5.1. Strength Development of Phosphate-Bonded Al2O3-MgAl2O4 High-Temperature Ceramics
Generating sufficient early stage strength, that allows ceramic components to be shaped and processed, is of utmost importance in ceramic manufacturing. By adding phosphate binders to the ceramic mix, the bonding strength of ceramics can be improved. Through extensive investigation of structural changes of the bonding phase, differences and changes in the bonding strengths can be explained analytically. For example, structure–property correlations can be achieved by comparing the cold modulus of rupture of phosphate bonded Al
2O
3-MgAl
2O
4-ceramics with structural changes in the bonding phase after a defined thermal treatment monitored, for instance, by
31P MAS NMR (
Figure 4).
The strength development of the ceramic components varies depending on the phosphate used. Early stage strength is achieved by applying water soluble phosphates as binders (water solubility at 25 °C: Al(H2PO4)3: >250 g/L; Mg(H2PO4)2: 70 g/L; Ca(H2PO4)2: 20 g/L; CaHPO4 < 1 g/L). Further heating generally leads to an increased strength of the components, but the strength developments as well as the end values after thermal treatment at T = 1500 °C vary significantly.
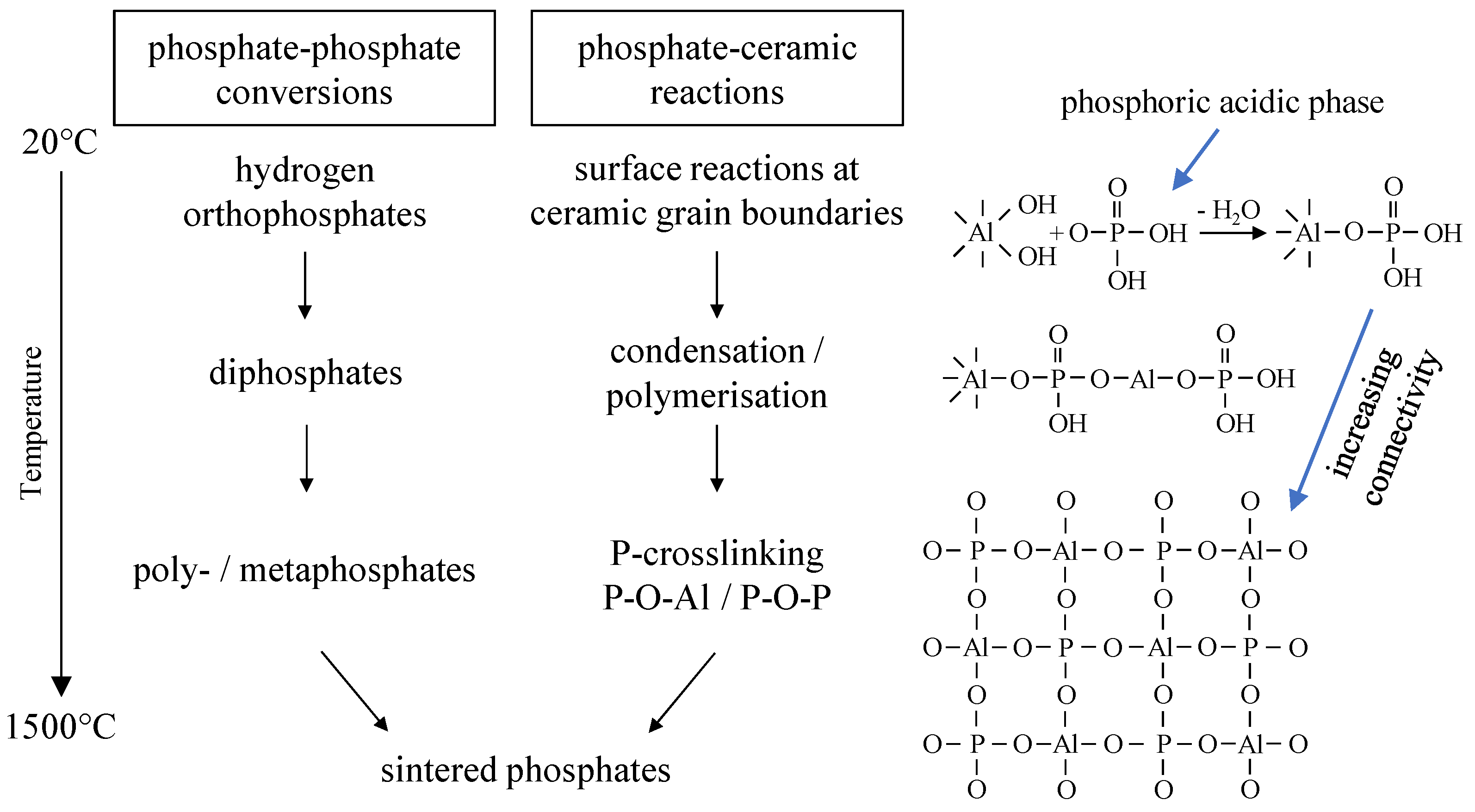
The strength development of the phosphate-bonded ceramics can be explained by evaluating the structure of phosphatic bonding phases. Investigating the phosphate connectivity by solid-state
31P NMR allows conclusions to be drawn about the bonding capacity of the developing phosphate structures. It can be stated that the formation of a P-crosslinked aluminum phosphate bonding network leads to an increasing strength of the ceramic components. Aluminum is incorporated in the glass structure and stabilizes crosslinked phosphate bonding networks. Within the refractory body, the binder creates a branched three-dimensional network because of chemical reactions, polymerization and polycondensation. With increasing temperature, the degree of P-crosslinking continuously increases via multiple crosslinking reactions (P–O–Al and P–O–P bonds) within the initialization and network-forming stages The bonding effect is based on adhesive and cohesive forces of the amorphous aluminum phosphate network thus leading to the strength development seen in
Figure 4. The development of aluminum phosphates has been verified by solid-state
27Al NMR and PXRD investigations.
When using water-insoluble phosphates as binders, no early stage strength is achieved since no acid–base reactions are initiated. However, bonding strength can be generated by heating (T > 600 °C) resulting in a thermally-induced development of aluminum phosphates, thus, bonding strength.
Besides aluminum phosphates, other phosphate species (such as calcium or magnesium phosphates) do not generate bonding effects, since they do not react with ceramic oxides at T < 600 °C and generally are not capable to form broad phosphate networks because of non-bridging-oxygens [
10,
11,
16].
At temperatures T ≥ 600 °C the phosphate phases react with MgAl2O4 to form Mg3(PO4)2, CaxMgy(PO4)6 and other crystalline ortho phosphates. The formation of these low-melting high-temperature phases leads to a partial break in the bonding network. Since high temperature processes include the sintering of ceramic particles, in total, temperature treatment at T > 1000 °C leads to a significant increase in bonding strength.
In conclusion, phosphate binders actively influence the chemistry of the ceramic components (e.g., cation exchange, spinel decomposition). By monitoring structural changes in the bonding phase, property developments can be explained. Ultimately, this correlation leads to a better understanding of phosphate bonding mechanisms and, consequently, the mode of action of phosphate binders.
5.2. Insights in the Setting Process of Sodium Water Glasses Using a Combination of Dynamic Mechanical Analysis, NMR and PXRD
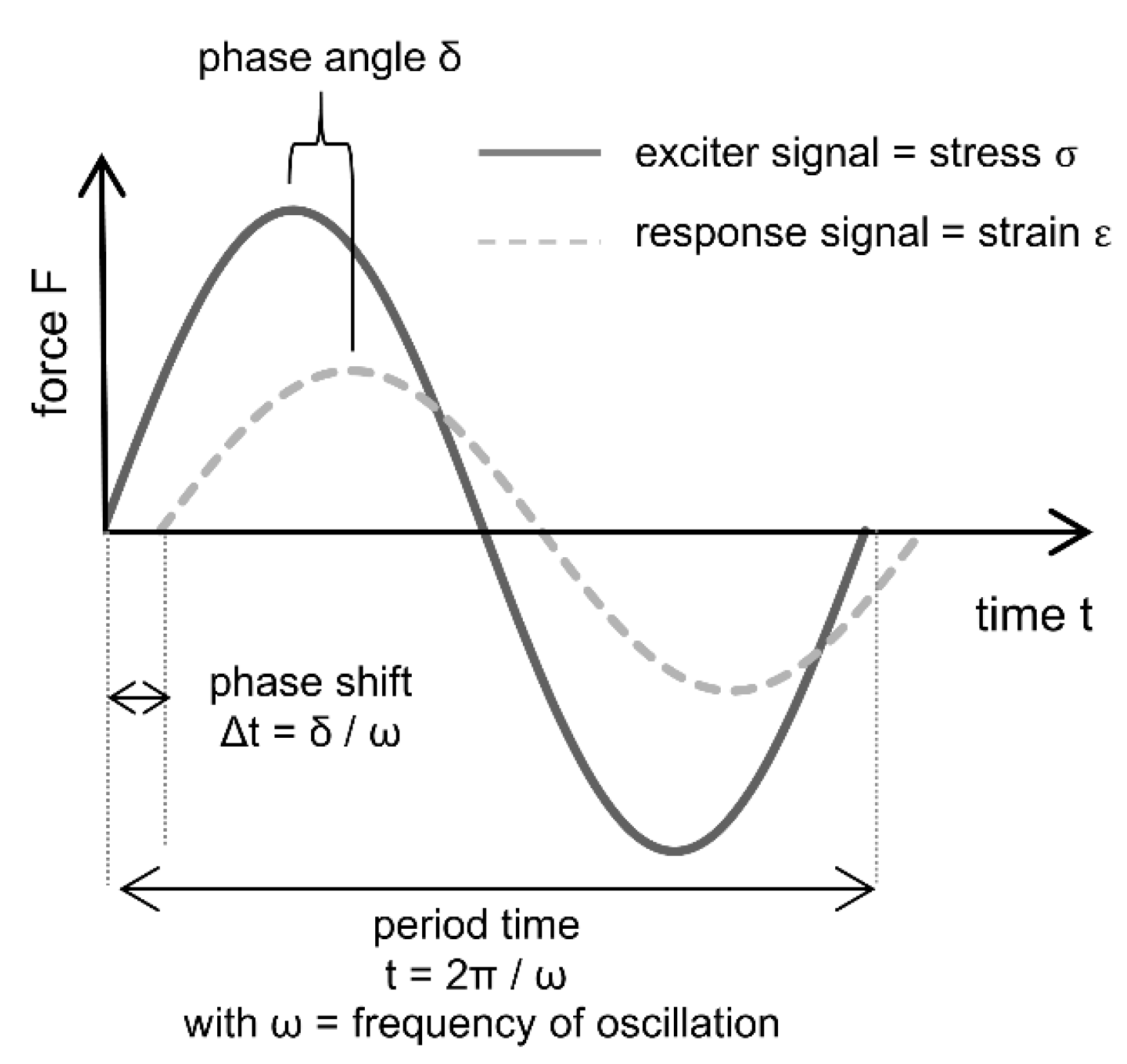
For the kinetic description of the setting process itself, the dynamic mechanical analysis (DMA) can obtain valuable information by measuring the viscoelastic properties of a material as a function of temperature, frequency and time. This allows time-dependent tracking of the strength development during setting. When combined with the structure information obtained by the above-mentioned methods, insights in the mechanism of the setting process are gained. During the measurement stress is put onto the material by an oscillating movement of a feeler stamp, the so-called exciter signal. This leads to a response signal of the sample, that is material-specific and depends on the viscoelastic behavior (
Figure 5). DMA describes the changes in the materials rigidity by the complex modulus of elasticity (MOE), which is composed of the storage modulus E
I representing the elastic part of energy and the loss modulus E
II standing for the energy that is lost due to plastic deformation. The ratio between E
II and E
I is defined by the loss factor tanδ [
17]. Specifically for the investigations of sol-gel-based binder systems, DMA offers the possibility to determine the gel and the glass point during the setting mechanism [
18].
Dynamic Mechanical Analysis investigations of a sol-gel-based pure binder system consisting of a sodium water glass hardened by either aluminum or boron orthophosphate were carried out with the aim to determine the influence of the attendant cation thus leading to a better understanding of the setting process of the alkali silicate systems [
19]. The results of the DMA as shown in
Figure 6 exhibit the overall setting of the water glass to be much faster when using boron orthophosphate as hardening agent instead of aluminum orthophosphate. Three different phases of the setting process can be discriminated. In an initial phase the storage modulus increases only very moderate until reaching the gel point, which is very similar for both hardeners. This point is followed by a much more intense increase of the storage modulus. This phase now differs highly using the two phosphates. The use of boron phosphate instead of aluminum phosphate leads to a significant faster increase and achievement of the final plateau that can be seen as the third stage of setting.
The increase in the storage modulus in the initial phase is quite similar for both hardeners. Investigations with different hardener portions (10, 20, 30 mass-%) and at different starting temperatures (25 °C, 35 °C, 45 °C) show that this phase of setting is more determined by the temperature and the solid to liquid ratio then by the chemistry of the hardener [
20]. Due to the very low solid to liquid ratio in the initial phase, the distances between the engaged species are very high leading to a very low influence of the network building processes of the different phosphate hardeners. Instead, condensation reactions due to the temperature induced release of water and the electrochemical destabilization caused by the change of both the pH value and the surface charge of the silicate particles, determine the setting progress.
When reaching the gel point, the distances between the different species in the sol are small enough to cause the differences in the hardening mechanisms of aluminum and boron phosphate becoming noticeable: The samples hardened with aluminum phosphate show a significant slower increase in the storage modulus and reach a later but clearly higher final level. NMR of silicon
29Si, aluminum
27Al and boron
11B nuclei explain the reasons for this in the structure of the three-dimensional network, specifically in the coordination of the boron and aluminum compounds integrated in the silicon network. Whereas Al is represented by high coordinated Al species (4,5,6) in the silicon network, boron compounds show a lower grade coordination (3,4) (
Figure 7). This is congruent with the observation of higher n-values for Si in the water glass network when using aluminum phosphate instead of boron orthophosphate, indicating a higher linking degree [
20]. Hardening with boron orthophosphate leads thus to a fast formed but less interconnected and wide-meshed network. The longer setting of the water glass gained by the use of aluminum orthophosphate results in a higher connected and denser network that leads to the observed higher values of E
I and thereby in a higher strength.
PXRD measurements of fully hardened samples (
Figure 7, 2nd row) indicate an additional aspect with regard to the different speed of the storage modulus’ increase. Besides the amorphous silicate network both mechanisms lead to the formation of crystalline structures. The use of boron orthophosphate causes the formation of short-chained hydrogen phosphates whereas the addition of the aluminum phosphate lead to the development of water-free long-chained phosphates. In combination with extensive STA measurements, it could be seen, that the two mechanisms cause differences in the release of water. In the case of hardening using AlPO
4, the samples show a higher weight loss due to the loss of water. However, the results indicated that the hardening mechanism of AlPO
4 do not generate more free water than the mechanism of BPO
4, but the mechanism of BPO
4 to cause a stronger prevention of water what results in the formation of the hydrogen phosphates. The reason for this different retention of water is seen in the inner structure of the originated silicate network with regard to the arrangement of the linking points. The essential aspect here is not the connectivity itself but the location in the network where the linking points are formed. It is known that different energy barriers of the condensation reactions lead to different locations of preferred interaction. This leads to the hypothesis that the network-forming reactions using boron orthophosphate as hardening agent exhibit lower energy barriers and thus induce condensation reactions in the periphery of the originating network. The result is a very fast formation of a far-reaching network that on the one hand hinders free water from release and one the other hand causes a fast hardening and increase in storage modulus. Instead, aluminum phosphate shows a higher energy barrier and thus needs more attempt for a successful condensation. This is even more likely in network areas with a higher density. This results in smaller, but higher linked domains which are surrounded by a certain content of water that thus can easily leave the samples. These structures result in the lower increase in storage modulus of the aluminum phosphate samples.
In an additional test series based on thermally induced linear change, melting behavior indicate a strong impact of the mechanism and the structure on the material properties. Therefore, it is important to identify the determining factors on the setting mechanism for the development of tailor-made material solutions. The outcome of this work points out the combination of the DMA with the methods for structure analysis and the thermo analysis as a powerful tool to connect the setting mechanism itself with the resulting structures and thus with the properties.
5.3. Inorganic Phosphate Hardeners in Chemical Setting of Potassium Water Glass
In the case of water glass as silicate binders the setting procedure can be conditioned by the admission of inorganic aluminum phosphates as hardening agents. Introducing cyclic aluminum metaphosphate modifications effects significantly the phase formation and the bonding properties, which can be determined by different measurement and spectroscopy techniques like PXRD, solid-state NMR, FT-IR, and Raman spectroscopy. As a monitor of the polycondensation progress of water glass systems the common Q
n notation is applied. The Q
n distribution in pure sodium water glasses can be considered in
Figure 8 left in terms of
29Si liquid NMR spectra. The signals for the Q
0 to Q
4 can be clearly distinguished and assigned. The spectra in
Figure 8 on the right-hand side illustrates the
29Si MAS NMR spectra of chemically hardened potassium water glass with aluminum tetrametaphosphate. Due to the nature of solid-state NMR technique and especially of the
29Si experiments very broad signals are detected with high overlap. The deconvolution of the signal region resolves four signals, where two can be assigned to Q
3 and Q
4 of pure water glass systems. The other two signals are formed by the introduction of aluminum ions into the silicate framework creating alumo-silicate phases. Therefore, it can be demonstrated that the polycondensation shifts the Q
n distribution to higher coordinated silicate specimen, since no Q
0 to Q
2 signals can be detected.
The impact of the aluminum in the formation of alumo-silicate phases in respect to the alkali modulus (molar ratio of SiO
2 in respect to M
2O) of the potassium water glass and the modification of the aluminum metaphosphate on the
29Si NMR signals is shown in
Table 2. The existence of newly formed Si-O-Al connections is additionally verified spectroscopically by FT-IR and Raman measurements.
Since the mechanical resistance towards deformation is mainly reasoned in the condensation degree of the silicate framework the formation of alumo-silicate sections has an impact on the mechanical properties.
The quantification of the assigned Q
n(Al
m) groups by deconvolution of the solid-state
29Si NMR spectra characterizes the degree of alumo-silicate content inside the sample. It can be demonstrated that in case of aluminum hexametaphosphate as hardening agent an increase in alumo-silicate content is detected. Therefore, comparing the alumo-silicate content to the mechanical properties like the cold bending strength and the Young’s modulus by resonance frequency damping analysis (RFDA) a decrease in mechanical stability is obtained (
Figure 9). Additionally, the water glass’ alkali modulus influences the mechanical properties, since the samples with a higher K
2O content show the highest cold bending strength values with the same hardener.
As a result, samples with potassium water glass with a lower alkali modulus and the application of aluminum tetrametaphosphate as hardening agent show the highest values in the cold bending strength tests. This can be correlated to the increase in polycondensation of the silicate framework and a lower degree in formed alumo-silicate phases due to the stronger interception of the aluminum ions by ion-exchange reaction.
Additionally, the solubility of aluminum ions in the alkaline water glass environment and existing ion-exchange reaction of aluminum-ions from the aluminum metaphosphate and the alkali ions of the water glass are mainly responsible for the formation of alumo-silicate phases. Dissolved aluminum ions are the main source of the alumo-silicate formation. The ion exchange reaction reduces the available aluminum ions and therefore reduces its impact on integrating into the silicate framework.
For the correct interpretation of the phase formation while setting procedure
31P MAS NMR experiments give insights into the depolymerization mechanism of the hardener. In
Figure 10 all samples show crystalline potassium hydrogen phosphate as a result of the depolymerizing metaphosphate ring structure. The incrementally decomposition can be found in the of amorphous di- and oligophosphates ranging the Q
1 area. Crystalline potassium tetrametaphosphate dihydrate was determined as a set of four signals as Q
2 which proves the existence of an ion-exchange reaction between the aluminum ions from the metaphosphate and the potassium ions from the water glass. As a result, in the case of aluminum tetrametaphosphate as chemical hardener an ion-exchange reaction affects the amount of aluminum ions for a potential formation of alumo-silicate frameworks. Due to the stepwise depolymerization of the cyclic phosphate structure potassium ions are consumed by the formation of alkali phosphate and alkali hydrogen phosphate structures affecting the condensation progress due the lowering of the alkali concentration. As a consequence, the polycondensation of the silicate framework is forced towards highly coordinated networks.
The chemical resistance of water glasses towards acidic attack (see
Figure 11) is a function of the alkali concentration of the silicate and alumo-silicate structures, since it is the region of attack. Therefore, the influence of the alkali modulus of the water glass has the strongest impact on the chemical resistance of the water glass system.
Using aluminum metaphosphates as hardening agents for alkali silicate binders increases the chemical stability towards acidic attack, since the available alkali ions are incorporated into alkali phosphate and alkali hydrogen phosphate structures as a result of the incrementally depolymerization of the cyclic metaphosphate structure.
Therefore, aluminum metaphosphate, especially aluminum tetrametaphosphate as hardening agent increases the chemical resistance towards acidic attack in the setting procedure of water glass binder system.
As a summary the chemical setting of potassium water glass binders with aluminum metaphosphates as hardening agents proceeds in a multi-step reaction mechanism involving the incrementally depolymerization of the cyclic metaphosphate structures, the polycondensation of the amorphous three-dimensional silicate network, an ion-exchange reaction, and the incorporation of alkali ions into the silicate framework. All reaction steps mutually influence each other and define the ultimate material properties.
By the structural investigation of the samples in terms of spectroscopic and diffraction techniques, information about the different phase contents are generated while chemical setting. Combining the results of the reaction mechanism with macroscopic properties like cold-bending strength, young’s modulus and chemical resistance towards acidic attack structure–property correlations can be derived. This can be used as a powerful tool in predicting sample properties based on the starting compounds and brings the ability in optimizing the sample matrix for required functionalities.