Modifications of FLC Physical Properties through Doping with Fe2O3 Nanoparticles (Part I)
Abstract
1. Introduction
2. Materials and Methods
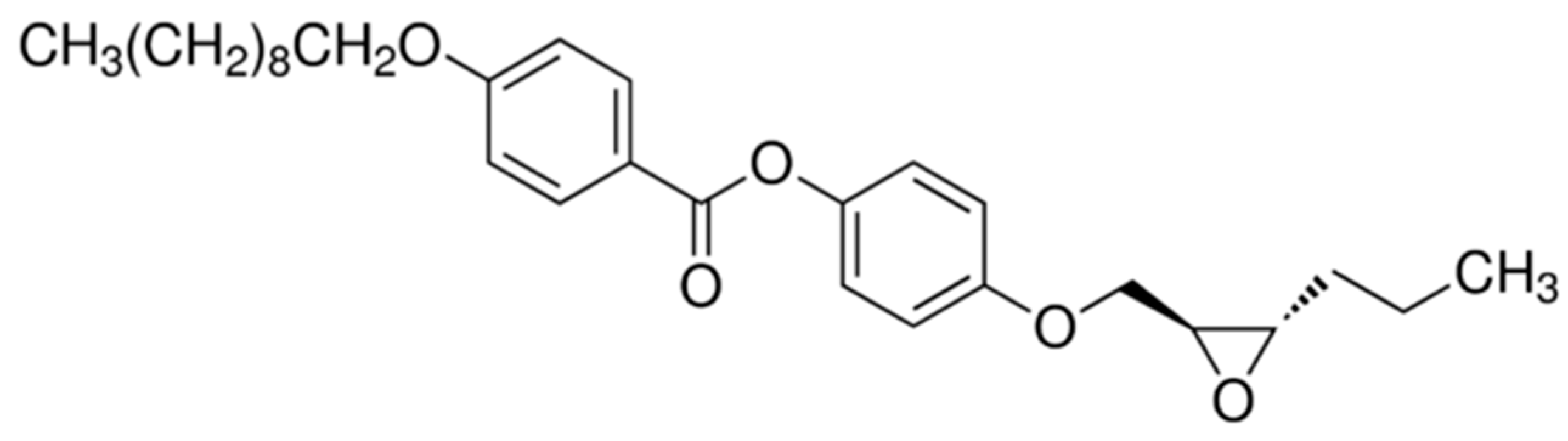
2.1. Samples
- The adequate amounts of pure EHPDB and Fe2O3 nanoparticles were weighed into separate vials (see Table 1).
- 1.5 mL of chloroform was added to the weighed amounts of nanoparticles at room temperature, and the solution was sonicated in 50 s(on)/10 s(off) mode for 45 min (A = 30%, ice blanket).
- The nanoparticle solution was sequentially poured into a vial with pure matrix EHPDB and chloroform was added to 2 mL, then the solution was left for 15 min to dissolve the EHPDB.
- The solution (EHPDB + Fe2O3 + chloroform) was sonicated in 50 s(on)/10 s(off) mode for 30 min (A = 30%, ice blanket).
- The solution (EHPDB + Fe2O3 + chloroform) was kept on the heating plate (80 °C) for about 150 min in order to obtain about 1 mL of solution.
- The solution (EHPDB + Fe2O3 + chloroform) was sonicated in 50 s(on)/10 s(off) mode for 15 min (A = 30%, ice blanket).
- The prepared solution was poured into microscopic slides and dried for at least 36 h at room temperature to evaporate the chloroform.
- Obtained composites were peeled off with a blade and used for measurements.
2.2. Powder X-ray Diffraction (PXRD)
2.3. Optical Microscopy (OM)
2.4. Scanning Electron Microscopy (SEM)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Polarizing Optical Microscopy (POM) and Electro-Optic Measurements (EOM)
2.7. Fluorescent Confocal Polarizing Microscopy (FCPM)
2.8. FT-MIR (Fourier Transform-Middle Infrared Spectroscopy)
3. Results and Discussion
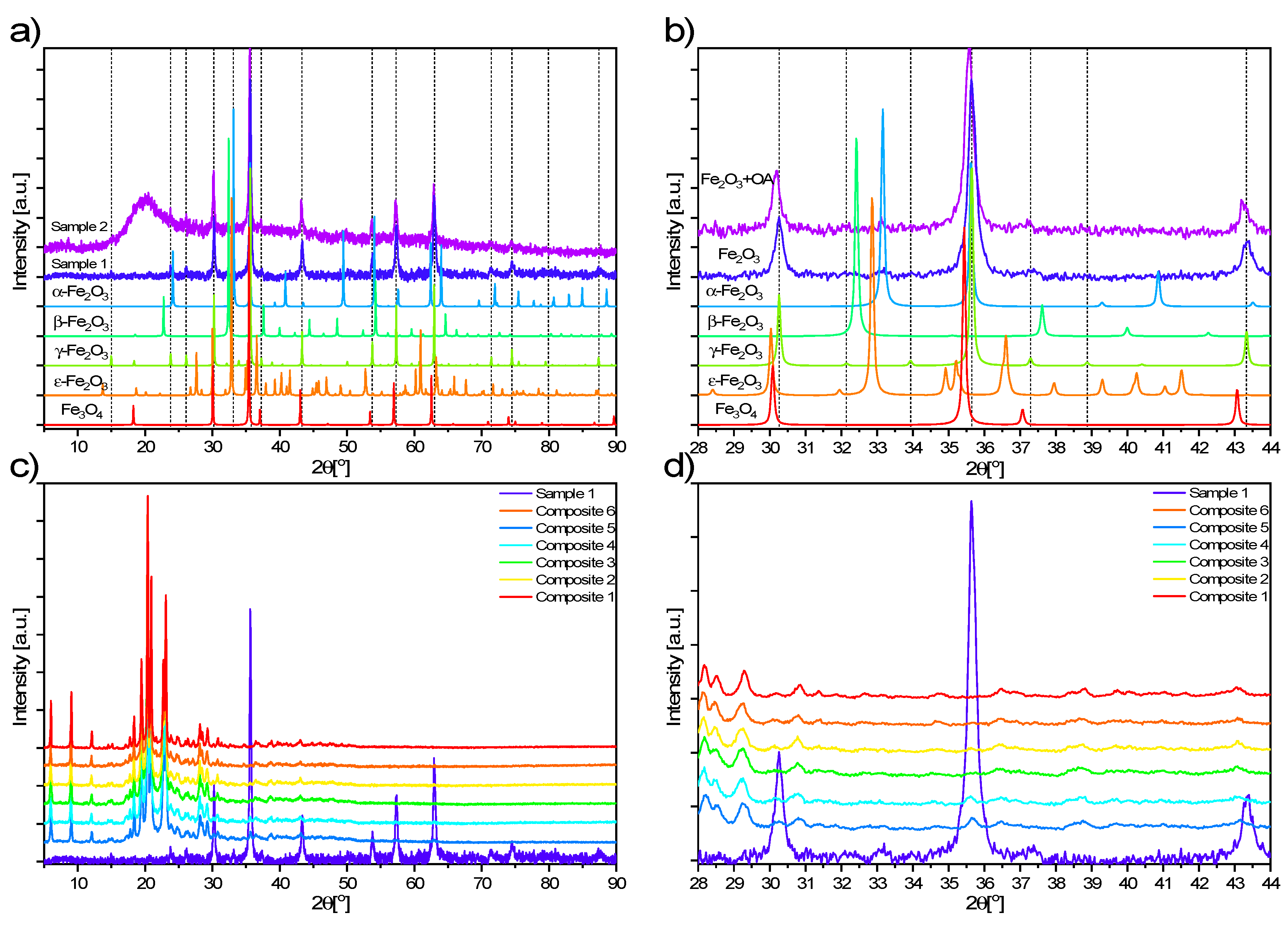
3.1. PXRD Results
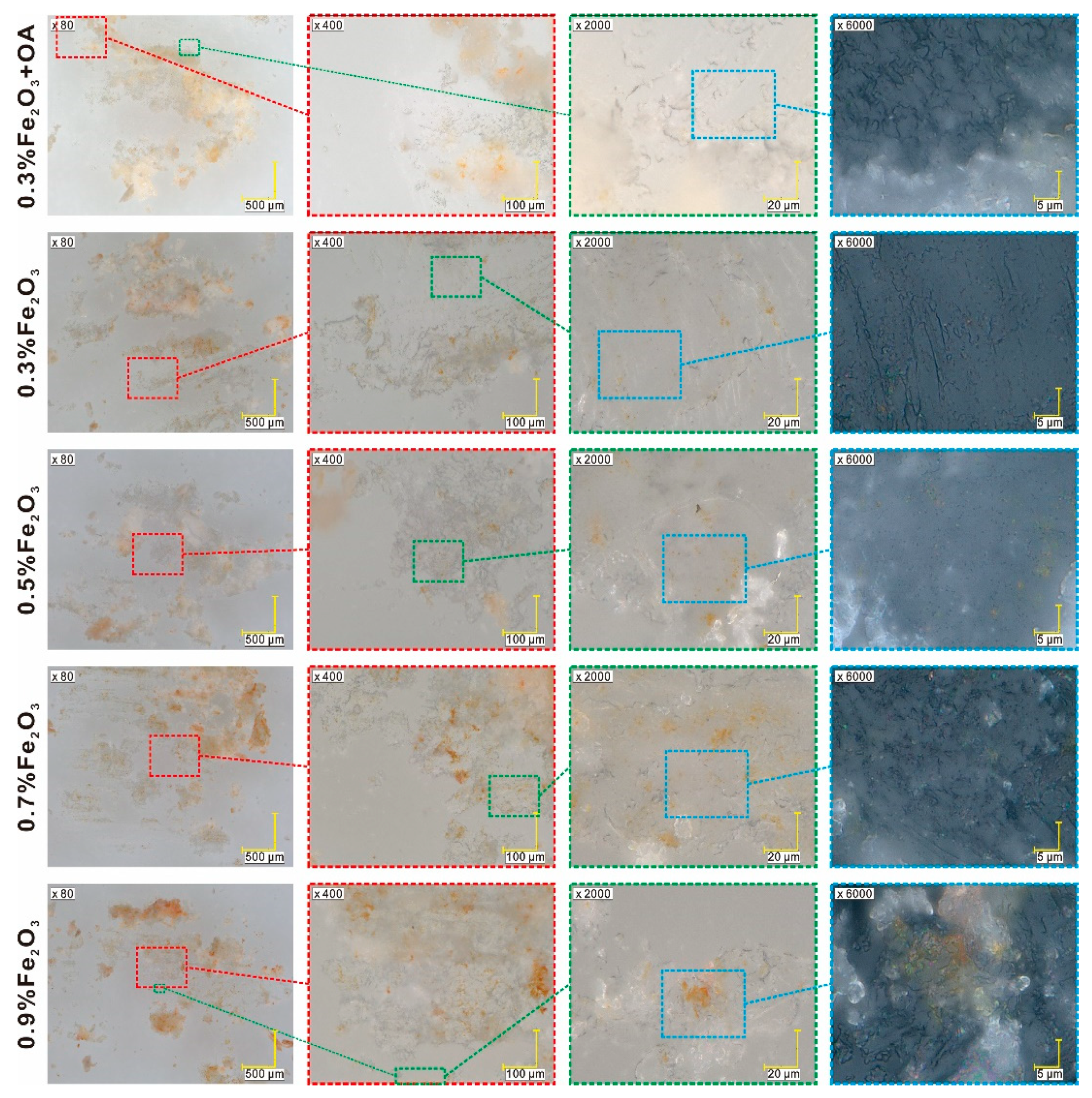
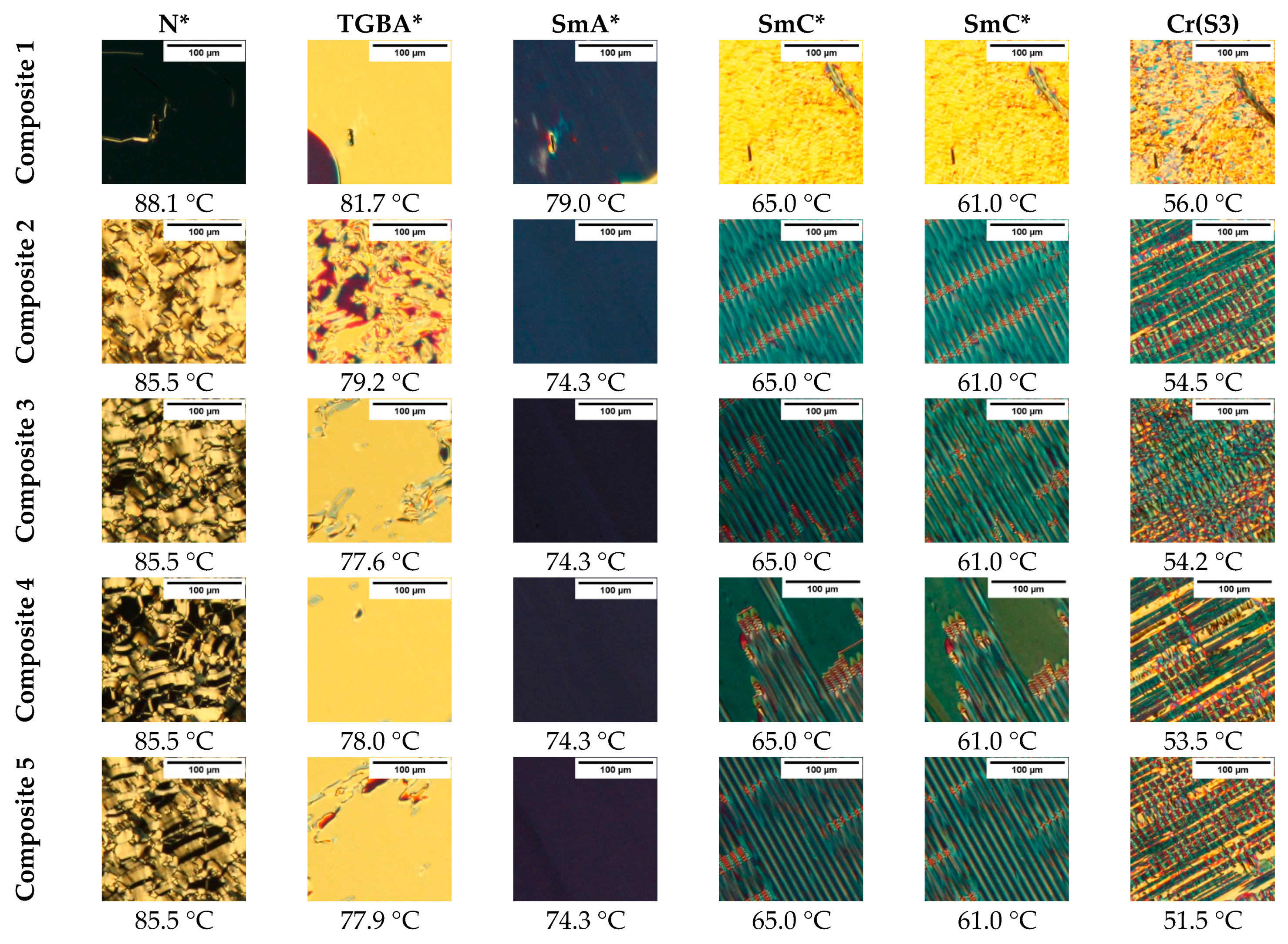
3.2. OM Results
3.3. SEM Results
3.4. FCPM Results
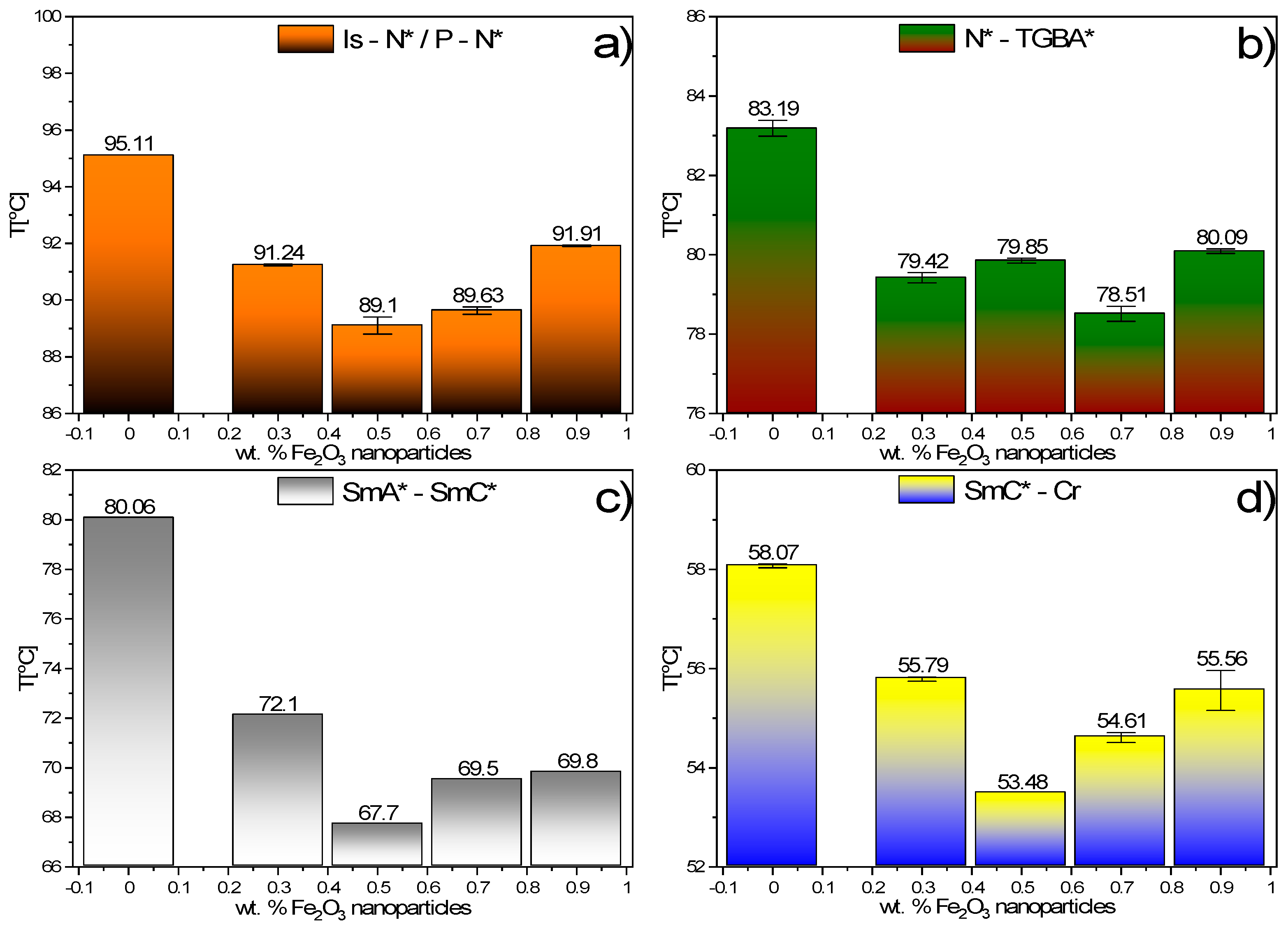
3.5. DSC Results
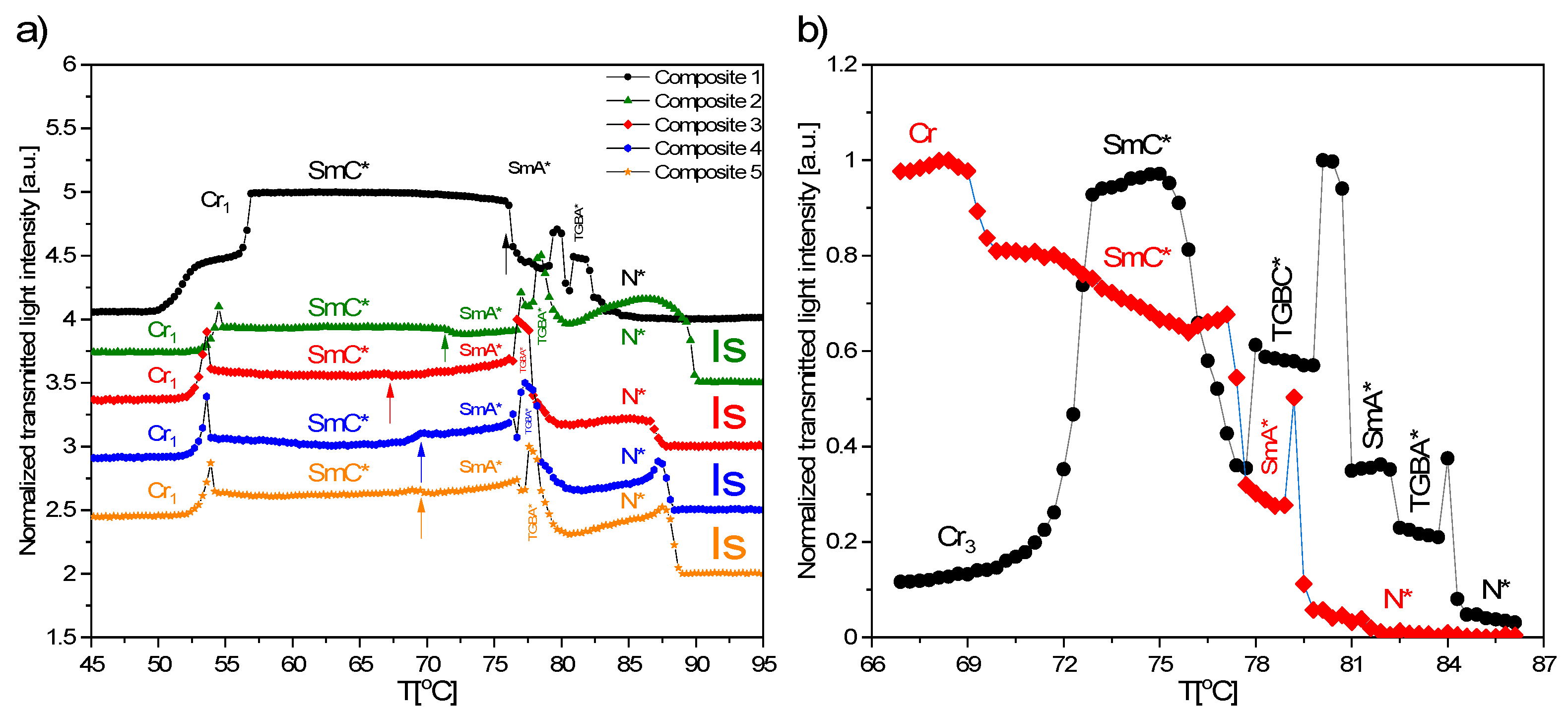
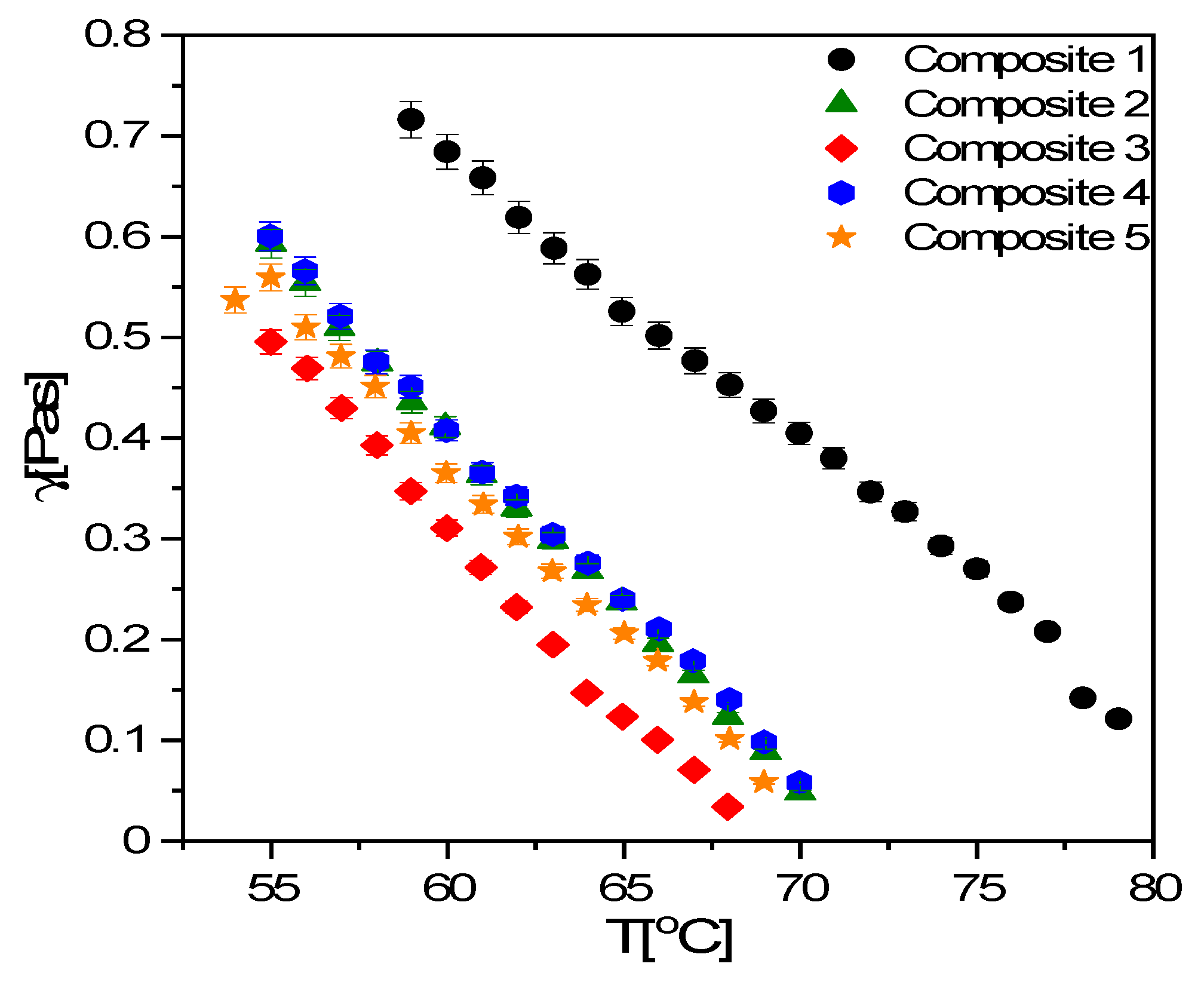
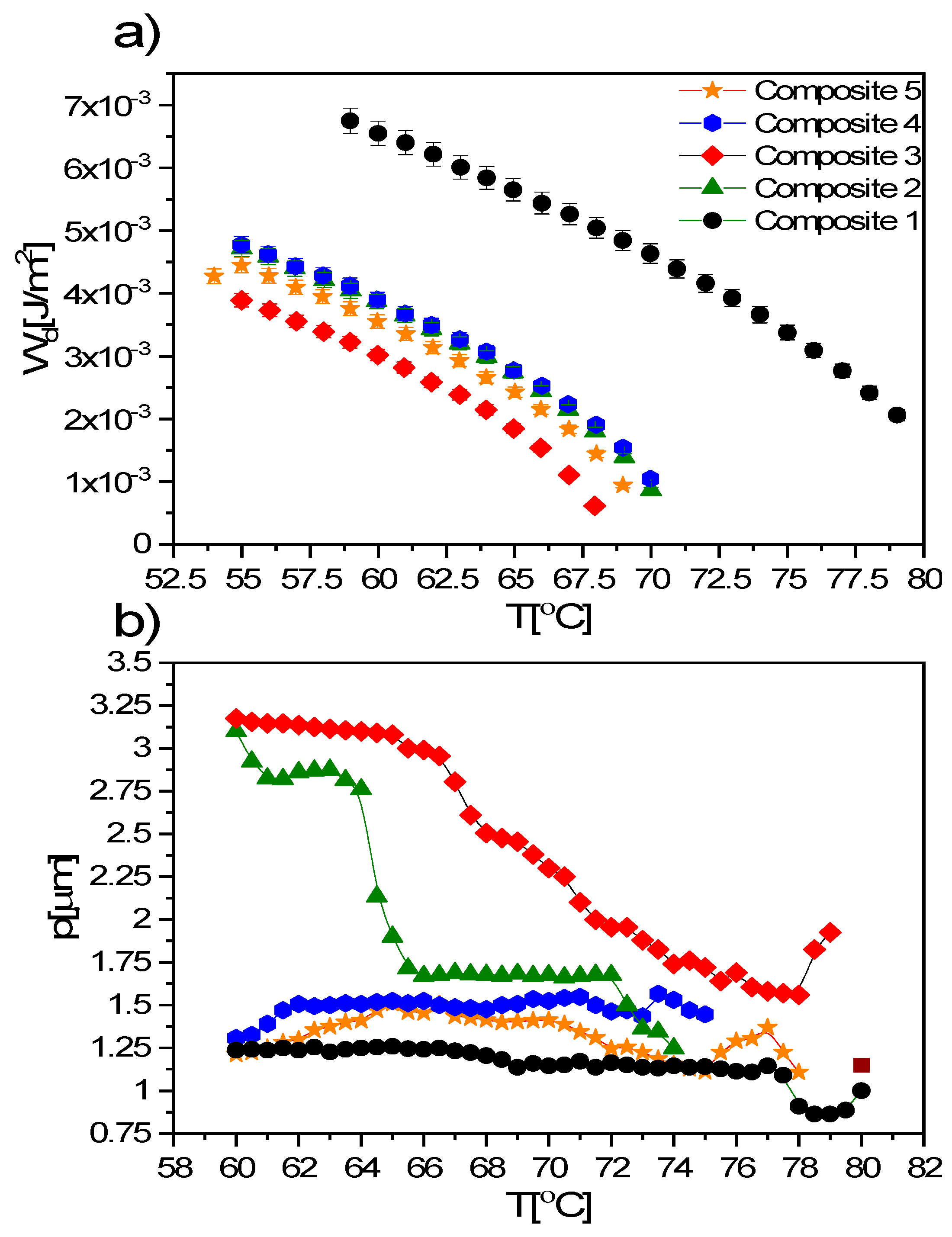
3.6. TLI Results
3.7. POM Results
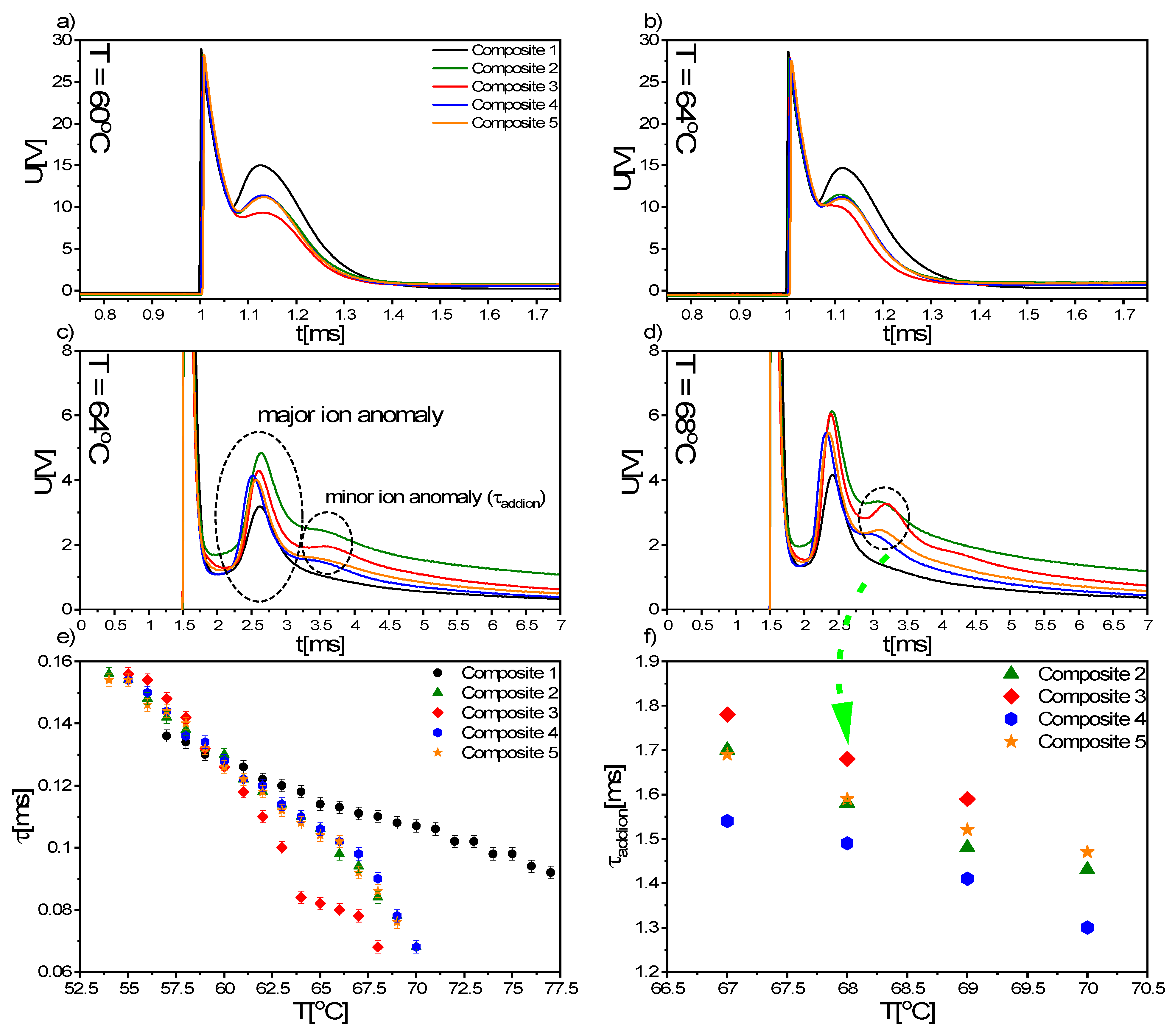
3.8. EOM Results
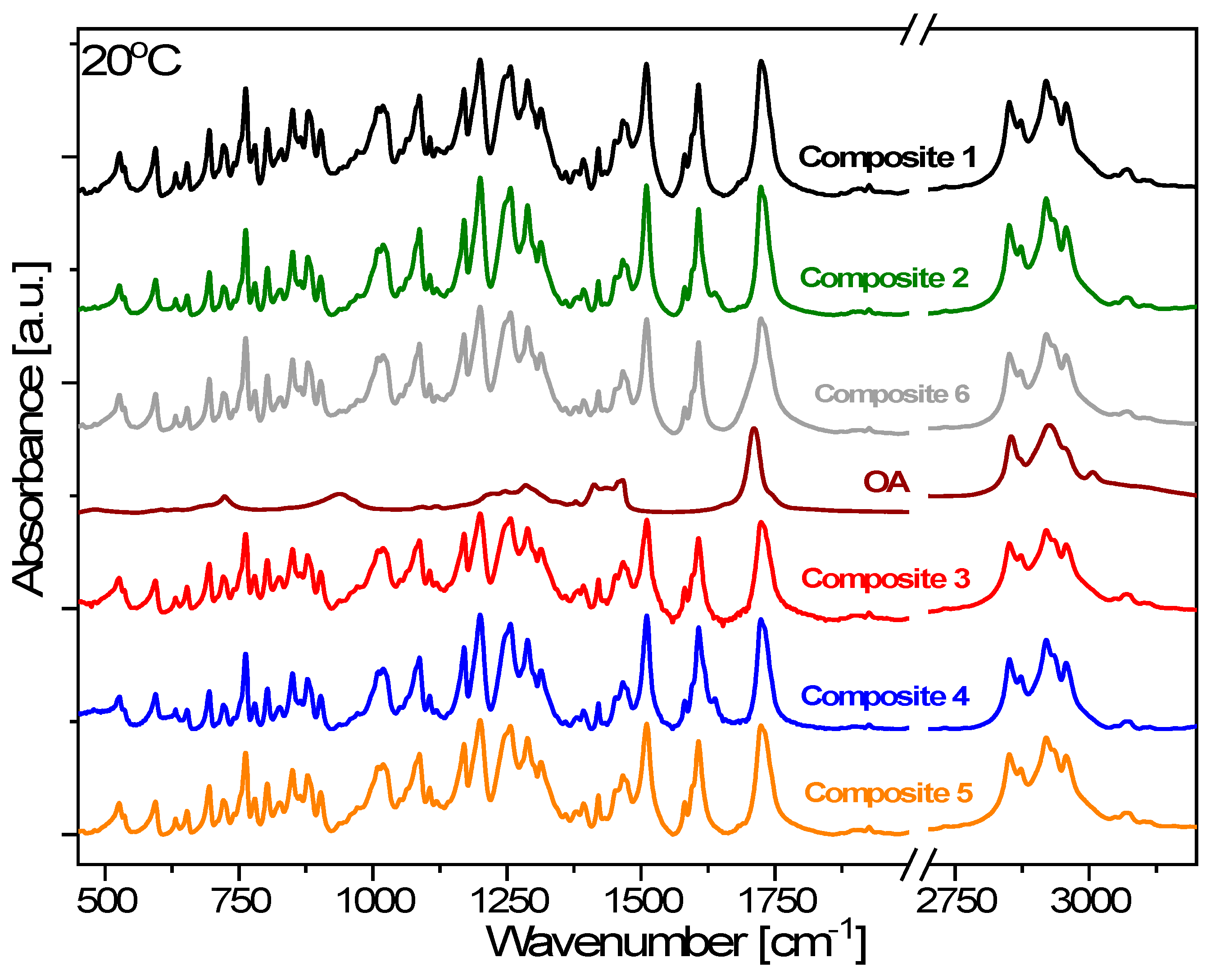
3.9. FTIR Results
4. Conclusions
- The structure of the EHPDB matrix did not change after doping. The diffraction patterns of the nanocomposites are practically the same as in the case of pure EHPDB, only the low-intensity reflections coming from Fe2O3 nanoparticles exist in addition. The surface morphology of the nanocomposites also did not change after doping (without oleic acid).
- The following phases during cooling were registered for studied composites: BP, N*, TGBA*, SmA*, TGBC* and SmC*. The shift of the phase transition temperatures to lower values, the disappearance of BP and the polymorphism of crystal phases, as well as the broadening of the SmA* versus the narrowing of the SmC* phase after the addition of Fe2O3 nanoparticles, were observed. What is interesting is that a very narrow TGBA* phase existed in all nanocomposites. The broadening of the SmA* phase in favor of SmC* was explained by modifying the mobility of nanoparticles. A very strong influence of the admixture on the texture of the SmC* phase in nanocomposites was observed as a strong striation.
- A very strong influence of the admixture on the electrical parameters was noted. The spontaneous polarization, tilt angle, switching time and rotational viscosity decreased after doping. The additional voltage response registered for nanocomposites was assigned to the additional ions introduced with nanoparticles into the EHPDB matrix. A slightly different modification of the SmC * phase parameters for nanocomposites with higher concentrations of nanoparticles (0.7 and 0.9 wt.%) was explained by the strong influence of Fe2O3 aggregation.
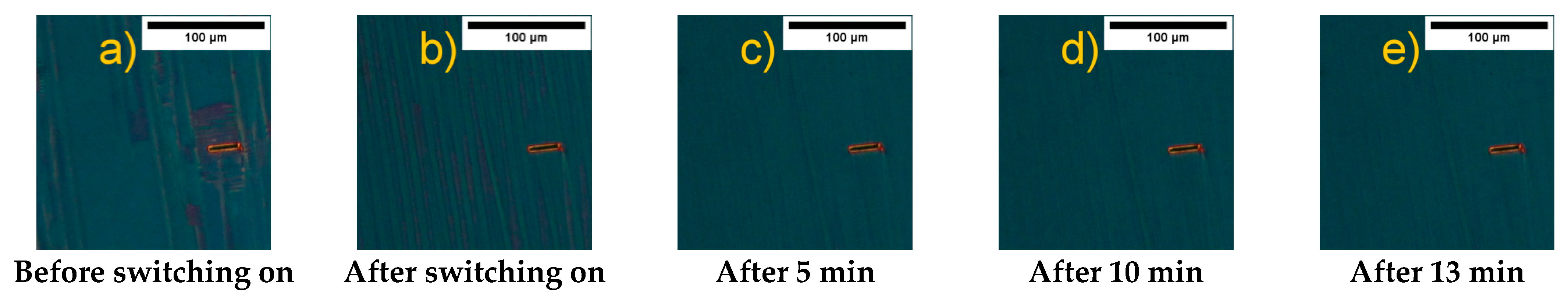
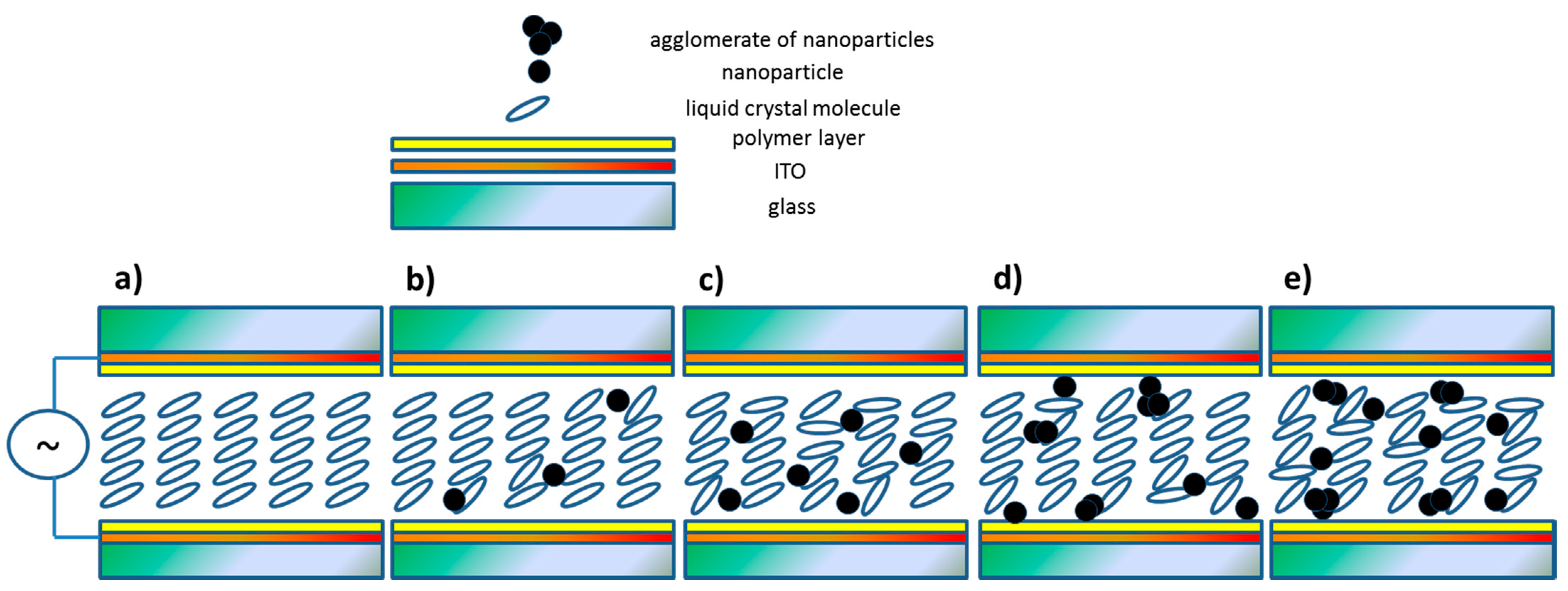
- The addition of oleic acid greatly narrowed the range of the mesophases with cooling and practically caused the disappearance of the liquid crystal polymorphism during heating. It was also responsible for the formation of ripples on the surface and changing the orientation of organic molecules from planar to homeotropic in a quite specific way (depending on the diameter of cholesteric droplets).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sugita, A.; Suzuki, K.; Kubono, A.; Tasaka, S. Electrical properties of trihexyl benzene tricarboxiamides. Jpn. J. Appl. Phys. 2008, 47, 1355–1358. [Google Scholar] [CrossRef]
- Kohn, H.; Ohshima, Y.; Manaka, T.; Iwamoto, M. Induced optical chirality of poly(diacetylene) film by circularly polarized light and its control by changing substrate temperature. Jpn. J. Appl. Phys. 2008, 47, 1359–1362. [Google Scholar] [CrossRef]
- Kurp, K.; Tykarska, M.; Drzewicz, A.; Lapanik, V.; Sasnouski, G. Effect of ferroelectric liquid crystalline quaterphenyl structure and handedness on helical pitch length in bicomponent mixtures. Liq. Cryst. 2017, 44, 618–627. [Google Scholar] [CrossRef]
- Ong, L.K.; Ha, S.T.; Yeap, G.Y.; Lin, H.C. Heterocyclic pyridine–based liquid crystals: Synthesis and mesomorphic properties. Liq. Cryst. 2018, 45, 1574–1584. [Google Scholar] [CrossRef]
- Bubnov, A.; Novotná, V.; Hamplová, V.; Kašpar, M.; Glogarová, M. Effect of multilactate chiral part of liquid crystalline molecule on mesomorphic behaviour. J. Mol. Struct. 2008, 892, 151–157. [Google Scholar] [CrossRef]
- Shell, P.; Chen, C.P.; Kim, D.S.; Jhun, C.G. Electro–Optical Effects of a Color Polymer–Dispersed Liquid Crystal Device by Micro–Encapsulation with a Pigment–Doped Shell. Crystals 2019, 9, 364. [Google Scholar]
- Zribi, O.; Garbovskiy, Y.; Glushchenko, A. Single–step colloidal processing of stable aqueous dispersions of ferroelectric nanoparticles for biomedical imaging. Mater. Res. Express 2015, 1, 045401. [Google Scholar] [CrossRef]
- Rejmer, W.; Czupryński, K.; Żurowska, M. An influence of the fluorosubstitution and the spacer length on helical twisting power of chiral three ring esters with perfluorinated alkanoyloxy unit in a terminal chain. Mol. Cryst. Liq. Cryst. 2011, 547, 3/[1693]–9/[1699]. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Toshima, N.; Maeda, K.; Yoshikawa, H.; Xu, J.; Kobayashi, S. Frequency modulation response of a liquid–crystal electro–optic device doped with nanoparticles. Appl. Phys. Lett. 2002, 81, 2845–2847. [Google Scholar] [CrossRef]
- Joshi, T.; Kumar, A.; Prakash, J.; Biradar, A.M. Low power operation of ferroelectric liquid crystal system dispersed with zinc oxide nanoparticles. Appl. Phys. Lett. 2010, 96, 2008–2011. [Google Scholar] [CrossRef]
- Kumar, A.; Prakash, J.; Mehta, D.S.; Biradar, A.M.; Haase, W. Enhanced photoluminescence in gold nanoparticles doped ferroelectric liquid crystals. Appl. Phys. Lett. 2009, 95, 28–31. [Google Scholar] [CrossRef]
- Prakash, J.; Choudhary, A.; Kumar, A.; Mehta, D.S.; Biradar, A.M. Nonvolatile memory effect based on gold nanoparticles doped ferroelectric liquid crystal. Appl. Phys. Lett. 2008, 93, 1–4. [Google Scholar] [CrossRef]
- Li, F.; West, J.; Reznikov, Y. Ferroelectric nanoparticle/liquid–crystal colloids for display applications. J. Soc. Inf. Disp. 2006, 14, 523–527. [Google Scholar] [CrossRef]
- Lalik, S.; Deptuch, A.; Jaworska-Gołąb, T.; Fryń, P.; Dardas, D.; Stefańczyk, O.; Urbańska, M.; Marzec, M. Modification of AFLC Physical Properties by Doping with BaTiO3 Particles. J. Phys. Chem. B 2020, 124, 6055–6073. [Google Scholar] [CrossRef]
- Manepalli, R.K.N.R.; Giridhar, G.; Pardhasaradhi, P.; Jayaprada, P.; Tejaswi, M.; Sivaram, K.; Kumar, C.M.; Pisipati, V.G.K.M. Influence of ZnO nanoparticles dispersion in Liquid Crystalline compounds—Experimental studies. Mater. Today Proc. 2018, 5, 2666–2676. [Google Scholar] [CrossRef]
- Kopčansky, P.; Koval’chuk, A.; Gornitska, O.; Vovk, V.; Koval’chuk, T.; Tomasovicov, N.; Koneracká, M.; Timko, M.; Zavisova, V.; Jadżyn, J. Dielectric spectroscopy of liquid crystal doped with Fe3O4 Nanoparticles. Phys. Procedia 2010, 9, 36–40. [Google Scholar] [CrossRef]
- Manna, S.K. Development of a Phenomenological Model on Surface Stabilized Ferroelectric Liquid Crystal Nanocomposite. IOSR J. Appl. Phys. 2012, 1, 33–38. [Google Scholar] [CrossRef]
- Raina, K.K. Nickel nanoparticles doped ferroelectric liquid crystal composites. Opt. Mater. 2013, 35, 531–535. [Google Scholar] [CrossRef]
- Roy, J.S.; Majumder, T.P.; Dąbrowski, R. Enhanced photoluminescence of antiferroelectric liquid crystals doped with sodium titanate nanoparticles. Funct. Mater. Lett. 2014, 7, 10–13. [Google Scholar] [CrossRef]
- Rudzki, A. Electro–optical properties of nanodispersions based on ferroelectric thiobenzoates. Phase Trans. 2015, 88, 513–520. [Google Scholar] [CrossRef]
- Rožič, B.; Jagodič, M.; Gyergyek, S.; Drofenik, M.; Kralj, S.; Jagličić, Z.; Kutnjak, Z. Mixtures of magnetic nanoparticles and the ferroelectric liquid crystal: New soft magnetoelectrics. Ferroelectrics 2012, 431, 150–153. [Google Scholar] [CrossRef]
- Rožič, B.; Jagodič, M.; Gyergyek, S.; Drofenik, M.; Kralj, S.; Cordoyiannis, G.; Kutnjak, Z. Multiferroic behaviour in mixtures of the ferroelectric liquid crystal and magnetic nanoparticles. Mol. Cryst. Liq. Cryst. 2011, 545, 99/[1323]–104/[1328]. [Google Scholar] [CrossRef]
- Chatterjee, J.; Haik, Y.; Chen, C.J. Size dependent magnetic properties of iron oxide nanoparticles. J. Magn. Magn. Mater. 2003, 257, 113–118. [Google Scholar] [CrossRef]
- Yadav, G.; Roy, A.; Agrahari, K.; Katiyar, R.; Chandel, V.S.; Manohar, R. Influence of Fe2O3 nanoparticles on the birefringence property of weakly polar nematic liquid crystal. Mol. Cryst. Liq. Cryst. 2019, 680, 65–74. [Google Scholar] [CrossRef]
- Gao, L.; Dai, Y.; Li, T.; Tang, Z.; Zhao, X.; Li, Z.; Meng, X.; He, Z.; Li, J.; Cai, M.; et al. Enhancement of image quality in LCD by doping γ–Fe2O3 nanoparticles and reducing friction torque difference. Nanomaterials 2018, 8, 911. [Google Scholar] [CrossRef]
- Koo, W.S.; Chung, H.K.; Park, H.G.; Han, J.J.; Jeong, H.C.; Cho, M.J.; Kim, D.H.; Seo, D.S. Enhanced switching behavior of iron oxide nanoparticle–doped liquid–crystal display. J. Nanosci. Nanotechnol. 2014, 14, 8609–8614. [Google Scholar] [CrossRef]
- Katiyar, R.; Pathak, G.; Agrahari, K.; Srivastava, A.; Garbat, K.; Manohar, R. Investigation of dielectric and electro–optical parameters of high birefringent nematic liquid crystal doped with TiO2 nanoparticles and its applicability toward liquid crystal displays. Mol. Cryst. Liq. Cryst. 2019, 691, 50–61. [Google Scholar] [CrossRef]
- Sivasri, J.; Rao, M.C.; Giridhar, G.; Madav, B.T.P.; Divakar, T.E.; Manepalli, R.K.N.R. Influence of Fe3O4 nanoparticles dispersed in liquid crystalline compounds—Spectroscopic characterization. Rasayan J. Chem. 2016, 9, 556–565. [Google Scholar]
- Goel, P.; Arora, M.; Biradar, A.M. Electro–optic switching in iron oxide nanoparticle embedded paramagnetic chiral liquid crystal via magneto–electric coupling. J. Appl. Phys. 2014, 115, 124905. [Google Scholar] [CrossRef]
- Novotná, V.; Vejpravová, J.; Hamplová, V.; Prokleška, J.; Górecka, E.; Pociecha, D.; Podoliak, N.; Glogarová, M. Nanocomposite of superparamagnetic maghemite nanoparticles and ferroelectric liquid crystal. RSC Adv. 2013, 3, 10919–10926. [Google Scholar] [CrossRef][Green Version]
- Romero–Hasler, P.N.; Kurihara, L.K.; Mair, L.O.; Weinberg, I.N.; Soto–Bustamante, E.A.; Martínez–Miranda, L.J. Nanocomposites of ferroelectric liquid crystals and FeCo nanoparticles: Towards a magnetic response via the application of a small electric field. Liq. Cryst. 2020, 47, 169–178. [Google Scholar] [CrossRef]
- Gathania, A.K.; Singh, B.; Raina, K.K. Dielectric relaxations in a room temperature ferroelectric liquid crystal mixture. J. Phys. Condens. Matter 1999, 11, 3813–3822. [Google Scholar] [CrossRef]
- Suber, L.; Santiago, A.G.; Fiorani, D.; Imperatori, P.; Maria Testa, A.; Angiolini, M.; Montone, A.; Dormann, J.L. DormannStructural and magnetic properties of α-Fe2O3 nanoparticles. Appl. Organomet. Chem. 1998, 12, 347–351. [Google Scholar] [CrossRef]
- Suman, S.; Sharma, V.; Devi, S.; Chahal, S.; Singh, J.P.; Chae, K.H.; Kumar, A.; Asokan, K.; Kumar, P. Phase transformation in Fe2O3 nanoparticles: Electrical properties with local electronic structure. Physica B Condens. Matter 2021, 620, 413275. [Google Scholar] [CrossRef]
- Al-Hakkani, M.F.; Gouda, G.A.; Hassan, S.H.A. A review of green methods for phyto-fabrication of hematite (α-Fe2O3) nanoparticles and their characterization, properties, and applications. Heliyon 2021, 7, e05806. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. Do Iron Oxide Nanoparticles Have Significant Antibacterial Properties? Antibiotics 2021, 10, 884. [Google Scholar] [CrossRef]
- Pozhidaev, E.; Minchenko, M.; Molkin, V.; Torgova, S.; Chigrinov, V.G.; Srivastava, A.K.; Kwok, H.S.; Vashenko, V.; Krivoshey, A. High frequency low voltage shock–free ferroelectric liquid crystal: A new electro–optical mode with electrically suppressed helix. In Proceedings of the 31st International Display Research Conference, Arcachon, France, 19–22 September 2011. [Google Scholar]
- Hemine, J.; Daoudi, A.; Legrand, C.; Isaert, N.; Kaaouachi, A.E.; Nafidi, A.; Nguyen, H.T. Dielectric spectroscopy of the goldstone–mode relaxation in the surface–stabilized chiral smectic C phase in ferroelectric liquid crystals. Ferroelectrics 2008, 371, 104–109. [Google Scholar] [CrossRef]
- Alla, R.A.; Hegde, G.; Komitov, L.; Morelli, A.; Chiellini, E.; Galli, G. Composite Materials Containing Perfluorinated and Siloxane Units for Vertical Alignment of Liquid Crystals. Soft Nanosci. Lett. 2013, 3, 11–13. [Google Scholar] [CrossRef][Green Version]
- Piecek, W.; Perkowski, P.; Raszewski, Z.; Morawiak, P.; Żurowska, M.; Dąbrowski, R.; Czupryński, K. Long pitch orthoconic antiferroelectric binary mixture for display applications. Mol. Cryst. Liq. Cryst. 2010, 525, 140–152. [Google Scholar] [CrossRef]
- Bawa, S.; Biradar, A.M.; Saxena, K.; Chandra, S. Direct pulse technique for spontaneous polarization dynamics and molecular reorientation processes in ferroelectric liquid crystals. Rev. Sci. Instrum. 1988, 59, 2023–2030. [Google Scholar] [CrossRef]
- Lee, J.; Ani, A.D.L.C.; Itoh, K.; Ouchi, Y.; Takezoe, H.; Fukuda, A. Frequency–Dependent Switching Behavior under Triangular Waves in Antiferroelectric and Ferrielectric Chiral Smectic Phases. Jpn. J. Appl. Phys. 1990, 29, 1122–1127. [Google Scholar] [CrossRef]
- Hou, J.; Schacht, J.; Gießelmann, F.; Zugenmaier, P. Investigations on the field–induced switching behaviour in an antiferroelectric liquid crystal. Liq. Cryst. 1997, 22, 401–407. [Google Scholar] [CrossRef]
- Dwivedi, A.; Dhar, R.; Dąbrowski, R.; Tykarska, M. Characteristic switching and dielectric parameters of ferro- and anti-ferroelectric phases of (S)-(+)-(1-methylheptyloxycarbonyl) phenyl 4′-(6-perfluoropentanoyloxyhex-1-oxy) biphenyl-4-carboxylate. J. Phys. D Appl. Phys. 2009, 42, 095402. [Google Scholar] [CrossRef]
- Dardas, D. Electro–optic and viscoelastic properties of a ferroelectric liquid crystalline binary mixture. Phase Trans. 2016, 89, 368–375. [Google Scholar] [CrossRef]
- Lavrentovich, O.D.; Pasini, P.; Zannoni, C.; Zumer, S. Defects in Liquid Crystals: Computer Simulations, Theory and Experiments; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Smalyukh, I.I.; Shiyanovskii, S.V.; Lavrentovich, O.D. Three–dimensional imaging of orientational order by fluorescence confocal polarizing microscopy. Chem. Phys. Lett. 2001, 336, 88–96. [Google Scholar] [CrossRef]
- Nowicka, K.; Bielejewska, N.; Kuczyński, W.; Knapkiewicz, M.; Hoffmann, J. Exploration of liquid crystal structures using fluorescent confocal polarizing microscopy. Phase Trans. 2014, 87, 1073–1079. [Google Scholar] [CrossRef]
- Ohkoshi, S.I.; Namai, A.; Imoto, K.; Yoshikiyo, M.; Tarora, W.; Nakagawa, K.; Komine, M.; Miyamoto, Y.; Nasu, T.; Oka, S.; et al. Nanometer–size hard magnetic ferrite exhibiting high optical–transparency and nonlinear optical–magnetoelectric effect. Sci. Rep. 2015, 5, 14414. [Google Scholar] [CrossRef]
- Sakurai, S.; Namai, A.; Hashimoto, K.; Ohkoshi, S.I. First observation of phase transformation of all four Fe2O3 phases (γ → ε → β → α–phase). J. Am. Chem. Soc. 2009, 131, 18299–18303. [Google Scholar] [CrossRef]
- Pandey, K.B.; Shahi, A.K.; Shah, J.; Kotnala, R.K.; Gopal, R. Optical and magnetic properties of Fe2O3 nanoparticles synthesized by laser ablation/fragmentation technique in different liquid media. Appl. Surf. Sci. 2014, 289, 462–471. [Google Scholar] [CrossRef]
- Malina, O.; Tuček, J.; Jakubec, P.; Kašlík, J.; Medřík, I.; Tokoro, H.; Yoshikiyo, M.; Namai, A.; Ohkoshi, S.I.; Zbořil, R. Magnetic ground state of nanosized β–Fe2O3 and its remarkable electronic features. RSC Adv. 2015, 5, 49719–49727. [Google Scholar] [CrossRef]
- Shokrollahi, H. A review of the magnetic properties, synthesis methods and applications of maghemite. J. Magn. Magn. Mater. 2016, 426, 74–81. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, X.H.; Zhang, S.F.; Peng, T.C.; Zhou, J.; Ren, F.; Jiang, C.Z. Synthesis and magnetic properties of maghemite (γ–Fe2O3) short–nanotubes. Nanoscale Res. Lett. 2010, 5, 1474–1479. [Google Scholar] [CrossRef]
- Ohkoshi, S.I.; Namai, A.; Yamaoka, T.; Yoshikiyo, M.; Imoto, K.; Nasu, T.; Anan, S.; Umeta, Y.; Nakagawa, K.; Tokoro, H. Mesoscopic bar magnet based on ϵ–Fe2O3 hard ferrite. Sci. Rep. 2016, 6, 27212. [Google Scholar] [CrossRef] [PubMed]
- Tuček, J.; Zbořil, R.; Namai, A.; Ohkoshi, S.I. ε–Fe2O3: An advanced nanomaterial exhibiting giant coercive field, millimeter–wave ferromagnetic resonance, and magnetoelectric coupling. Chem. Mater. 2010, 22, 6483–6505. [Google Scholar] [CrossRef]
- Tuček, J.; Machala, L.; Ono, S.; Namai, A.; Yoshikiyo, M.; Imoto, K.; Tokoro, H.; Ohkoshi, S.I.; Zbořil, R. Zeta–Fe2O3—A new stable polymorph in iron(III) oxide family. Sci. Rep. 2015, 5, 15091. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, M.; Romero, C.; McCloy, J. Magnetic analysis of commercial hematite, magnetite, and their mixtures. AIP Adv. 2018, 8, 056807. [Google Scholar] [CrossRef]
- Kundu, S.; Majumder, T.P.; Roy, S.K.; Darius, M.; Haase, W. Studies on the dielectric behavior of ferroelectric liquid crystal material having a TGBA phase. Ferroelectrics 2000, 244, 39–47. [Google Scholar] [CrossRef]
- Da Cruz, C.; Sandre, O.; Cabuil, V. Phase behavior of nanoparticles in a thermotropic liquid crystal. J. Phys. Chem. B 2005, 109, 14292–14299. [Google Scholar] [CrossRef] [PubMed]
- Sivasri, J.; Pardhasaradhi, P.; Madhav, B.T.P.; Tejaswi, M.; Manepalli, R.K.N.R. Birefringence studies on alkoxy benzoic acids with dispersed Fe3O4 nanoparticles. Liq. Cryst. 2020, 47, 330–344. [Google Scholar] [CrossRef]
- Chakraborty, S.; Das, M.K.; Bubnov, A.; Weissflog, W.; Wȩgłowska, D.; Dąbrowski, R. Induced frustrated twist grain boundary liquid crystalline phases in binary mixtures of achiral hockey stick–shaped and chiral rod–like materials. J. Mater. Chem. C 2019, 7, 10530–10543. [Google Scholar] [CrossRef]
- Dierking, I. Textures of Liquid Crystals; WILEY–VCH: Hoboken, NJ, USA, 2003. [Google Scholar]
- Blinov, L.; Barnik, M.; Lazareva, V.; Trufanov, A. Electrohydrodynamic instabilities in the liquid crystalline phases with smectic ordering. Journal de Physique Colloques 1979, 40, C3-C263–C3-C268. [Google Scholar] [CrossRef]
- Chiba, S.; Yoshizawa, A. Electrooptical properties of liquid crystal oligomer possessing both lateral and terminal polar groups. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 2008, 47, 6386–6389. [Google Scholar] [CrossRef]
- Haase, W.; Hiller, S.; Pfeiffer, M.; Beresnev, L.A. The domain mode in ferroelectric liquid crystals; electrooptical and dielectric investigations. Ferroelectrics 1993, 140, 37–44. [Google Scholar] [CrossRef]
- Misra, A.K.; Tripathi, P.K.; Pandey, K.K.; Pandey, F.P.; Singh, S.; Singh, A. Electro–optic switching and memory effect in suspension of ferroelectric liquid crystal and iron oxide nanoparticles. Mater. Res. Express 2019, 6, 1050d2. [Google Scholar] [CrossRef]
- Marzec, M.; Wróbel, S.; Hiller, S.; Biradar, A.M.; Dąbrowski, R.; Gestblom, B.; Haase, W. Dynamical properties of two ferroelectric phases of epoxy compound. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1997, 302, 35–40. [Google Scholar] [CrossRef]
- Zakerhamidi, M.S.; Shoarinejad, S.; Mohammadpour, S. Fe3O4 nanoparticle effect on dielectric and ordering behavior of nematic liquid crystal host. J. Mol. Liq. 2014, 191, 16–19. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Buchnev, O.; Nandhakumar, I. Ferroelectric nanoparticles in low refractive index liquid crystals for strong electro–optic response. Appl. Phys. Lett. 2008, 92, 67–70. [Google Scholar] [CrossRef]
- Gökçen, M.; Yildirim, M.; Köysal, O. Dielectric and AC electrical conductivity characteristics of liquid crystal doped with graphene. EPJ Appl. Phys. 2012, 60, 1–5. [Google Scholar] [CrossRef]
- Khushboo Sharma, P.; Malik, P.; Raina, K.K. Electro–optic, dielectric and optical studies of NiFe2O4–ferroelectric liquid crystal: A soft magnetoelectric material. Liq. Cryst. 2016, 43, 1671–1681. [Google Scholar] [CrossRef]
- Prodanov, M.F.; Popova, E.V.; Gamzaeva, S.A.; Fedoryako, A.P.; Vashchenko, V.V. Ferromagnetic nanoparticles in a ferroelectric liquid crystal: Properties of stable colloids in homogeneous cells. J. Mol. Liq. 2018, 267, 353–362. [Google Scholar] [CrossRef]
- Popova, E.V.; Gamzaeva, S.A.; Krivoshey, A.I.; Kryshtal, A.P.; Fedoryako, A.P.; Prodanov, M.F.; Kolosov, M.A.; Vashchenko, V.V. Dielectric properties of magnetic nanoparticles’ suspension in a ferroelectric liquid crystal. Liq. Cryst. 2015, 42, 334–343. [Google Scholar] [CrossRef]
- Steimle, T.C.; Gengler, J.; Hodges, P.J. The permanent electric dipole moments of iron monoxide, FeO. J. Chem. Phys. 2004, 121, 12303–12307. [Google Scholar] [CrossRef]
- Goel, P.; Upadhyay, P.L.; Biradar, A.M. Induced dielectric relaxation and enhanced electro–optic parameters in Ni nanoparticles—ferroelectric liquid crystal dispersions. Liq. Cryst. 2013, 40, 45–51. [Google Scholar] [CrossRef]
- Lalik, S.; Deptuch, A.; Fryń, P.; Jaworska–Gołąb, T.; Dardas, D.; Pociecha, D.; Urbańska, M.; Tykarska, M.; Marzec, M. Systematic study of the chiral smectic phases of a fluorinated compound. Liq. Cryst. 2019, 46, 2256–2268. [Google Scholar] [CrossRef]
- Vimal, T.; Pandey, S.; Gupta, S.K.; Singh, D.P.; Agrahari, K.; Pathak, G.; Kumar, S.; Tripathi, P.K.; Manohar, R. Manifestation of strong magneto–electric dipolar coupling in ferromagnetic nanoparticles−FLC composite: Evaluation of time–dependent memory effect. Liq. Cryst. 2018, 45, 687–697. [Google Scholar] [CrossRef]
- Yadav, G.; Pathak, G.; Agrahari, K.; Kumar, M.; Khan, M.S.; Chandel, V.S.; Manohar, R. Improved dielectric and electro–optical parameters of nematic liquid crystal doped with magnetic nanoparticles. Chin. Phys. B 2019, 28, 034209. [Google Scholar] [CrossRef]
- Blinov, L.M.; Chigrinov, V.G. Electrooptic Effects in Liquid Crystal Materials; Springer: Berlin, Germany, 1994. [Google Scholar]
- Gear, C.; Diest, K.; Liberman, V.; Rothschild, M. Engineered liquid crystal anchoring energies with nanopatterned surfaces. Opt. Express 2015, 23, 807. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, C.H.; Ye, Y.C. Effects of mechanical rubbing on surface tension of polyimide thin films. Jpn. J. Appl. Phys. 2008, 47, 1651–1656. [Google Scholar] [CrossRef]
- Lyuu, J.F.; Chen, C.C.; Lee, J.Y. Anchoring energy coefficient of dye guest–host ferroelectric liquid crystals. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1999, 329, 711–724. [Google Scholar] [CrossRef]
- Nepal, S.; Mondal, S.; Sinha, A.; Das, B.; Das, M.K. Fast switching behaviour and dielectric parameters of two chiral ferroelectric mesogens. Liq. Cryst. 2020, 47, 1464–1472. [Google Scholar] [CrossRef]
- Misra, A.K.; Srivastava, A.K.; Shukla, J.P.; Manohar, R. Dielectric and electro–optical parameters of two ferroelectric liquid crystals: A comparative study. Phys. Scr. 2008, 78, 065602. [Google Scholar] [CrossRef]
- Middha, M.; Kumar, R.; Raina, K.K. Improved Electro–Optical Response of Induced Chiral Nematic Liquid Crystal Doped with Multi–Walled Carbon Nanotubes. Ferroelectrics 2016, 495, 75–86. [Google Scholar] [CrossRef]




| Composite | Amount of EHPDB | Amount of Fe2O3 | Amount of Oleic Acid |
|---|---|---|---|
| Composite 1 (0.0 wt.%) | 40.52 mg | 0.00 mg | – |
| Composite 2 (0.3 wt.%) | 40.32 mg | 0.12 mg | – |
| Composite 3 (0.5 wt.%) | 40.20 mg | 0.20 mg | – |
| Composite 4 (0.7 wt.%) | 40.91 mg | 0.29 mg | – |
| Composite 5 (0.9 wt.%) | 40.20 mg | 0.37 mg | – |
| Composite 6 (0.3 wt.% + OA) | 40.07 mg | 0.12 mg | 0.5 μL |
| Amount of Fe2O3 | Amount of Oleic Acid | ||
| Sample 1 | 36.00 mg 36.01 mg | – | |
| Sample 2 | 150.0 μL | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalik, S.; Stefańczyk, O.; Dardas, D.; Górska, N.; Ohkoshi, S.-i.; Marzec, M. Modifications of FLC Physical Properties through Doping with Fe2O3 Nanoparticles (Part I). Materials 2021, 14, 4722. https://doi.org/10.3390/ma14164722
Lalik S, Stefańczyk O, Dardas D, Górska N, Ohkoshi S-i, Marzec M. Modifications of FLC Physical Properties through Doping with Fe2O3 Nanoparticles (Part I). Materials. 2021; 14(16):4722. https://doi.org/10.3390/ma14164722
Chicago/Turabian StyleLalik, Sebastian, Olaf Stefańczyk, Dorota Dardas, Natalia Górska, Shin-ichi Ohkoshi, and Monika Marzec. 2021. "Modifications of FLC Physical Properties through Doping with Fe2O3 Nanoparticles (Part I)" Materials 14, no. 16: 4722. https://doi.org/10.3390/ma14164722
APA StyleLalik, S., Stefańczyk, O., Dardas, D., Górska, N., Ohkoshi, S.-i., & Marzec, M. (2021). Modifications of FLC Physical Properties through Doping with Fe2O3 Nanoparticles (Part I). Materials, 14(16), 4722. https://doi.org/10.3390/ma14164722








