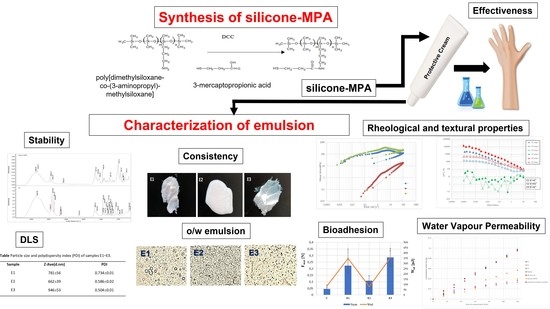
Thiolated Silicone Oils as New Components of Protective Creams in the Prevention of Skin Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Thiolated Silicone Oil
2.3. Determination of Thiol Groups (–SH)
2.4. Obtaining Protective Creams
2.5. Analysis of the Physicochemical Properties of Protective Preparations
2.5.1. pH and Conductivity of Emulsion
2.5.2. Stability of Emulsion
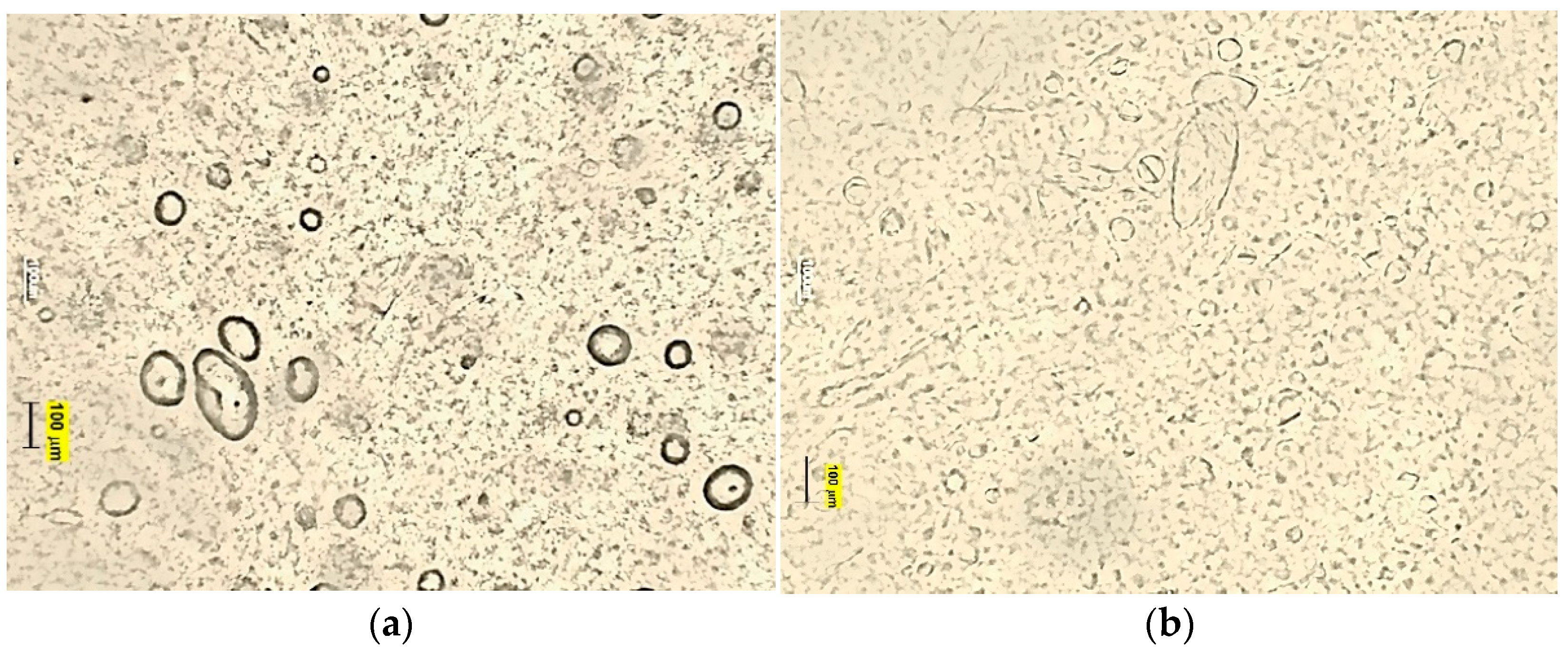
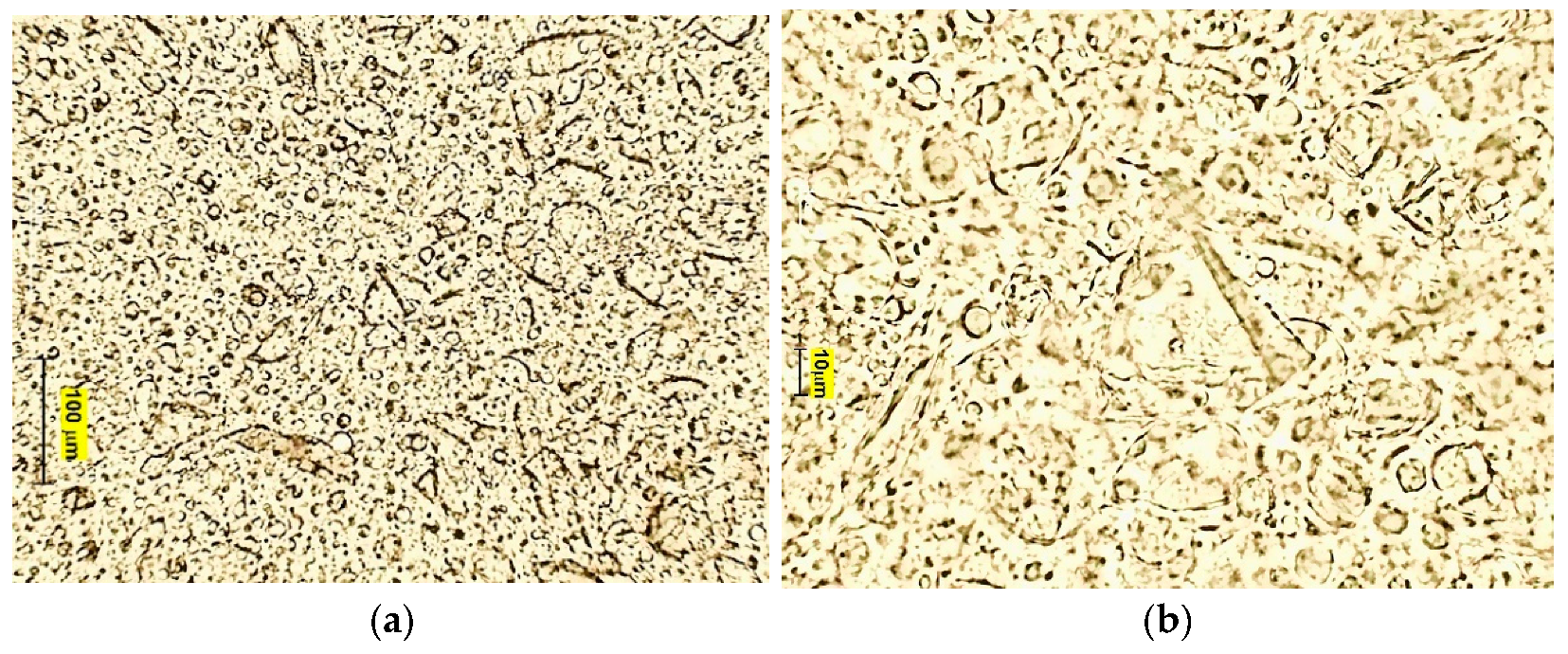
2.5.3. Droplet Size Distribution and Microscopic Structure
2.6. Rheological Studies
2.6.1. Study of Viscoelastic Properties
2.6.2. Flow Curves
2.6.3. Determination of Yield Stress
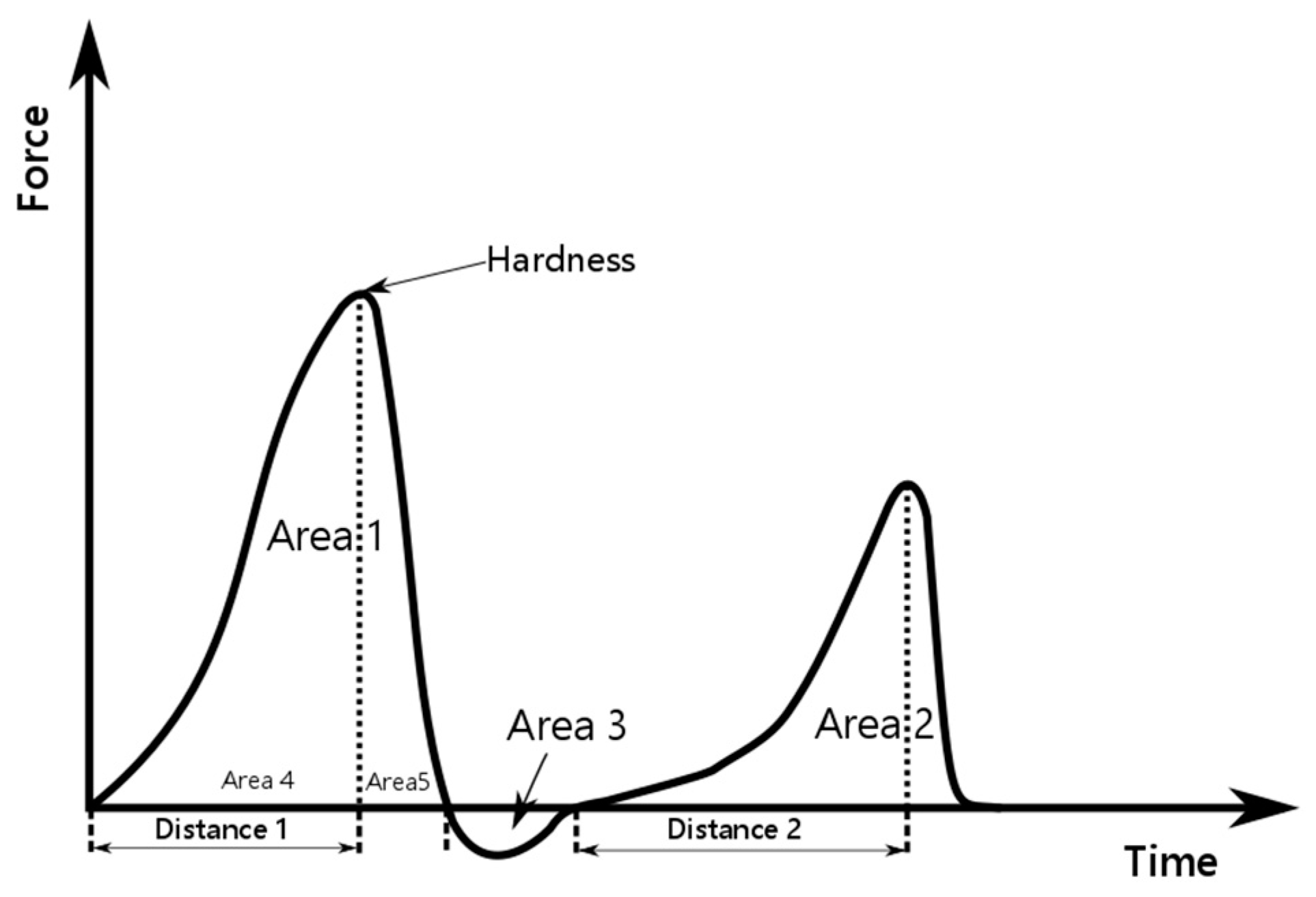
2.7. Texture Analysis
2.8. Efficacy
2.8.1. Parallel-Plate Method: Spreadability
2.8.2. Payne Cup Occlusivity Test
2.8.3. Absorbability
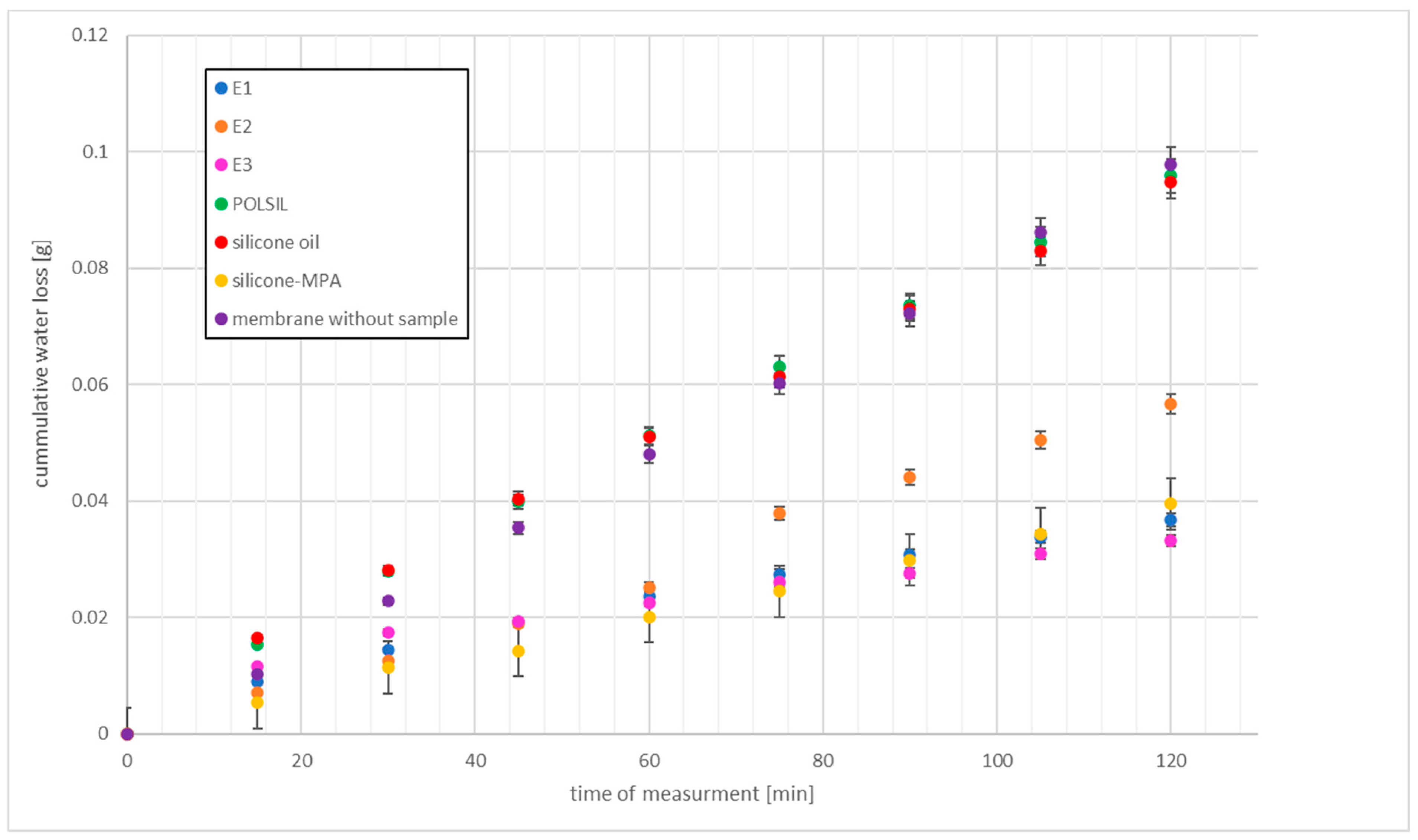
2.8.4. Water Washability
2.8.5. Ex Vivo Bioadhesive Properties Using Hairless Mice Skin as Adhesive Layer
2.8.6. Statistical Analysis
3. Results
3.1. Synthesis of the Thiolated Silicone Oil
3.2. Characteristics of Emulsion
3.3. Rheological Properties of Emulsions
3.3.1. Study of Viscoelastic Properties
3.3.2. Flow Curves
3.4. Texture Profile
3.5. Efficacy of Protective Products
4. Discussion
4.1. Spreadability
4.2. Washability and Absorbability
4.3. Occlusion Ability
4.4. Adhesion
4.5. Limitations of Use of Thiolated Silicone Oils in Protective Preparations. Stability Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hines, J.; Wilkinson, S.M.; John, S.M.; Diepgen, T.L.; English, J.; Rustemeyer, T.; Wasilew, S.; Kezic, S.; Maibach, H.I. The tree moment of skin cream application: An evidence-based proposal for use of skin creams in the prevention of irritant contact dermatitis in work place. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 53–64. [Google Scholar] [CrossRef]
- Schliemann, S.; Elsner, P. Skin Protection Practical Applications in the Occupational Settings, Current Problems in Dermatology, 1st ed.; Karger: Basel, Switzerland, 2007; Volume 34, pp. 1–10. [Google Scholar] [CrossRef]
- European Risk Observation Report Occupational Skin Diseases and Dermal Exposure in the European Union: Policy and Practice Overview; European Agency for Safety and Health at Work: Belgium, 2008; pp. 1–244.
- Antonov, D.; Schliemann, S.; Elsner, P. Occupational Skin Products, In Kanerva’s Occupational Dermatology, 1st ed.; John, S.M., Johansen, J.D., Rustemeyer, T., Elsner, P., Maibach, H.I., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Ptaszek, A.; Kruk, J. Effective tool for assessment of the quality of barrier creams—Relationships between rheological, textural and sensory properties. Regul. Toxicol. Pharm. 2019, 103, 113–123. [Google Scholar] [CrossRef]
- Garg, A.; Aggarwal, D.; Garg, S.; Singla, A.K. Spreading of semisolid formulations. Pharm. Technol. 2002, 26, 84–102. [Google Scholar]
- Sadhra, S.S.; Kurmi, O.P.; Mohammed, N.I.; Foulds, I.S. Protection afforded by controlled application of barrier cream: A study in a workplace setting. Br. J. Dermatol. 2014, 171, 813–818. [Google Scholar] [CrossRef]
- Partenhauser, A.; Zupančič, O.; Rohrer, J.; Bonengel, S.; Bernkop-Schnürch, A. Thiolated silicone oils as adhesive skin protectants for improved barrier function. Int. J. Cosmet. Sci. 2016, 38, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.; Kung, J.S.C.; Ng, W.G.G.; Leung, T.F. Emollient treatment of atopic dermatitis: Latest evidence and clinical considerations. Drugs Context 2018, 17, 212530. [Google Scholar] [CrossRef] [Green Version]
- Aliyar, H.; Schalau, G. Recent developments in silicones for topical and transdermal drug delivery. Ther. Deliv. 2015, 6, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Chularojanamontri, L.; Tuchinda, P.; Kulthanan, K.; Pongparit, K. Moisturizers for Acne: What are their Constituents? J. Clin. Aesthet. Dermatol. 2014, 7, 36–44. [Google Scholar]
- Glombitza, B.; Müller-Goymann, C.C. Influence of different ceramides on the structure of in vitro model lipid systems of the stratum corneum lipid matrix. Chem. Phys. Lipids 2002, 117, 29–44. [Google Scholar] [CrossRef]
- Oborska, A.; Sikora, M. Silikonowa kosmetyka. Les Nouvalies Esthet. 2006, 1, 82–83. [Google Scholar]
- Partenhauser, A.; Zupančič, O.; Podričnik, S.; Rohrer, J.; Leichner, C.; Bernkop-Schnürch, A. Entirley S-protected silicone oil as second generation mucoadhesive agent. Eur. Polym. J. 2016, 76, 53–62. [Google Scholar] [CrossRef]
- Hanif, M.; Zaman, M.; Qureshi, S. Thiomers: A blessing to Evaluating Era of Pharmaceuticals. Int. J. Polym. Sci. 2015. [Google Scholar] [CrossRef] [Green Version]
- Bernkop-Schnürch, A. Thiomers: A new generation of mucoadhesive polymers. Adv. Drug Deliv. Rev. 2005, 57, 1569–1582. [Google Scholar] [CrossRef]
- Partenhauser, A. Silicone Thiomers Conqueros of Innovative Drug Delivery Systems, 1st ed.; Scholar’s Press: Saarbrücken, Germany, 2016; pp. 1–188. [Google Scholar]
- Grießinger, J.A.; Bonengel, S.; Partenhauser, A.; Ijaz, M.; Bernkop-Schnürch, A. Thiolated polymers: Evaluation of their potential as dermoadhesive excipients. Drug Dev. Ind. Pharm. 2017, 43, 204–212. [Google Scholar] [CrossRef]
- Partenhauser, A.; Laffleur, F.; Rohrer, J.; Bernkop-Schnürch, A. Thiolated silicone oils: Synthesis, gelling and mucoadhesive properties. Acta Biomater. 2015, 16, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Leichner, C.; Jelkmann, M.; Bernkop-Schnürch, A. Thiolated polymers: Bioinspired polymers utilizing one of the most important bridging structures in nature. Adv. Drug Deliv. Rev. 2019, 151–152, 191–221. [Google Scholar] [CrossRef] [PubMed]
- Valenta, C.; Walzer, A.; Clausen, A.E.; Bernkop-Schnürch, A. Thiolated Polymers: Development and Evaluation of Transdermal Delivery Systems for Progesterone. Pharm. Res. 2001, 18, 211–216. [Google Scholar] [CrossRef]
- Sakloetsakun, D.; Hombach, J.; Bernkop-Schnürch, A. In situe gelling properties of chitosan-thioglycolic acid conjugate in the presence of oxidizing agents. Biomaterials 2009, 30, 6151–6157. [Google Scholar] [CrossRef] [PubMed]
- Egwim, I.O.; Gruber, H.J. Spectrophometric measurment of mercaptants with 4,4′-dithiodipyridyne. Anal. Biochem. 2001, 288, 188–194. [Google Scholar] [CrossRef]
- Cosmetics Europe Guidelines on Stability Testing of Cosmetic Products. 2004. Available online: https://www.cosmeticseurope.eu/files/5914/6407/8121/Guidelines_on_Stability_Testing_of_Cosmetics_CECTFA_-_2004.pdf (accessed on 22 July 2021).
- Surmacka-Szcześniak, A. Classification of textural characteristics. J. Food Sci. 1962, 28, 385–389. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Potykanowicz, A. Determining the quality of hydrophobic barrier creams by rheological measurements, sensory analysis, pH determination and permeation time measurements. Chemom. Intell. Lab. Syst. 2016, 15, 14–20. [Google Scholar] [CrossRef]
- Kurpiewska, J.; Liwkowicz, J. Skin protection measures for protecting human skin against water and aqueous solutions of detergents, acids and bases. Podstawy I Metod. Oceny Sr. Pr. 2014, 1, 151–160. [Google Scholar]
- Casiraghi, A.; Ranzini, F.; Musazii, U.M.; Franze, S.; Meloni, M.; Minghetti, P. In Vivo method to evaluate the barrier properties of medical devices for cutaneus use. Regul. Toxicol. Pharmacol. 2017, 90, 42–50. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Klimaszewska, E.; Ogorzałek, M.; Rożnawska, K.; Ruman, J. Effectiveness of protective preparations: Impact of vegetable oil additives to recipes. Eur. J. Lipid Sci. Tech. 2020, 122, 2000130. [Google Scholar] [CrossRef]
- ASTM Standard E 96/E 96M—05: Standard Test Methods for Water Vapor Transmission of Materials; ASTM International: West Conshohocken, PA, USA, 2005.
- Treffel, P.; Gabard, B.; Juch, R. Evaluation of barrier creams: An in vitro technique on human skin. Acta Derm.-Venereol. 1994, 74, 7–11. [Google Scholar] [CrossRef]
- Fürst, A.; Baus, R.A.; Lupo, N.; Bernkop-Schnürch, A. Entirely S-Protected Thiolated Silicone: A novel Hydrophobic Mucoadhesive and Skin Adhesive. J. Pharm. Sci. 2019, 108, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.C.; López-Mesas, M.; Lacorte, M.T.; Cegarra, J. Infrared Analysis of the Amino Group Content in Functional Aminopolydimethylsiloxanes. Fibers Polym. 2009, 10, 437–441. [Google Scholar] [CrossRef]
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: https://ec.europa.eu/health/sites/default/files/endocrine_disruptors/docs/cosmetic_1223_2009_regulation_en.pdf (accessed on 20 August 2021).
- Binks, B.P.; Dong, J. Emulsions and equilibrium phase behaviour in silicone oil+ water+ nonionic surfactant mixtures. Colloids Surf. A Physicochem. Eng. Asp. 1998, 132, 289–301. [Google Scholar] [CrossRef]
- Purhoit, P.S.; Kulkarni, R.; Somasundaran, P. Investigantion of colloidal properties of modified silicone polymers emulsified by nonionic surfactants. J. Colloid Interface Sci. 2012, 383, 49–54. [Google Scholar] [CrossRef]
- Hu, Z.; Liao, M.; Chen, Y.; Cai, Y.; Meng, L.; Liu, Y.; Lv, N.; Liu, Z.; Yuan, W. A novel preparation method for silicone oil nanoemulsion and its application for coating hair with silicone. Int. J. Nanomed. 2012, 7, 5719–5724. [Google Scholar] [CrossRef] [Green Version]
- Somasundaran, P.; Mehta, S.C.; Purohit, P. Silicone emulsions. Adv. Colloid Interface Sci. 2006, 128–130, 103–109. [Google Scholar] [CrossRef]
- Nazir, H.; Zhang, W.; Liu, Y.; Chen, X.; Wang, L.; Naseer, M.M.; Ma, G. Silicone oil emulsions: Strategies to improve their stability and applications in hair care products. Int. J. Cosmet. Sci. 2014, 36, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Partenhauser, A.; Netsomboon, K.; Leobaviciute, G.; Bernkop-Schnürch, A. Evaluation of thiolated silicone oil as advances mucoadhesive antifoaming agent. Drug Deliv. 2016, 23, 2711–2719. [Google Scholar] [CrossRef]
- Sikora, E. Cosmetic Emulsions; Wydawnictwo Politechniki Krakowskiej: Kraków, Poland, 2019; pp. 1–118. [Google Scholar]
- Personal Care Product Guide 5th Edition, Croda. Available online: https://www.crodapersonalcare.com/en-gb/technical-library/brochures (accessed on 22 July 2021).
- Danila, A.; Ibanescu, S.-A.; Zaharia, C.; Muresan, E.I.; Popescu, A.; Danu, M.; Rotaru, V. Eco-friendly O/W emulsions with potential application in skincare products. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125969. [Google Scholar] [CrossRef]
- Kim, K.-P.; Jeon, S.; Kim, M.-J.; Cho, Y. Borage oil restores acidic skin pH by up-regulating the activity or expression of filaggrin and enzymes involved in epidermal lactate, free fatty acid, and acidic free amino acid metabolism in essential fatty acid-deficient Guinea pigs. Nutr. Res. 2018, 58, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.C.; Somasundaran, P.; Kulkarni, R. Variation in emulsion stabilization behavior of hybrid silicone polymers with change in molecular structure: Phase diagram study. J. Colloid Interface Sci. 2009, 333, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Kulawik-Pióro, A.; Gibas, K.; Osak, E. Consistency as a quality parameter of hydrophobic skin protection preparations. Pol. J. Cosmetol. 2020, 23, 27–34. [Google Scholar]
- Savary, G.; Grisel, M.; Picard, C. Impact of emollients on the spreading properties of cosmetic products: A combined sensory and instrumental characterization. Colloid Surf. B 2013, 102, 371–378. [Google Scholar] [CrossRef]
- Gore, E.; Picard, C.; Savary, G. Spreading behavior of cosmetic emulsion: Impact of the oil phase. Biotribology 2018, 16, 17–24. [Google Scholar] [CrossRef]
- Miastkowska, M.; Kulawik-Pióro, A.; Szczurek, M. Nanoemulsion gel formulation optimization for burn wounds: Analysis of rheological and sensory properties. Processes 2020, 8, 1416. [Google Scholar] [CrossRef]
- Kurpiewska, J.; Liwkowicz, J. The composition of waterproof barrier creams’ ingredients and their barrier properties. Chemik 2012, 66, 991–996. [Google Scholar]
- Wiedersberg, S.; Leopold, C.S.; Guy, R.H. Dermatopharmacokinetics of betamethasone 17-valerate: Influence of formulation viscosity and skin surface cleasing procedure. Eur. J. Pharm. Biopharm. 2009, 71, 362–366. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, L.; Picard, C.; Savary, G.; Grisel, M. Rheological and textural characterization of cosmetic emulsions containing natural and synthetic polymers: Relationships between both data. Colloids Surf. A 2013, 421, 150–163. [Google Scholar] [CrossRef]
- Barnes, H.A. The Rheology of Emulsion. In Emulsions Structure Stability Interaction 4; Petsev, D.N., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 721–759. [Google Scholar]
- Lukic, M.; Jaksic, I.; Krstonsic, V.; Cekic, N.; Savic, S. A combined approach in characterization of an effective w/o hand cream: The influence of emollient on textural, sensorial and in vivo skin performance. Int. J. Cosmet. Sci. 2012, 34, 140–149. [Google Scholar] [CrossRef]
- Karavana, S.Y.; Güneri, P.; Ertan, G. Benzydamine hydrochloride buccal bioadhesive gels, designed for oral ulcers: Preparation, rheological, textural, mucoadhesive and release properties. Pharm. Dev. Technol. 2009, 14, 623–631. [Google Scholar] [CrossRef]
- Vitorino, C.; Alves, L.; Antunes, F.E.; Sousa, J.J.; Pais, A.A. Design of a dual nanostructured lipid carrier formulation based on psychicochemical rheological, and mechanical properties. J. Nanopart. Res. 2013, 15, 1–14. [Google Scholar] [CrossRef]
- Wróblewska, M.; Szekalska, M.; Hafner, A.; Winnicka, K. Oleogels and bigels as topical drug carriers for ketoconazole—Development and in vitro characterization. Acta Pol. Pharm. 2018, 75, 777–786. [Google Scholar]
- Halkier-Sorensen, L. Occupational skin diseases. Contact Dermat. 1996, 35, 1–120. [Google Scholar]
- Kucharekova, M.; Schalkwijk, J.; Van De Kerkhof, P.C.; Van De Valk, P.G. Effect of a lipid-rich emollient containing ceramide 3 in experimentally induced skin barrier dysfunction. Contact Dermat. 2002, 46, 331–338. [Google Scholar] [CrossRef]
- Lautenschläger, H. Membrane-containing barrier creams—Protecting the skin with skin related-substances. Kosmet. Prax. 2006, 4, 12–14. [Google Scholar]
- Igielska-Kalwat, J.; Nowak, I. Uwalnianie substancji aktywnych z emulsji kosmetycznych. Chemik 2015, 69, 498–504. [Google Scholar]
- Płocica, J.; Tal-Figiel, B.; Figiel, W. Badania reologiczne i sensoryczne stosowane do oceny preparatów kosmetycznych. Świat Przem. Kosm. 2014, 1, 68–73. [Google Scholar]
- Brummer, R.; Godersky, S. Rheological studies to objectify sensations occurring when cosmetic emulsions are applied to the skin. Colloids Surf. A 1999, 152, 89–94. [Google Scholar] [CrossRef]
- Sene, C. Silicone excipients for aesthically superior and substantive topical pharmaceutical formulations. Pharm. Chem. 2003, 2, 17–20. [Google Scholar]
- Reeth, I.V.; Wilson, A. Understanding Factors Which Influence Permeability of silicones and their derivatives. Cosmet. Toiltries 1994, 109, 87–92. [Google Scholar]
- De Paepe, K.; Sieg, A.; Le Meur, M.; Rogiers, V. Silicone as Nonocclusive Topical Agents. Ski. Pharm. Physiol. 2014, 27, 164–171. [Google Scholar] [CrossRef]
- Jamrógiewicz, M.; Żebrowska, M.; Łukasiak, J.; Sznitowska, M. Silikonowe preparaty do leczenia powierzchniowego blizn. Farm. Pol. 2010, 66, 437–442. [Google Scholar]
- Glombitza, B.; Müller-Goymann, C.C. Investigation of interactions between silicones and stratum corneum lipids. Int. J. Cosmet. Sci. 2001, 23, 25–34. [Google Scholar] [CrossRef]
- Andriot, M.; De Groot, J.V.; Meeks, R.; Gerlach, E.; Jungk, M.; Wolf, A.T.; Cray, S.; Easton, S.; Mountney, A.; Leadley, S.; et al. Chapter 2—Silicones in Industrial Applications. In Inorganic Polymers; Nova Science Pub. Inc.: Hauppauge, NY, USA, 2007; pp. 61–161. [Google Scholar]
- Mintel GNPD Data Base. 26 January 2016. Available online: https://www.mintel.com/global-new-products-database (accessed on 22 July 2021).
- Łubkowska, M.; Stańczyk, W. Aminoalkyl functionalized silioxanes. Polimery 2014, 59, 763–768. [Google Scholar] [CrossRef]
- Fernanda, M.; Dias, R.G. Hair Cosmetics: An Overview. Int. J. Trichol. 2015, 7, 2–15. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A. Strategies to overcome the polycation dilemma in drug delivery. Adv. Drug Deliv. Rev. 2018, 136–137, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Pasieczna-Patkowska, S.; Olejnik, T. Analysis of cosmetic products using different IR spectroscopy techniques. Ann. Univ. Mariae Curie-Skłodowska Sect. AA–Chem. 2013, 68, 95–106. [Google Scholar] [CrossRef] [Green Version]








| Trademark | Abbreviation Used at Work | Producer |
|---|---|---|
| poly[dimethylsiloxane-co-(3-aminopropyl)methylsiloxane with a functional group equivalent of 4400 Da, viscosity 100 cSt | silicone oil | Sigma Aldrich |
| 3-merkaptopropionic acid | MPA | Sigma Aldrich |
| N,N′-dicyclohexylcarbodiimide | DCC | Sigma Aldrich |
| dichloromethane | DCM | POCH |
| pyridine | − | POCH |
| dimethyl sulfoxide | DMSO | POCH |
| triethylamine | − | POCH |
| Aldrithiol-4 | DTDP | Sigma Aldrich |
| chloroform | − | POCH |
| neat acetic acid | − | POCH |
| stearic acid | − | Chempur |
| POLSIL OM 100 | methyl silicone oil | Silikony Polskie |
| glycerin | − | POCH |
| Arlamol™ PS15 | − | Croda Inc. |
| Crodamol™ PMP | − | Croda Inc. |
| Cithrol™ 4MS | − | Croda Inc. |
| sodium benzoate | − | POCH |
| Phase | Ingredient | Function in Recipe | Concentration of Components (% Mass) | ||
|---|---|---|---|---|---|
| E1 | E2 | E3 | |||
| A | stearic acid | emulsifier in combination with triethanolamine, barrier, and lubricating substance | 24.0 | 24.0 | 24.0 |
| A | methyl silicone oil | creates a protective, occlusive film on the surface which prevent water loss | 10.0 | − | − |
| A | silicone oil | − | 10.0 | − | |
| A | silicone-MPA | − | − | 10.0 | |
| B | Glycerin | moisturizing substance | 8.5 | 8.5 | 8.5 |
| B | Triethanolamine | with stearic acid creates emulsifier, pH regulator | 1.2 | 1.2 | 1.2 |
| A | PPG-15-Stearyl Ether | emollient | 1.0 | 1.0 | 1.0 |
| B | PPG-2-Myristyl Ether Propionate | emollient conditioning agent | 1.0 | 1.0 | 1.0 |
| B | PEG-8 Stearate | humectant, rheology modifier, emulsifier | 1.0 | 1.0 | 1.0 |
| B | Sodium Benzoate | preservative | 0.2 | 0.2 | 0.2 |
| B | Deionized water | solvent | 53.1 | 53.1 | 53.1 |
| Sample | Visual Evaluation | pH | Stability | Conductivity mV |
|---|---|---|---|---|
| E1 | white, characteristic smell of stearic acid, soft, ointment-like consistency, | 6.8 ± 0.1 | + | 152 ± 0 |
| E2 | white, slight smell of stearic acid, thin product, lotion-like consistency | 8.5 ± 0.0 | + | 158 ± 0 |
| E3 | white with a characteristic unpleasant odor from the thiolated derivative, semi-solid consistency | 8.2 ± 0.0 | + | 135 ± 0 |
| Sample | Z-Ave (d.nm) | PDI |
|---|---|---|
| E1 | 781 ± 56 | 0.734 ± 0.008 |
| E2 | 662 ± 39 | 0.586 ± 0.017 |
| E3 | 946 ± 53 | 0.504 ± 0.009 |
| Sample | E1 | E2 | E3 | |
|---|---|---|---|---|
| Basic rheological properties | Apparent Viscosity, Pas | |||
| 1.0 | 133.00 ± 6.73 | 1.02 ± 0.05 | 214.70 ± 12.81 | |
| 10.0 | 18.99 ± 11.41 | 0.59 ± 0.04 | 28.22 ± 1.72 | |
| 50.0 | 1.22 ± 0.07 | 0.30 ± 0.02 | 4.41 ± 0.27 | |
| 100.0 | 1.22 ± 0.07 | 0.18 ± 0.01 | 2.09 ± 0.12 | |
| Yield stress, Pa | ||||
| 138.3 ± 15.0 | 1.4 ± 0.3 | 226.3 ± 12.0 | ||
| Energy dissipated E, J | ||||
| 5.24 ± 0.76 | 0.54 ± 0.05 | 9.40 ± 0.93 | ||
| Texture profile | Texture parameters | |||
| Hardness, N | 0.866 ± 0.036 | 0.075 ± 0.003 | 3.726 ± 0.185 | |
| Compressibility, N⋅s | 4.307 ± 0.194 | 0.285 ± 0.008 | 22.423 ± 0.895 | |
| Adhesiveness, N·s | 3.687 ± 0.116 | − | 12.803 ± 0.526 | |
| Cohesiveness, - | 0.741 ± 0.029 | 0.936 ± 0.039 | 0.497 ± 0.024 | |
| Sample | Washability | Resistance to Water | Resistance to Detergents | Spreadability 25 °C/32 °C cm2 | Occlusion Ability g/m2⋅d | ||
|---|---|---|---|---|---|---|---|
| Nav [%] | Assessment | Nav [%] | Assessment | ||||
| E1 | partially washed off, a greasy deposit remains on the skin | 35.07 ± 1.70 | low resistance | 11.64 ± 0.90 | good resistance | 10.4 ± 0.4 18.8 ± 0.6 | 478.8 ± 13.9 |
| E2 | washed off | 18.74 ± 0.89 | medium resistance | 28.38 ± 1.47 | medium resistance | 40.7 ± 0.2 78.5 ± 2.0 | 680.2 ± 15.2 |
| E3 | partially washed off, a greasy deposit and the smell of thiol remains on the skin | 37.16 ± 1.50 | low resistance | 29.57 ± 0.37 | medium resistance | 4.7 ± 0.5 7.5 ± 0.1 | 378.6 ± 12.5 |
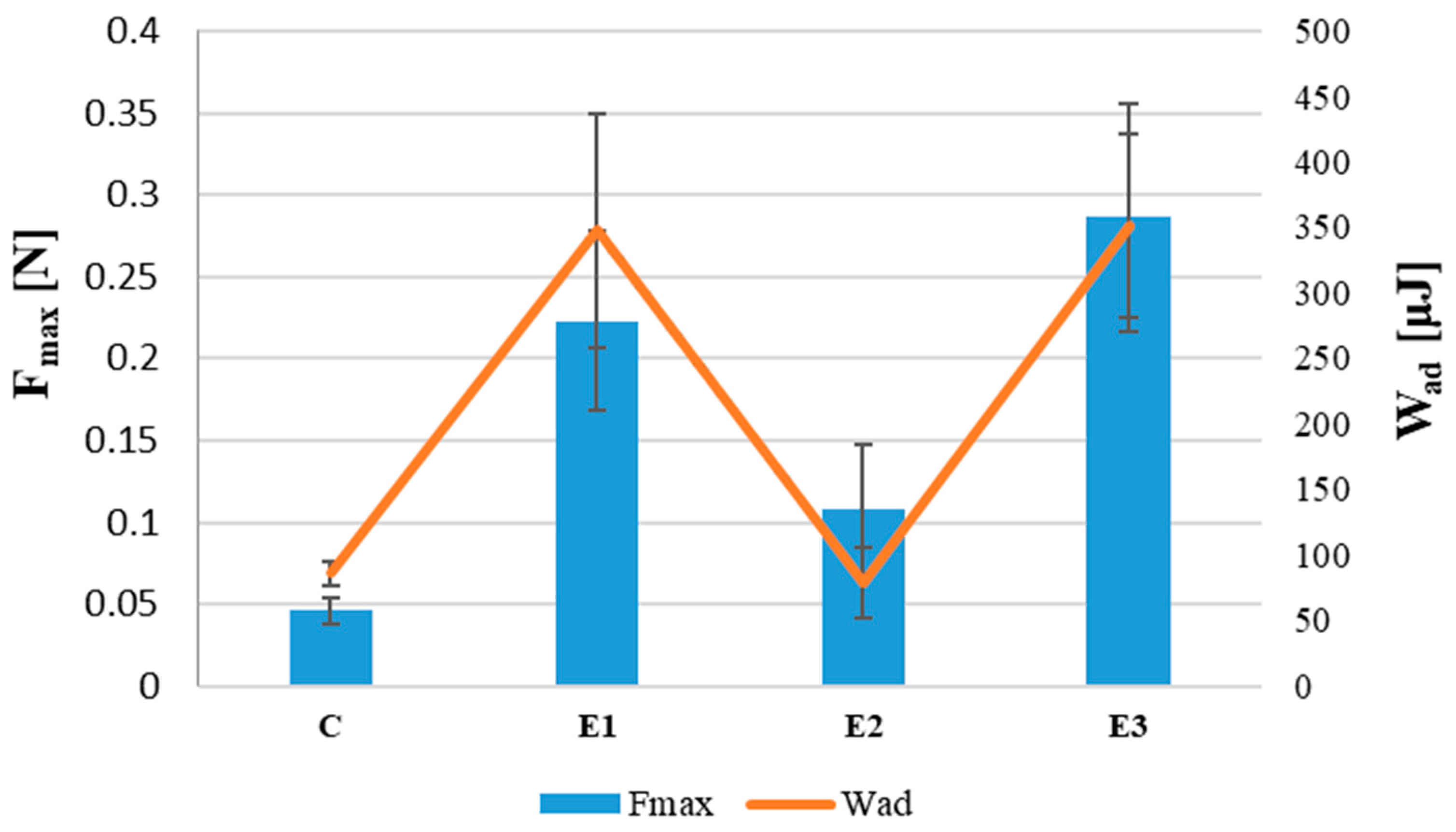
| Fmax | |||||
|---|---|---|---|---|---|
| Groups | Count | Sum | Mean | Variance | Post hoc |
| E1 | 7 | 1.563 | 0.223 | 0.003 | a |
| E2 | 7 | 0.758 | 0.108 | 0.002 | b |
| E3 | 7 | 2.002 | 0.286 | 0.005 | and |
| Source of Variation | SS | df | MS | F | p-value |
| Between Groups | 0.1137 | 2 | 0.057 | 18.161 | 0.00005 |
| Within Groups | 0.0563 | 18 | 0.003 | ||
| Total | 0.1700 | 20 | |||
| Wad | |||||
| Groups | Count | Sum | Mean | Variance | Post hoc |
| E1 | 6 | 2085 | 347.5 | 7998.8 | and |
| E2 | 6 | 551.2 | 78.7 | 713.8 | b |
| E3 | 6 | 2108 | 351.3 | 4805.4 | a |
| Source of Variation | SS | df | MS | F | p-value |
| Between Groups | 323,829.0 | 2 | 161,914.5 | 37.927 | 0.0000008 |
| Within Groups | 68,304.4 | 16 | 4269.0 | ||
| Total | 392,133.4 | 18 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulawik-Pióro, A.; Drabczyk, A.K.; Kruk, J.; Wróblewska, M.; Winnicka, K.; Tchórzewska, J. Thiolated Silicone Oils as New Components of Protective Creams in the Prevention of Skin Diseases. Materials 2021, 14, 4723. https://doi.org/10.3390/ma14164723
Kulawik-Pióro A, Drabczyk AK, Kruk J, Wróblewska M, Winnicka K, Tchórzewska J. Thiolated Silicone Oils as New Components of Protective Creams in the Prevention of Skin Diseases. Materials. 2021; 14(16):4723. https://doi.org/10.3390/ma14164723
Chicago/Turabian StyleKulawik-Pióro, Agnieszka, Anna K. Drabczyk, Joanna Kruk, Magdalena Wróblewska, Katarzyna Winnicka, and Justyna Tchórzewska. 2021. "Thiolated Silicone Oils as New Components of Protective Creams in the Prevention of Skin Diseases" Materials 14, no. 16: 4723. https://doi.org/10.3390/ma14164723
APA StyleKulawik-Pióro, A., Drabczyk, A. K., Kruk, J., Wróblewska, M., Winnicka, K., & Tchórzewska, J. (2021). Thiolated Silicone Oils as New Components of Protective Creams in the Prevention of Skin Diseases. Materials, 14(16), 4723. https://doi.org/10.3390/ma14164723