Influence of Manufacturing Regimes on the Phase Transformation of Dental Zirconia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
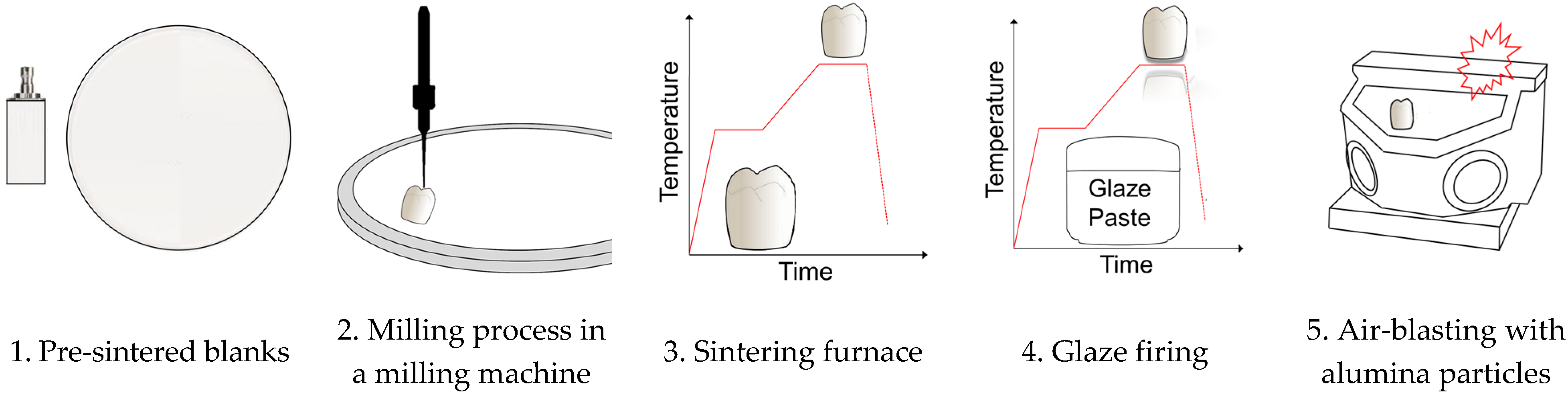
2.2. Methods
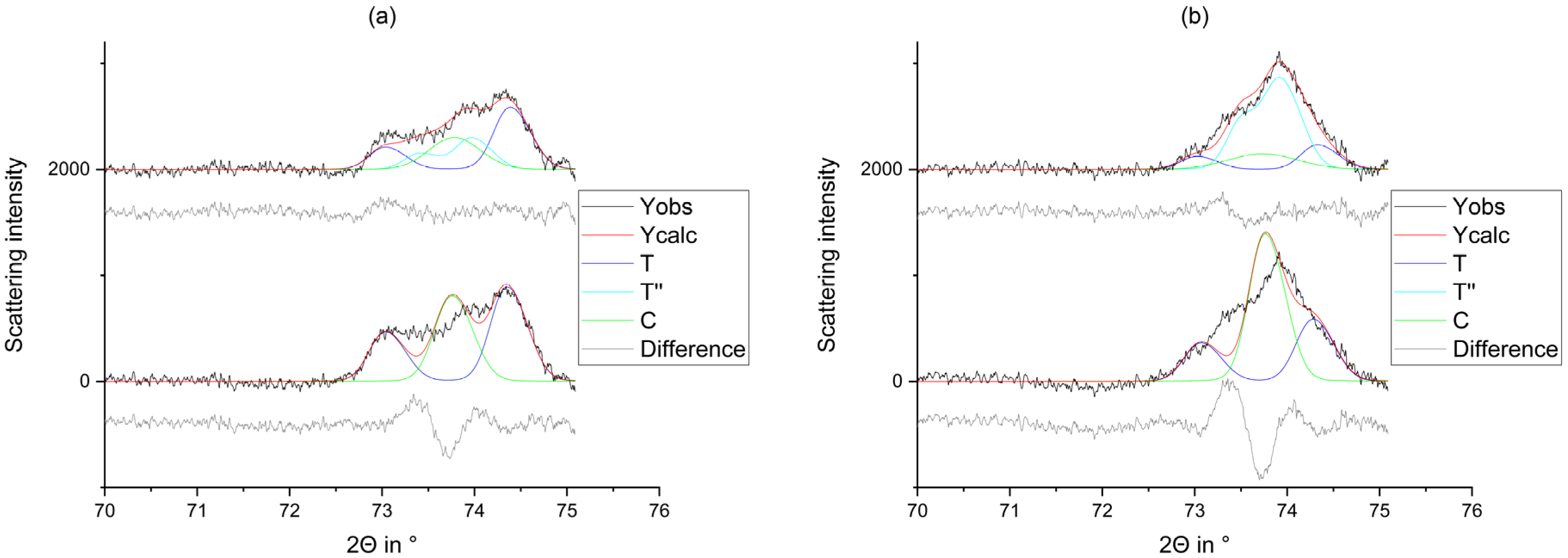
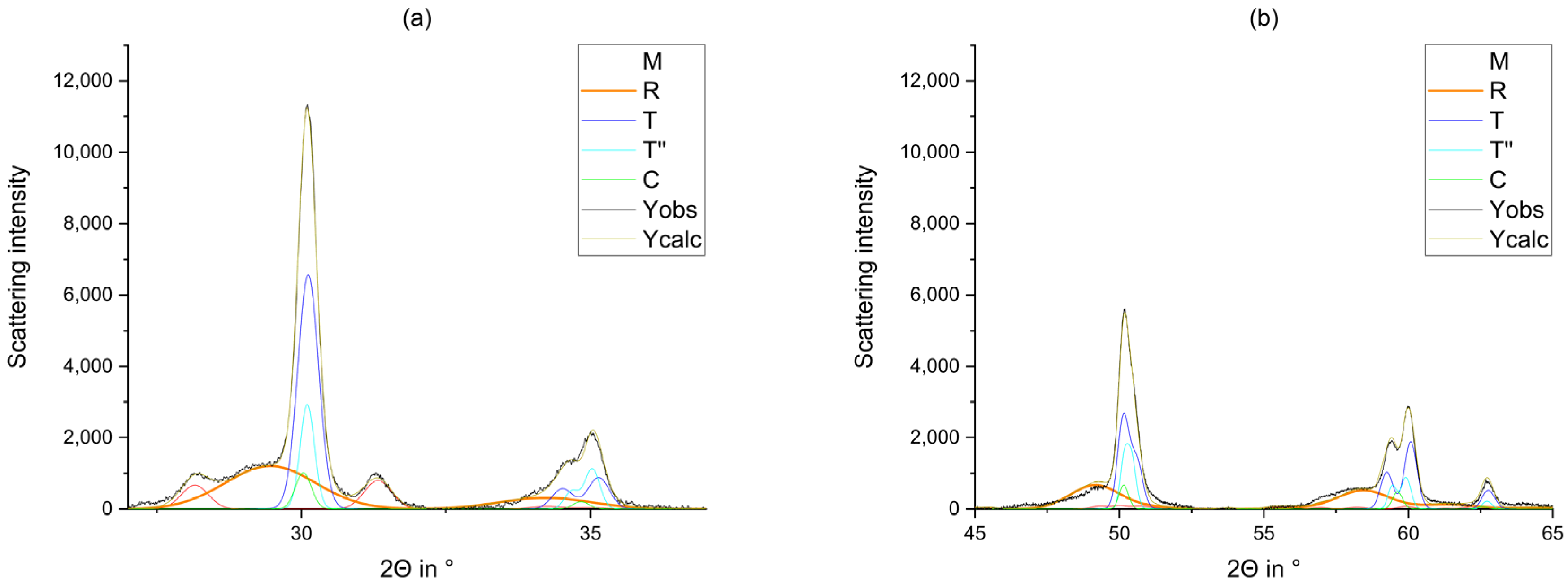
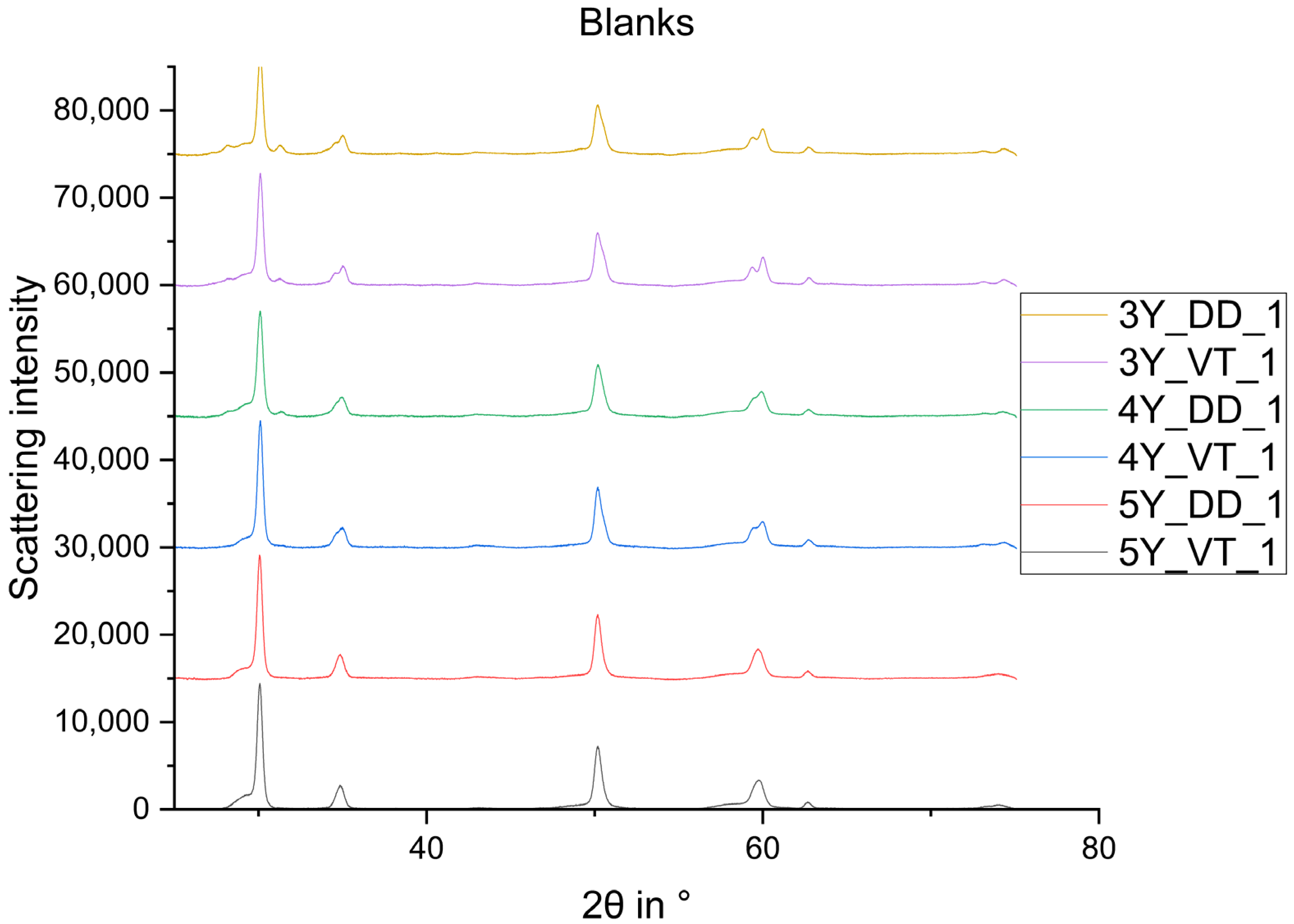
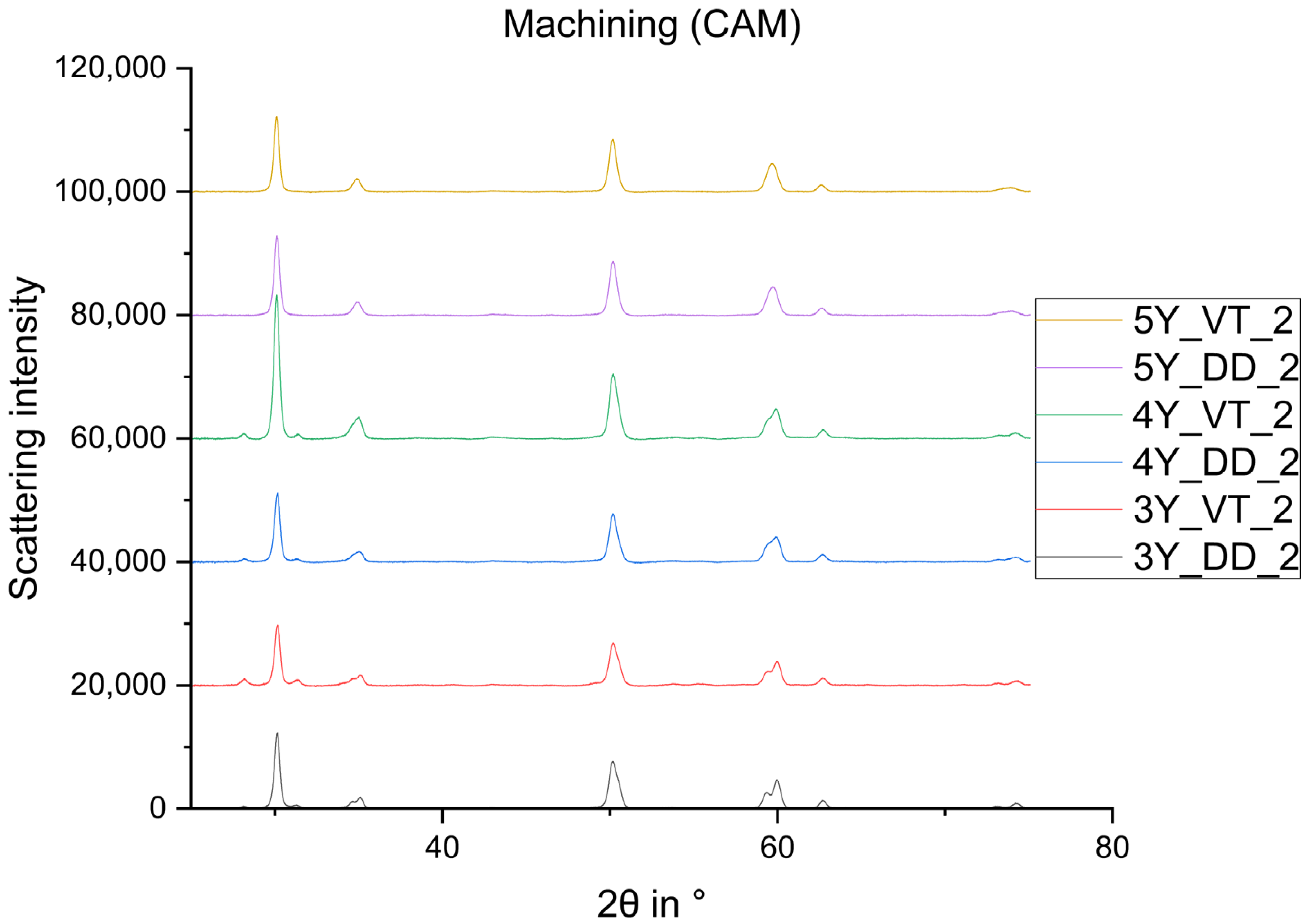
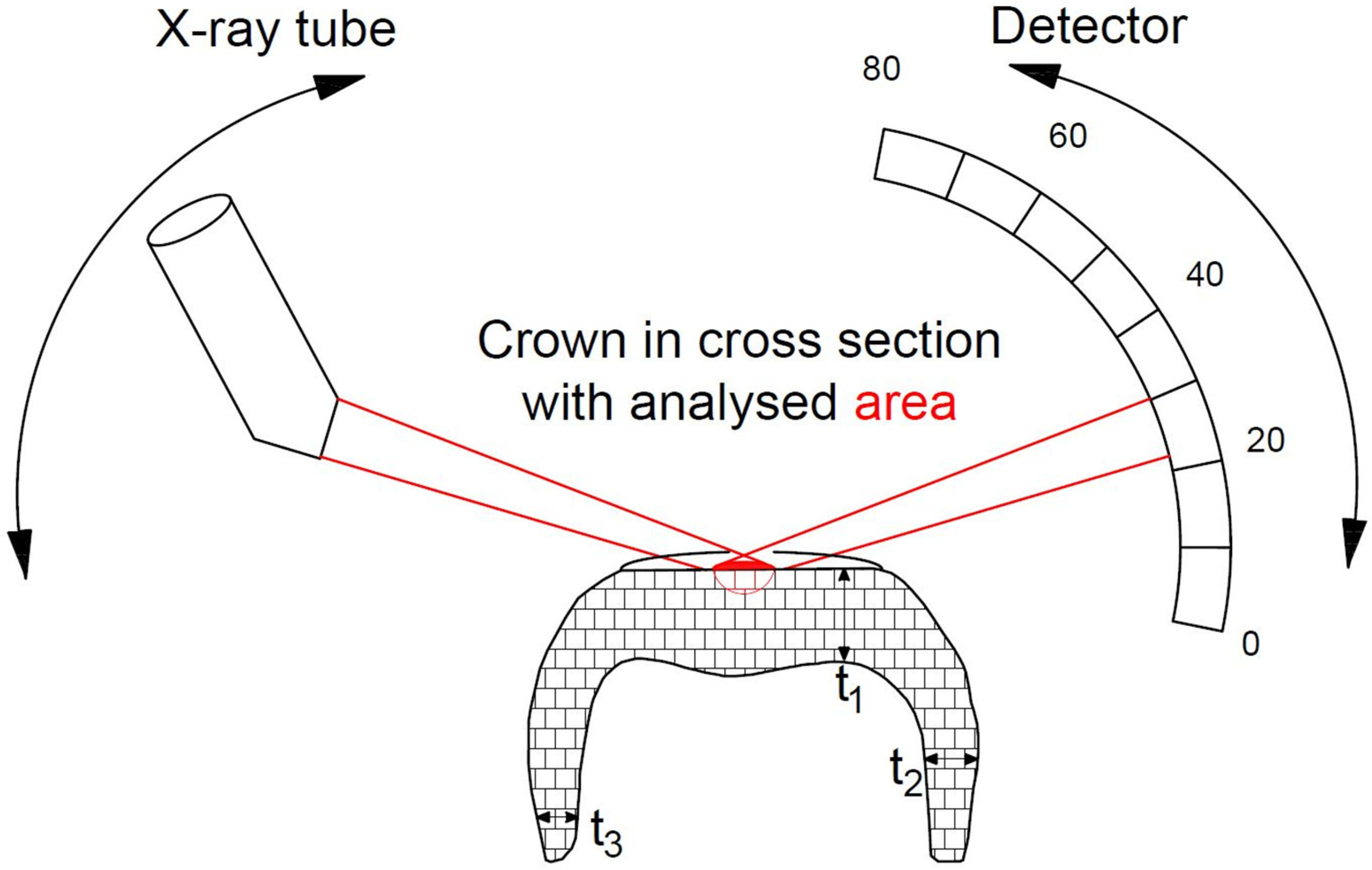
2.2.1. X-ray Diffraction
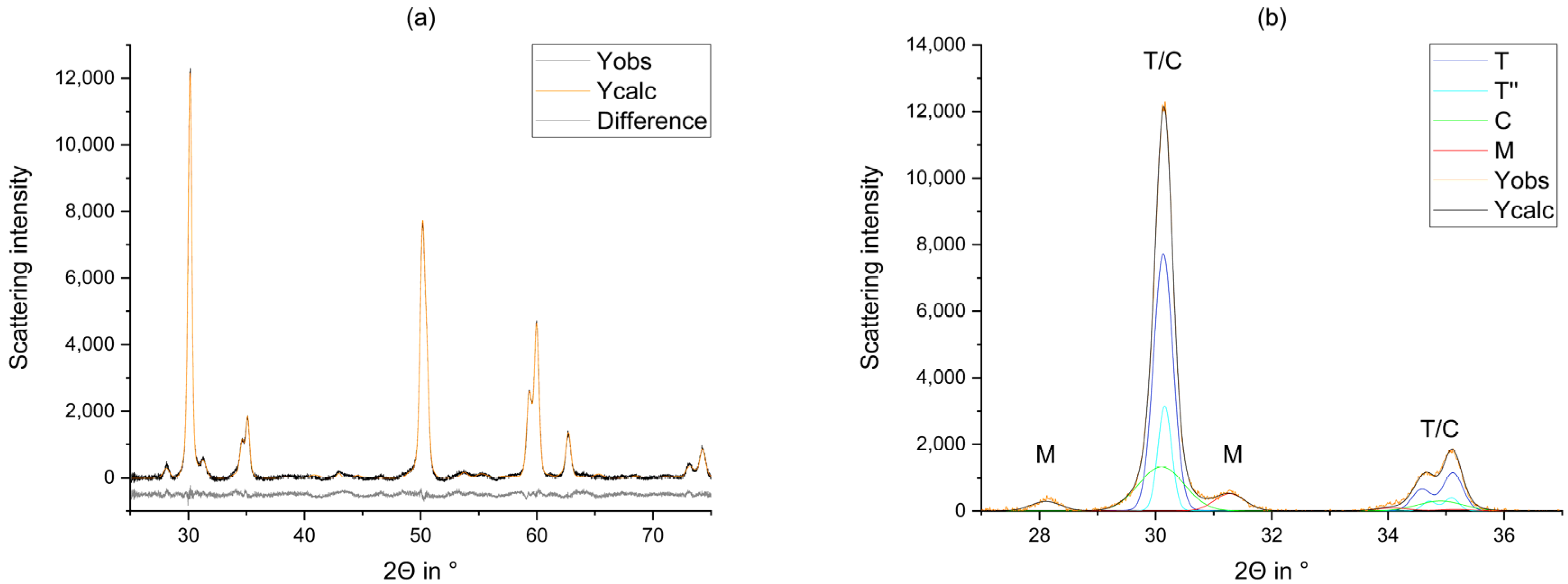
2.2.2. Rietveld Refinement
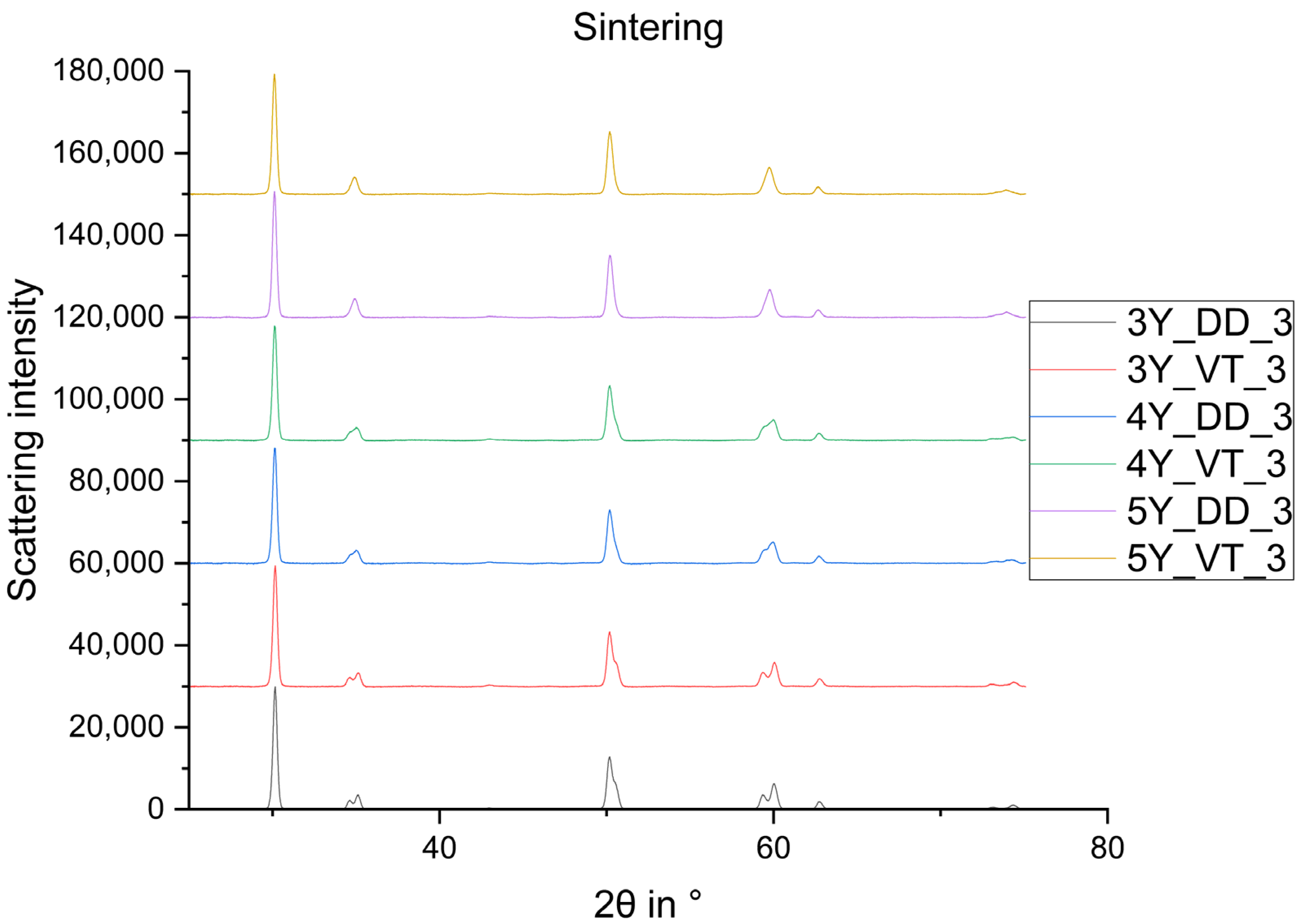
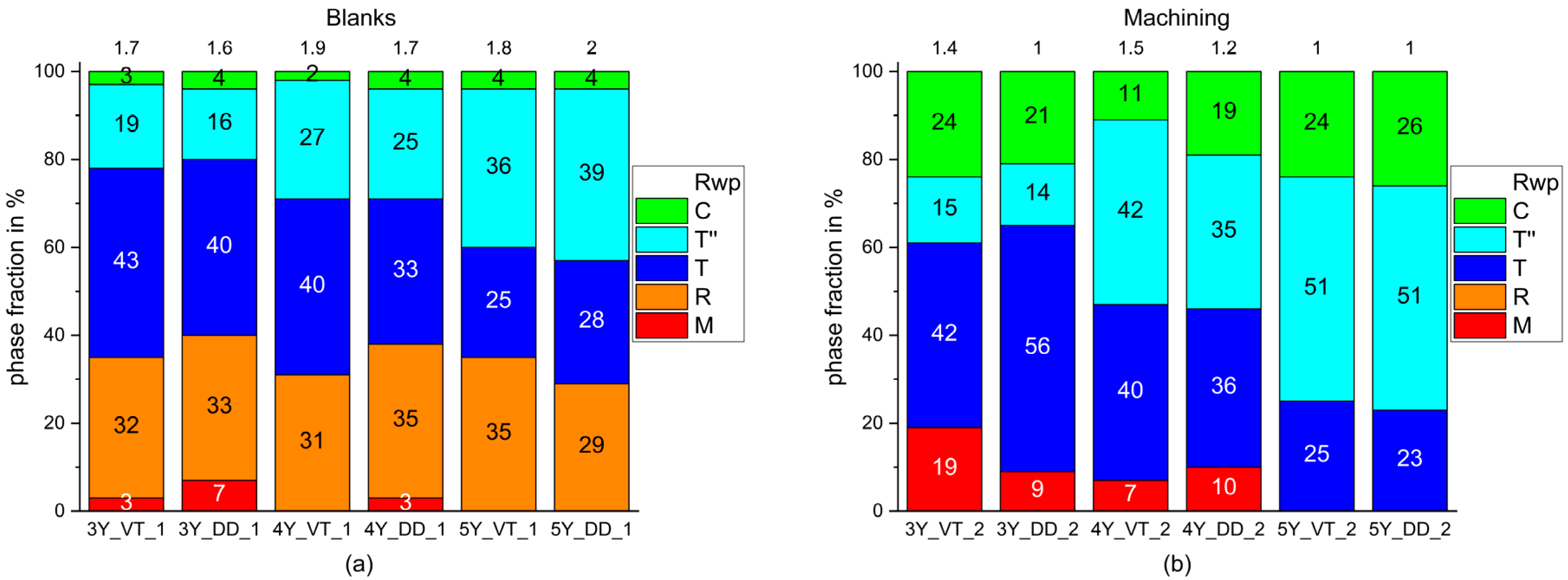
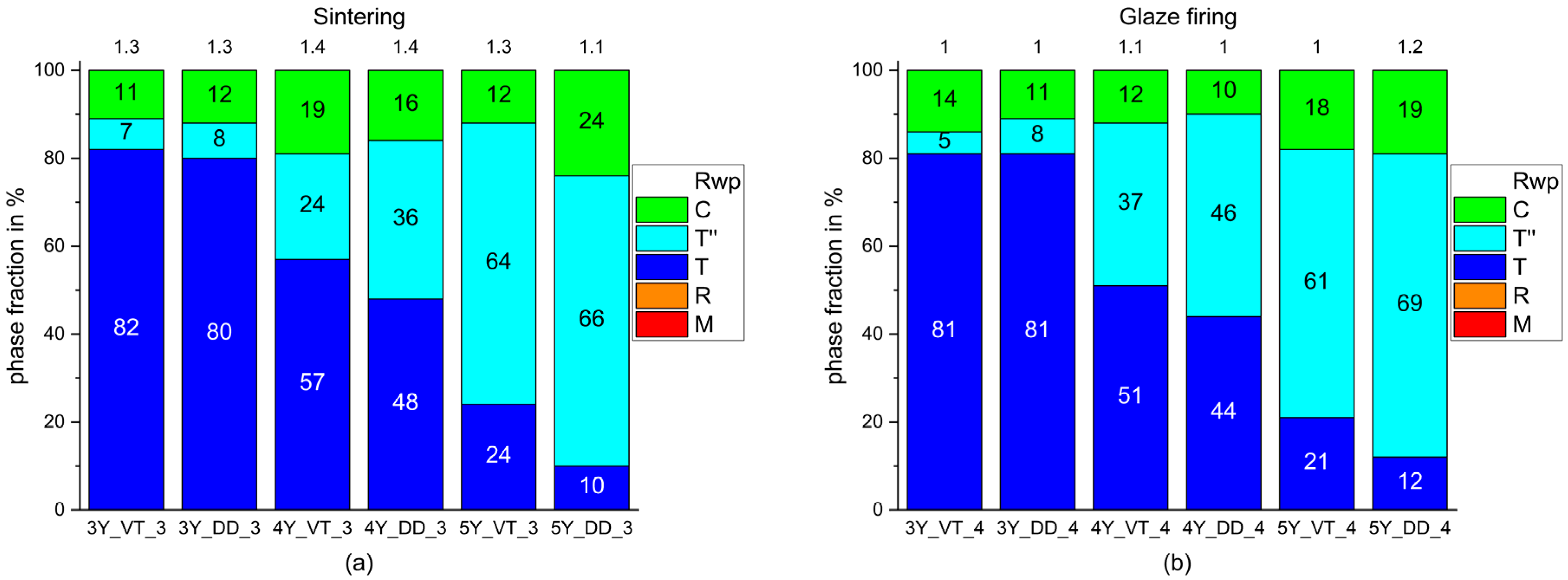
3. Results
4. Discussion
5. Conclusions
- The phase composition of the blanks feature a proportion of a rhombohedral phase fraction with very small crystallites.
- The monoclinic phase fraction increases massively after milling and diminishes with the sintering for both the 3Y- and 4Y-specimens. The cubic phase fraction simultaneously increases.
- Glaze firing and alumina-particle air-blasting cause little to no changes in phase composition. The signal to noise ratio decreases as a result of the glaze layer and increasing surface roughness.
- The tetragonal phase is highly dependent on the yttria content. 3Y-TZPs prefer the tetragonal phase T and 5Y-TZPs prefer the tetragonal phase T”. The 4Y-TZPs prefer both phases, with a slightly higher proportion of the tetragonal phase T.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Ban, S. Chemical durability of high translucent dental zirconia. Dent. Mater. J. 2020, 39, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lawn, B.R. Novel Zirconia Materials in Dentistry. J. Dent. Res. 2018, 97, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Denry, I. Stabilized zirconia as a structural ceramic: An overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef]
- Shen, J.; Xie, H.; Wu, X.; Yang, J.; Liao, M.; Chen, C. Evaluation of the effect of low-temperature degradation on the translucency and mechanical properties of ultra-transparent 5Y-TZP ceramics. Ceram. Int. 2020, 46, 553–559. [Google Scholar] [CrossRef]
- Vila-Nova, G.d.C. Effect of finishing/polishing techniques and low temperature degradation on the surface topography, phase transformation and flexural strength of ultra-translucent ZrO2 ceramic. Dent. Mater. 2020, 36, e126–e139. [Google Scholar] [CrossRef] [PubMed]
- Kolakarnprasert, N.; Kaizer, M.R.; Kim, D.K.; Zhang, Y. New multi-layered zirconias: Composition, microstructure and translucency. Dent. Mater. 2019, 35, 797–806. [Google Scholar] [CrossRef]
- Garvie, R.C. Ceramic steel? Nature 1975, 703–704. [Google Scholar] [CrossRef]
- Rosentritt, M.; Preis, V.; Behr, M.; Strasser, T. Fatigue and wear behaviour of zirconia materials. J. Mech. Behav. Biomed. Mater. 2020, 110, 103970. [Google Scholar] [CrossRef] [PubMed]
- Pecho, O.E.; Ghinea, R.; Ionescu, A.M.; La Cardona, J.d.C.; Paravina, R.D.; Pérez, M.D.M. Color and translucency of zirconia ceramics, human dentine and bovine dentine. J. Dent. 2012, 40 (Suppl. 2), e34–e40. [Google Scholar] [CrossRef]
- Mao, L.; Kaizer, M.R.; Zhao, M.; Guo, B.; Song, Y.F.; Zhang, Y. Graded Ultra-Translucent Zirconia (5Y-PSZ) for Strength and Functionalities. J. Dent. Res. 2018, 97, 1222–1228. [Google Scholar] [CrossRef]
- Atkinson, H.V.; Davies, S. Fundamental Aspects of Hot Isostatic Pressing: An Overview. Metall. Mater. Trans. A 2000, 31, 2981–3000. [Google Scholar] [CrossRef]
- Kitano, Y.; Mori, Y.; Ishitani, A.; Masaki, T. Rhombohedral Phase in Y2O3-Partially-Stabilized ZrO2. J. Amer. Ceram. Soc. 1988, 1, C34–C36. [Google Scholar] [CrossRef]
- Krogstad, J.A.; Gao, Y.; Bai, J.; Wang, J.; Lipkin, D.M.; Levi, C.G. In Situ Diffraction Study of the High-Temperature Decomposition of t′-Zirconia. J. Am. Ceram. Soc. 2015, 98, 247–254. [Google Scholar] [CrossRef]
- Kisi, E.H.; Howard, C.J. Crystal Structures of Zirconia Phases and their Inter-Relation. Key Eng. Mater. 1998, 153–154, 1–36. [Google Scholar] [CrossRef]
- Lipkin, D.M.; Krogstad, J.A.; Gao, Y.; Johnson, C.A.; Nelson, W.A.; Levi, C.G. Phase Evolution upon Aging of Air-Plasma Sprayed t′-Zirconia Coatings: I-Synchrotron X-ray Diffraction. J. Am. Ceram. Soc. 2013, 96, 290–298. [Google Scholar] [CrossRef]
- Ohtaka, O. Stability of Monoclinic and Orthorhombic Zirconia: Studies by High-Pressure Phase Equilibria and Calorimetry. J. Am. Ceram. Soc. 1991, 3, 505–509. [Google Scholar] [CrossRef]
- Kim, D.-J.; Jung, H.-J.; Kim, H.-J. t → r phase transformation of tetragonal zirconia alloys by grinding. Mat. Sci. Lett. 1995, 14, 285–288. [Google Scholar] [CrossRef]
- Juškevičius, K.; Audronis, M.; Subačius, A.; Drazdys, R.; Juškėnas, R.; Matthews, A.; Leyland, A. High-rate reactive magnetron sputtering of zirconia films for laser optics applications. Appl. Phys. A 2014, 116, 1229–1240. [Google Scholar] [CrossRef]
- Yoshida, K. Influence of alumina air-abrasion for highly translucent partially stabilized zirconia on flexural strength, surface properties, and bond strength of resin cement. J. Appl. Oral Sci. 2020, 28, e20190371. [Google Scholar] [CrossRef]
- Tobias, K. Einfluss Externer Spannungen auf Phasenumwandlungen in Tetragonalem Zirconiumdioxid. Ph.D Thesis, Eberhard Karls Universität, Tübingen, Germany, 2017. [Google Scholar]
- Li, P.; Chen, I.W.; Penner-Hahn, J.E. Effect of Dopants on Zirconia Stabilization—An X-ray Absorption Study: II, Tetravalent Dopants. J. Am. Ceram. Soc. 1994, 77, 1281–1288. [Google Scholar] [CrossRef]
- Luthardt, R.G.; Holzhüter, M.S.; Rudolph, H.; Herold, V.; Walter, M.H. CAD/CAM-machining effects on Y-TZP zirconia. Dent. Mater. 2004, 20, 655–662. [Google Scholar] [CrossRef]
- Alao, A.-R.; Stoll, R.; Song, X.-F.; Miyazaki, T.; Hotta, Y.; Shibata, Y.; Yin, L. Surface quality of yttria-stabilized tetragonal zirconia polycrystal in CAD/CAM milling, sintering, polishing and sandblasting processes. J. Mech. Behav. Biomed. Mater. 2017, 65, 102–116. [Google Scholar] [CrossRef]
- Lawson, N.C.; Maharishi, A. Strength and translucency of zirconia after high-speed sintering. J. Esthet. Restor. Dent. 2020, 32, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Jerman, E.; Wiedenmann, F.; Eichberger, M.; Reichert, A.; Stawarczyk, B. Effect of high-speed sintering on the flexural strength of hydrothermal and thermo-mechanically aged zirconia materials. Dent. Mater. 2020, 36, 1144–1150. [Google Scholar] [CrossRef]
- Amat, N.F.; Raji, S.L.; Muchtar, A.; Amril, M.S.; Ghazali, M.J.; Yahaya, N. Influence of Presintering Parameters on the Mechanical Properties of Presintered Dental Zirconia Block. IJIE 2018, 10. [Google Scholar] [CrossRef]
- Hatanaka, G.R.; Polli, G.S.; Fais, L.M.G.; Reis, J.M.D.S.N.; Pinelli, L.A.P. Zirconia changes after grinding and regeneration firing. J. Prosthet. Dent. 2017, 118, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-K. Effect of A Rapid-Cooling Protocol on the Optical and Mechanical Properties of Dental Monolithic Zirconia Containing 3–5 mol% Y2O3. Materials 2020, 13, 1923. [Google Scholar] [CrossRef] [Green Version]
- Kumchai, H.; Juntavee, P.; Sun, A.F.; Nathanson, D. Effect of Glazing on Flexural Strength of Full-Contour Zirconia. Int. J. Dent. 2018, 2018, 8793481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guazzato, M.; Quach, L.; Albakry, M.; Swain, M.V. Influence of surface and heat treatments on the flexural strength of Y-TZP dental ceramic. J. Dent. 2005, 33, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Keuper, M.; Berthold, C.; Nickel, K.G. Long-time aging in 3 mol.% yttria-stabilized tetragonal zirconia polycrystals at human body temperature. Acta Biomater. 2014, 10, 951–959. [Google Scholar] [CrossRef]
- Garvie, R.C.; Nicholson, P.S. Phase Analysis in Zirconia Systems. J. Amer. Ceram. Soc. 1972, 6, 303–305. [Google Scholar] [CrossRef]
- Toraya, H. Calibration Curve for Quantitative Analysis of the Monoclinic-Tetragonal ZrO2 System by X-ray Diffraction. J. Amer. Ceram. Soc. 1984, 6, C119–C121. [Google Scholar]
- Wertz, M.; Fuchs, F.; Hoelzig, H.; Wertz, J.M.; Kloess, G.; Hahnel, S.; Rosentritt, M.; Koenig, A. The Influence of Surface Preparation, Chewing Simulation, and Thermal Cycling on the Phase Composition of Dental Zirconia. Materials 2021, 14, 2133. [Google Scholar] [CrossRef]
- Howard, C.J.; Hill, R.J. The polymorphs of zirconia: Phase abundance and crystal structure by Rietveld analysis of neutron and X-ray diffraction data. J. Mater. Sci. 1991, 26, 127–134. [Google Scholar] [CrossRef]
- Yashima, M.; Sasaki, S.; Kakihana, M.; Yamaguchi, Y.; Arashi, H.; Yoshimura, M. Oxygen-induced structural change of the tetragonal phase around the tetragonal–cubic phase boundary in ZrO2–YO1.5 solid solutions. Acta Crystallogr. B Struct. Sci. 1995, 51, 381. [Google Scholar] [CrossRef]
- Krogstad, J.A.; Lepple, M.; Gao, Y.; Lipkin, D.M.; Levi, C.G. Effect of Yttria Content on the Zirconia Unit Cell Parameters. J. Am. Ceram. Soc. 2011, 94, 4548–4555. [Google Scholar] [CrossRef]
- Pitschke, W.; Hermann, H.; Mattern, N. The influence of surface roughness on diffracted X-ray intensities in Bragg–Brentano geometry and its effect on the structure determination by means of Rietveld analysis. Powder Diffr. 1993, 8, 74–83. [Google Scholar] [CrossRef]
- Dollase, W.A. Correction of intensities for preferred orientation in powder diffractometry: Application of the March model. J. Appl. Crystallogr. 1986, 19, 267–272. [Google Scholar] [CrossRef]
- March, A. Mathematische Theorie der Regelung nach der Korngestah bei affiner Deformation. Z. Krist. 1932, 81, 285–297. [Google Scholar] [CrossRef]
- Gibson, I.R.; Irvine, J.T.S. Qualitative X-ray Diffraction Analysis of Metastable Tetragonal (t′) Zirconia. J. Am. Ceram. Soc. 2001, 84, 615–618. [Google Scholar] [CrossRef]
- Bučevac, D.; Kosmač, T.; Kocjan, A. The influence of yttrium-segregation-dependent phase partitioning and residual stresses on the aging and fracture behaviour of 3Y-TZP ceramics. Acta Biomater. 2017, 62, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.; Barloi, A.; Yang, B. Surface conditioning influences zirconia ceramic bonding. J. Dent. Res. 2009, 88, 817–822. [Google Scholar] [CrossRef]
- Eichler, J.; Eisele, U.; Rödel, J. Mechanical Properties of Monoclinic Zirconia. J. Am. Ceram. Soc. 2004, 87, 1401–1403. [Google Scholar] [CrossRef]
- Shukla, S.; Seal, S. Mechanisms of room temperature metastable tetragonal phase stabilisation in zirconia. Int. Mater. Rev 2005, 50, 45–64. [Google Scholar] [CrossRef]
- Lughi, V.; Sergo, V. Low temperature degradation -aging- of zirconia: A critical review of the relevant aspects in dentistry. Dent. Mater. 2010, 26, 807–820. [Google Scholar] [CrossRef]
- Edalati, K.; Toh, S.; Ikoma, Y.; Horita, Z. Plastic deformation and allotropic phase transformations in zirconia ceramics during high-pressure torsion. Scr. Mater. 2011, 65, 974–977. [Google Scholar] [CrossRef]


| Abbreviation | Product | Manufacturer | Yttria Content (mol%) | Flexural Strength (MPa) 1 |
|---|---|---|---|---|
| 3Y_VT | VITA YZ HT | VT | 3 | 1200 |
| 3Y_DD | DD Bio ZX2 | DD | 3 | 1250 |
| 4Y_VT | VITA YZ ST | VT | 4 | >850 |
| 4Y_DD | DD cube ONE | DD | 4 | >1250 |
| 5Y_VT | VITA YZ XT | VT | 5 | >600 |
| 5Y_DD | DD cubeX2 | DD | 5 | >750 |
| Dental Direct | Sintering Program |
| 1 | Heating up to 900 °C with 8 K/min |
| 2 | Dwell at 900 °C for 30 min |
| 3 | Heating up to 1450 °C (1530 °C for DD cube ONE (4Y_DD)) |
| 4 | Dwell at 1450 °C (1530 °C) for 120 min |
| 5 | Cooling to 200 °C with 10 K/min |
| VITA | Sintering Program |
| 1 | Heating up to 1430 °C (1530 °C for VT YZ ST (4Y_VT)) |
| with 17 K/min for VT YZ HT (3Y_VT)), with 8 K/min for VT YZ ST (4Y_VT)) or with 4 K/min for VT YZ XT (5Y_VT)) | |
| 2 | Dwell at 1450 °C (1530 °C for VT YZ ST (4Y_VT)) for 120 min |
| 3 | Cooling to 200 °C |
| Glaze Firing Program | |
|---|---|
| 1 | Heating up to 500 °C |
| 2 | Heating up to 900 °C with 80 K/min |
| 3 | Dwell at 900 °C for 1 min |
| 4 | Cooling to 600 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wertz, M.; Hoelzig, H.; Kloess, G.; Hahnel, S.; Koenig, A. Influence of Manufacturing Regimes on the Phase Transformation of Dental Zirconia. Materials 2021, 14, 4980. https://doi.org/10.3390/ma14174980
Wertz M, Hoelzig H, Kloess G, Hahnel S, Koenig A. Influence of Manufacturing Regimes on the Phase Transformation of Dental Zirconia. Materials. 2021; 14(17):4980. https://doi.org/10.3390/ma14174980
Chicago/Turabian StyleWertz, Markus, Hieronymus Hoelzig, Gert Kloess, Sebastian Hahnel, and Andreas Koenig. 2021. "Influence of Manufacturing Regimes on the Phase Transformation of Dental Zirconia" Materials 14, no. 17: 4980. https://doi.org/10.3390/ma14174980
APA StyleWertz, M., Hoelzig, H., Kloess, G., Hahnel, S., & Koenig, A. (2021). Influence of Manufacturing Regimes on the Phase Transformation of Dental Zirconia. Materials, 14(17), 4980. https://doi.org/10.3390/ma14174980







