Formamidinium Lead Iodide Perovskite Films with Polyvinylpyrrolidone Additive for Active Layer in Perovskite Solar Cells, Enhanced Stability and Electrical Conductivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation Procedures
2.2. Methods of Characterization
3. Results and Discussion
3.1. Structure and Morphology
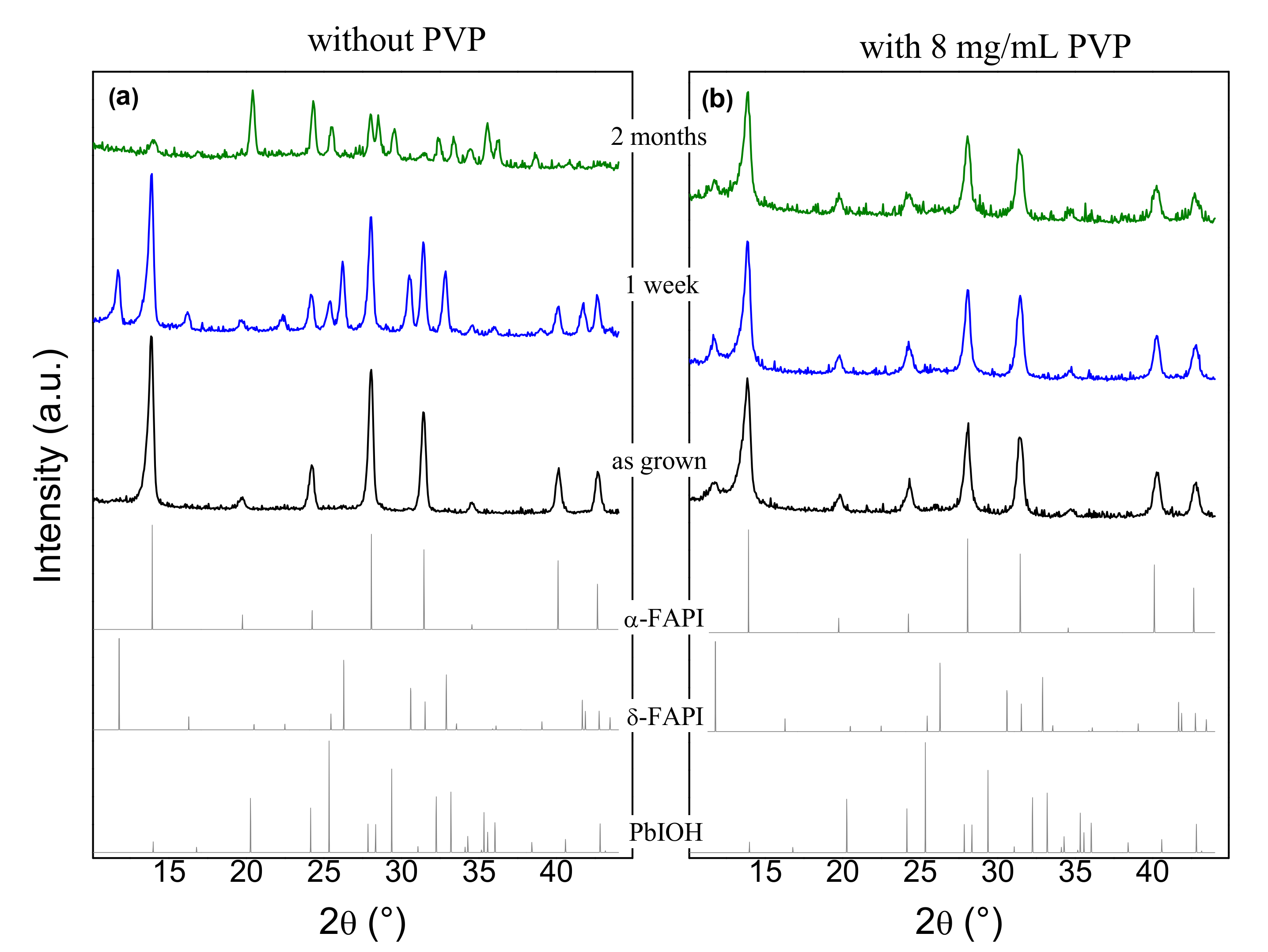
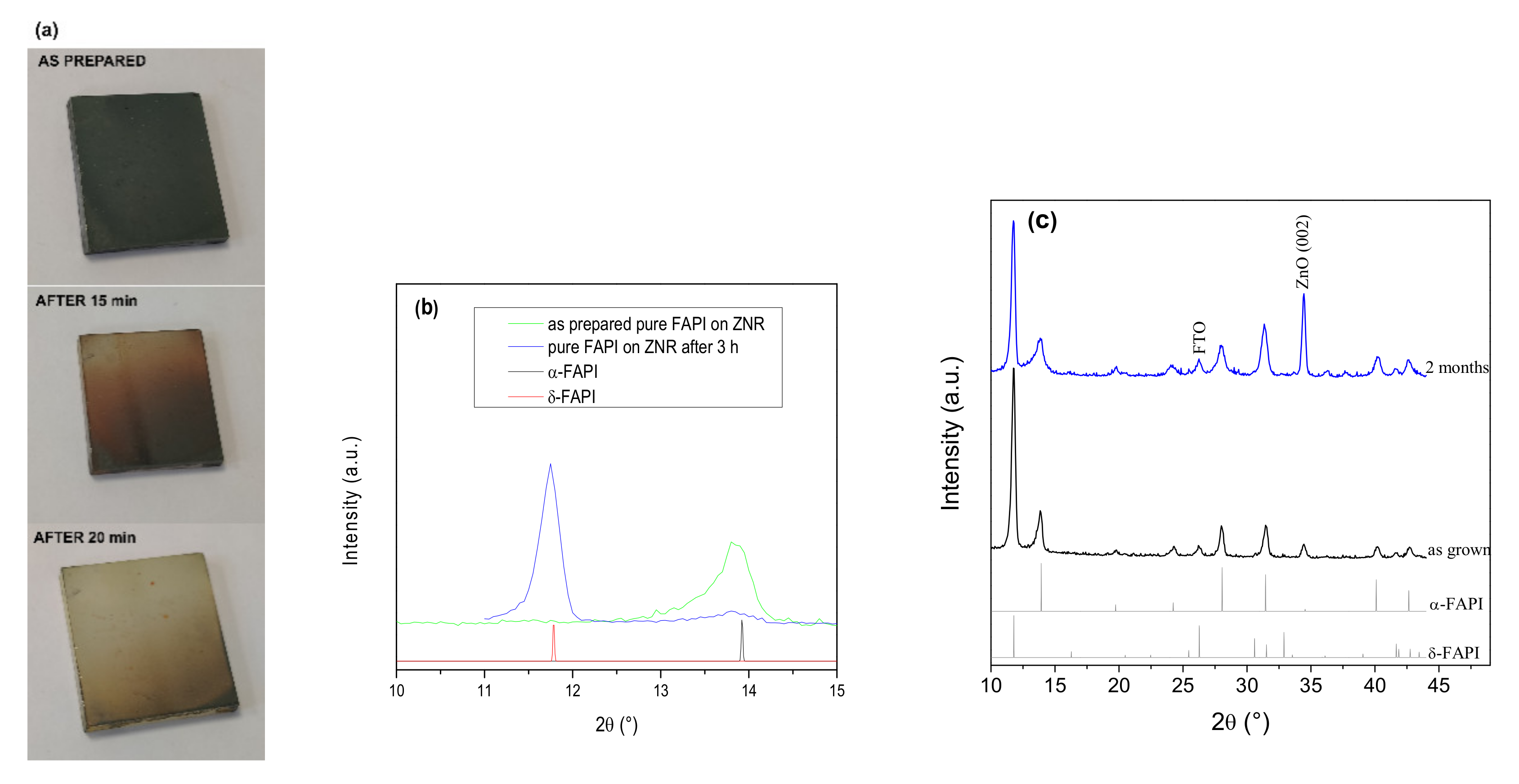
3.1.1. X-ray Diffraction
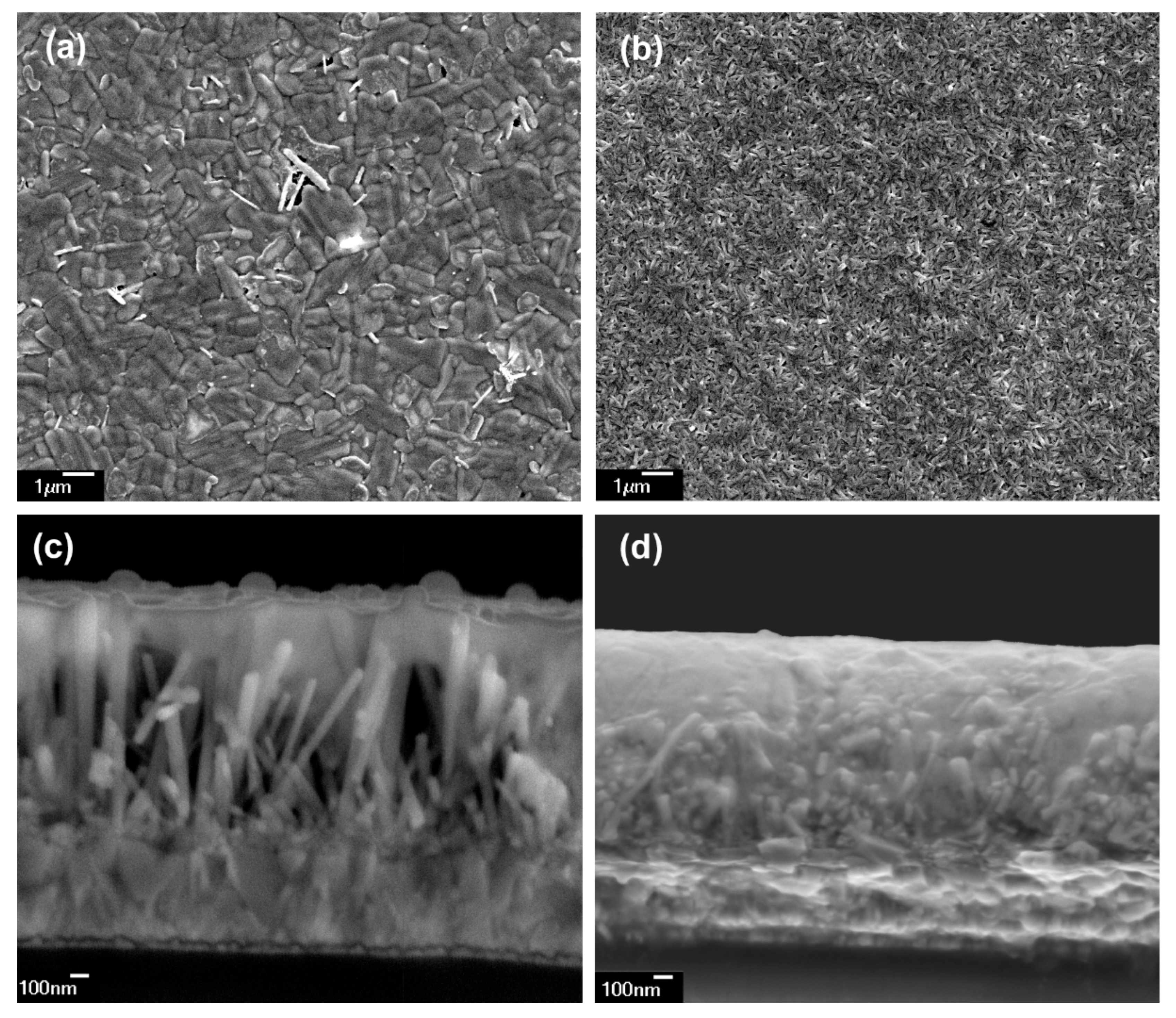
3.1.2. Scanning Electron Microscopy (SEM)
3.2. Surface Tension and Contact Angle
3.3. Optical Properties
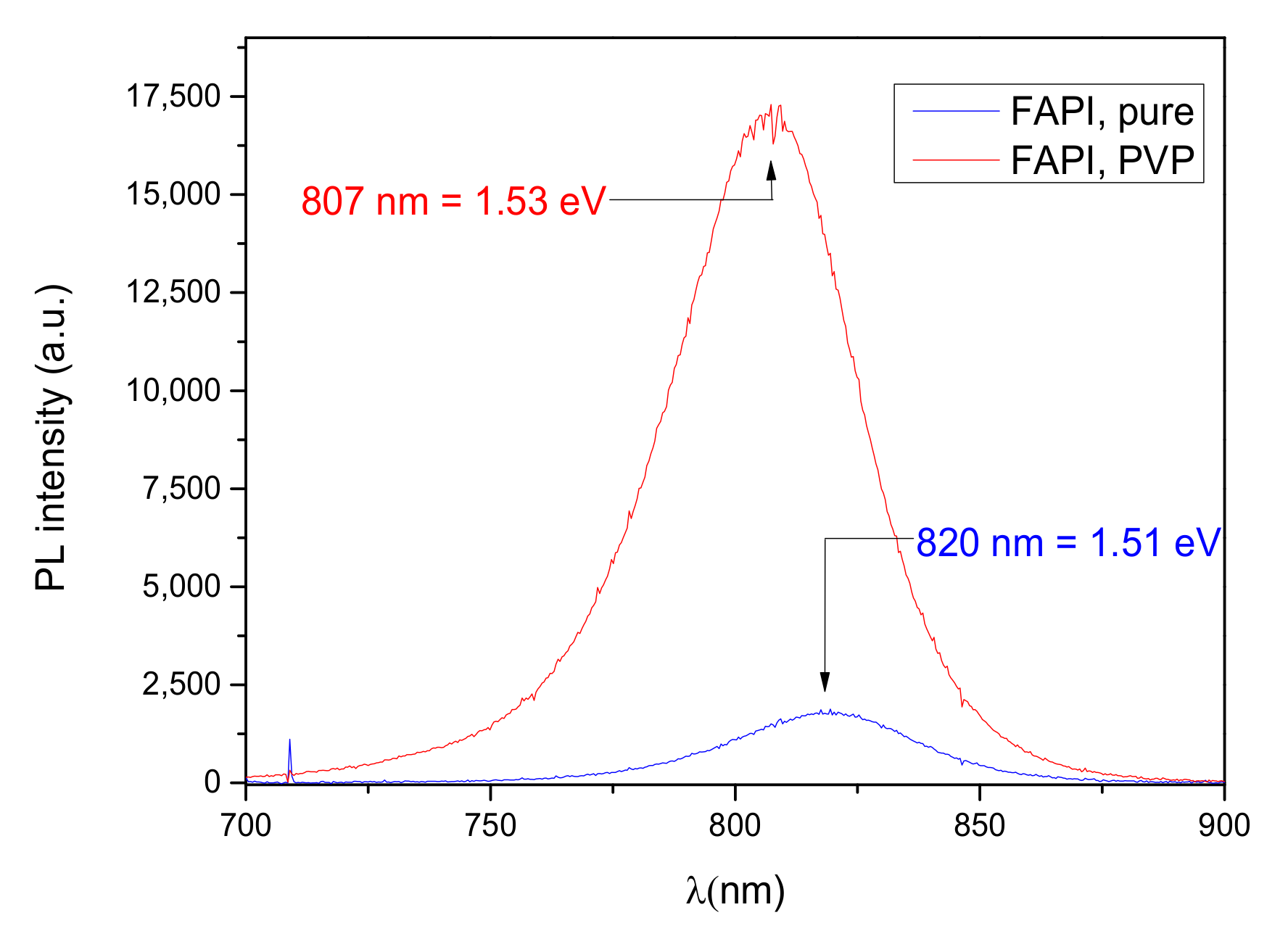
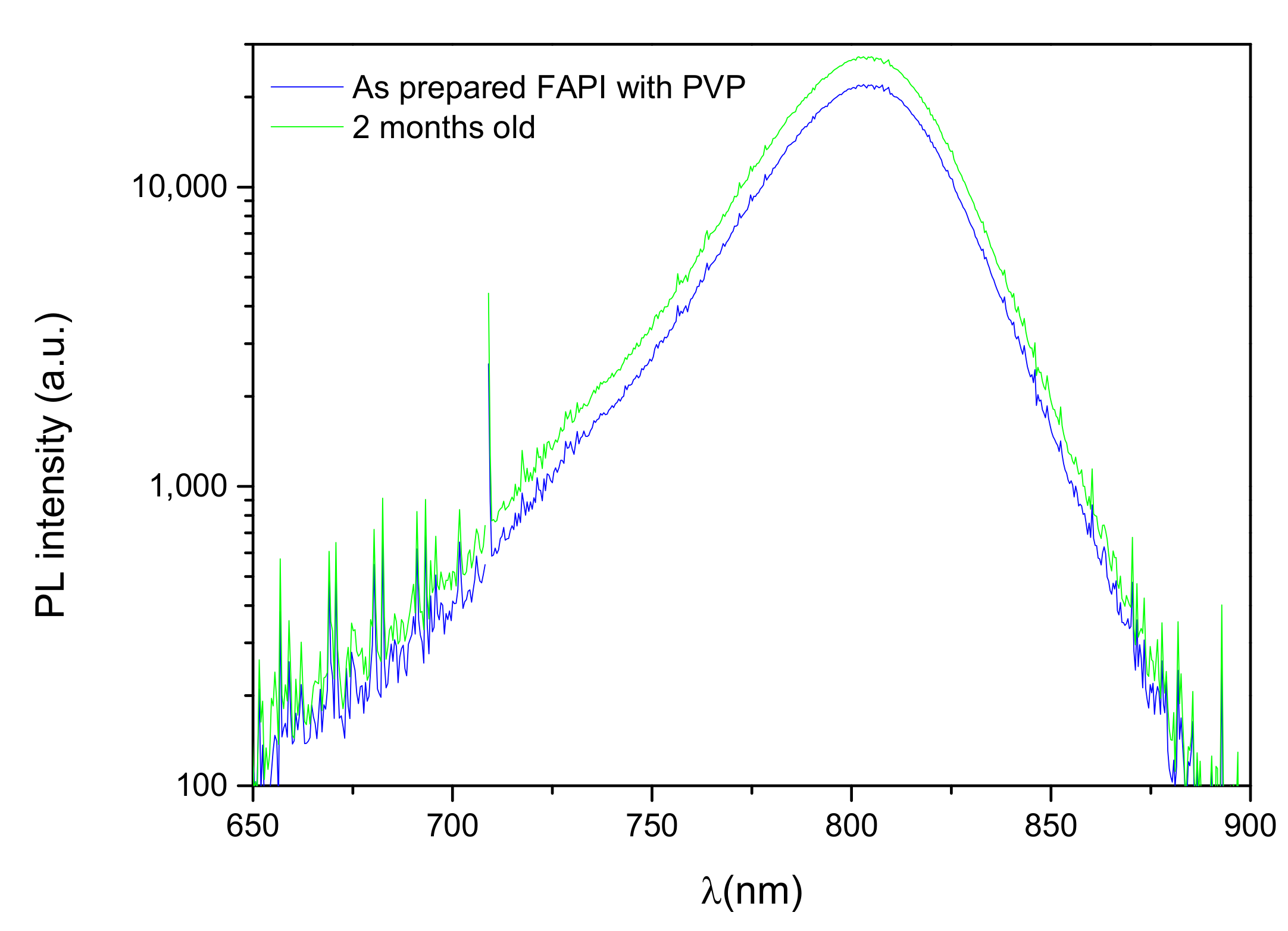
3.3.1. Photoluminescence Measurements
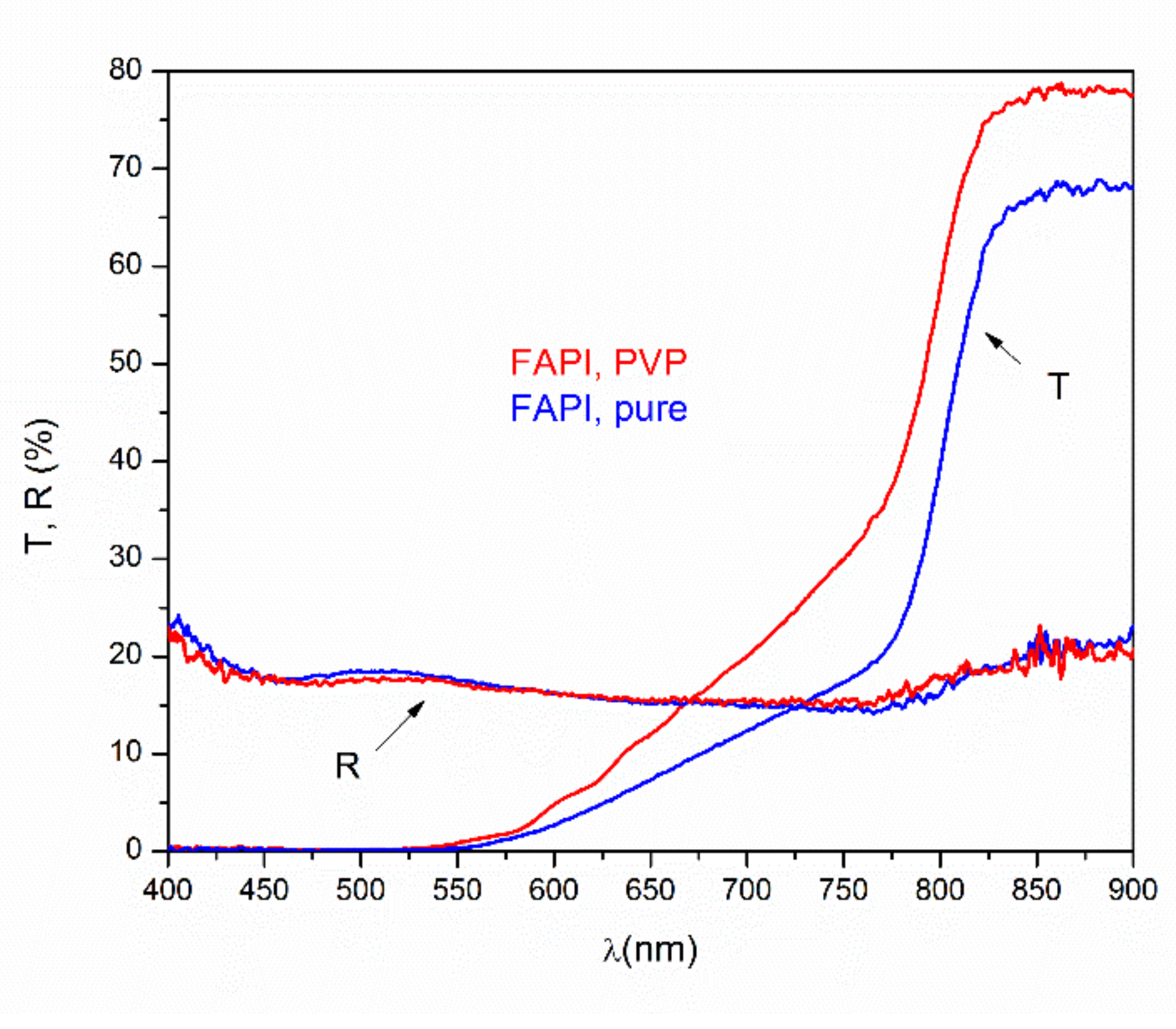
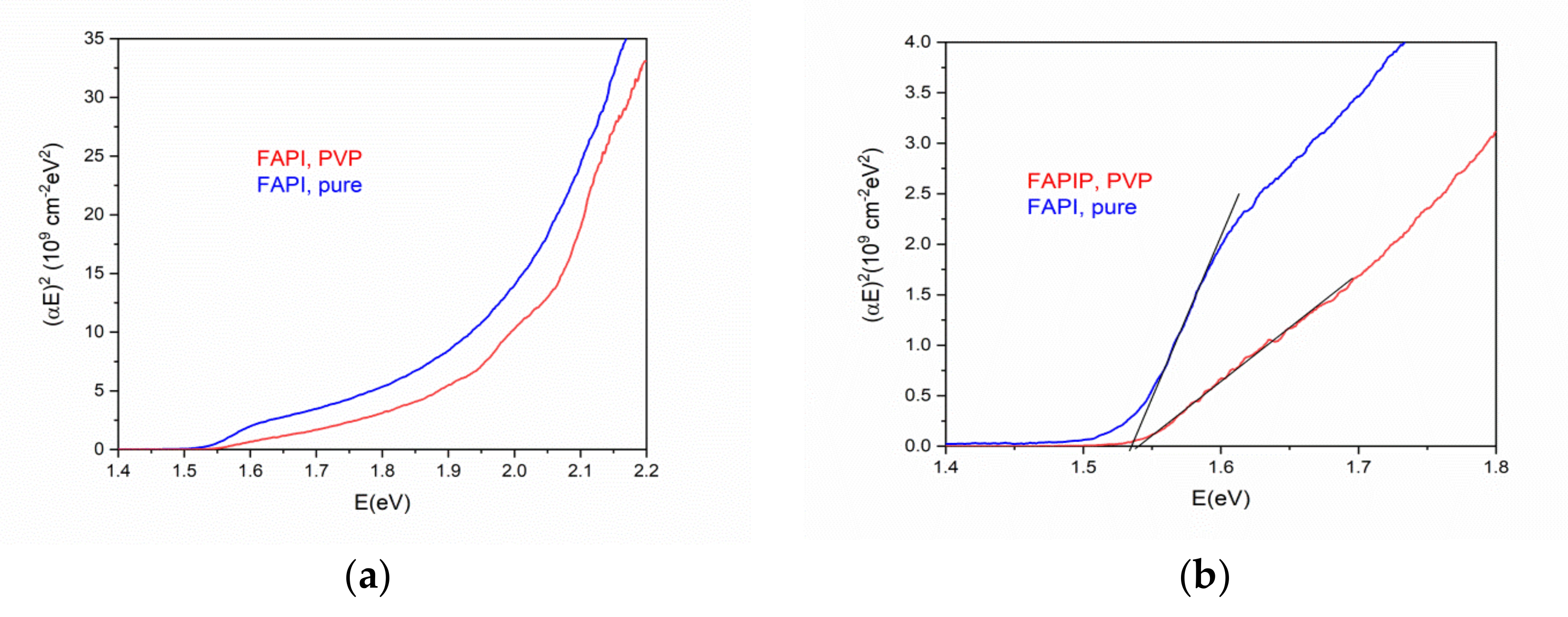
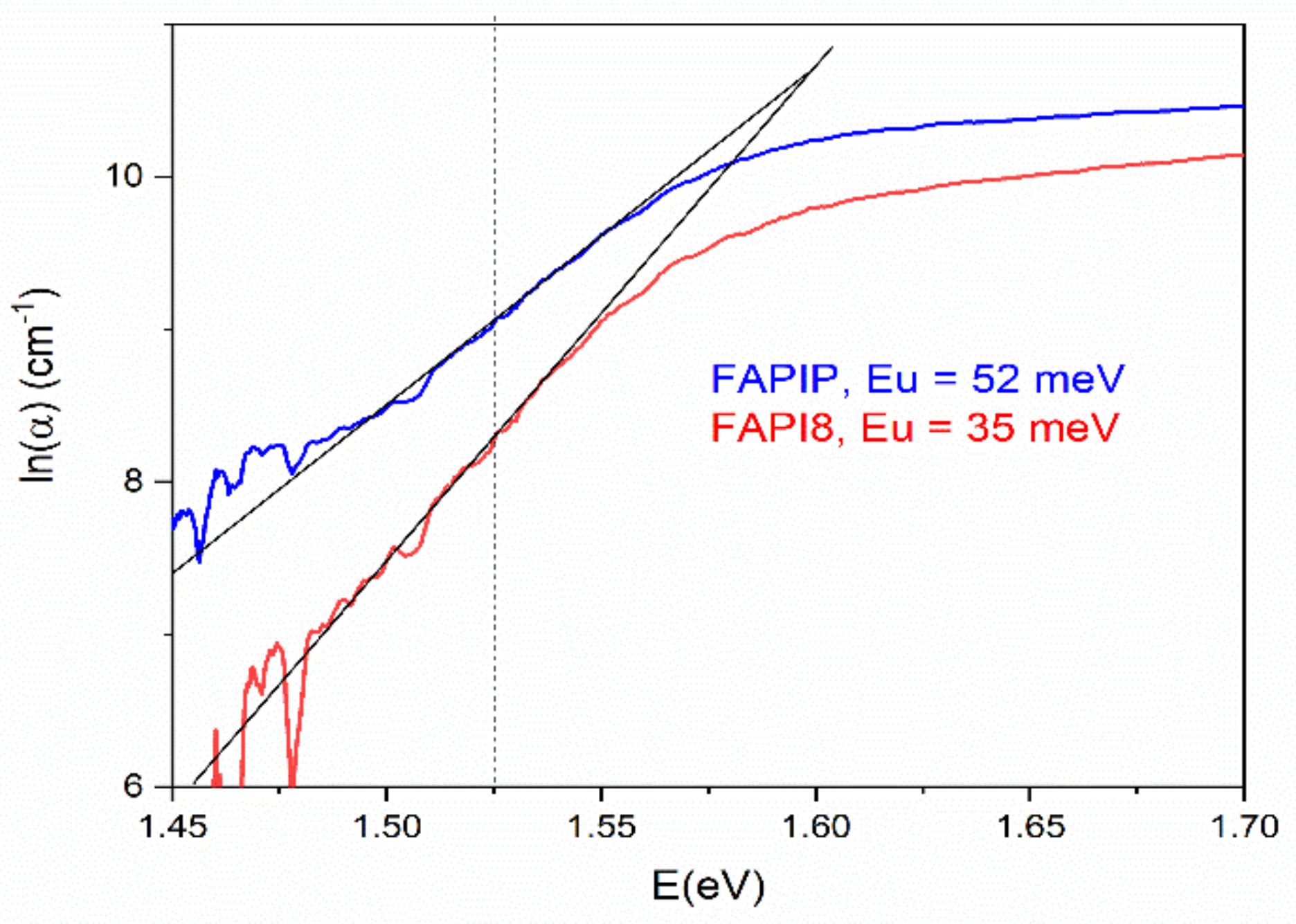
3.3.2. UV-Vis Measurements
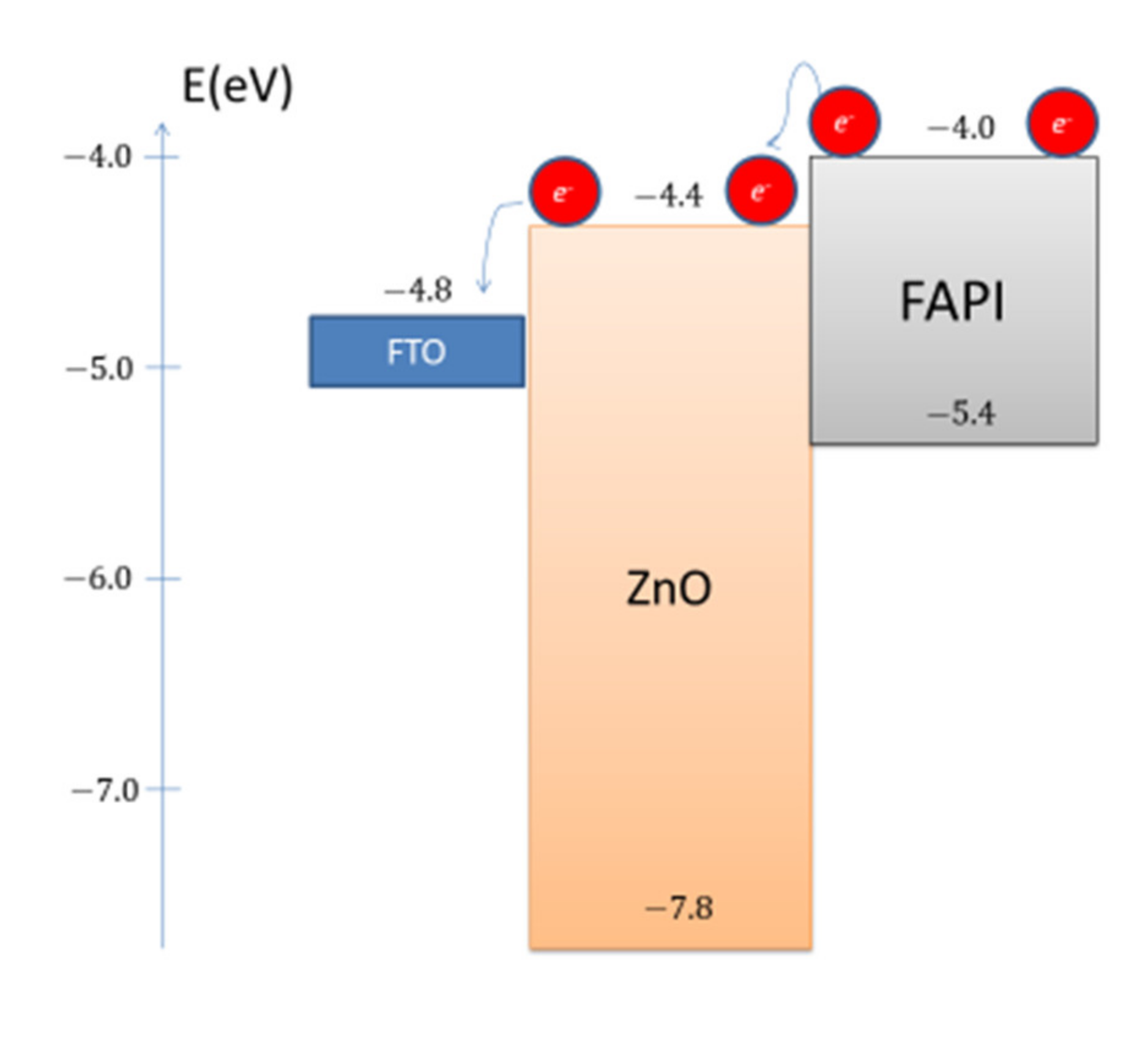
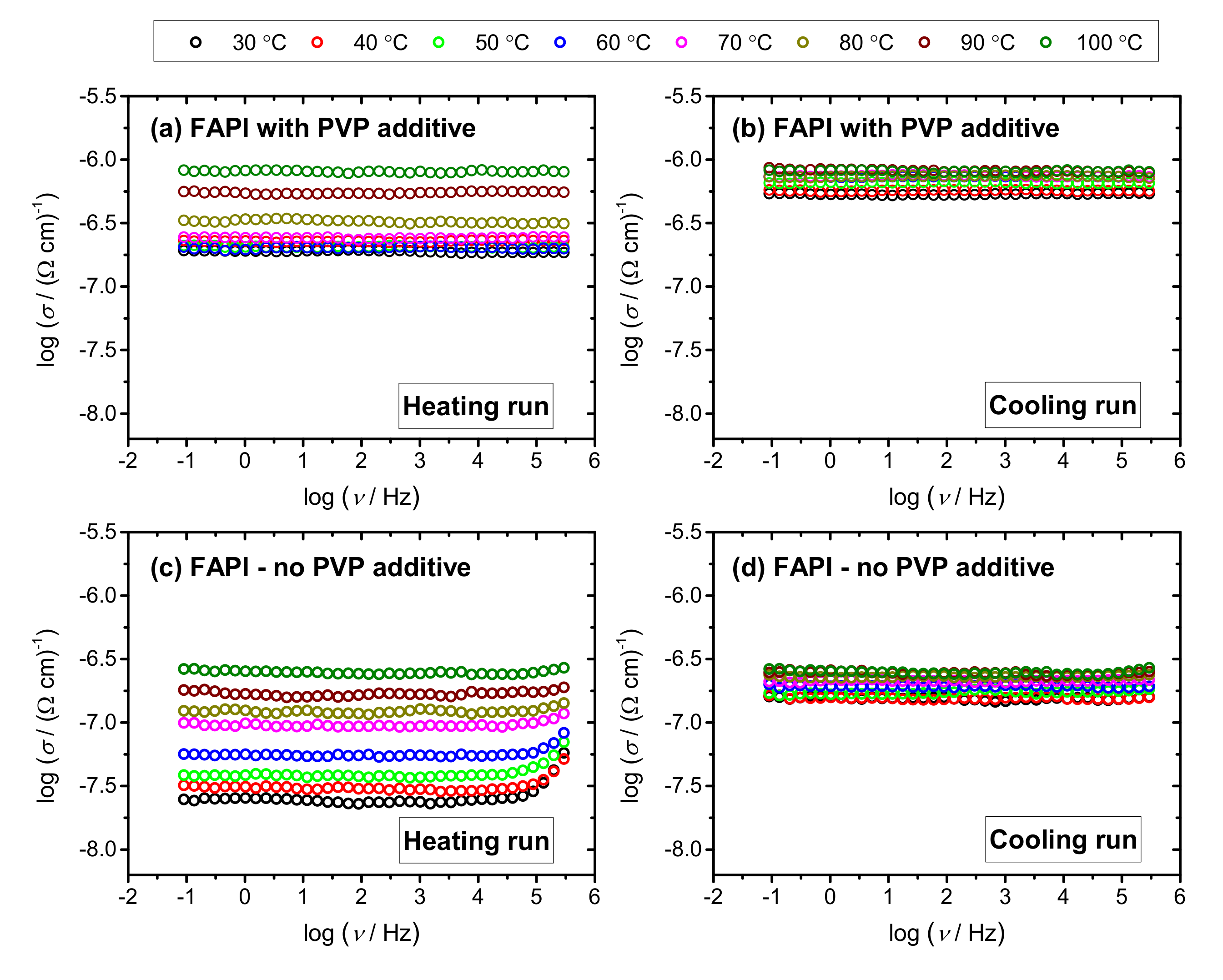
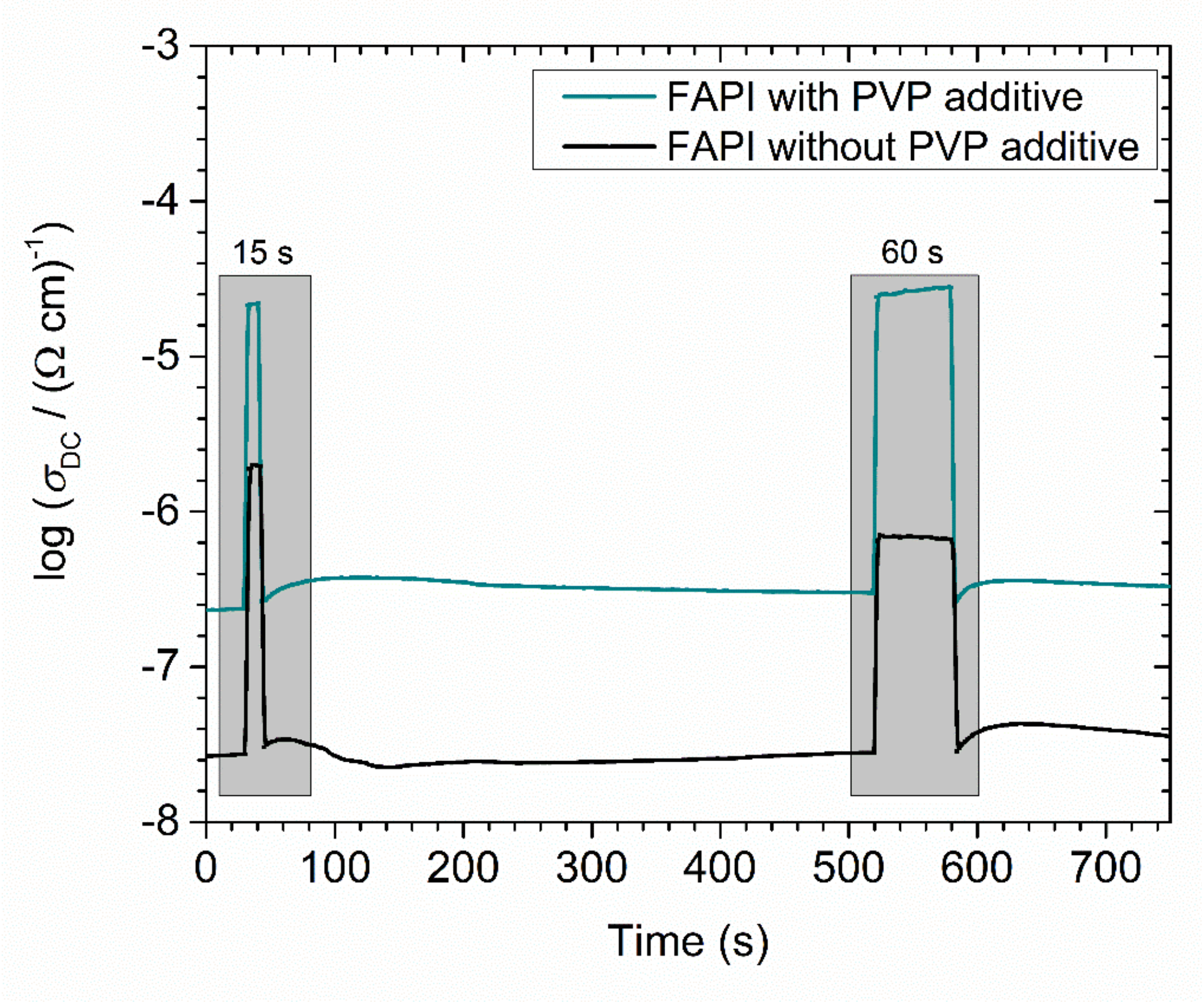
3.4. Electrical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitisers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Ava, T.T.; Mamun, A.A.; Marsillac, S.; Namkoong, G. A review: Thermal stability of methylammonium lead halied based perovskite solar cells. Appl. Sci. 2019, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Correa-Baena, J.; Saliba, M.; Buonassisi, T.; Gratzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and challenges of perovskite solar cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef] [Green Version]
- Pang, S.; Hu, H.; Zhang, J.; Lv, S.; Yu, Y.; Wei, F.; Qin, T.; Xu, H.; Liu, Z.; Cui, G. NH2CH = NH2PbI3: An alternative organolead iodide perovskite sensitizer for mesoscopic solar cells. Chem. Mater. 2014, 26, 1485–1491. [Google Scholar] [CrossRef]
- Ruhle, S. Tabulaterd values of the Shockley-Queisser limit for single junction solar cells. Sol. Energy 2016, 130, 139–147. [Google Scholar] [CrossRef]
- Ma, F.; Li, J.; Li, W.; Lin, N.; Wang, L.; Qiao, J. Stable α/δ junction of formamidinium lead iodide perovskites for enhanced near—Infrared emission. Chem. Sci. 2017, 8, 800–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salado, M.; Calio, L.; Berger, R.; Kazim, S.; Ahmad, S. Influence of the mixed organic cation ratio in lead iodide based perovskite on the performance of solar cells. Phys. Chem. Chem. Phys. 2016, 18, 27148–27157. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Wu, C.; Jha, S.K.; Li, Z.; Zhu, K.; Priya, S. Improved phase stability of Formamidinium Lead Triiodide Perovskite by Strain Relaxation. ACS Energy Lett. 2016, 1, 1014–1020. [Google Scholar] [CrossRef]
- Sui, M.; Li, S.; Gu, X. Improved performance of perovskite solar cells through using (FA)x(MA)1–xPbI3 optical absorber layer. Optoelectron. Lett. 2019, 15, 117–121. [Google Scholar] [CrossRef]
- Ji, F.; Wang, L.; Pang, S.; Gao, P.; Xu, H.; Xie, G.; Zhang, J.; Cui, G. A balanced cation exchange reaction toward highly uniform and pure phase FA1-xMAxPbI3 perovskite films. J. Mater. Chem. A 2016, 4, 14437–14443. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, M.; Pang, S.; Zhu, K.; Padture, N.P. Exceptional morphology-preserving evolution of formamidinium lead triiodide perovskite thin films via organic-cation displacement. J. Am. Chem. Soc. 2016, 138, 5535–5538. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.S.; Wei, S.H.; Berry, J.J.; Zhu, K. Stabilizing perovskite structures by tuning tolerance factor: Formation of formamidinium and cesium lead iodide solid-state alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Yi, C.; Luo, J.; Meloni, S.; Boziki, A.; Ashari-Astani, N.; Gratzel, C.; Zakeeruddin, U.; Rothlisberger, S.M.; Gratzel, M. Entropic stabilization of mixed A-cation ABX3 metal halide perovskites for high performance perovskite solar cells. Energy Environ. Sci. 2016, 9, 656–662. [Google Scholar] [CrossRef]
- Mcmeekin, D.P.; Sadoughi, G.; Rehman, W.; Eperon, G.E.; Saliba, M.; Horantner, A.; Haghighirad, M.T.; Sakai, N.; Korte, L.; Rech, B.; et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 2016, 351, 151–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Que, M.; Dai, Z.; Yang, H.; Zhu, H.; Zong, Y.; Que, W.; Padture, N.P.; Zhou, Y.; Chen, O. Quantum-dot-induced cesium-rich surface imparts enhanced stability to formamidinium lead iodide perovskite solar cells. ACS Energy Lett. 2019, 4, 1970–1975. [Google Scholar] [CrossRef]
- Kim, M.; Lee, T.K.; Choi, I.W.; Choi, H.W.; Jo, Y.; Lee, J.; Kim, G.H.; Kwak, S.K.; Kim, D.S. Effects of cation size and concentration of cationic chlorides on the properties of formamidinium lead iodide based perovskite solar cells. Sustain. Energy Fuels 2020, 4, 3753–3763. [Google Scholar] [CrossRef]
- Lee, J.W.; Dai, Z.; Han, T.H.; Choi, C.; Chang, S.Y.; Lee, S.J.; De Marco, N.; Zhao, H.; Sun, P.; Hunag, Y.; et al. 2D perovskite stabilized phase-pure formamidinium perovskite solar cells. Nat. Commun. 2018, 9, 3021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, T.N.; Lu, J.; Tang, M.C.; Barrit, D.; Smilgies, D.M.; Yang, Z.; Li, J.; Fan, Y.; Luo, T.; McCulloch, I.; et al. High performance ambient-air-stabile FAPbI3 perovskite solar cells with molecule-passivated Ruddlesden-Popper/3D heterostructured film. Energy Environ. Sci. 2018, 11, 3358–3366. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, H.S.; Park, N.G. Lewis acid-base adduct approach for high efficiency perovskite solar cells. Acc. Chem. Res. 2016, 49, 311–319. [Google Scholar] [CrossRef]
- Lee, J.W.; Dai, Z.; Lee, C.; Lee, H.M.; Han, T.H.; De Marco, N.; Lin, O.; Choi, C.S.; Dunn, B.; Koh, J.; et al. Tuning molecular interactions for highly reproducible and efficient formamidinium perovskite solar cells via adduct aproach. J. Am. Chem. Soc. 2018, 140, 6317–6324. [Google Scholar] [CrossRef]
- Tan, H.; Jain, A.; Voznyy, O.; Lan, X.; Pelayo Garcia, F.; Fan, J.Z.; Bermudez, R.Q.; Yuan, M.; Zhang, B.; Zhao, Y.; et al. Efficient and stable solution processed planar perovskite solar cells via contact passivation. Science 2017, 355, 722–726. [Google Scholar] [CrossRef]
- Meng, L.; Sun, C.; Wang, R.; Huang, W.; Zhao, Z.; Sun, P.; Huang, T.; Xue, J.; Lee, J.W.; Zhu, C.; et al. Tailored phase conversion under conjugated polymer enables thermally stable perovskite solar cells with efficiency exceeding 21%. J. Am. Chem. Soc. 2018, 140, 17255–17262. [Google Scholar] [CrossRef]
- Yan, J.; Li, N.; Ai, Y.; Wang, Z.; Yang, W.; Zhao, M.; Shou, C.; Yan, B.; Sheng, J.; Ye, J. Enhanced perovskite crystallization by the polyvinylpyrrolidone additive for high efficiency solar cells. Sustain. Energy Fuels 2019, 3, 3448–3454. [Google Scholar] [CrossRef]
- Xiong, H.; DeLuca, G.; Rui, Y.; Zhang, B.; Li, Y.; Zhang, Q.; Wang, H.; Reichmanis, E. Modifying perovskite films with polyvinylpyrrolidone for ambient-air-stable highly bendable solar cells. ACS Appl. Mater. Interfaces 2018, 10, 35385–35394. [Google Scholar] [CrossRef]
- Veeramutuhu, L.; Liang, F.C.; Zhang, Z.X.; Cho, C.J.; Ercan, E.; Chueh, C.C.; Chen, W.C.; Borsali, R.; Kuo, C.C. Improving the performance and stability of perovskite light-emitting diodes by a polymeric nanothick interlayer-assisted grain control process. ACS Omega 2020, 5, 8972–8981. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Fu, L.; Yu, T.; Zhou, S.; Zhang, L.; Yin, L. Surface passivation engineering strategy to fully-inorganic cubic CsPbI3 perovskites for high-performance solar cells. Nat. Commun. 2018, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Fang, Z.; Zhou, W.; Qiao, Q.; Wang, M.; Chen, T.; Yang, S. Nonconjugated polymer poly(vinylpyrrolidone) as an efficient interlayer promoting electron transport for perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 32957–32964. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Zhong, M.; Wang, Y. Polyvinylpyrrolidone/polyvinyl alcohol blends modification on light absorbing layer to improve the efficiency and stability of perovskite solar cells. Mat. Sci. Semicon. Proc. 2021, 133, 105941. [Google Scholar] [CrossRef]
- Zhong, M.; Chai, L.; Wang, Y.; Di, J. Enhanced efficiency and stability of perovskite solar cell by adding polymer mixture in perovskite photoactive layer. J. Alloys Compd. 2021, 864, 158793. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, X.; You, J. SnO2: A wonderful electron transport layer for Perovskite solar cells. Small 2018, 14, 1801154. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Elumalai, N.K.; Upama, M.B.; Wang, D.; Chan, K.H.; Wright, M.; Xu, C.; Haque, F.; Uddin, A. Low temperature processed ZnO thin film as electron transport layer for efficient perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 159, 251–264. [Google Scholar] [CrossRef]
- Shaikh, J.S.; Shaikh, N.S.; Shaikh, A.; Mali, S.S.; Kale, A.J.; Kanjanaboosb, P.; Hong, C.J.; Kim, J.H.; Patil, P.S. Perovskite solar cells: In pursuit of efficiency and stability. Mater. Des. 2017, 136, 54–80. [Google Scholar] [CrossRef]
- Hu, W.; Yang, S.; Yang, S. Surface modification of TiO2 for perovskite solar cells. Trends Chem. 2020, 2, 148–162. [Google Scholar] [CrossRef]
- Son, D.Y.; Im, J.H.; Kim, H.S.; Park, N.G. 11% efficient perovskite solar cell based on ZnO nanorods: An effective charge collection system. J. Phys. Chem. C 2014, 118, 16567–16573. [Google Scholar] [CrossRef]
- Son, D.Y.; Bae, K.H.; Kim, H.S.; Park, N.G. Effects of seed layer on growth of ZnO nanorod and performance of perovskite solar cell. J. Phys. Chem. C 2015, 119, 10321–10328. [Google Scholar] [CrossRef]
- Bi, D.; Boschloo, G.; Schwartzmuller, S.; Yang, L.; Johansson, E.M.J.; Hagfeldt, A. Efficient and stable CH3NH3PbI3-sensitized ZnO nanorod array solid-state solar cell. Nanoscale 2013, 5, 11686–11691. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Siempelkamp, B.D.; Mosconi, E.; De Angelis, F.; Kelly, T.L. Origin of the thermal instability in CH3NH3PbI3 thin films deposited on ZnO. Chem. Mater. 2015, 27, 4229–4236. [Google Scholar] [CrossRef]
- Song, J.; Hu, W.; Wang, X.F.; Chen, G.; Tian, W.; Miyasaka, T. HC(NH2)2PbI3 as a thermally stable absorber for efficient ZnO-based perovskite solar cells. J. Mater. Chem. A 2016, 4, 8435–8443. [Google Scholar] [CrossRef]
- Foo, K.L.; Hashim, U.; Muhammad, K.; Voon, C.H. Sol-gel synthesized zinc oxide nanorods and their structural and optical investigation for optoelectronic application. Nanoscale Res. Lett. 2014, 9, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panžić, I.; Juraić, K.; Krstulović, N.; Šantić, A.; Belić, D.; Blažeka, D.; Plodinec, M.; Mandić, V.; Macan, J.; Hammud, A.; et al. ZnO@TiO2 core shell nanorod arrays with tailored structural, electrical and optical properties for photovoltaic application. Molecules 2019, 24, 3965. [Google Scholar] [CrossRef] [Green Version]
- Niu, G.; Guo, X.; Wang, L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Kalin, M.; Polajnar, M. The correlation between the surface energy, the contact angle and the spreading parameter, and their relevance for the wetting behaviour of DLC with lubricating oils. Tribol. Int. 2013, 66, 225–233. [Google Scholar] [CrossRef]
- Heavens, O.S. Optical Properties of Thin Solid Films; Dover publications: New York, NY, USA, 1991. [Google Scholar]
- Tauc, J.; Menth, A.J. States in the gap. J. Non-Cryst. Solids 1972, 8, 569–585. [Google Scholar] [CrossRef]
- Urbach, F. The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Cody, G.D. Urbach edge of crystalline and amorphous silicon: A personal review. J. Non-Cryst. Solids 1992, 141, 3–15. [Google Scholar] [CrossRef]
- Skumanich, A.; Frova, A.; Amer, N.M. Urbach tail and gap states in hydrogenated a-SiC and a-SiGe alloys. Solid State Commun. 1958, 54, 597–601. [Google Scholar] [CrossRef]
- Studenyak, I.; Kranjec, M.; Kurik, M. Urbach rule in solid state physics. Int. J. Opt. 2014, 4, 76–83. [Google Scholar]
- Abe, S.; Toyozawa, Y. Interband absorption spectra of disordered semiconductors in the coherent potential approximation. J. Phys. Soc. Jpn. 1981, 50, 2185–2194. [Google Scholar] [CrossRef]
- Li, W.; Zhao, K.; Zhou, H.; Yu, W.; Zhu, J.; Hu, Z.; Chu, J. Precursor solution temperature dependance of the optical constants, band gap and Urbach tail in organic-inorganic hybrid halide perovskite films. J. Phys. D Appl. Phys. 2018, 52, 045103. [Google Scholar] [CrossRef]
- Wu, J.M.; Chen, Y.R.; Lin, Y.H. Rapidly synthesized ZnO nanowires by ultraviolet decomposition process in ambient air for flexible photodetector. Nanoscale 2011, 3, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Oktik, S. Low cost, non-vacuum techniques for the preparation of thin/thick films for photovoltaic applications. Prog. Cryst. Growth Charact. 1988, 17, 171–240. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.G.; Yang, J.M.; Yang, Y.; Park, N.G. Verification and mitigation of ion migration in perovskite solar cells. APL Mater. 2019, 7, 041111. [Google Scholar] [CrossRef] [Green Version]
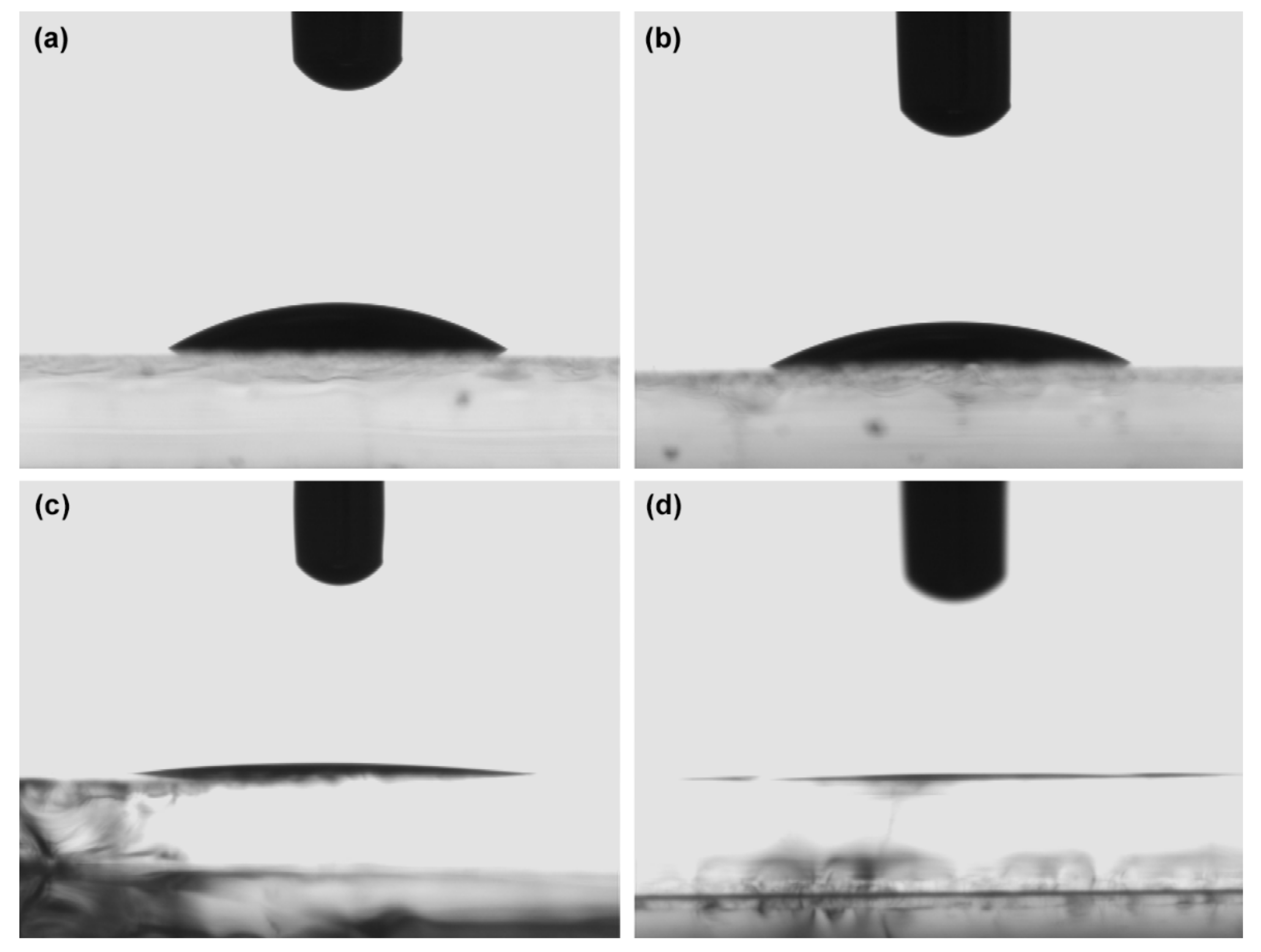

| Sample | CA(Glass)/(°) | CA(ZnO)/(°) |
|---|---|---|
| DMF/DMSO | 32.73 ± 0.85 | 3.96 ± 0.38 |
| PVP + DMF/DMSO | 29.10 ± 1.02 | 1.62 ± 0.38 |
| Sample | σDCa/(Ω cm)−1 ±0.5% | σDCb/(Ω cm)−1 ±0.5% | EDC,Heating/kJ mol−1 ±0.5% | EDC,Cooling/kJ mol−1 ±0.5% |
|---|---|---|---|---|
| With PVP | 1.9 × 10−7 | 5.4×10−7 | 18 | 6 |
| Without PVP | 2.5×10−8 | 1.5×10−7 | 32 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojić, V.; Bohač, M.; Bafti, A.; Pavić, L.; Salamon, K.; Čižmar, T.; Gracin, D.; Juraić, K.; Leskovac, M.; Capan, I.; et al. Formamidinium Lead Iodide Perovskite Films with Polyvinylpyrrolidone Additive for Active Layer in Perovskite Solar Cells, Enhanced Stability and Electrical Conductivity. Materials 2021, 14, 4594. https://doi.org/10.3390/ma14164594
Kojić V, Bohač M, Bafti A, Pavić L, Salamon K, Čižmar T, Gracin D, Juraić K, Leskovac M, Capan I, et al. Formamidinium Lead Iodide Perovskite Films with Polyvinylpyrrolidone Additive for Active Layer in Perovskite Solar Cells, Enhanced Stability and Electrical Conductivity. Materials. 2021; 14(16):4594. https://doi.org/10.3390/ma14164594
Chicago/Turabian StyleKojić, Vedran, Mario Bohač, Arijeta Bafti, Luka Pavić, Krešimir Salamon, Tihana Čižmar, Davor Gracin, Krunoslav Juraić, Mirela Leskovac, Ivana Capan, and et al. 2021. "Formamidinium Lead Iodide Perovskite Films with Polyvinylpyrrolidone Additive for Active Layer in Perovskite Solar Cells, Enhanced Stability and Electrical Conductivity" Materials 14, no. 16: 4594. https://doi.org/10.3390/ma14164594
APA StyleKojić, V., Bohač, M., Bafti, A., Pavić, L., Salamon, K., Čižmar, T., Gracin, D., Juraić, K., Leskovac, M., Capan, I., & Gajović, A. (2021). Formamidinium Lead Iodide Perovskite Films with Polyvinylpyrrolidone Additive for Active Layer in Perovskite Solar Cells, Enhanced Stability and Electrical Conductivity. Materials, 14(16), 4594. https://doi.org/10.3390/ma14164594












