4982. https://doi.org/10.3390/ma14174982
Sikkema R, Keohan B, Zhitomirsky I. Hyaluronic-Acid-Based Organic-Inorganic Composites for Biomedical Applications. Materials. 2021; 14(17):4982. https://doi.org/10.3390/ma14174982
Chicago/Turabian StyleSikkema, Rebecca, Blanca Keohan, and Igor Zhitomirsky. 2021. "Hyaluronic-Acid-Based Organic-Inorganic Composites for Biomedical Applications" Materials 14, no. 17: 4982. https://doi.org/10.3390/ma14174982
APA StyleSikkema, R., Keohan, B., & Zhitomirsky, I. (2021). Hyaluronic-Acid-Based Organic-Inorganic Composites for Biomedical Applications. Materials, 14(17), 4982. https://doi.org/10.3390/ma14174982





