ToF-SIMS Analysis of Demineralized Dentin Biomodified with Calcium Phosphate and Collagen Crosslinking: Effect on Marginal Adaptation of Class V Adhesive Restorations
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Thermo Mechanical Loading
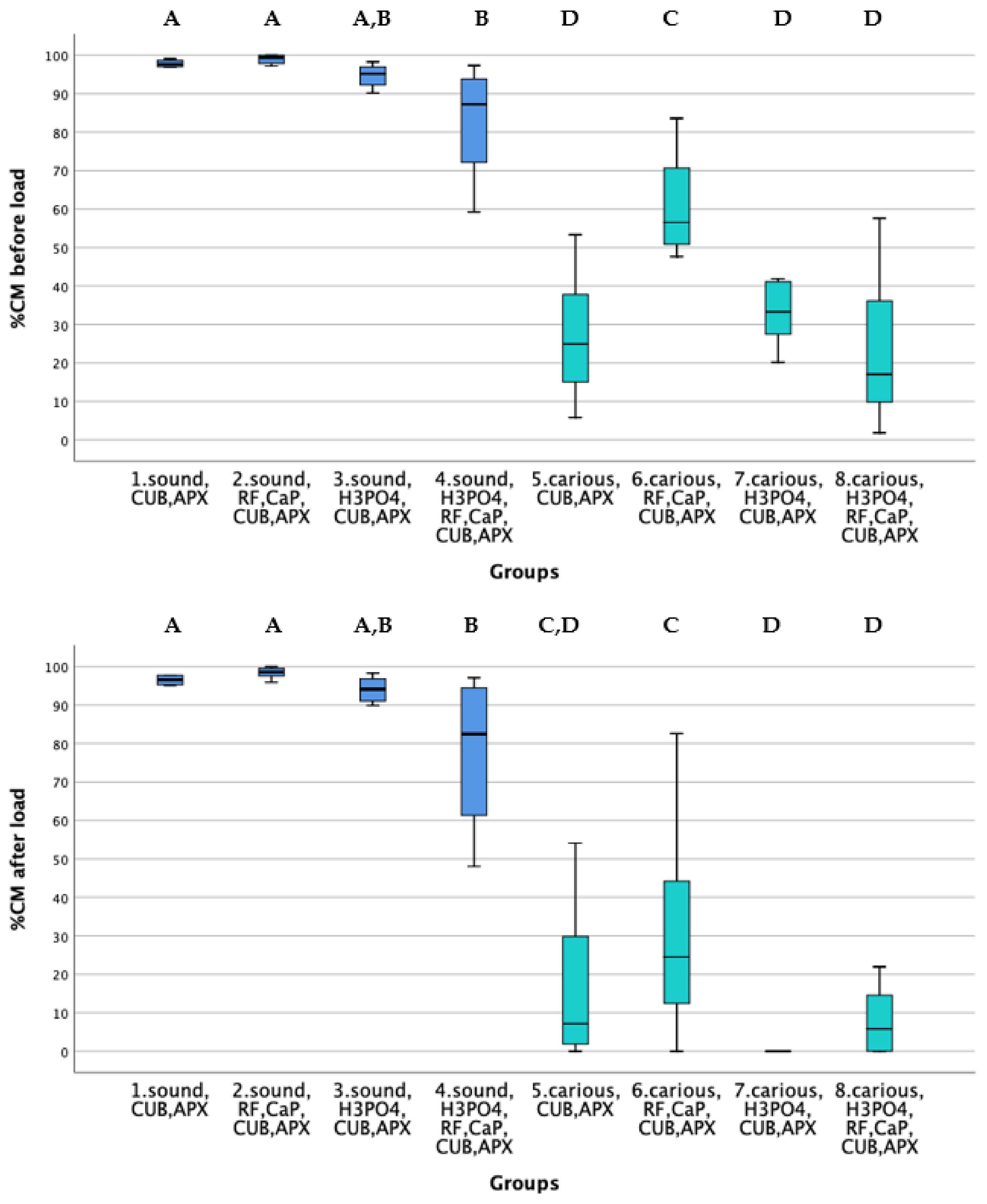
2.3. Quantitative Margin Analysis
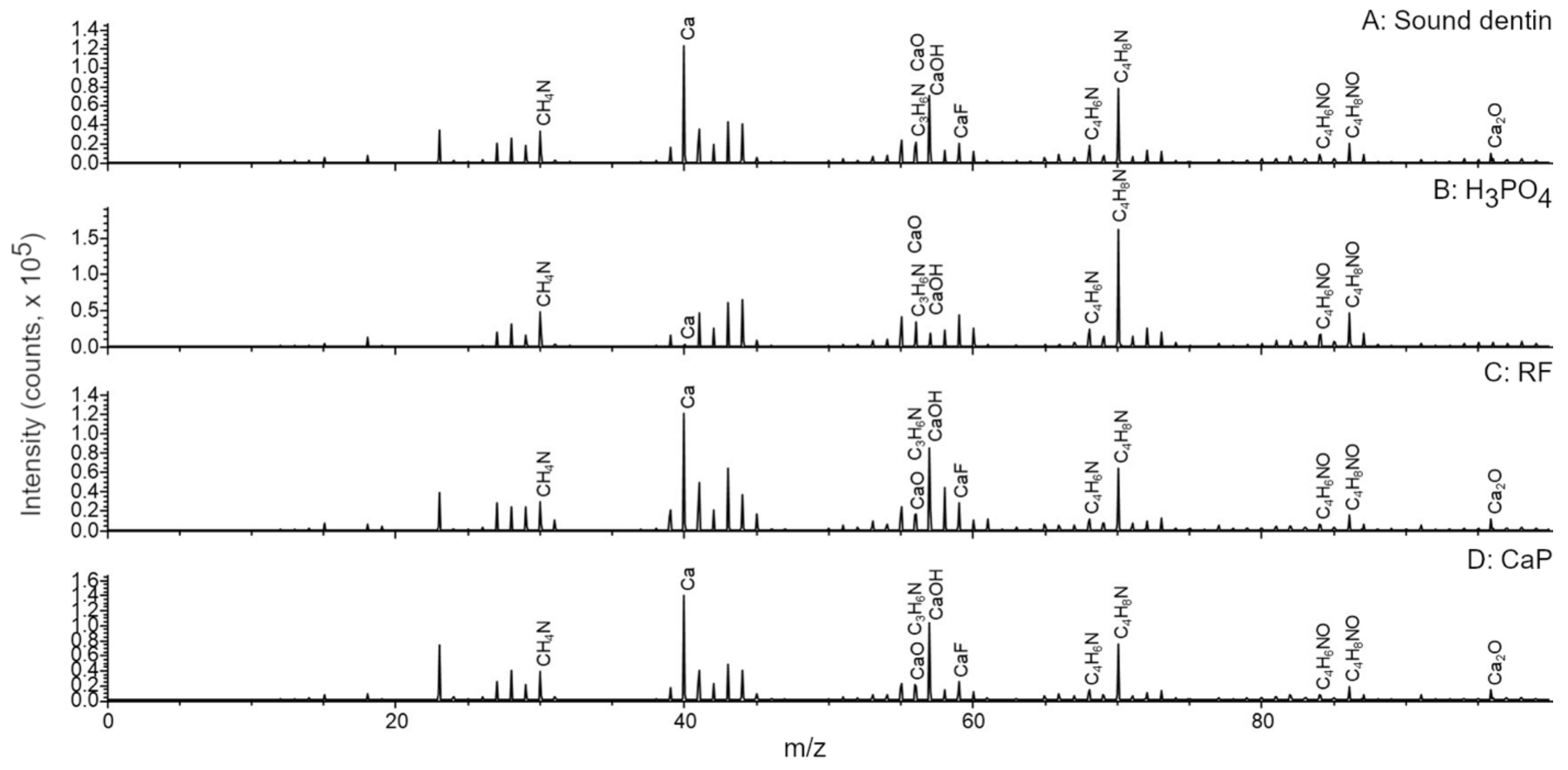
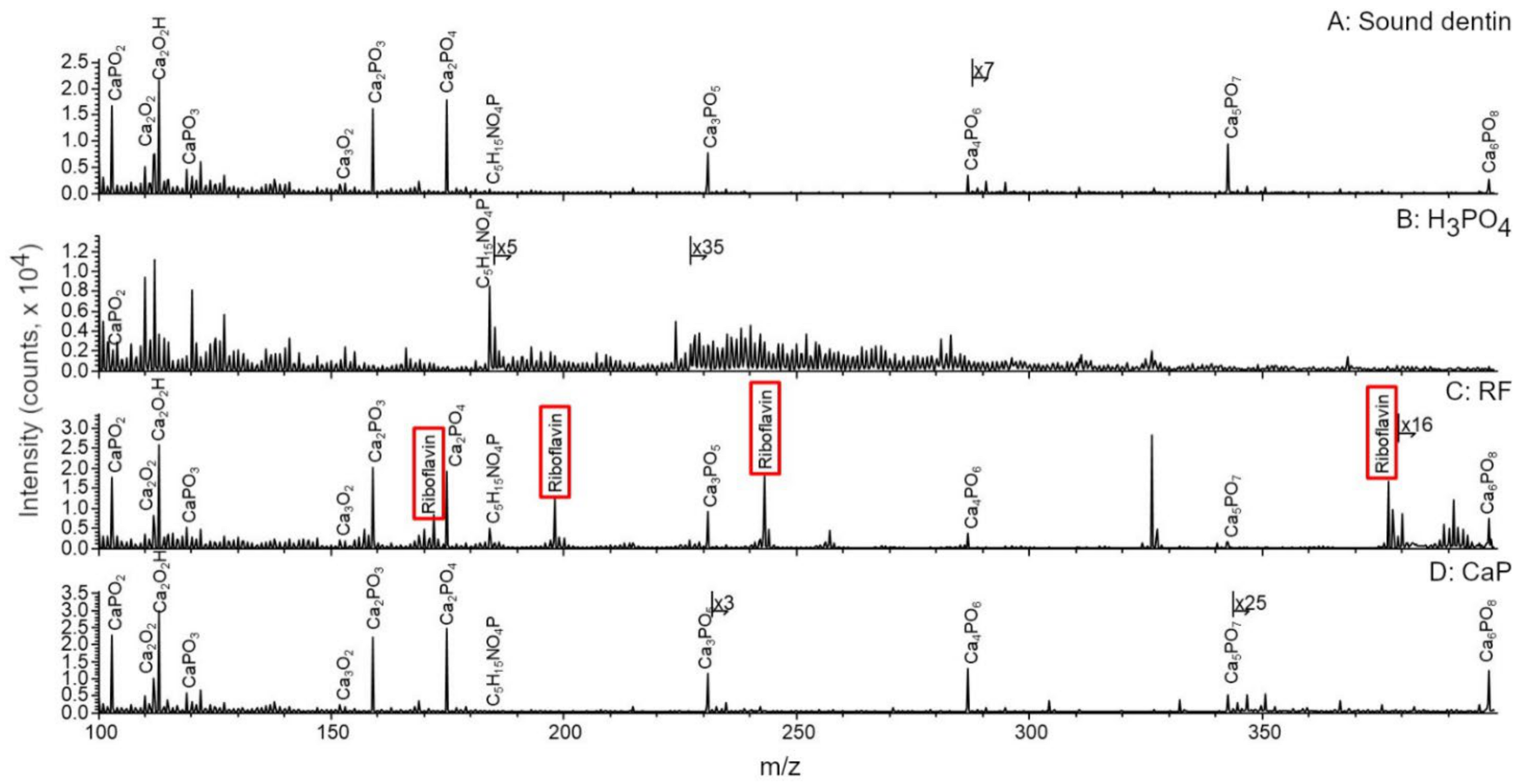
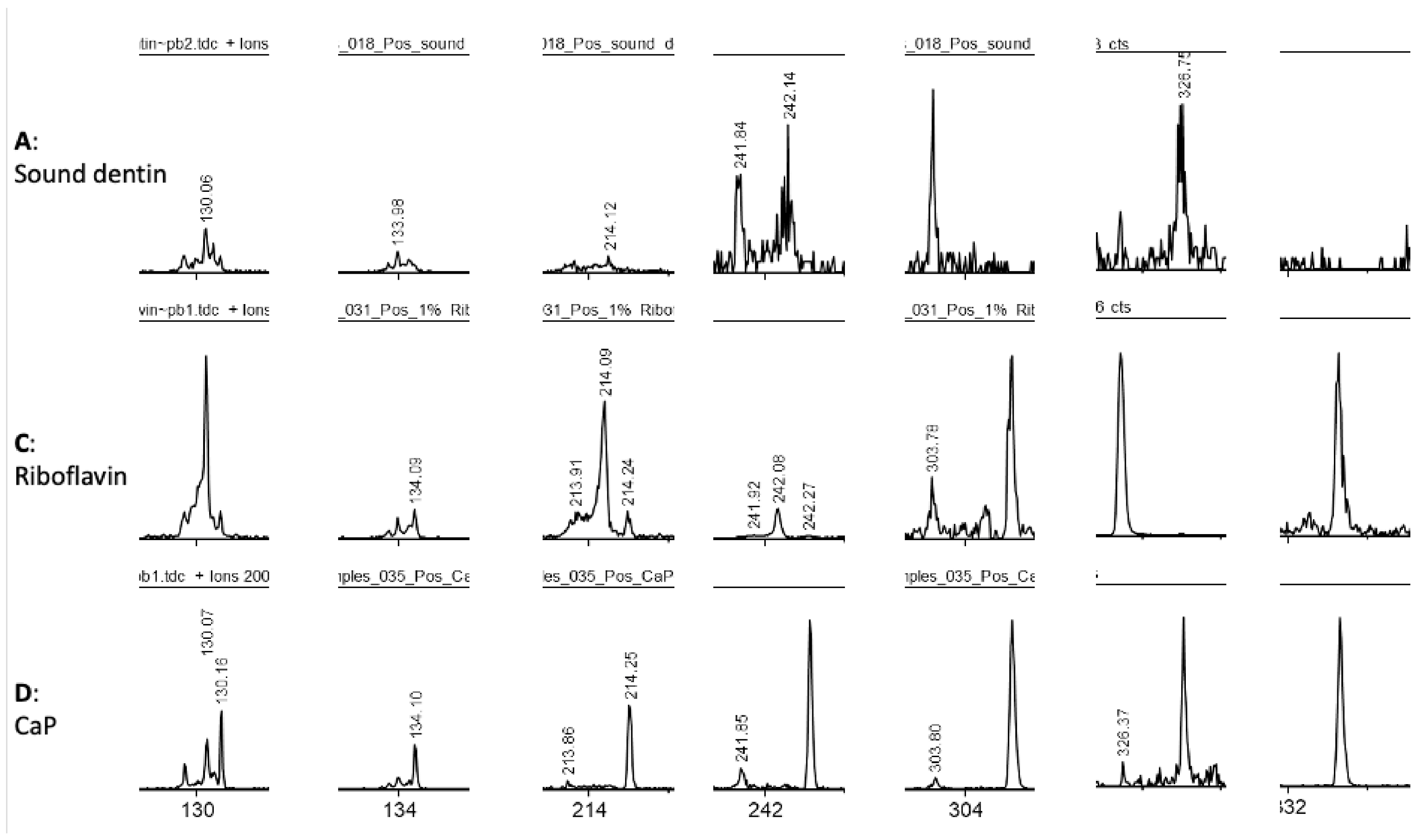
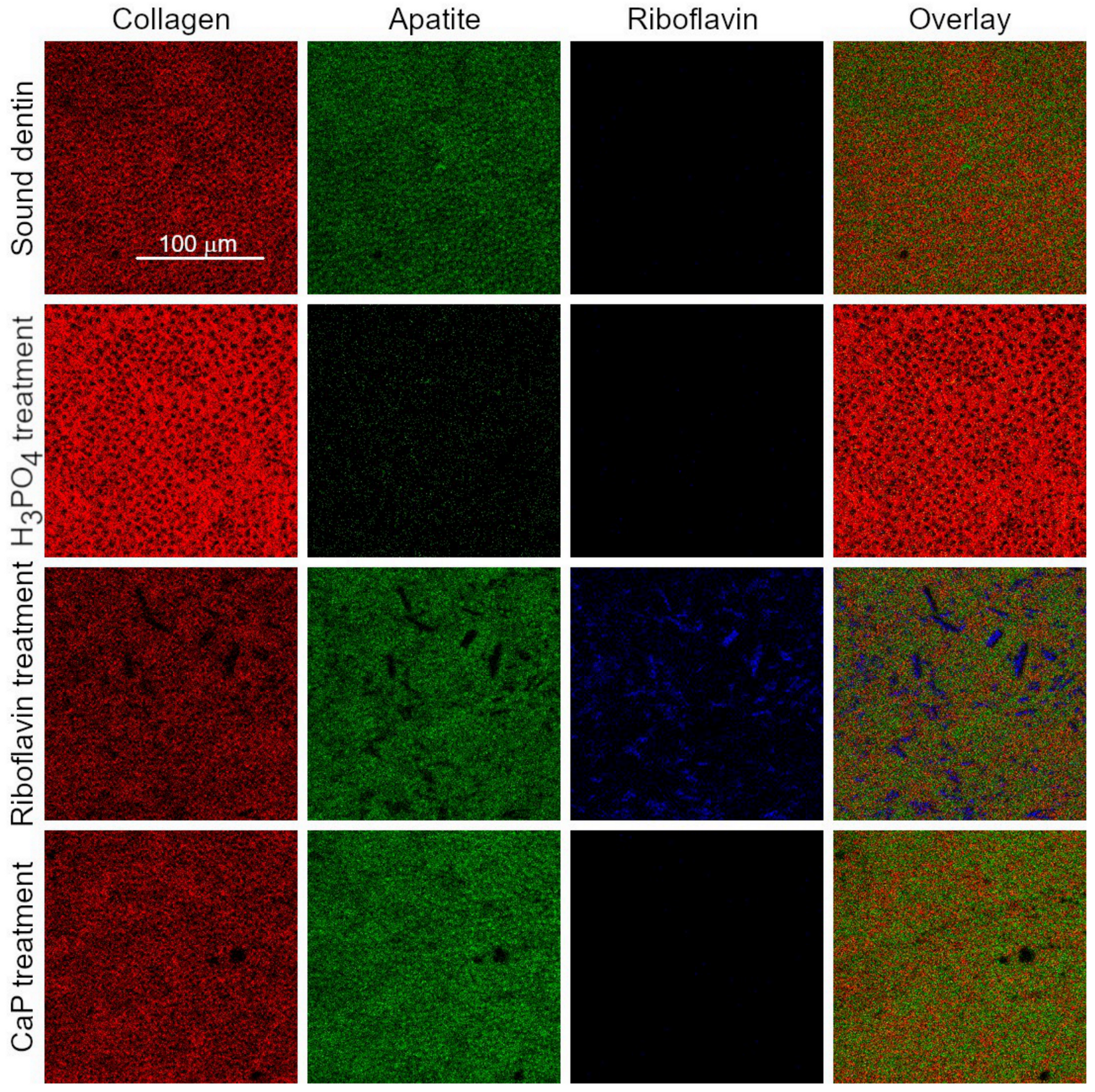
2.4. ToF-SIMS Molecular Characterization
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hannas, A.R.; Kato, M.T.; Cardoso Cde, A.; Magalhaes, A.C.; Pereira, J.C.; Tjaderhane, L.; Buzalaf, M.A. Preventive effect of toothpastes with MMP inhibitors on human dentine erosion and abrasion in vitro. J. Appl. Oral Sci. 2016, 24, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Seemann, R.; Flury, S.; Pfefferkorn, F.; Lussi, A.; Noack, M.J. Restorative dentistry and restorative materials over the next 20 years: A Delphi survey. Dent. Mater. 2014, 30, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Gluzman, R.; Katz, R.V.; Frey, B.J.; McGowan, R. Prevention of root caries: A literature review of primary and secondary preventive agents. Spec. Care Dent. 2013, 33, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Wierichs, R.J.; Meyer-Lueckel, H. Systematic review on noninvasive treatment of root caries lesions. J. Dent. Res. 2015, 94, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Caneppele, T.M.; Jeronymo, R.D.; Di Nicolo, R.; de Araujo, M.A.; Soares, L.E. In Vitro assessment of dentin erosion after immersion in acidic beverages: Surface profile analysis and energy-dispersive X-ray fluorescence spectrometry study. Braz. Dent. J. 2012, 23, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Bortolotto, T.; Mileo, A.; Krejci, I. Strength of the bond as a predictor of marginal performance: An in vitro evaluation of contemporary adhesives. Dent. Mater. 2010, 26, 242–248. [Google Scholar] [CrossRef]
- Bortolotto, T.; Doudou, W.; Kunzelmann, K.H.; Krejci, I. The competition between enamel and dentin adhesion within a cavity: An in vitro evaluation of class V restorations. Clin. Oral Investig. 2012, 16, 1125–1135. [Google Scholar] [CrossRef]
- Bahillo, J.; Roig, M.; Bortolotto, T.; Krejci, I. Self-etching aspects of a three-step etch-and-rinse adhesive. Clin. Oral Investig. 2013, 17, 1893–1900. [Google Scholar] [CrossRef]
- Bortolotto, T.; Dagon, C.; Krejci, I. Light polymerization during cavity filling: Effect of ‘exposure reciprocity law’ and the resulted shrinkage forces on restoration margins. Acta Odontol. Scand. 2013, 71, 1296–1302. [Google Scholar] [CrossRef]
- Bortolotto, T.; Prando, F.; Dietschi, D.; Krejci, I. Light polymerization during cavity filling: Influence of total energy density on shrinkage and marginal adaptation. Odontology 2014, 102, 184–188. [Google Scholar] [CrossRef]
- Bortolotto, T.; Bahillo, J.; Richoz, O.; Hafezi, F.; Krejci, I. Failure analysis of adhesive restorations with SEM and OCT: From marginal gaps to restoration loss. Clin. Oral Investig. 2015, 19, 1881–1890. [Google Scholar] [CrossRef]
- MÜLler, C.; Rosa, G.C.d.; Teixeira, G.S.; Krejci, I.; Bortolotto, T.; Susin, A.H. Effect of caries-affected dentin on one-step universal and multi-step etch-and-rinse adhesives’ bond strength. Rev. Odontol. UNESP 2017, 46, 273–277. [Google Scholar] [CrossRef]
- Pretty, I.A.; Ellwood, R.P. The caries continuum: Opportunities to detect, treat and monitor the re-mineralization of early caries lesions. J. Dent. 2013, 41 (Suppl. 2), S12–S21. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; He, L.H.; Kataoka, Y.; Miyazaki, T.; Swain, M.V. Micromechanical property recovery of human carious dentin achieved with colloidal nano-beta-tricalcium phosphate. J. Dent. Res. 2008, 87, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Mjor, I.A. Dentin permeability: The basis for understanding pulp reactions and adhesive technology. Braz. Dent. J. 2009, 20, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Palamara, J.E.A.; Burrow, M.F. Effects of Collagen Crosslinkers on Dentine: A Literature Review. Calcif. Tissue Int. 2018, 102, 265–279. [Google Scholar] [CrossRef]
- Bedran-Russo, A.K.; Pereira, P.N.; Duarte, W.R.; Drummond, J.L.; Yamauchi, M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 268–272. [Google Scholar] [CrossRef]
- Bedran-Russo, A.K.; Pashley, D.H.; Agee, K.; Drummond, J.L.; Miescke, K.J. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Bedran-Russo, A.K.; Pauli, G.F.; Chen, S.N.; McAlpine, J.; Castellan, C.S.; Phansalkar, R.S.; Aguiar, T.R.; Vidal, C.M.; Napotilano, J.G.; Nam, J.W.; et al. Dentin biomodification: Strategies, renewable resources and clinical applications. Dent. Mater. 2014, 30, 62–76. [Google Scholar] [CrossRef]
- Cecchin, D.; Farina, A.P.; Bedran-Russo, A.K. Efficacy of Natural Collagen Crosslinkers on the Compromised Adhesive Bond Strength to NaOCl-treated Pulp Chamber Dentin. J. Adhes. Dent. 2018, 20, 365–369. [Google Scholar] [CrossRef]
- Hafezi, F.; Mrochen, M.; Iseli, H.P.; Seiler, T. Collagen crosslinking with ultraviolet-A and hypoosmolar riboflavin solution in thin corneas. J. Cataract Refract. Surg. 2009, 35, 621–624. [Google Scholar] [CrossRef]
- Spoerl, E.; Huhle, M.; Seiler, T. Induction of cross-links in corneal tissue. Exp. Eye Res. 1998, 66, 97–103. [Google Scholar] [CrossRef]
- Bortolotto, T.; Ryabova, A.; Nerushay, I.; Kling, S.; Hafezi, F.; Garcia-Godoy, F.; Krejci, I. Effects of riboflavin, calcium-phosphate layer and adhesive system on stress-strain behavior of demineralized dentin. Am. J. Dent. 2017, 30, 179–184. [Google Scholar] [PubMed]
- Ahgilan, A.; Sabaratnam, V.; Periasamy, V. Antimicrobial Properties of Vitamin B2. Int. J. Food Prop. 2015, 19, 1173–1181. [Google Scholar] [CrossRef]
- Watson, T.F.; Atmeh, A.R.; Sajini, S.; Cook, R.J.; Festy, F. Present and future of glass-ionomers and calcium-silicate cements as bioactive materials in dentistry: Biophotonics-based interfacial analyses in health and disease. Dent. Mater. 2014, 30, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Atmeh, A.R.; Chong, E.Z.; Richard, G.; Boyde, A.; Festy, F.; Watson, T.F. Calcium silicate cement-induced remineralisation of totally demineralised dentine in comparison with glass ionomer cement: Tetracycline labelling and two-photon fluorescence microscopy. J. Microsc. 2015, 257, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R. Calcium phosphates as ion-releasing fillers in restorative resin-based materials. Dent. Mater. 2019, 35, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Spagnuolo, G.; Siboni, F.; Procino, A.; Rivieccio, V.; Pelliccioni, G.A.; Prati, C.; Rengo, S. Calcium silicate/calcium phosphate biphasic cements for vital pulp therapy: Chemical-physical properties and human pulp cells response. Clin. Oral Investig. 2015, 19, 2075–2089. [Google Scholar] [CrossRef] [PubMed]
- Gotliv, B.A.; Robach, J.S.; Veis, A. The composition and structure of bovine peritubular dentin: Mapping by time of flight secondary ion mass spectroscopy. J. Struct. Biol. 2006, 156, 320–333. [Google Scholar] [CrossRef]
- Malmberg, P.; Nygren, H. Methods for the analysis of the composition of bone tissue, with a focus on imaging mass spectrometry (TOF-SIMS). Proteomics 2008, 8, 3755–3762. [Google Scholar] [CrossRef]
- Henss, A.; Rohnke, M.; El Khassawna, T.; Govindarajan, P.; Schlewitz, G.; Heiss, C.; Janek, J. Applicability of ToF-SIMS for monitoring compositional changes in bone in a long-term animal model. J. R. Soc. Interface 2013, 10, 20130332. [Google Scholar] [CrossRef] [PubMed]
- Kolosowski, K.P.; Sodhi, R.N.; Kishen, A.; Basrani, B.R. Qualitative analysis of precipitate formation on the surface and in the tubules of dentin irrigated with sodium hypochlorite and a final rinse of chlorhexidine or QMiX. J. Endod. 2014, 40, 2036–2040. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.; Sodhi, R.N.S.; Kishen, A. Interfacial Characterization of Dentin Conditioned with Chitosan Hydroxyapatite Precursor Nanocomplexes Using Time-of-flight Secondary Ion Mass Spectrometry. J. Endod. 2019, 45, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.; Nitisusanta, L.; Iqbal, K.; Daood, U.; Beng, L.T.; Neo, J. Characterization of riboflavin-modified dentin collagen matrix. J. Dent. Res. 2012, 91, 1049–1054. [Google Scholar] [CrossRef]
- Krejci, I.; Kuster, M.; Lutz, F. Influence of dentinal fluid and stress on marginal adaptation of resin composites. J. Dent. Res. 1993, 72, 490–494. [Google Scholar] [CrossRef]
- Bortolotto, T.; Betancourt, F.; Krejci, I. Marginal integrity of resin composite restorations restored with PPD initiatorcontaining resin composite cured by QTH, monowave and polywave LED units. Dent. Mater. J. 2016, 35, 869–875. [Google Scholar] [CrossRef][Green Version]
- Li, X.; De Munck, J.; Van Landuyt, K.; Pedano, M.; Chen, Z.; Van Meerbeek, B. How effectively do hydraulic calcium-silicate cements re-mineralize demineralized dentin. Dent. Mater. 2017, 33, 434–445. [Google Scholar] [CrossRef]
- Krejci, I.; Albertoni, M.; Lutz, F. An in-vitro test procedure for evaluating dental restoration systems. 2. Toothbrush/toothpaste abrasion and chemical degradation. Schweiz. Monatsschr. Zahnmed. 1990, 100, 1164–1168. [Google Scholar]
- Kawecki, M.; Bernard, L. Database of proteinogenic amino acid reference spectra for Bismuth-cluster ToF-SIMS. II. Positive polarity. Surf. Sci. Spectra 2018, 25. [Google Scholar] [CrossRef]
- Banerjee, A.; Frencken, J.E.; Schwendicke, F.; Innes, N.P.T. Contemporary operative caries management: Consensus recommendations on minimally invasive caries removal. Br. Dent. J. 2017, 223, 215–222. [Google Scholar] [CrossRef]
- Frencken, J.E.; Peters, M.C.; Manton, D.J.; Leal, S.C.; Gordan, V.V.; Eden, E. Minimal intervention dentistry for managing dental caries—A review: Report of a FDI task group. Int. Dent. J. 2012, 62, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; van Noort, R.; Martin, N. Infiltration of demineralized dentin with silica and hydroxyapatite nanoparticles. Dent. Mater. 2012, 28, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Isolan, C.P.; Sarkis-Onofre, R.; Lima, G.S.; Moraes, R.R. Bonding to Sound and Caries-Affected Dentin: A Systematic Review and Meta-Analysis. J. Adhes. Dent. 2018, 20, 7–18. [Google Scholar] [CrossRef]
- Hass, V.; da Maceno Oliveira, T.B.; Cardenas, A.F.M.; de Siqueira, F.S.F.; Bauer, J.R.; Abuna, G.; Sinhoreti, M.A.C.; de Souza, J.J.; Loguercio, A.D. Is it possible for a simultaneous biomodification during acid etching on naturally caries-affected dentin bonding? Clin. Oral Investig. 2020, 25, 3543–3553. [Google Scholar] [CrossRef]
- Vidal, C.M.; Tjaderhane, L.; Scaffa, P.M.; Tersariol, I.L.; Pashley, D.; Nader, H.B.; Nascimento, F.D.; Carrilho, M.R. Abundance of MMPs and cysteine cathepsins in caries-affected dentin. J. Dent. Res. 2014, 93, 269–274. [Google Scholar] [CrossRef]
- Seseogullari-Dirihan, R.; Apollonio, F.; Mazzoni, A.; Tjaderhane, L.; Pashley, D.; Breschi, L.; Tezvergil-Mutluay, A. Use of crosslinkers to inactivate dentin MMPs. Dent. Mater. 2016, 32, 423–432. [Google Scholar] [CrossRef]
- Makdoumi, K.; Backman, A.; Mortensen, J.; Crafoord, S. Evaluation of antibacterial efficacy of photo-activated riboflavin using ultraviolet light (UVA). Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 207–212. [Google Scholar] [CrossRef]
- Hass, V.; de Paula, A.M.; Parreiras, S.; Gutierrez, M.F.; Luque-Martinez, I.; de Paris Matos, T.; Bandeca, M.C.; Loguercio, A.D.; Yao, X.; Wang, Y.; et al. Degradation of dentin-bonded interfaces treated with collagen cross-linking agents in a cariogenic oral environment: An in situ study. J. Dent. 2016, 49, 60–67. [Google Scholar] [CrossRef]
- Uemura, R.; Miura, J.; Ishimoto, T.; Yagi, K.; Matsuda, Y.; Shimizu, M.; Nakano, T.; Hayashi, M. UVA-activated riboflavin promotes collagen crosslinking to prevent root caries. Sci. Rep. 2019, 9, 1252. [Google Scholar] [CrossRef]
- Lodha, E.; Hamba, H.; Nakashima, S.; Sadr, A.; Nikaido, T.; Tagami, J. Effect of different desensitizers on inhibition of bovine dentin demineralization: Micro-computed tomography assessment. Eur. J. Oral Sci. 2014, 122, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.C. Next generation calcium phosphate-based biomaterials. Dent. Mater. J. 2009, 28, 1–10. [Google Scholar] [CrossRef]
- Daood, U.; Omar, H.; Qasim, S.; Nogueira, L.P.; Pichika, M.R.; Mak, K.K.; Steier, L.; Cky, Y.; Lin, S.L.; Fawzy, A.S. New antimicrobial and collagen crosslinking formulated dentin adhesive with improved bond durability. J. Mech. Behav. Biomed. Mater. 2020, 110, 103927. [Google Scholar] [CrossRef]
- Ahn, Y.; Lamy, R.; Darling, C.L.; Stewart, J.M.; Pinzon, L.M. Photochemical crosslinking of caries-affected dentin combined with total- or self-etch systems. Am. J. Transl. Res. 2018, 10, 2990–2995. [Google Scholar] [PubMed]
- Reis, A.; Carrilho, M.; Breschi, L.; Loguercio, A.D. Overview of clinical alternatives to minimize the degradation of the resin-dentin bonds. Oper. Dent. 2013, 38, E1–E25. [Google Scholar] [CrossRef] [PubMed]
- Epasinghe, D.J.; Yiu, C.; Burrow, M.F. Synergistic effect of proanthocyanidin and CPP-ACFP on remineralization of artificial root caries. Aust. Dent. J. 2015, 60, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.C.; Bresciani, E.; Barata, T.J.; Fagundes, T.C.; Navarro, R.L.; Navarro, M.F.; Dickens, S.H. In vivo dentin remineralization by calcium-phosphate cement. J. Dent. Res. 2010, 89, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Deyhle, H.; Bunk, O.; Muller, B. Nanostructure of healthy and caries-affected human teeth. Nanomedicine 2011, 7, 694–701. [Google Scholar] [CrossRef]
| Sound Dentin | Caries-Simulated Dentin | H3PO4 etch | RF + CaP | CUB | APX | |
|---|---|---|---|---|---|---|
| Gr 1 | x | x | x | |||
| Gr 2 | x | x | x | x | ||
| Gr 3 | x | x | x | x | ||
| Gr 4 | x | x | x | x | x | |
| Gr 5 | x | x | x | |||
| Gr 6 | x | x | x | x | ||
| Gr 7 | x | x | x | x | ||
| Gr 8 | x | x | x | x | x |
| Gr 1: CUB + APX in sound dentin |
| CUB was applied on dentin surface, air-dried and light-cured for 10 s with a LED Curing Light (VALO, Ultradent, Cologne, Germany, power output: 1000 mW/cm2, wavelength: 450–470 nm) at a distance of 1 mm from the light source output. |
| Gr 2: RF + CaP + CUB + APX in sound dentin |
| Dentin was brushed with RF for 5 min, gently air-dried and UV-exposed with the same light-curing unit as mentioned above for 60 s. Then the CaP-containing material was applied for 2 min, rinsed and air-dried. CUB was applied on dentin surface, air-dried and light-cured for 10 s. |
| Gr 3: H3PO4 + CUB + APX in sound dentin |
| Dentin was etched with H3PO4 for 10 s, rinsed and air-dried. CUB was applied on dentin surface, air-dried and light-cured for 10 s. |
| Gr 4: H3PO4 + RF + CaP + CUB + APX in sound dentin |
| Dentin was etched with H3PO4 for 10 s, rinsed and air-dried. Dentin was then brushed with RF for 5 min, gently air-dried and UV-exposed for 60 s. Then the CaP-containing material was applied for 2 min, rinsed and air-dried. CUB was applied on dentin surface, air-dried and light-cured for 10 s. |
| Gr 5: CUB + APX in caries-simulated dentin |
| CUB was applied on dentin surface, air-dried and light-cured for 10 s. |
| Gr 6: RF+ CaP + CUB + APX in caries-simulated dentin |
| Dentin was brushed with RF for 5 min, gently air-dried and UV-exposed for 60 s. Then the CaP-containing material was applied for 2 min, rinsed and air-dried. CUB was applied on dentin surface, air-dried and light-cured for 10 s. |
| Gr 7: H3PO4 + CUB + APX in caries-simulated dentin |
| Dentin was etched with H3PO4 for 10 s, rinsed and air-dried. CUB was applied on dentin surface, air-dried and light-cured for 10 s. |
| Gr 8: H3PO4 + RF + CaP + CUB + APX in caries-simulated dentin |
| Dentin was etched with H3PO4 for 10 s, rinsed and air-dried. Dentin was then brushed with RF for 5 min, gently air-dried and UV-exposed for 60 s. Then the CaP-containing material was applied for 2 min, rinsed and air-dried. CUB was applied on dentin surface, air-dried and light-cured for 10 s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betancourt, F.; Kiss, A.; Krejci, I.; Bortolotto, T. ToF-SIMS Analysis of Demineralized Dentin Biomodified with Calcium Phosphate and Collagen Crosslinking: Effect on Marginal Adaptation of Class V Adhesive Restorations. Materials 2021, 14, 4535. https://doi.org/10.3390/ma14164535
Betancourt F, Kiss A, Krejci I, Bortolotto T. ToF-SIMS Analysis of Demineralized Dentin Biomodified with Calcium Phosphate and Collagen Crosslinking: Effect on Marginal Adaptation of Class V Adhesive Restorations. Materials. 2021; 14(16):4535. https://doi.org/10.3390/ma14164535
Chicago/Turabian StyleBetancourt, Francisco, Andràs Kiss, Ivo Krejci, and Tissiana Bortolotto. 2021. "ToF-SIMS Analysis of Demineralized Dentin Biomodified with Calcium Phosphate and Collagen Crosslinking: Effect on Marginal Adaptation of Class V Adhesive Restorations" Materials 14, no. 16: 4535. https://doi.org/10.3390/ma14164535
APA StyleBetancourt, F., Kiss, A., Krejci, I., & Bortolotto, T. (2021). ToF-SIMS Analysis of Demineralized Dentin Biomodified with Calcium Phosphate and Collagen Crosslinking: Effect on Marginal Adaptation of Class V Adhesive Restorations. Materials, 14(16), 4535. https://doi.org/10.3390/ma14164535







