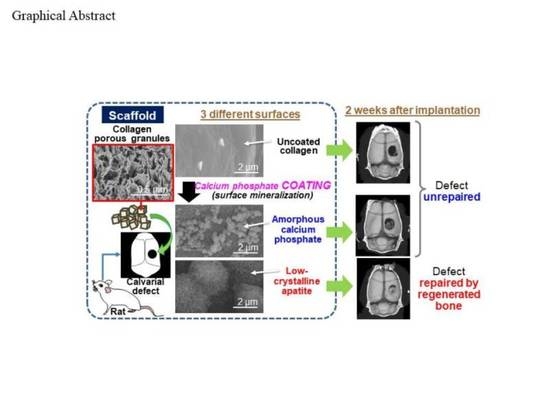
Bone Tissue Regeneration by Collagen Scaffolds with Different Calcium Phosphate Coatings: Amorphous Calcium Phosphate and Low-Crystalline Apatite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Scaffolds
2.2. Physicochemical Analyses
2.3. In Vivo Study
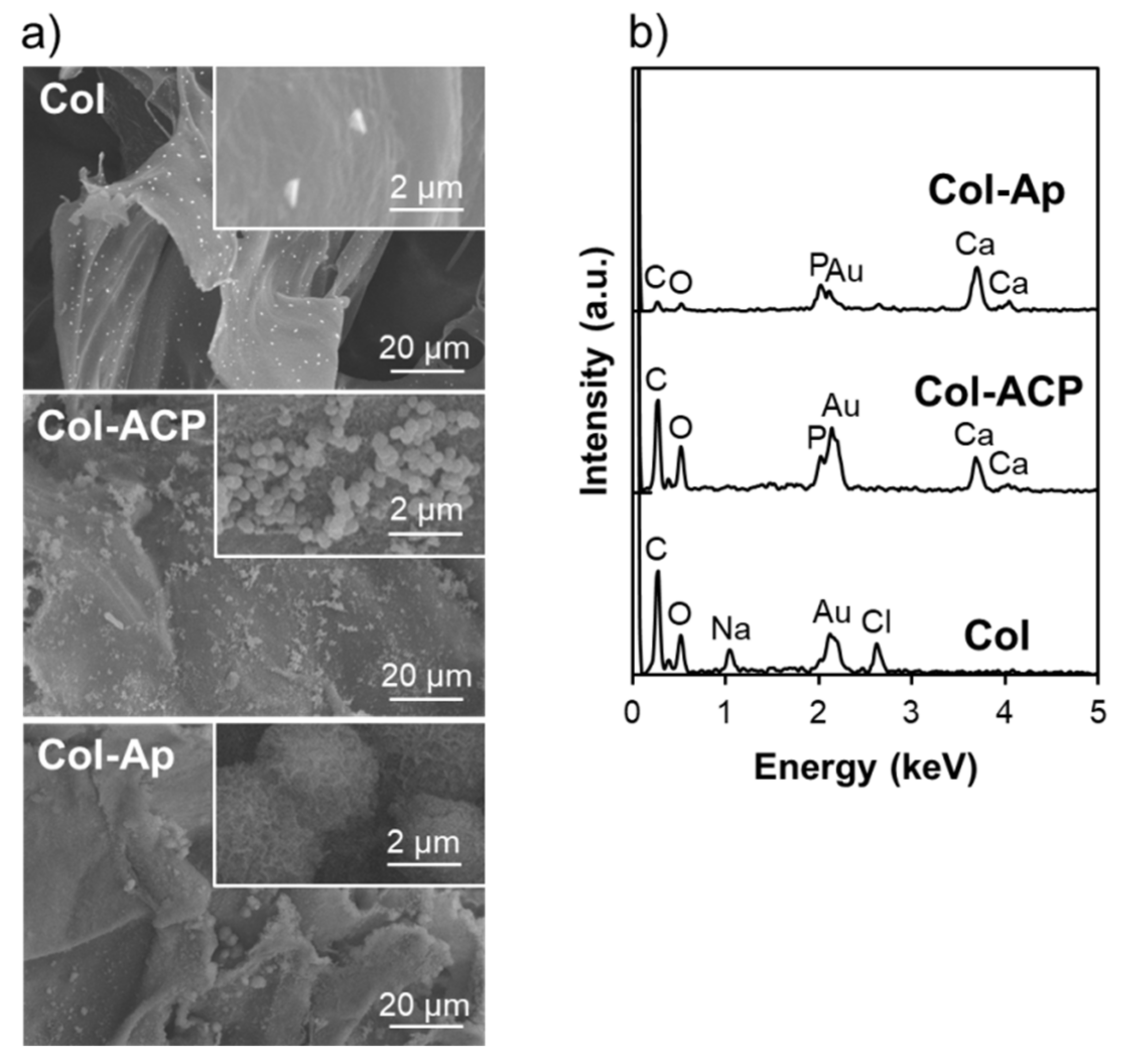
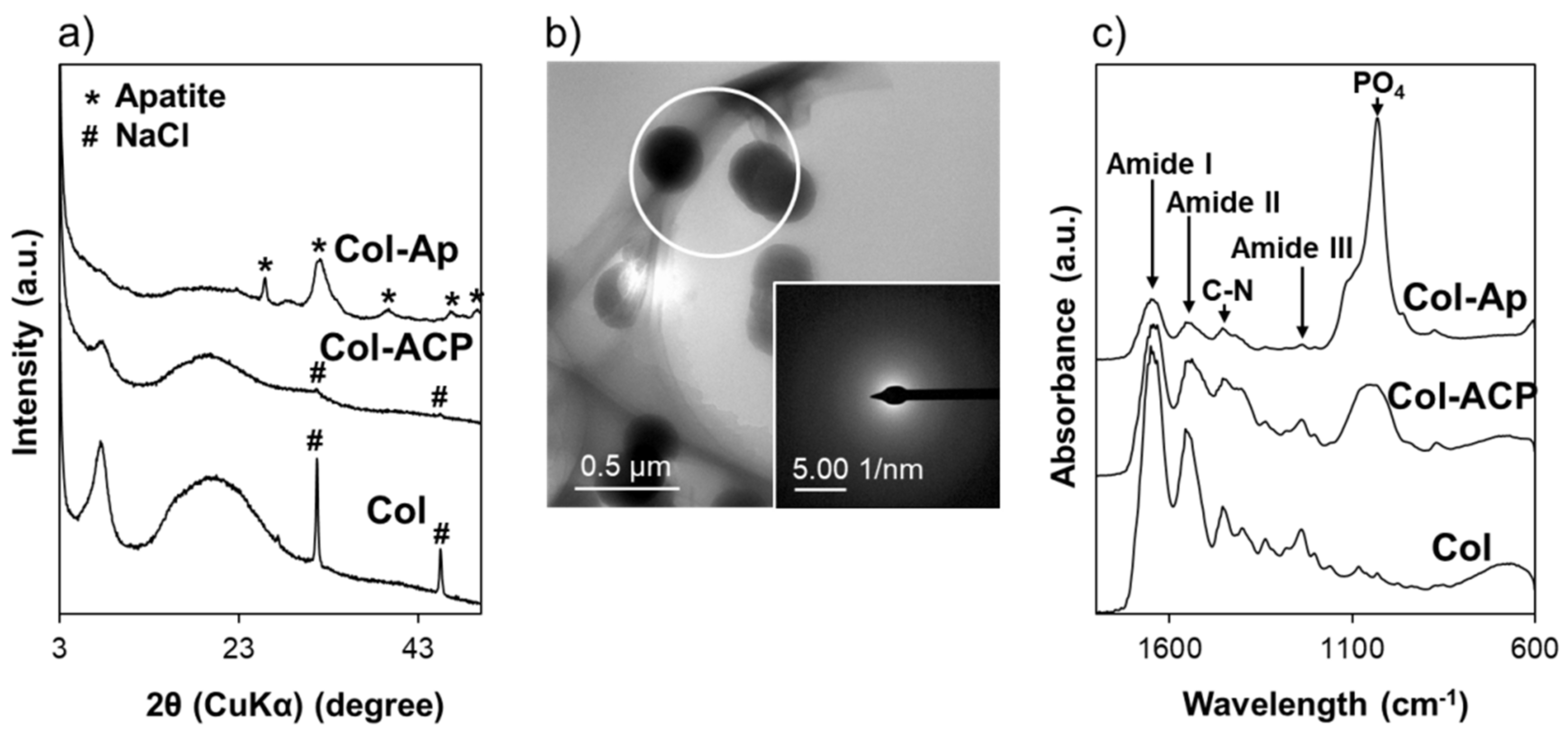
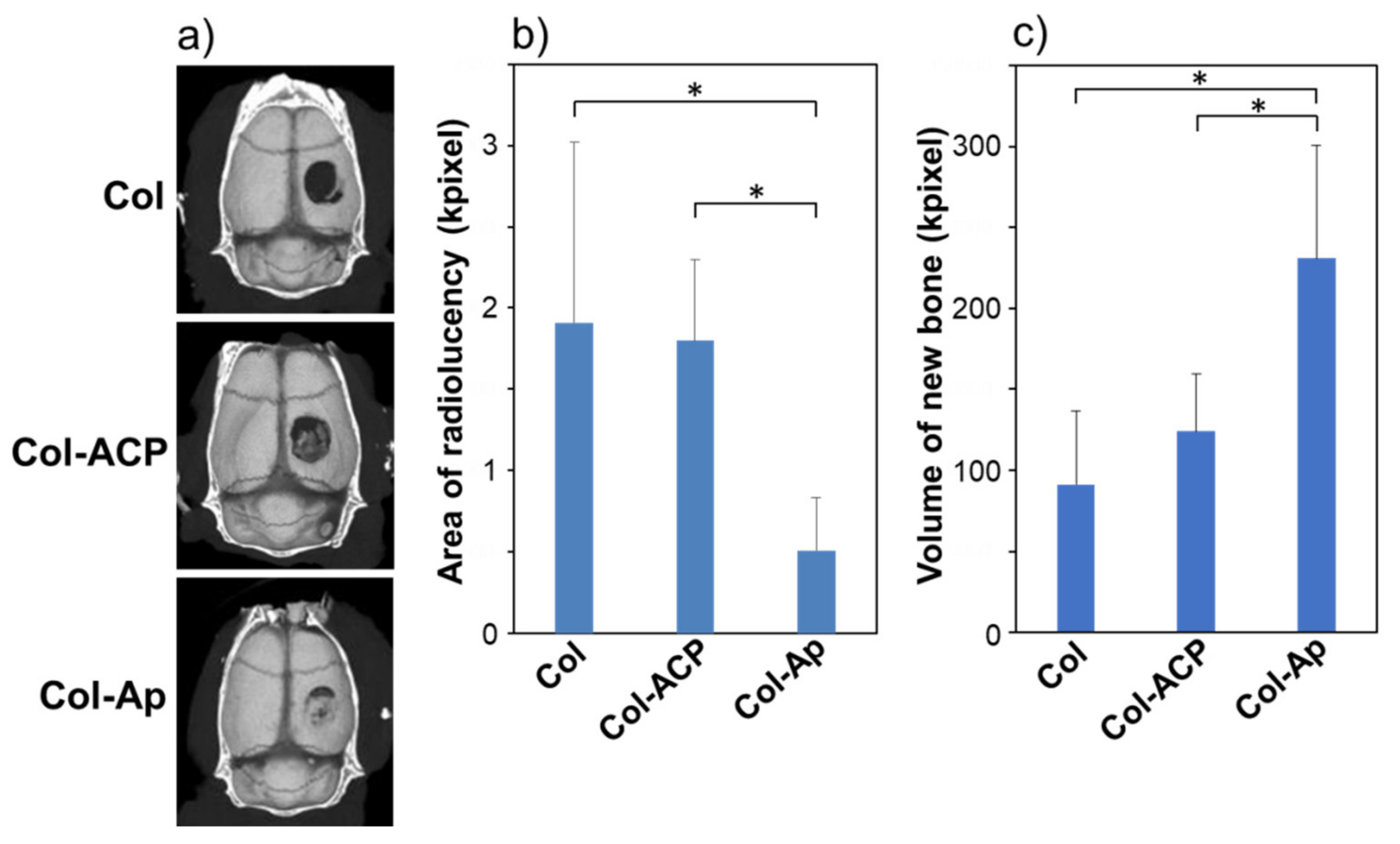
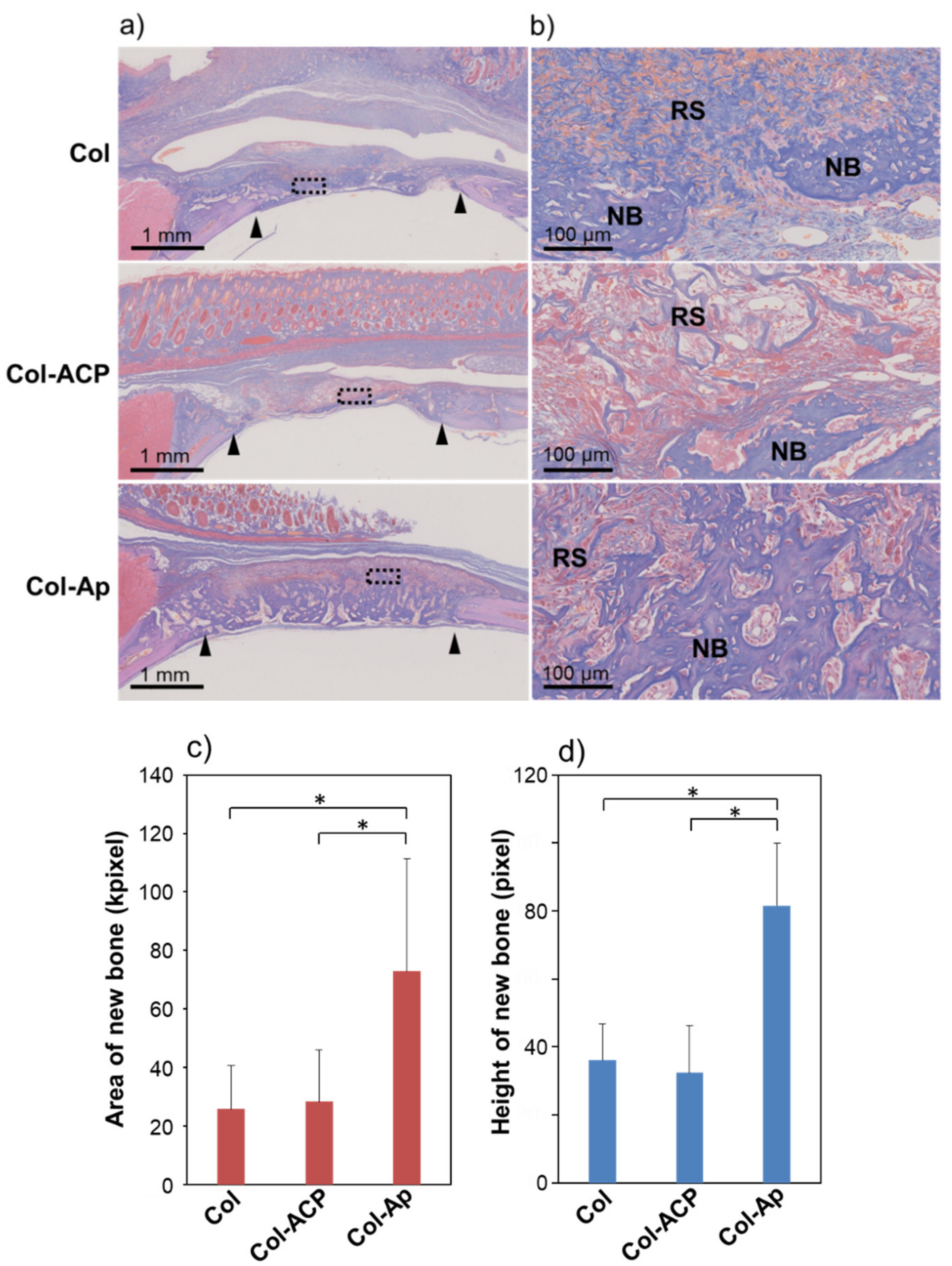
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jee, W.S.S. The skeletal tissue. In Histology, Cell and Tissue Biology, 5th ed.; Weiss, L., Ed.; The MacMillan Press, Ltd.: London, UK, 1983; pp. 225–228. [Google Scholar]
- Fukui, N.; Sato, T.; Kuboki, Y.; Aoki, H. Bone tissue reaction of nano-hydroxyapatite/collagen composite at the early stage of implantation. Biomed. Mater. Eng. 2008, 18, 25–33. [Google Scholar]
- Yang, H.S.; La, W.G.; Park, J.; Kim, C.S.; Im, G.I.; Kim, B.S. Efficient bone regeneration induced by bone morphogenetic protein-2 released from apatite-coated collagen scaffolds. J. Biomater. Sci. Polym. Ed. 2012, 23, 1659–1671. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y.; Fu, Y.; Chang, D.; Fu, C.; Wang, G.; Liu, Y.; Tay, F.R.; Zhou, Y. Bioinspired collagen-apatite nanocomposites for bone regeneration. J. Endod. 2016, 42, 1226–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, J.L.; Eanes, E.D. A thermodynamic analysis of the amorphous to crystalline calcium phosphate transformation. Calcif. Tiss. Res. 1978, 25, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Eanes, E.D. Amorphous calcium phosphate: Thermodynamic and kinetic considerations. In Calcium Phosphates in Biological and Industrial Systems; Amjad, Z., Ed.; Springer: Boston, MA, USA, 1998; pp. 21–39. [Google Scholar]
- Mahamid, J.; Sharir, A.; Addadi, L.; Weiner, S. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: Indications for an amorphous precursor phase. Proc. Natl. Acad. Sci. USA 2008, 105, 12748–12753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahamid, J.; Sharir, A.; Gur, D.; Zelzer, E.; Addadi, L.; Weiner, S. Bone mineralization proceeds through intracellular calcium phosphate loaded vesicles: A cryo-electron microscopy study. J. Struct. Biol. 2011, 174, 527–535. [Google Scholar] [CrossRef]
- Boonrungsiman, S.; Gentleman, E.; Carzaniga, R.; Evans, N.D.; McComb, D.W.; Porter, A.E.; Stevens, M.M. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14170–14175. [Google Scholar] [CrossRef] [Green Version]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans, P.H.; Friedrich, H.; Brylka, L.J.; Hilbers, P.A.; de With, G.; Sommerdijk, N.A. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef] [Green Version]
- Mahamid, J.; Aichmayer, B.; Shimoni, E.; Ziblat, R.; Li, C.; Siegel, S.; Paris, O.; Fratzl, P.; Weiner, S.; Addadi, L. Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. Proc. Natl. Acad. Sci. USA 2010, 107, 6316–6321. [Google Scholar] [CrossRef] [Green Version]
- Du, C.; Cui, F.Z.; Zhang, W.; Feng, Q.L.; Zhu, X.D.; de Groot, K. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. J. Biomed. Mater. Res. 2000, 50, 518–527. [Google Scholar] [CrossRef]
- Vranceanu, M.D.; Saban, R.; Albu, M.G.; Antoniac, I. Preparation and characterization of collagen: Amorphous calcium phosphate composites. Revista de Pielarie Incaltaminte Leather Footwear J. 2012, 12, 215–222. [Google Scholar]
- Nathanael, A.J.; Oyane, A.; Nakamura, M.; Sakamaki, I.; Nishida, E.; Kanemoto, Y.; Miyaji, H. In vitro and in vivo analysis of mineralized collagen-based sponges prepared by a plasma- and precursor-assisted biomimetic process. ACS Appl. Mater. Interfaces 2017, 9, 22185–22194. [Google Scholar] [CrossRef]
- Santhakumar, S.; Oyane, A.; Nakamura, M.; Koga, K.; Miyata, S.; Muratsubaki, K.; Miyaji, H. In situ precipitation of amorphous calcium phosphate nanoparticles within 3D porous collagen sponges for bone tissue engineering. Mater. Sci. Eng. C 2020, 116, 111194. [Google Scholar] [CrossRef] [PubMed]
- Kanemoto, Y. Assessment of Bone Forming Ability of Apatite-Coated Collagen Scaffold Prepared by a Precursor-Assisted Biomimetic Process. Ph.D. Thesis, Hokkaido University, Hokkaido, Japan, 2020. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Anada, T.; Handa, T.; Kanda, N.; Yoshinari, M.; Takahashi, T.; Suzuki, O. Osteoconductive property of a mechanical mixture of octacalcium phosphate and amorphous calcium phosphate. ACS Appl. Mater. Interfaces 2014, 6, 22602–22611. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Z.; Tian, F.; Chen, S.; Lei, L.; Jiang, T.; Feng, Q.; Fan, Y. Sustained delivery of calcium and orthophosphate ions from amorphous calcium phosphate and poly(L-lactic acid)-based electrospinning nanofibrous scaffold. Sci. Rep. 2017, 7, 45655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.; Tan, Z.; Liu, Y.; Tao, J.; Cai, Y.; Zhang, M.; Pan, H.; Xua, X.; Tang, R. Effect of crystallinity of calcium phosphate nanoparticles on adhesion, proliferation, and differentiation of bone marrow mesenchymal stem cells. J. Mater. Chem. 2007, 17, 4690–4698. [Google Scholar] [CrossRef]
- Clèries, L.; Fernández-Pradas, J.M.; Morenza, J.L. Bone growth on and resorption of calcium phosphate coatings obtained by pulsed laser deposition. J. Biomed. Mater. Res. 2000, 49, 43–52. [Google Scholar] [CrossRef]
- Walsh, W.R.; Morberg, P.; Yu, Y.; Yang, J.L.; Haggard, W.; Sheath, P.C.; Svehla, M.; Bruce, W.J.M. Response of a calcium sulfate bone graft substitute in a confined cancellous defect. Clin. Orthop. Relat. Res. 2003, 406, 228–236. [Google Scholar] [CrossRef]
- Frayssinet, P.; Gineste, L.; Conte, P.; Fages, J.; Rouquet, N. Short-term implantation effects of a DCPD-based calcium phosphate cement. Biomaterials 1998, 19, 971–977. [Google Scholar] [CrossRef] [Green Version]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, Y.C.; Park, S.S.; Subieta, A.R.; Brennan, T.J. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology 2004, 101, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317–332. [Google Scholar]
- Dorozhkin, S.V. Amorphous calcium (ortho)phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef] [PubMed]
- Burke, E.M.; Lucas, L.C. Dissolution kinetics of calcium phosphate coatings. Implant Dent. 1998, 7, 323–330. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, T.; Ueno, T.; Kagawa, T.; Sakata, Y.; Sugahara, T. Comparison of bone formation ingrafted periosteum harvested from tibia and calvaria. Microsc. Res. Technol. 2006, 69, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lee, J.; Yun, H.S.; Shin, H.I.; Park, E.K. Comparison of bone regeneration rate in flat and long bone defects: Calvarial and tibial bone. Tissue Eng. Regen. Med. 2013, 10, 336–340. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santhakumar, S.; Oyane, A.; Nakamura, M.; Yoshino, Y.; Alruwaili, M.K.; Miyaji, H. Bone Tissue Regeneration by Collagen Scaffolds with Different Calcium Phosphate Coatings: Amorphous Calcium Phosphate and Low-Crystalline Apatite. Materials 2021, 14, 5860. https://doi.org/10.3390/ma14195860
Santhakumar S, Oyane A, Nakamura M, Yoshino Y, Alruwaili MK, Miyaji H. Bone Tissue Regeneration by Collagen Scaffolds with Different Calcium Phosphate Coatings: Amorphous Calcium Phosphate and Low-Crystalline Apatite. Materials. 2021; 14(19):5860. https://doi.org/10.3390/ma14195860
Chicago/Turabian StyleSanthakumar, Syama, Ayako Oyane, Maki Nakamura, Yuto Yoshino, Mohammed Katib Alruwaili, and Hirofumi Miyaji. 2021. "Bone Tissue Regeneration by Collagen Scaffolds with Different Calcium Phosphate Coatings: Amorphous Calcium Phosphate and Low-Crystalline Apatite" Materials 14, no. 19: 5860. https://doi.org/10.3390/ma14195860
APA StyleSanthakumar, S., Oyane, A., Nakamura, M., Yoshino, Y., Alruwaili, M. K., & Miyaji, H. (2021). Bone Tissue Regeneration by Collagen Scaffolds with Different Calcium Phosphate Coatings: Amorphous Calcium Phosphate and Low-Crystalline Apatite. Materials, 14(19), 5860. https://doi.org/10.3390/ma14195860