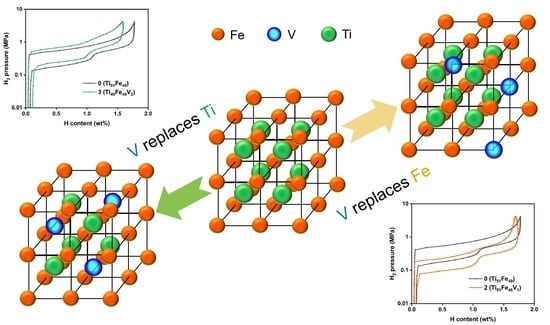
Design of V-Substituted TiFe-Based Alloy for Target Pressure Range and Easy Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Synthesis
2.2. Structure and Chemical Composition Analysis
2.3. Hydrogen Sorption Property
3. Results and Discussion
3.1. Alloy Design and Phase Study
3.2. Activation and PCI Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reilly, J.J.; Wiswall, R.H. Formation and properties of iron titanium hydride. Inorg. Chem. 1974, 13, 218–222. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, Y.S.; Suh, J.Y.; Huh, J.Y.; Cho, Y.W. Tailoring the equilibrium hydrogen pressure of TiFe via vanadium substitution. J. Alloys Compd. 2021, 854, 157263–157272. [Google Scholar] [CrossRef]
- Lee, S.M.; Perng, T.P. Correlation of substitutional solid solution with hydrogenation properties of TiFe1-xMx (M = Ni, Co, Al) alloys. J. Alloys Compd. 1999, 291, 254–261. [Google Scholar] [CrossRef]
- Bououdina, M.; Fruchart, D.; Jacquet, S.; Pontonnier, L.; Soubeyroux, J.L. Effect of nickel alloying by using ball milling on the hydrogen absorption properties of TiFe. Int. J. Hydrogen Energy 1999, 24, 885–890. [Google Scholar] [CrossRef]
- Lee, S.M.; Perng, T.P. Effect of the second phase on the initiation of hydrogenation of TiFe1−xMx (M = Cr, Mn) alloys. Int. J. Hydrogen Energy 1994, 19, 259–263. [Google Scholar] [CrossRef]
- Oguro, K.; Osumi, Y.; Suzuki, H.; Kato, A.; Imamura, Y.; Tanaka, H. Hydrogen storage properties of TiFe1−xNiyMz alloys. J. Less-Common. Met. 1983, 89, 275–279. [Google Scholar] [CrossRef]
- Qu, H.; Du, J.; Pu, C.; Niu, Y.; Huang, T.; Li, Z.; Lou, Y.; Wu, Z. Effects of Co introduction on hydrogen storage properties of Ti–Fe–Mn alloys. Int. J. Hydrogen Energy 2015, 40, 2729–2735. [Google Scholar] [CrossRef]
- Patel, A.K.; Duguay, A.; Tougas, B.; Neumann, B.; Schade, C.; Sharma, P.; Huot, J. Study of the Microstructural and First Hydrogenation Properties of TiFe Alloy with Zr, Mn and V as Additives. Processes 2021, 9, 1217–1228. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Dreistadt, D.M.; Capurso, G.; Jepsen, J.; Cuevas, F.; Latroche, M. Fundamental hydrogen storage properties of TiFe-alloy with partial substitution of Fe by Ti and Mn. J. Alloys Compd. 2021, 874, 159925–159936. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Berti, N.; Cuevas, F.; Latroche, M.; Baricco, M. Substitutional effects in TiFe for hydrogen storage: A comprehensive review. Mater. Adv. 2021, 2, 2524–2560. [Google Scholar] [CrossRef]
- Zeng, L.; Xu, G.; Liu, L.; Bai, W.; Zhang, L. Experimental investigation of phase equilibria in the Ti-Fe-Zr system. Calphad 2018, 61, 20–32. [Google Scholar] [CrossRef]
- Gosselin, C.; Santos, D.; Huot, J. First hydrogenation enhancement in TiFe alloys for hydrogen storage. J. Phys. D Appl. Phys 2017, 50, 375303–375308. [Google Scholar] [CrossRef]
- Lv, P.; Huot, J. Hydrogen storage properties of Ti0.95FeZr0.05, TiFe0.95Zr0.05 and TiFeZr0.05 alloys. Int. J. Hydrogen Energy 2016, 41, 22128–22133. [Google Scholar] [CrossRef]
- Gosselin, C.; Huot, J. Hydrogenation Properties of TiFe Doped with Zirconium. Materials 2015, 8, 7864–7872. [Google Scholar] [CrossRef] [Green Version]
- Jain, P.; Gosselin, C.; Huot, J. Effect of Zr, Ni and Zr7Ni10 alloy on hydrogen storage characteristics of TiFe alloy. Int. J. Hydrogen Energy 2015, 40, 16921–16927. [Google Scholar] [CrossRef]
- Lv, P.; Huot, J. Hydrogenation improvement of TiFe by adding ZrMn2. Energy 2017, 138, 375–382. [Google Scholar] [CrossRef]
- Faisal, M.; Suh, J.Y.; Lee, Y.S. Understanding first cycle hydrogenation properties of Ti–Fe–Zr ternary alloys. Int. J. Hydrogen Energy 2021, 46, 4241–4251. [Google Scholar] [CrossRef]
- Kim, H.; Faisal, M.; Lee, S.I.; Jung, J.Y.; Kim, H.J.; Hong, J.; Lee, Y.S.; Shim, J.H.; Cho, Y.W.; Kim, D.H.; et al. Activation of Ti–Fe–Cr alloys containing identical AB2 fractions. Alloy. Compd. 2021, 864, 158876–158883. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.I.; Faisal, M.; Kim, H.; Lee, Y.S.; Suh, J.Y.; Shim, J.H.; Huh, J.Y.; Cho, Y.W. Effect of Cr addition on room temperature hydrogenation of TiFe alloys. Int. J. Hydrogen Energy 2021, 46, 19478–19485. [Google Scholar] [CrossRef]
- Park, K.B.; Ko, W.S.; Fadonougbo, J.O.; Na, T.W.; Im, H.T.; Park, J.Y.; Kang, J.W.; Kang, H.S.; Park, C.S.; Park, H.K. Effect of Fe substitution by Mn and Cr on first hydrogenation kinetics of air-exposed TiFe-based hydrogen storage alloy. Mater. Charact. 2021, 178, 111246–111251. [Google Scholar] [CrossRef]
- Bronca, V.; Bergman, P.; Ghaemmaghami, V.; Khatamian, D.; Manchester, F.D. Hydrogen absorption characteristics of an FeTi + misch metal alloy. J Less-Common Met. 1985, 108, 313–325. [Google Scholar] [CrossRef]
- Wang, X.; Chen, R.; Chen, C.; Wang, Q. Hydrogen storage properties of TixFe + y wt.% La and its use in metal hydride hydrogen compressor. J. Alloys Compd. 2006, 425, 291–295. [Google Scholar] [CrossRef]
- Singh, B.K.; Singh, A.K.; Srivastava, O.N. Improved hydrogen sorption characteristics in FeTi1 + xMm material. Int. J. Hydrogen Energy 1996, 21, 111–117. [Google Scholar] [CrossRef]
- Ma, J.; Pan, H.; Wang, X.; Chen, C.; Wang, Q. Hydrogen storage properties of FeTi1.3+x wt%Mm (x=0.0, 1.5, 3.0, 4.5, 6.0) hydrogen storage alloys. Int. J. Hydrogen Energy 2000, 25, 779–782. [Google Scholar] [CrossRef]
- Leng, H.; Yu, Z.; Yin, J.; Li, Q.; Wu, Z.; Chou, K.-C. Effects of Ce on the hydrogen storage properties of TiFe0.9Mn0.1 alloy. Int. J. Hydrogen Energy 2017, 42, 23731–23736. [Google Scholar] [CrossRef]
- Nagai, H.; Kitagaki, K.; Shoji, K. Microstructure and hydriding characteristics of FeTi alloys containing manganese. J. Less-Common Met. 1987, 134, 275–286. [Google Scholar] [CrossRef]
- Liu, X.P.; Cuevas, F.; Jiang, L.J.; Latroche, M.; Li, Z.N.; Wang, S.M. Improvement of the hydrogen storage properties of Ti–Cr–V–Fe BCC alloy by Ce addition. J. Alloys Compd. 2009, 476, 403–407. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, Y.; Liang, H.; Zhou, X.; Wu, C.; Tao, M. Effect of Ce on the structure and hydrogen storage properties of V55Ti22.5Cr16.1Fe6.4. J. Alloys Compd. 2007, 429, 301–305. [Google Scholar] [CrossRef]
- Bruzzone, G.; Costa, G.; Ferretti, M.; Olcese, G.L. Hydrogen storage in a beryllium substituted TiFe compound. Int. J. Hydrogen Energy 1980, 5, 317–322. [Google Scholar] [CrossRef]
- Yang, T.; Wang, P.; Xia, C.; Liu, N.; Liang, C.; Yin, F.; Li, Q. Effect of chromium, manganese and yttrium on microstructure and hydrogen storage properties of TiFe-based alloy. Int. J. Hydrogen Energy 2020, 45, 12071–12081. [Google Scholar] [CrossRef]
- Zadorozhnyy, V.Y.; Klyamkin, S.N.; Zadorozhnyy, M.Y.; Bermesheva, O.V.; Kaloshkin, S.D. Mechanical alloying of nanocrystalline intermetallic compound TiFe doped by aluminum and chromium. J. Alloys Compd. 2014, 586, S56–S60. [Google Scholar] [CrossRef]
- Berdonosova, E.A.; Zadorozhnyy, V.Y.; Zadorozhnyy, M.Y.; Geodakian, K.V.; Zheleznyi, M.V.; Tsarkov, A.A.; Kaloshkin, S.D.; Klyamkin, S.N. Hydrogen storage properties of TiFe-based ternary mechanical alloys with cobalt and niobium. A thermochemical approach. Int. J. Hydrogen Energy 2019, 44, 29159–29165. [Google Scholar] [CrossRef]
- Johnson, J.R.; Reilly, J.J. The Use of Manganese Substituted Ferrotitanium Alloys for Energy Storage. In Proceedings of the International Conference on Alternative Energy Sources, Miami Beach, FL, USA, 5–7 December 1977. [Google Scholar]
- Mintz, M.H.; Vaknin, S.; Biderman, S.; Hadari, Z. Hydrides of ternary TiFexM1−x (M=Cr, Mn, Co, Ni) intermetallics. J. Appl. Phys 1981, 52, 463–467. [Google Scholar] [CrossRef]
- Liu, J.; Lundin, C.E. Alloys for Hydrogen Storage. U.S. Patent 4358316, 1982. [Google Scholar]
- Mitrokhin, S.V.; Verbetsky, V.N.; Kajumov, R.R.; Cunmao, H.; Yufen, Z. Hydrogen sorption peculiarities in FeTi-type Ti-Fe-V-Mn alloys. J. Alloys Compd. 1993, 199, 155–160. [Google Scholar] [CrossRef]
- Massicot, B. Étude du Système Fe–Ti–V et de ses Applications au Stockage de Lߣhydrogène. Ph.D. Thesis, Université Paris-Est, Thiais, France, 2009. [Google Scholar]
- Massicot, B.; Joubert, J.M.; Latroche, M. Phase equilibria in the Fe–Ti–V system. Int. J. Mat. Res. 2010, 101, 1414–1423. [Google Scholar] [CrossRef]
- TOPAS Version 5; Bruker AXS GmbH: Karlsruhe, Germany, 2014.
- Ko, W.S.; Park, K.B.; Park, H.K. Density functional theory study on the role of ternary alloying elements in TiFe-based hydrogen storage alloys. J. Mater. Sci. Technol. 2021, 92, 148–158. [Google Scholar] [CrossRef]
- Wenzl, H.; Lebsanft, E. Phase diagram and thermodynamic parameters of the quasibinary interstitial alloy Fe0.5Ti0.5Hx in equilibrium with hydrogen gas. J. Phys. F Met. Phys 1980, 10, 2147–2156. [Google Scholar] [CrossRef]
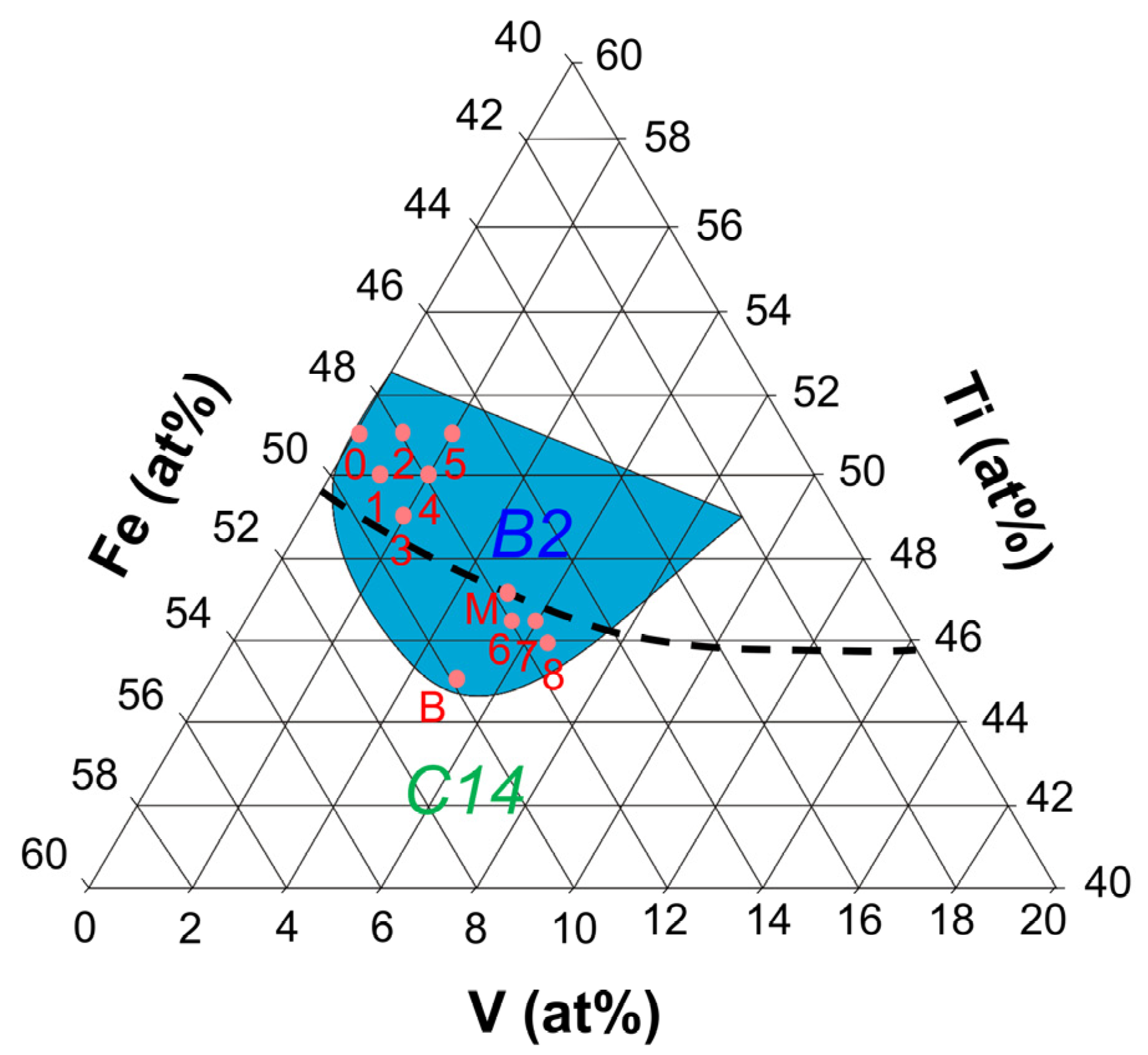

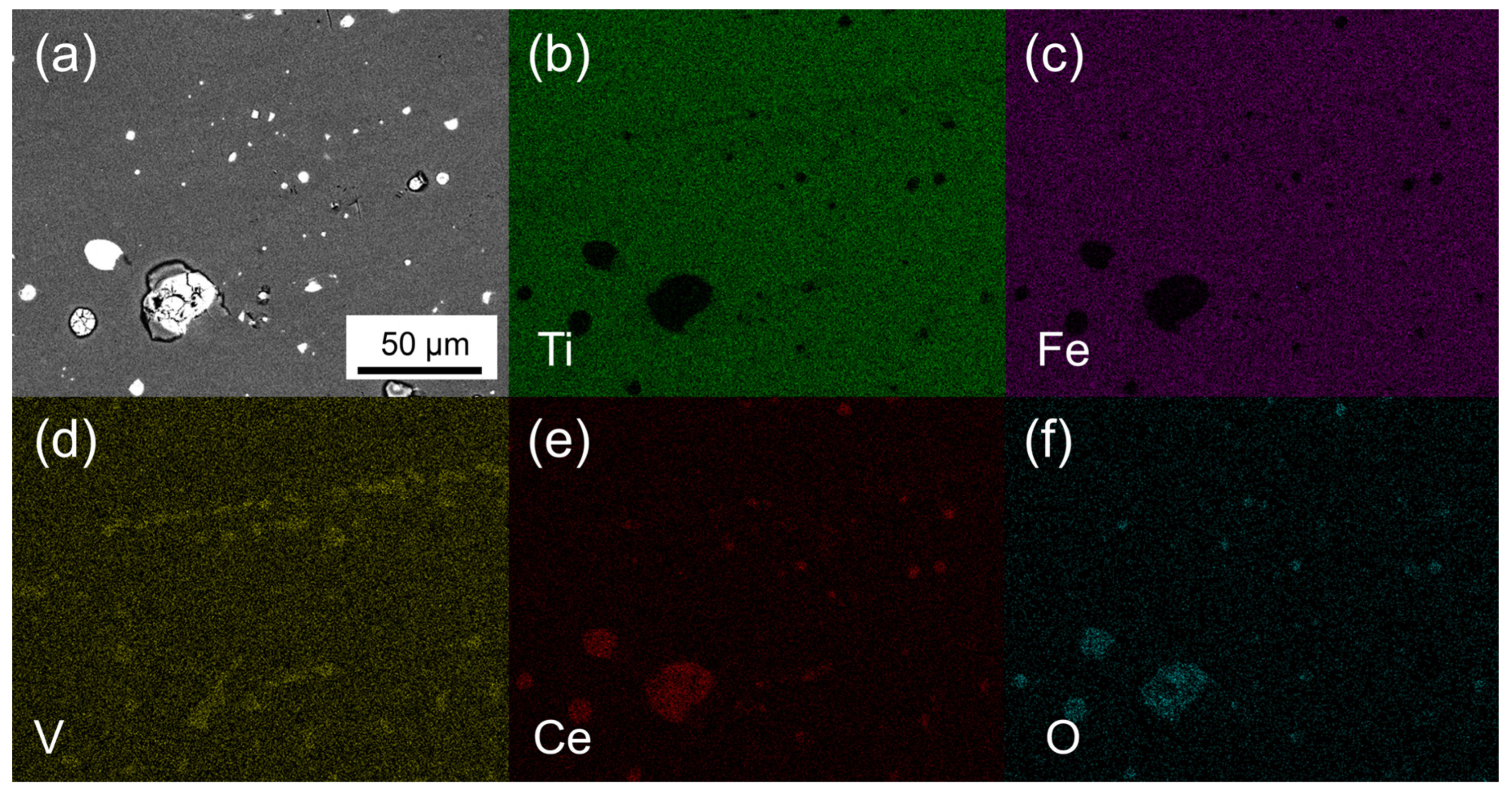
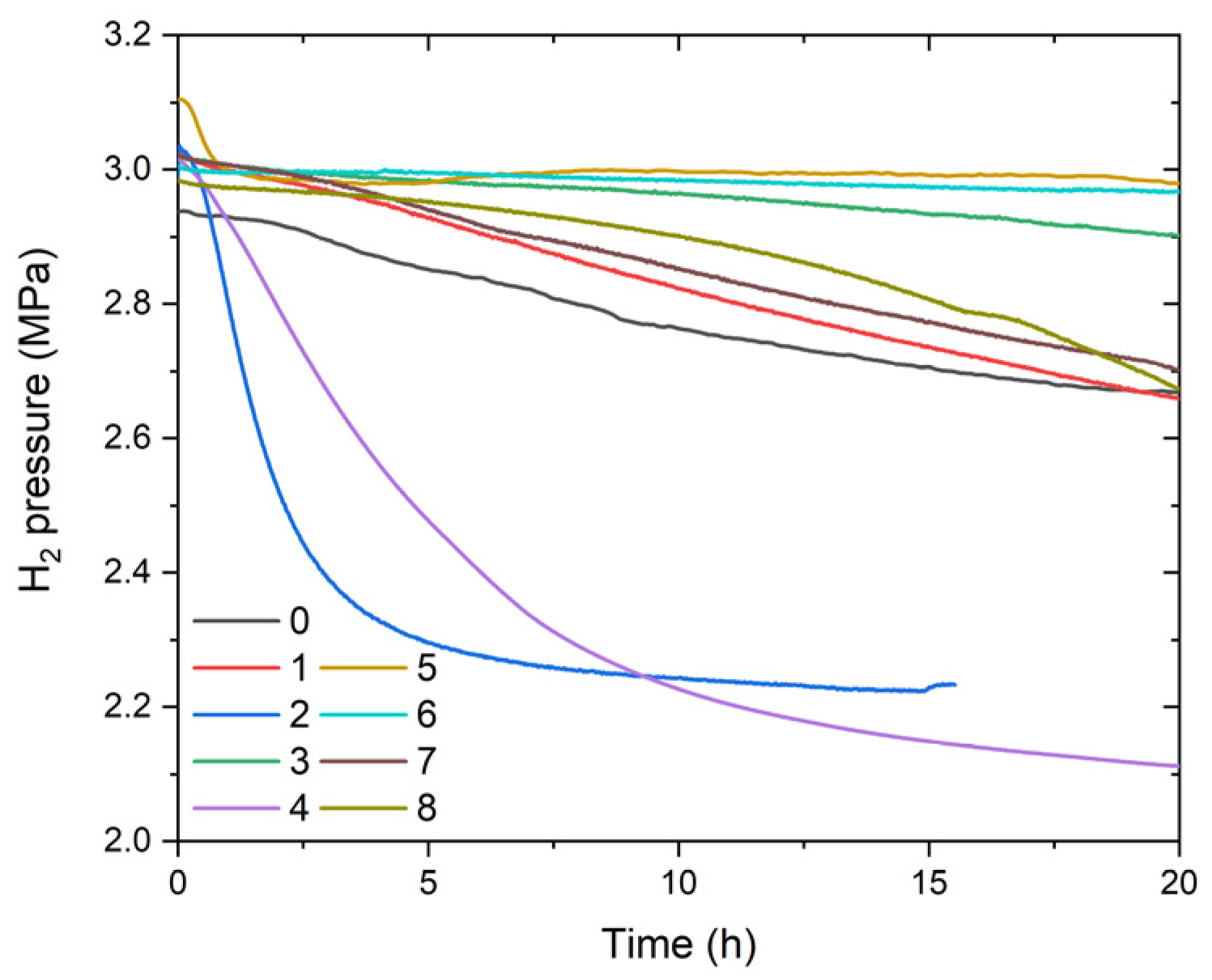
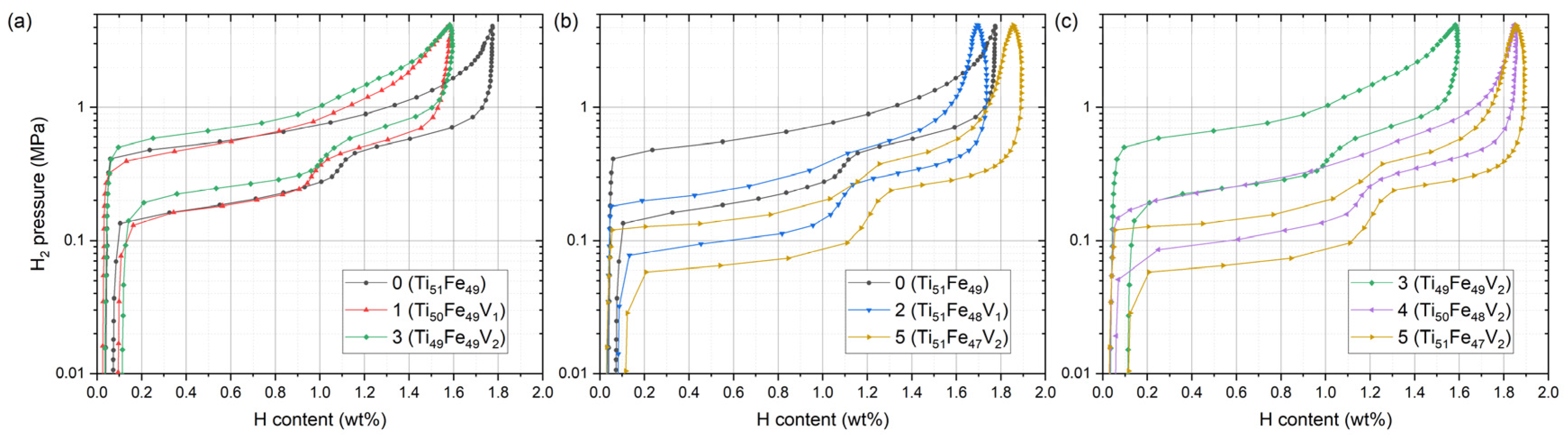


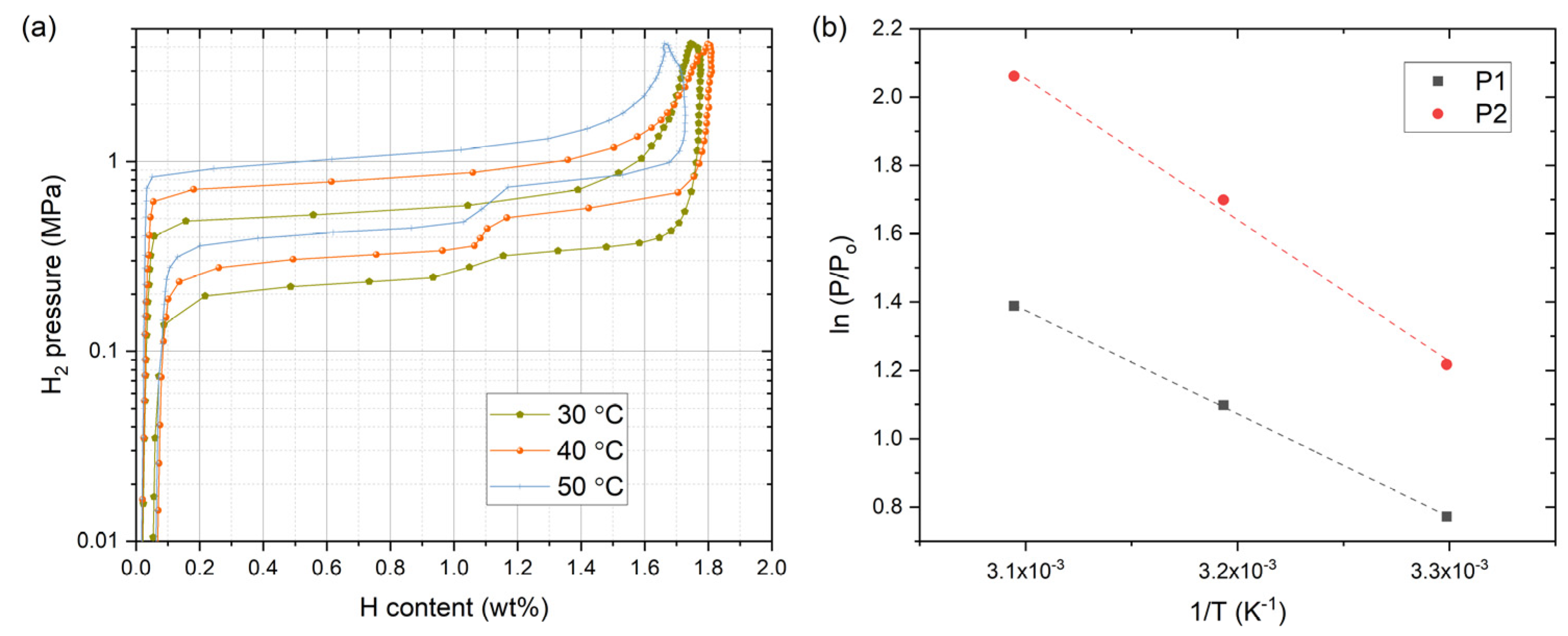
| Sample ID | Nominal Composition | Ti Sublattice | Fe Sublattice |
|---|---|---|---|
| 0 | Ti51Fe49 | Ti50 | Fe49Ti1 |
| 1 | Ti50Fe49V1 | Ti50 | Fe49V1 |
| 2 | Ti51Fe48V1 | Ti50 | Fe48Ti1V1 |
| 3 | Ti49Fe49V2 | Ti49V1 | Fe49V1 |
| 4 | Ti50Fe48V2 | Ti50 | Fe48V2 |
| 5 | Ti51Fe47V2 | Ti50 | Fe47Ti1V2 |
| 6 | Ti46.5Fe48V5.5 | Ti46.5V3.5 | Fe48V2 |
| 7 | Ti46.5Fe47.5V6 | Ti46.5V3.5 | Fe47.5V2.5 |
| 8 | Ti46Fe47.5V6.5 | Ti46V4 | Fe47.5V2.5 |
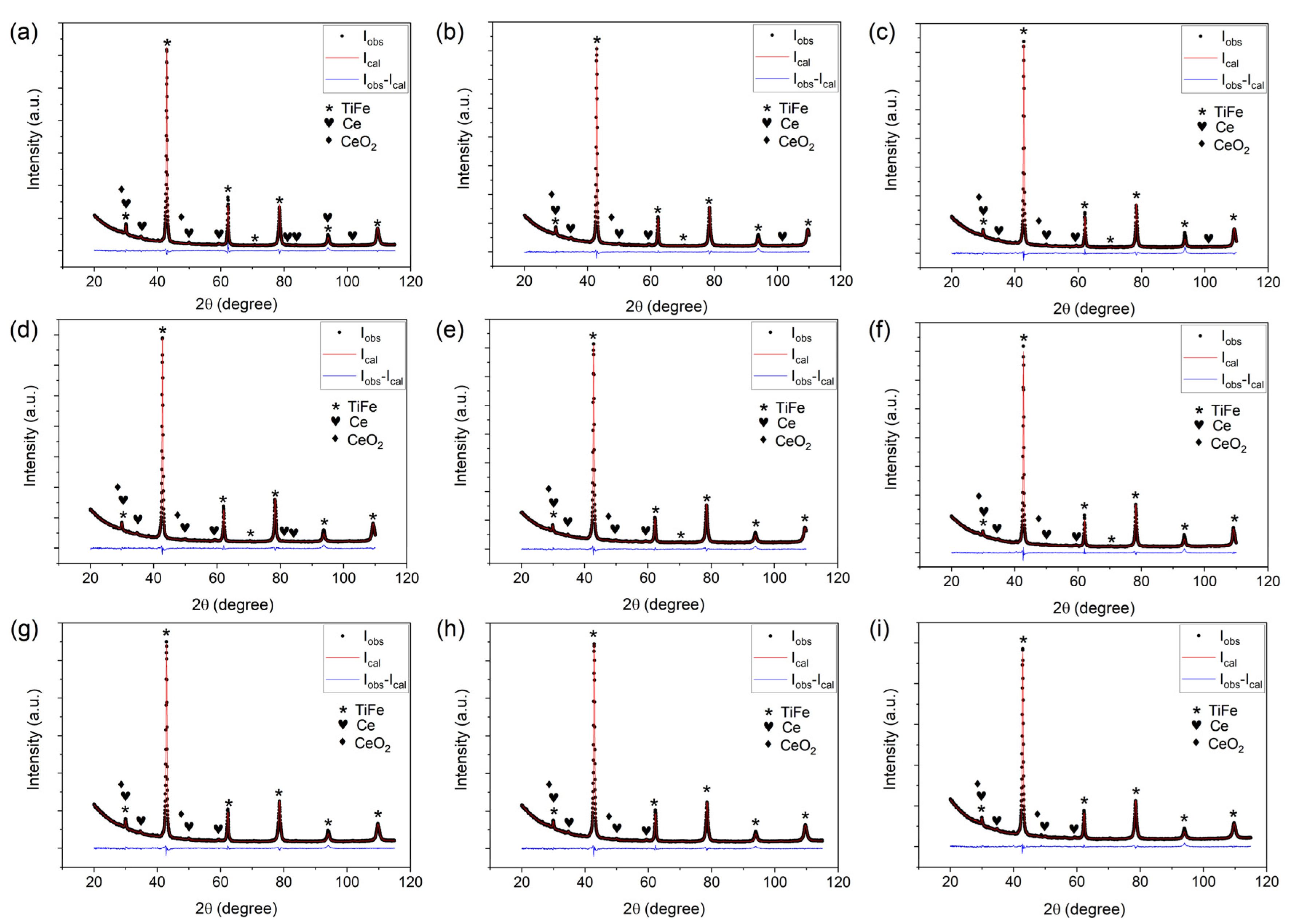
| Sample ID | Nominal Composition | Lattice Parameter (Å) | Phase Fraction (wt.%) | Rwp (%) | χ2 | ||
|---|---|---|---|---|---|---|---|
| TiFe | Ce | CeO2 | |||||
| 0 | Ti51Fe49 | 2.98327 (5) | 96.9 (2) | 1.3 (1) | 1.8 (2) | 3.52 | 2.83 |
| 1 | Ti50Fe49V1 | 2.98241 (7) | 97.2 (2) | 1.4 (1) | 1.4 (2) | 3.41 | 2.15 |
| 2 | Ti51Fe48V1 | 2.98764 (6) | 97.2 (3) | 1.1 (1) | 1.7 (3) | 4.07 | 2.57 |
| 3 | Ti49Fe49V2 | 2.98058 (7) | 97.5 (2) | 0.9 (1) | 1.6 (1) | 3.09 | 2.00 |
| 4 | Ti50Fe48V2 | 2.98690 (6) | 96.8 (3) | 1.3 (1) | 1.9 (3) | 3.28 | 2.10 |
| 5 | Ti51Fe47V2 | 2.99072 (6) | 97.5 (2) | 1.0 (1) | 1.5 (1) | 3.54 | 2.21 |
| 6 | Ti46.5Fe48V5.5 | 2.98008 (6) | 96.7 (2) | 1.2 (1) | 2.1 (2) | 3.29 | 1.93 |
| 7 | Ti46.5Fe47.5V6 | 2.98179 (6) | 96.9 (2) | 1.0 (1) | 2.1 (2) | 3.04 | 1.79 |
| 8 | Ti46Fe47.5V6.5 | 2.98122 (7) | 96.5 (2) | 1.1 (1) | 2.4 (2) | 3.26 | 1.89 |
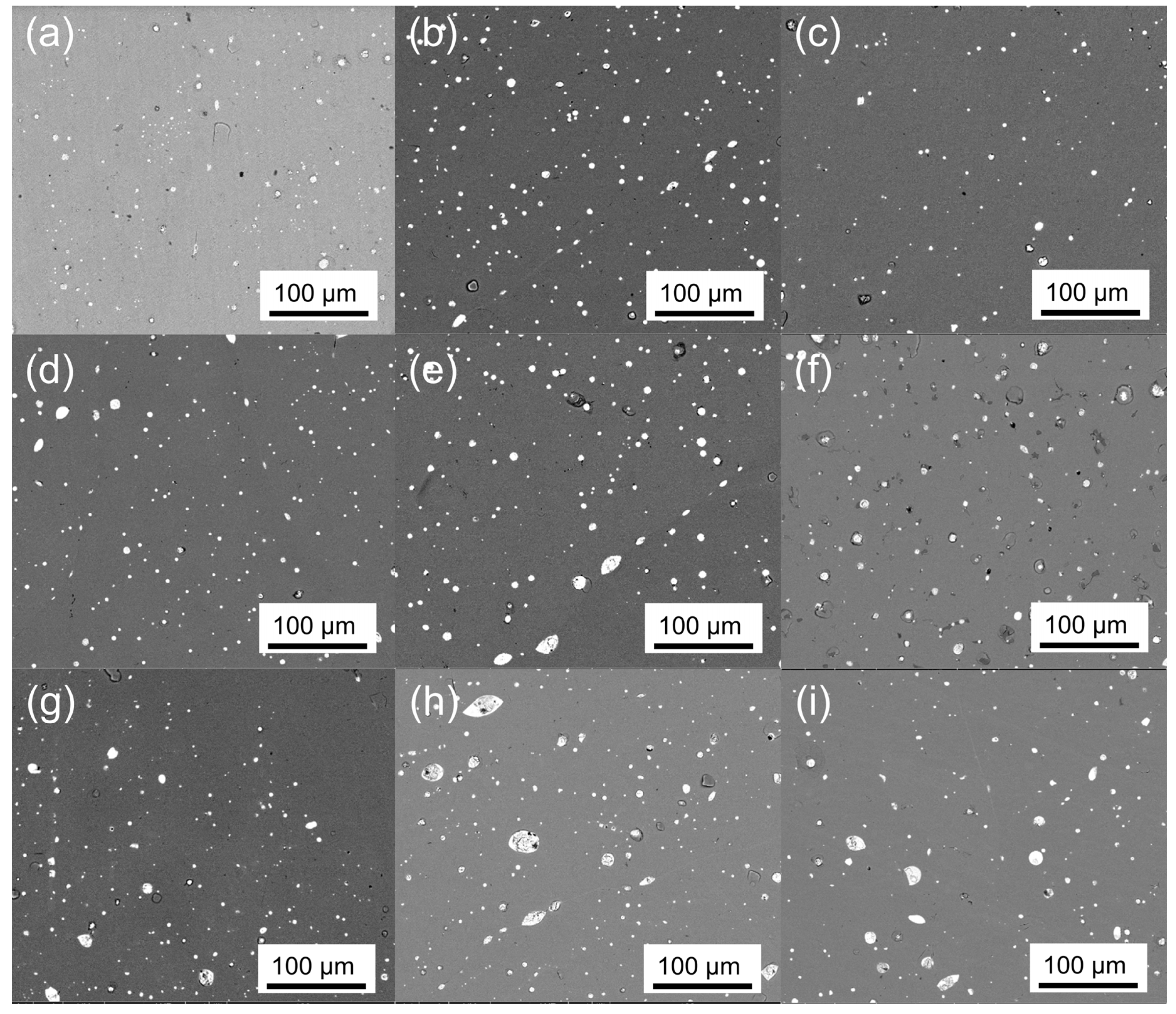
| Sample ID | Nominal Composition | Phase Fraction (Area%) | |
|---|---|---|---|
| Gray | White | ||
| 0 | Ti51Fe49 | 96.9 | 3.1 |
| 1 | Ti50Fe49V1 | 96.9 | 3.1 |
| 2 | Ti51Fe48V1 | 97.4 | 2.6 |
| 3 | Ti49Fe49V2 | 97.0 | 3.0 |
| 4 | Ti50Fe48V2 | 96.5 | 3.5 |
| 5 | Ti51Fe47V2 | 97.2 | 2.8 |
| 6 | Ti46.5Fe48V5.5 | 97.0 | 3.0 |
| 7 | Ti46.5Fe47.5V6 | 96.8 | 3.2 |
| 8 | Ti46Fe47.5V6.5 | 97.0 | 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faisal, M.; Kim, J.-H.; Cho, Y.W.; Jang, J.-i.; Suh, J.-Y.; Shim, J.-H.; Lee, Y.-S. Design of V-Substituted TiFe-Based Alloy for Target Pressure Range and Easy Activation. Materials 2021, 14, 4829. https://doi.org/10.3390/ma14174829
Faisal M, Kim J-H, Cho YW, Jang J-i, Suh J-Y, Shim J-H, Lee Y-S. Design of V-Substituted TiFe-Based Alloy for Target Pressure Range and Easy Activation. Materials. 2021; 14(17):4829. https://doi.org/10.3390/ma14174829
Chicago/Turabian StyleFaisal, Mohammad, June-Hyung Kim, Young Whan Cho, Jae-il Jang, Jin-Yoo Suh, Jae-Hyeok Shim, and Young-Su Lee. 2021. "Design of V-Substituted TiFe-Based Alloy for Target Pressure Range and Easy Activation" Materials 14, no. 17: 4829. https://doi.org/10.3390/ma14174829
APA StyleFaisal, M., Kim, J.-H., Cho, Y. W., Jang, J.-i., Suh, J.-Y., Shim, J.-H., & Lee, Y.-S. (2021). Design of V-Substituted TiFe-Based Alloy for Target Pressure Range and Easy Activation. Materials, 14(17), 4829. https://doi.org/10.3390/ma14174829