Industrial Thermal Insulation Properties above Sintering Temperatures
Abstract
:1. Introduction
2. Materials and Methods
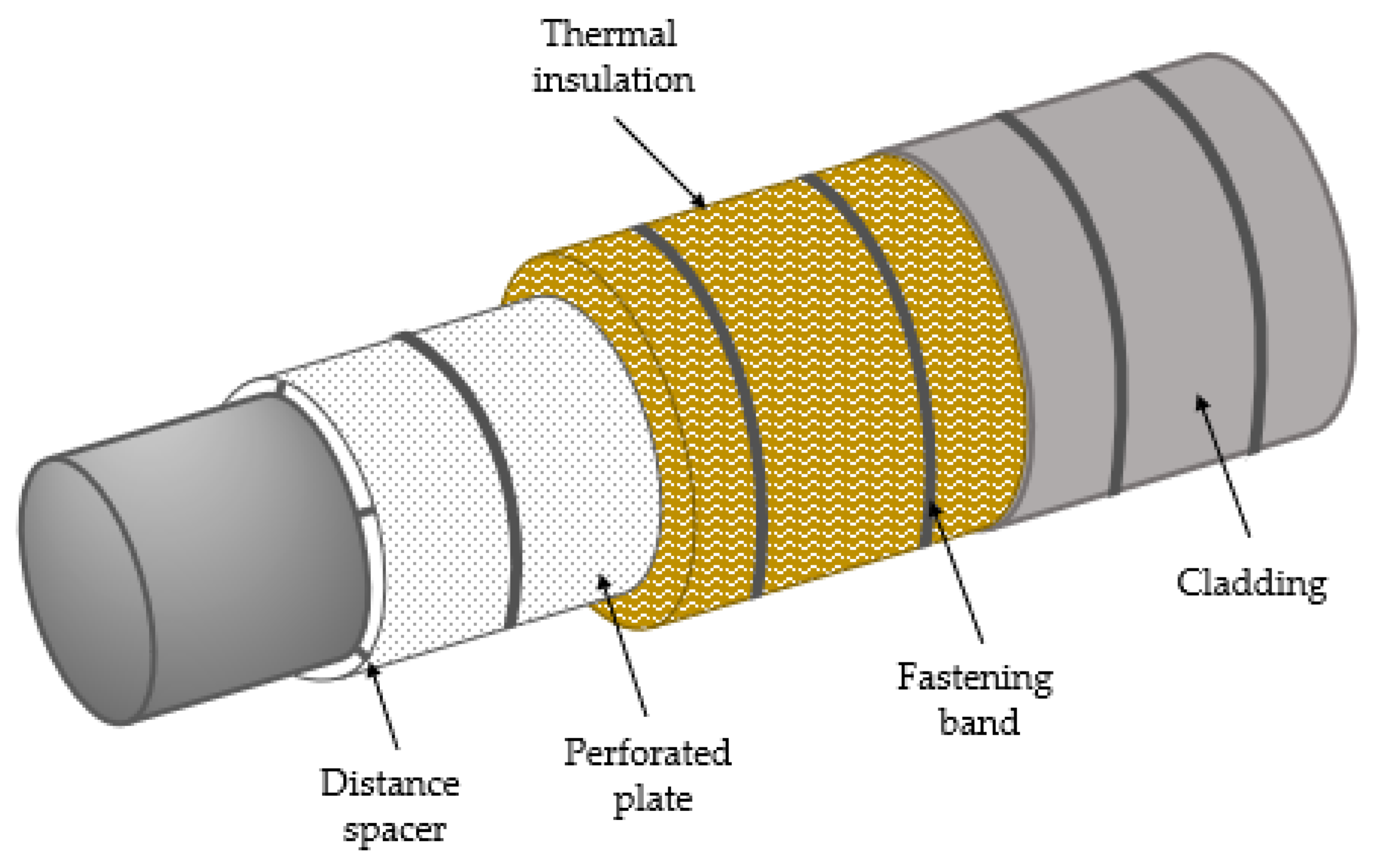
2.1. The Thermal Insulation Studied
2.2. Heat Treatment of Thermal Insulation Test Specimens
2.3. Thermal Conductivity Measurements
2.4. Density of Heat Treated Test Specimens
2.5. Specific Heat
3. Results
3.1. Dimensional Changes
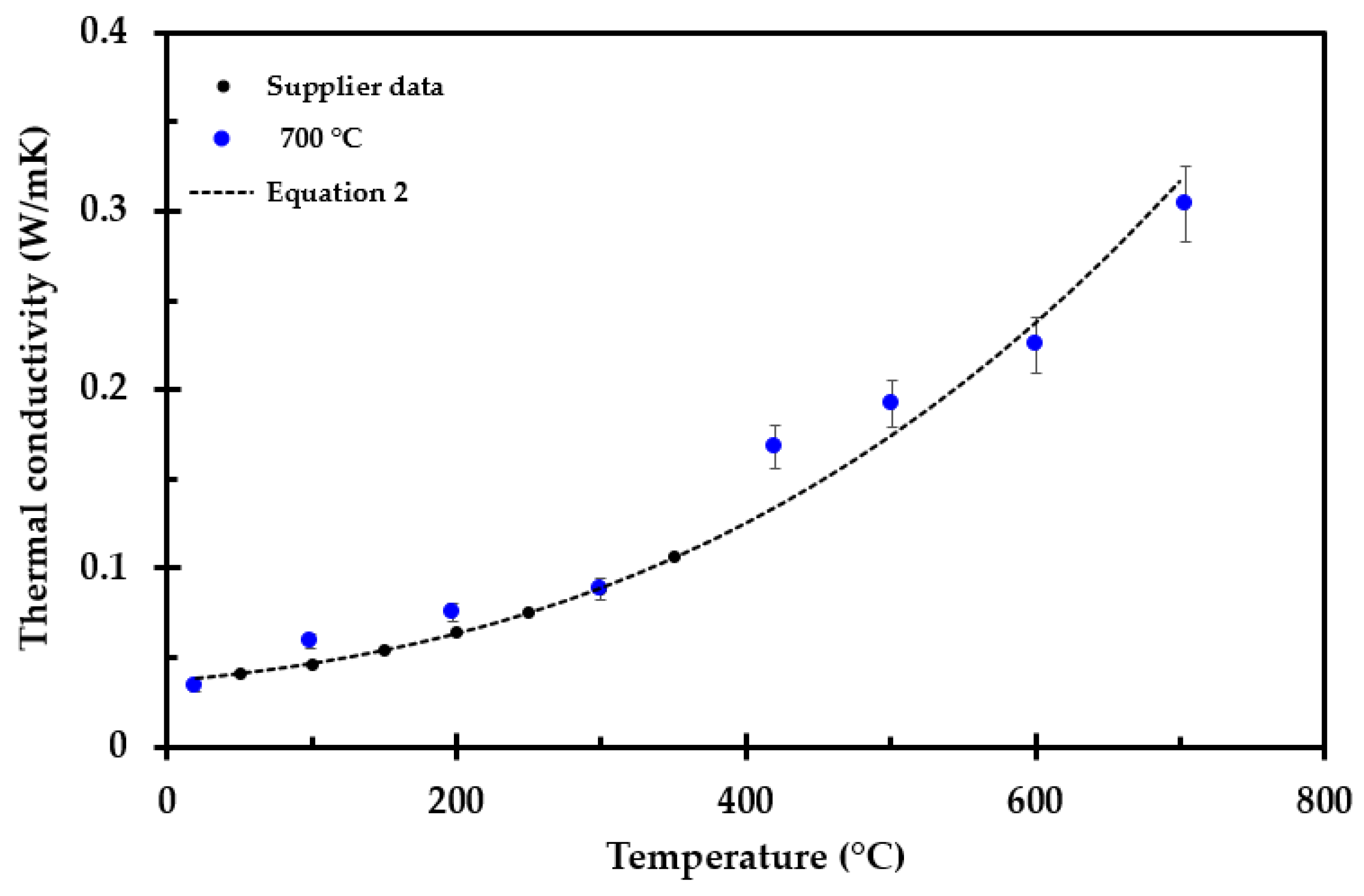
3.2. Thermal Conductivity Measurements
3.3. Volumetric Heat Capacity
3.4. Crack Formation
3.5. Internal Temperature Development during Heat Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Name | Description | |
| Material | Stone wool | |
| Operating range | −40 to 700 °C | |
| Name | Performance | Norms |
| Maximum service temperature | 700 °C | EN 14706 |
| Reaction to fire | Euroclass A1 | EN 13501-1 |
| Nominal density | 140 kg/m3 | EN 1602 |
| Water absorption | ≤1 kg/m2 ≤20 kg/m3 | EN 1609 |
| BP 172 | ||
| Water vapor diffusion resistance | Sd > 200 m | EN 12086 |
| Air flow resistivity | >60 kPa·s/m2 | |
| Designation code | MW EN 14303-T4-ST(+)700-WS1-MV2 | EN 14303 |
| Temperature (°C) | Thermal Conductivity (W/m∙K) |
|---|---|
| 50 | 0.041 |
| 100 | 0.046 |
| 150 | 0.054 |
| 200 | 0.064 |
| 250 | 0.075 |
| 300 | 0.088 |
| 350 | 0.106 |
| Name | Product | Percentage |
|---|---|---|
| Dust binder 1 | Oil product | <0.5% |
| Binder 1 | (C6H6O·CH2O)N | 2.5% (±0.4%) |
| Bulk oxide | SiO2 | 40.6–44.6% |
| Bulk oxide | Al2O3 | 17.4–20.4% |
| Bulk oxide | MgO + CaO | 23.9–27.9% |
| Bulk oxide | Fe2O3 | 5.5–8.5% |
| Bulk oxide | Na2O + K2O | 1.3–4.3% |
| Bulk oxide | TiO2 | 0.6–2.6% |
| Bulk oxide | P2O5 | Max. 1.2% |
Appendix B
Appendix C
| Percentage (%) | 42.54 | 19.33 | 1.64 | 7.16 | 13.24 | 13.24 | 1.43 | 1.43 |
| Product | SiO2 | Al2O3 | TiO2 | Fe2O3 | MgO | CaO | Na2O | K2O |
| Mw (g/mol) | 60.0843 | 101.9613 | 79.866 | 159.688 | 40.3044 | 56.077 | 61.9789 | 94.196 |
| Mw (kg/mol) | 0.0600843 | 0.1019613 | 0.079866 | 0.159688 | 0.0403044 | 0.056077 | 0.0619789 | 0.094196 |
| T (°C) | 298–847 | 298–2327 | 298–2000 | 298–950 | 298–3105 | 298–3200 | 298–1023 | 298–700 |
| A | −6.076591 | 106.918 | 67.2983 | 93.43834 | 47.25995 | 49.95403 | 25.5754 | 245.0104 |
| B | 251.6755 | 36.6219 | 18.7094 | 108.3577 | 5.681621 | 4.887916 | 177.71 | −567.0492 |
| C | −324.7964 | −13.9759 | −11.579 | −50.86447 | −0.872665 | −0.352056 | −166.335 | 778.7219 |
| D | 168.5604 | 2.15799 | 2.449561 | 25.58683 | 0.1043 | 0.046187 | 57.6116 | −346.2641 |
| E | 0.002548 | −3.157761 | −1.485471 | −1.61133 | −1.053955 | −0.825097 | 0.338149 | −4.653361 |
| T (°C) | 847–1996 | - | - | 950–1050 | - | - | 1023–1243 | 700–2000 |
| A | 58.7534 | 150.624 | −125.773 | 72.55098 | ||||
| B | 10.27925 | 0 | 302.074 | 41.39097 | ||||
| C | −0.131384 | 0 | −140.642 | −0.728497 | ||||
| D | 0.02521 | 0 | 21.324 | 0.218564 | ||||
| E | 0.025601 | 0 | 38.2831 | 0.066026 | ||||
| T (°C) | - | - | - | 1050–2500 | - | - | 1243–1405 | - |
| A | 110.9362 | 2240.95 | ||||||
| B | 32.04714 | −3209.97 | ||||||
| C | −9.192333 | 1803.69 | ||||||
| D | 0.901506 | −359.073 | ||||||
| E | 5.433677 | −386.248 |
References
- Bakka, M.S.; Eriksen, B.; Johansen, O.J.; Oltedal, H.L.; Log, T.; Rogde, S. Brann i Luftinntak på Gassturbin GTG4, Hammerfest LNG 20.09.2020 (Fire in Air Gas Turbine GTG4 Air Intake, Hammerfest LNG, 20.09.2020), Equinor Corporate Investigation Report, A MMP L2 2020-18, 131p. Available online: https://www.equinor.com/content/dam/statoil/documents/newsroom-additional-documents/news-attachments/endelig-rapport-coa-gransking-hlng-brann.pdf (accessed on 12 May 2021). (In Norwegian).
- Hallan, B.; Rundell, L.-R.; Thorsen, A.J.; Steinbakk, A.-H.; Sundby, T. Report of the Investigation of a Fire in the Air Intake of GTG4 at Hammerfest LNG, Melkøya, Activity 001901043, 45p. Available online: www.ptil.no/contentassets/3391c6686b2b4265abe8585294151335/2020_1862_rapport-equinor-hammerfest-lng-gransking-eng.pdf (accessed on 12 May 2021).
- Handal, E.; Sandvik, O.-I.; de Jong, H.; Bondevik, T.B.; Ovesen, R.V. Brann i Kompressorhus på Tjeldbergodden (Fire in Compressor House at Tjeldbergodden), Equinor Corporate Investigation report, A MMP L2 2020-18, 103p. Available online: https://www.equinor.com/content/dam/statoil/documents/newsroom-additional-documents/news-attachments/endelig-rapport-coa-gransking-tbo-brann.pdf (accessed on 12 May 2021). (In Norwegian).
- Landro, E.; Haug, B.A.; Mihajlovic, D.; Sande, E.; Steinbakk, A.H. Report Following the Investigation of a Turbine Breakdown with Subsequent Fire at Tjeldbergodden on 2 December 2020, Activity 001904026, 39p. Available online: https://www.ptil.no/contentassets/d7cb364377b741a597c8b85dd06c8ce9/2020_2201-rapport-eng-gransking-equinor-tjeldbergodden.pdf (accessed on 24 June 2021). (In Norwegian).
- Scandpower. Guidelines for the Protection of Pressurised Systems Exposed to Fire; Report No. 27.207.291/R1, Version 2; Scandpower: Kjeller, Norway, 2004. [Google Scholar]
- Norsk Standard. S-001 Technical Safety. NORSOK Standard, 5th ed.; Norwegian Technology Standards Institution: Oslo, Norway, 2018; Available online: http://www.standard.no (accessed on 13 June 2020).
- Bahadori, A. Thermal Insulation Handbook for the Oil, Gas, and Petrochemical Industries; Elsevier BV: Amsterdam, The Netherlands, 2014. [Google Scholar]
- API. API RP 583: Recommended Practice 583: Corrosion under Insulation and Fireproofing, 1st ed.; American Petroleum Institute: Washington, DC, USA, 2014. [Google Scholar]
- Choi, J.-Y.; Beyeon, S.-H. Overview of the Legal Design Basis for Passive Fire Protection in Korea and the Direction of Further Development. Sustainability 2021, 13, 1674. [Google Scholar] [CrossRef]
- API. API RP 2218: Recommended Practice 2218: Fireproofing Practices in Petroleum and Petrochemical Processing Plants, 3rd ed.; American Petroleum Institute: Washington, DC, USA, 2013. [Google Scholar]
- ASTM. ASTM E1725:19: Standard Test Methods for Fire Tests of Fire-Resistive Barrier Systems for Electrical System Components; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]
- Equinor. Performance Standards for Safety Systems and Barriers—Onshore; TR2237; Equinor: Tysværvåg, Norway, 2020; Volume 4, p. 15. [Google Scholar]
- Lovdata. Forskrift om Tekniske og Operasjonelle Forhold på Landanlegg i Petroleumsvirksomheten med mer (Teknisk og operasjonell forskrift). §35. Available online: https://lovdata.no/dokument/SF/forskrift/2010-04-29-612 (accessed on 18 June 2021).
- Sjöström, J.; Jansson, R. Measuring thermal material properties for structural fire engineering. In Proceedings of the 15th International Conference on Experimental Mechanics, ICEM15, Porto, Portugal, 22–27 July 2012. [Google Scholar]
- Olsen, H.; Sjöström, J.; Jansson, R.; Anderson, J. Thermal Properties of Heated Insulation Materials. In Proceedings of the 13th International Fire and Engineer Conference, INTERFLAM, London, UK, 24–26 June 2013. [Google Scholar]
- Livkiss, K.; Andres, B.; Bhargava, A.; Van Hees, P. Characterization of stone wool properties for fire safety engineering calculations. J. Fire Sci. 2018, 36, 202–223. [Google Scholar] [CrossRef]
- Moesgaard, M.; Pedersen, H.; Yue, Y.; Nielsen, E. Crystallization in stone wool fibres. J. Non-Cryst. Solids. 2007, 353, 1101–1108. [Google Scholar] [CrossRef]
- Liu, K.-S.; Zheng, X.-F.; Hsieh, C.-H.; Lee, S.-K. The Application of Silica-Based Aerogel Board on the Fire Resistance and Thermal Insulation Performance Enhancement of Existing External Wall System Retrofit. Energies 2021, 14, 4518. [Google Scholar] [CrossRef]
- Bjørge, J.S.; Metallinou, M.-M.; Kraaijeveld, A.; Log, T. Small Scale Hydrocarbon Fire Test Concept. Technologies 2017, 5, 72. [Google Scholar] [CrossRef] [Green Version]
- Bjørge, J.S.; Gunnarshaug, A.; Log, T.; Metallinou, M.-M. Study of Industrial Grade Thermal Insulation as Passive Fire Protection up to 1200 °C. Safety. 2018, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Rockwool. Available online: https://www.rockwool.co.uk/product-overview/hvac/pipe-section-mat-psm-en-gb/?selectedCat=downloads (accessed on 15 January 2021).
- Gunnarshaug, A.; Metallinou, M.-M.; Log, T. Study of industrial grade thermal insulation at elevated temperatures. Materials 2020, 13, 4613. [Google Scholar] [CrossRef] [PubMed]
- Keerthan, P.; Mahendran, M. Thermal Performance of Composite Panels under Fire Conditions Using Numerical Studies: Plasterboards, Rockwool, Glass Fibre and Cellulose Insulations. Fire Technol. 2012, 49, 329–356. [Google Scholar] [CrossRef] [Green Version]
- Thomas, G.C. Fire Resistance of Light Timber Framed Walls and Floors. Ph.D. Thesis, University of Canterbury, Christchurch, New Zeeland, 1996. [Google Scholar]
- Schleifer, V. Zum Verhalten von Raumabschliessenden Mehrschichtigen Holzbauteilen im Brandfall. Ph.D. Thesis, ETH Zurich University, Zurich, Switzerland, 2009. [Google Scholar]
- Equinor. The Insulation Handbook; TR1660; Equinor: Tysværvåg, Norway, 2019; Volume 6.02, Appendix A, Item 3. [Google Scholar]
- Rockwool. Available online: https://www.rockwool.com/west-virginia/factory-operations-and-production (accessed on 1 October 2020).
- Solyman, W.S.; Nagiub, H.M.; Alian, N.A.; Shaker, N.O.; Kandil, U.F. Synthesis and characterization of phenol/formaldehyde nanocomposites: Studying the effect of incorporating reactive rubber nanoparticles or Cloisite-30B nanoclay on the mechanical properties, morphology and thermal stability. J. Radiat. Res. Appl. Sci. 2017, 10, 72–79. [Google Scholar] [CrossRef]
- Log, T. Thermal Conductivity of Carbon Materials. Ph.D. Thesis, Institute of Inorganic Chemistry, National Institute of Technology and Science, Trondheim, Norway, 1989. [Google Scholar]
- Kingery, W.D. Thermal Conductivity: XII, Temperature Dependence of Conductivity for Single-Phase Ceramics. J. Am. Ceram. Soc. 1955, 38, 251–255. [Google Scholar] [CrossRef]
- Log, T.; Gustafsson, S.E. Transient plane source (TPS) technique for measuring thermal transport properties of building materials. Fire Mater. 1995, 19, 43–49. [Google Scholar] [CrossRef]
- International Organization for Standardization. Plastics—Determination of Thermal Conductivity and Thermal Diffusivity—Part 2: Transient Plane Heat Source (Hot Disc) Method; ISO 22007:2; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Chou, Y.-S.; Singh, B.; Chen, Y.-S.; Yen, S.-C. Study on Volume Reduction of Radioactive Perlite Thermal Insulation Wastes by Heat Treatment with Potassium Carbonate. Nuclear Eng. Technol. 2021, in press. [Google Scholar] [CrossRef]
- Seltveit, A. Ildfaste Materialer (Refractory materials), 1st ed.; Tapir: Trondheim, Norway, 1980. [Google Scholar]
- Rasmussen, S.N.; ROCKWOOL Inc., Hedehusene, Denmark. Personal communication, 2018.
- National Institute of Standards and Technology. Available online: http://webbook.nist.gov/cgi/cbook.cgi?ID=C14808607&Type =JANAFS&Table=on (accessed on 20 June 2021).
- National Institute of Standards and Technology. Available online: http://webbook.nist.gov/cgi/cbook.cgi?ID=C1344281&Unit s=SI&Mask=2#Thermo-Condensed (accessed on 20 June 2021).
- National Institute of Standards and Technology. Available online: http://webbook.nist.gov/cgi/cbook.cgi?ID=C1317802&Units= SI&Mask=2&Type=JANAFS&Plot=on#JANAFS (accessed on 20 June 2021).
- National Institute of Standards and Technology. Available online: http://webbook.nist.gov/cgi/cbook.cgi?ID=C1317608&Units= SI&Mask=2#Thermo-Condensed (accessed on 20 June 2021).
- National Institute of Standards and Technology. Available online: http://webbook.nist.gov/cgi/cbook.cgi?ID=C1309484&Units= SI&Mask=2#Thermo-Condensed (accessed on 20 June 2021).
- National Institute of Standards and Technology. Available online: http://webbook.nist.gov/cgi/cbook.cgi?ID=C1305788&Units= SI&Mask=2#Thermo-Condensed (accessed on 20 June 2021).
- National Institute of Standards and Technology. Available online: http://webbook.nist.gov/cgi/cbook.cgi?ID=C1313593&Units= SI&Mask=2#Thermo-Condensed (accessed on 20 June 2021).
- National Institute of Standards and Technology. Available online: http://webbook.nist.gov/cgi/cbook.cgi?ID=C12136457&Units =SI&Mask=2#Thermo-Condensed (accessed on 20 June 2021).
















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunnarshaug, A.; Metallinou, M.-M.; Log, T. Industrial Thermal Insulation Properties above Sintering Temperatures. Materials 2021, 14, 4721. https://doi.org/10.3390/ma14164721
Gunnarshaug A, Metallinou M-M, Log T. Industrial Thermal Insulation Properties above Sintering Temperatures. Materials. 2021; 14(16):4721. https://doi.org/10.3390/ma14164721
Chicago/Turabian StyleGunnarshaug, Amalie, Maria-Monika Metallinou, and Torgrim Log. 2021. "Industrial Thermal Insulation Properties above Sintering Temperatures" Materials 14, no. 16: 4721. https://doi.org/10.3390/ma14164721
APA StyleGunnarshaug, A., Metallinou, M.-M., & Log, T. (2021). Industrial Thermal Insulation Properties above Sintering Temperatures. Materials, 14(16), 4721. https://doi.org/10.3390/ma14164721









