Nonlinear Optical Properties of Porphyrin, Fullerene and Ferrocene Hybrid Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. EFISH Measurements
2.3. Computational Details
3. Results and Discussion
3.1. Synthesis
3.2. UV-Vis Spectroscopy
3.3. EFISH Investigation of the Second-Order NLO Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cariati, E.; Pizzotti, M.; Roberto, D.; Tessore, F.; Ugo, R. Coordination and organometallic compounds and inorganic-organic hybrid crystalline materials for second-order non-linear optics. Coord. Chem. Rev. 2006, 250, 1210–1233. [Google Scholar] [CrossRef]
- Di Bella, S.; Dragonetti, C.; Pizzotti, M.; Roberto, D.; Tessore, F.; Ugo, R. Coordination and organometallic complexes as second-order nonlinear optical molecular materials. Top. Organomet. Chem. 2010, 28, 1–55. [Google Scholar] [CrossRef]
- Tessore, F.; Orbelli Biroli, A.; Di Carlo, G.; Pizzotti, M. Porphyrins for second order nonlinear optics (NLO): An intriguing history. Inorganics 2018, 6, 81–97. [Google Scholar] [CrossRef]
- Suslick, K.S.; Chen, C.T.; Meredith, G.R.; Cheng, L.T. Push-Pull porphyrins as nonlinear optical materials. J. Am. Chem. Soc. 1992, 114, 6928–6930. [Google Scholar] [CrossRef]
- LeCours, S.M.; Guan, H.W.; Di Magno, S.G.; Wang, C.H.; Therien, M.J. Push-pull arylethynyl porphyrins: New chromophores that exhibit large molecular first-order hyperpolarizabilities. J. Am. Chem. Soc. 1996, 118, 1497–1503. [Google Scholar] [CrossRef]
- Yeung, M.; Ng, A.C.H.; Drew, M.G.E.; Vorpagel, E.; Breitung, E.M.; McMahon, R.J.; Ng, D.K. Facile synthesis and nonlinear optical properties of push-pull 5,15-diphenylporphyrins. J. Org. Chem. 1998, 63, 7143–7150. [Google Scholar] [CrossRef]
- Pizzotti, M.; Annoni, E.; Ugo, R.; Bruni, S.; Quici, S.; Fantucci, P.; Bruschi, M.; Zerbi, G.; Del Zoppo, M. A multitechnique investigation of the second order NLO response of 10,20-diphenylporphyrinato nickel(II) complex carrying a phenylethynyl based push-pull system in the 5- and 15-positions. J. Porphyr. Phthalocyanines 2004, 8, 1311–1324. [Google Scholar] [CrossRef]
- Annoni, E.; Pizzotti, M.; Ugo, R.; Quici, S.; Morotti, T.; Bruschi, M.; Mussini, P. Synthesis, electronic properties and significant second-order non-linear optical responses of meso-tetraphenylporphyrins and their ZnII complexes carrying a push or pull group in the β pyrrolic position. Eur. J. Inorg. Chem. 2005, 3857–3874. [Google Scholar] [CrossRef]
- Morotti, T.; Pizzotti, M.; Ugo, R.; Quici, S.; Bruschi, M.; Mussini, P.; Righetto, S. Electronic characterization and significant second-order NLO response of 10,20-diphenylporphyrins and their ZnII complexes substituted in the meso position with π-delocalized linkers carrying push or pull groups. Eur. J. Inorg. Chem. 2006, 1743–1757. [Google Scholar] [CrossRef]
- Lopez-Duarte, I.; Reeve, J.E.; Pérez-Moreno, J.; Boczarow, I.; Depotter, G.; Fleischhauer, J.; Clays, K.; Anderson, H.L. “Push-no-pull” porphyrins for second harmonic generation imaging. Chem. Sci. 2013, 4, 2024–2027. [Google Scholar] [CrossRef]
- Levine, B.F.; Bethea, C.G. Molecular hyperpolarizabilities determined from conjugated and nonconjugated organic liquids. Appl. Phys. Lett. 1974, 24, 445–447. [Google Scholar] [CrossRef]
- Singer, K.D.; Garito, A.F. Measurements of molecular second order optical susceptibilities using dc induced second harmonic generation. J. Chem. Phys. 1981, 75, 3572–3580. [Google Scholar] [CrossRef]
- De Angelis, F.; Fantacci, S.; Sgamellotti, A.; Pizzotti, M.; Tessore, F.; Orbelli Biroli, A. Time-dependent and coupled-perturbed DFT and HF investigations on the absorption spectrum and nonlinear optical properties of push-pull M(II)-porphyrin complexes (M=Zn, Cu, Ni). Chem. Phys. Lett. 2007, 447, 10–15. [Google Scholar] [CrossRef]
- Tessore, F.; Di Carlo, G.; Forni, A.; Righetto, S.; Limosani, F.; Orbelli Biroli, A. Second order nonlinear optical properties of 4-styrylpyridines axially coordinated to A4 ZnII porphyrins: A comparative experimental and theoretical investigation. Inorganics 2020, 8, 45. [Google Scholar] [CrossRef]
- Pizzotti, M.; Tessore, F.; Orbelli Biroli, A.; Ugo, R.; De Angelis, F.; Fantacci, S.; Sgamellotti, A.; Zuccaccia, D.; Macchioni, A. An EFISH, theoretical, and PGSE NMR investigation on the relevant role of aggregation on the second order response in CHCl3 of the push-pull chromophores [5-[[4′-(Dimethylamino)phenyl]ethynyl]-15-[(4″-nitrophenyl)ethynyl]-10,20-diphenylporphyrinate]M(II) (M=Zn, Ni). J. Phys. Chem. C 2009, 113, 11131–11141. [Google Scholar] [CrossRef]
- Orbelli Biroli, A.; Tessore, F.; Righetto, S.; Forni, A.; Macchioni, A.; Rocchigiani, L.; Pizzotti, M.; Di Carlo, G. Intriguing influence of −COOH-driven intermolecular aggregation and acid−base interactions with N,N-dimethylformamide on the second-order nonlinear-optical response of 5,15 push−pull diarylzinc(II) porphyrinates. Inorg. Chem. 2017, 56, 6438–6450. [Google Scholar] [CrossRef]
- Di Carlo, G.; Pizzotti, M.; Righetto, S.; Forni, A.; Tessore, F. Electric-field-induced second harmonic generation nonlinear optic response of A4 β-pyrrolic-substituted ZnII porphyrins: When cubic contributions cannot be neglected. Inorg. Chem. 2020, 59, 7561–7570. [Google Scholar] [CrossRef]
- Li, L.L.; Diau, E.W.G. Porphyrin-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 291–304. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef]
- Lu, J.; Liu, S.; Wang, M. Push-pull porphyrins as light-harvesters for efficient dye-sensitized solar cells. Front. Chem. 2018, 6, 541. [Google Scholar] [CrossRef]
- Di Carlo, G.; Orbelli Biroli, A.; Tessore, F.; Pizzotti, M.; Mussini, P.R.; Amat, A.; De Angelis, F.; Abbotto, A.; Trifiletti, V.; Ruffo, R. Physicochemical investigation of the panchromatic effect on β-substituted ZnII porphyrinates for DSSCs: The role of the π bridge between a dithienylethylene unit and the porphyrinic ring. J. Phys. Chem. C 2014, 118, 7307–7320. [Google Scholar] [CrossRef]
- Covezzi, A.; Orbelli Biroli, A.; Tessore, F.; Forni, A.; Marinotto, D.; Biagini, P.; Di Carlo, G.; Pizzotti, M. 4D-π-1A type β-substituted ZnII-porphyrins: Ideal green sensitizers for building-integrated photovoltaics. Chem. Commun. 2016, 52, 12642–12645. [Google Scholar] [CrossRef]
- Colombo, A.; Di Carlo, G.; Dragonetti, C.; Magni, M.; Orbelli Biroli, A.; Pizzotti, M.; Roberto, D.; Tessore, F.; Benazzi, E.; Bignozzi, C.A.; et al. Coupling of zinc porphyrin dyes and copper electrolytes: A springboard for novel sustainable dye-sensitized solar cells. Inorg. Chem. 2017, 56, 14189–14197. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, G.; Orbelli Biroli, A.; Pizzotti, M.; Tessore, F. Efficient sunlight harvesting by A4 β-pyrrolic substitued ZnII porphyrins: A mini-review. Front. Chem. 2019, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Antolovic, M.; Keyte, P.J.; Oliver, A.M.; Paddon-Row, M.N.; Kroon, J.; Verhoeven, J.W.; Jonker, S.A.; Warman, J.M. Modeling long-range photosynthetic electron transfer in rigidly bridged porphyrin-quinone systems. J. Phys. Chem. 1991, 95, 1933–1941. [Google Scholar] [CrossRef]
- Bell, T.D.M.; Smith, T.A.; Ghiggino, K.P.; Ranasinghe, M.G.; Shephard, M.J.; Paddon-Row, M.N. Long-lived photoinduced charge separation in a bridged C60-porphyrin dyad. Chem. Phys. Lett. 1997, 268, 223–228. [Google Scholar] [CrossRef]
- Guldi, D.M.; Kamat, P.V. Photophysical properties of pristine fullerenes, functionalized fullerenes, and fullerene-containing donor-bridge acceptor systems. In Fullerenes: Chemistry, Physics, and Technology, 1st ed.; Kadish, K.M., Ruoff, R.S., Eds.; John Wiley & Sons Inc.: New York, NY, USA, 2000; pp. 225–281. [Google Scholar]
- Armaroli, N. Photoinduced energy transfer processes in functionalized fullerenes. In Fullerenes: From Synthesis to Optoelectronic Properties, 1st ed.; Guldi, D.M., Martin, N., Eds.; Springer: Dordrecht, Switzerland, 2002; Volume 4, pp. 137–162. [Google Scholar]
- Signorini, R.; Bozio, R.; Prato, M. Optical limiting applications. In Fullerenes: From Synthesis to Optoelectronic Properties, 1st ed.; Guldi, D.M., Martin, N., Eds.; Springer: Dordrecht, Switzerland, 2002; Volume 4, pp. 295–326. [Google Scholar]
- Echegoyen, L.; Diederich, F.; Echegoyen, L.E. Electrochemistry of fullerenes. In Fullerenes: Chemistry, Physics, and Technology, 1st ed.; Kadish, K.M., Ruoff, R.S., Eds.; John Wiley & Sons Inc.: New York, NY, USA, 2000; pp. 1–51. [Google Scholar]
- Loboda, O.; Zalesny, R.; Avramopoulos, A.; Luis, J.M.; Kirtman, B.; Tagmatarchis, N.; Reis, H.; Papadopoulos, M.G. Linear and nonlinear optical properties of [60]fullerene derivatives. J. Phys. Chem. A 2009, 113, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Prato, M. [60]Fullerene chemistry for materials science applications. J. Mater. Chem. 1997, 7, 1097–1109. [Google Scholar] [CrossRef]
- Guldi, D.M. Fullerene-porphyrin architectures; photosynthetic antenna and reaction center models. Chem. Soc. Rev. 2002, 31, 22–36. [Google Scholar] [CrossRef]
- Imahori, H.; Hagiwara, K.; Aoki, M.; Akiyama, T.; Taniguchi, S.; Okada, T.; Shirakawa, M.; Sakata, Y. Linkage and solvent dependence of photoinduced electron transfer in zincporphyrin-C60 dyads. J. Am. Chem. Soc. 1996, 118, 11771–11782. [Google Scholar] [CrossRef]
- Reed, C.A.; Boyd, P.; Drovetskaya, T. A fullerene porphyrin conjugate. Tetrahedron Lett. 1995, 36, 7971–7974. [Google Scholar] [CrossRef]
- Lembo, A.; Tagliatesta, P.; Guldi, D.M. Synthesis and photophysical investigation of new porphyrin derivatives with β-pyrrole ethynyl linkage and corresponding dyad with [60]fullerene. J. Phys. Chem. A 2006, 110, 11424–11434. [Google Scholar] [CrossRef]
- Lembo, A.; Tagliatesta, P.; Guldi, D.M.; Wielopolski, M.; Nuccettelli, M. Porphyrin-β-oligo-ethynylenephenylene-[60]fullerene triads: Synthesis and electrochemical and photophysical characterization of the new porphyrin-oligo-PPE-[60]fullerene systems. J. Phys. Chem. A 2009, 113, 1779–1793. [Google Scholar] [CrossRef]
- Imahori, H.; Sakata, Y.; Nishimura, Y.; Yamazaki, I. Synthesis and photophysical properties of a diporphyrin-fullerene triad. Chem. Commun. 1999, 625–626. [Google Scholar] [CrossRef]
- Luo, C.; Guldi, D.M.; Imahori, H.; Tamaki, K.; Sakata, Y. Sequential energy and electron transfer in an artificial reaction center: Formation of a long-lived charge-separated state. J. Am. Chem. Soc. 2000, 122, 6535–6551. [Google Scholar] [CrossRef]
- Imahori, H.; Tamaki, K.; Guldi, D.M.; Luo, C.; Fujitsuka, M.; Ito, O.; Fukuzumi, S. Modulating charge separation and charge recombination dynamics in porphyrin-fullerene linked dyads and triads: Marcus-normal versus inverted region. J. Am. Chem. Soc. 2001, 123, 2607–2617. [Google Scholar] [CrossRef]
- Imahori, H.; Guldi, D.M.; Tamaki, K.; Yoshida, Y.; Luo, C.; Sakata, Y.; Fukuzumi, S. Charge separation in a novel artificial photosynthetic reaction center lives 380 ns. J. Am. Chem. Soc. 2001, 123, 6617–6628. [Google Scholar] [CrossRef] [PubMed]
- Imahori, H.; Sekiguchi, Y.; Kashiwagi, Y.; Sato, T.; Araki, Y.; Ito, O.; Fukuzumi, S. Long-lived charge-separated state generated in a ferrocene-meso,meso-linked porphyrin trimer-fullerene pentad with a high quantum yield. Chem. Eur. J. 2004, 10, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, A.; Galloni, P.; Floris, B.; Dudkin, S.V.; Nemykin, V.N. Metallocened meet porphyrinoid: Consequences of a “fusion”. Coord. Chem. Rev. 2015, 291, 95–171. [Google Scholar] [CrossRef]
- Curiel, D.; Ohkubo, K.; Reimers, J.R.; Fukuzumi, S.; Crossley, M.J. Photoinduced electron transfer in β, β’-pyrrolic fused ferrocene-(zinc porphyrin)-fullerene. Phys. Chem. Chem. Phys. 2007, 9, 5260–5266. [Google Scholar] [CrossRef] [PubMed]
- Mazzuca, C.; Di Napoli, B.; Lentini, S.; Cicero, D.O.; Gatto, E.; Tagliatesta, P.; Palleschi, A. β-substituted ferocenyl porphyrins: The role of the spacer and of the number of substituents on their structural and spectroscopic characteristics. J. Porphyr. Phthalocyanines 2016, 20, 234–244. [Google Scholar] [CrossRef]
- Tagliatesta, P.; Pizzoferrato, R. Synthesis and characterization of new ferrocene, porphyrin and C60 triads, connected by triple bonds. J. Organomet. Chem. 2015, 787, 27–32. [Google Scholar] [CrossRef]
- Limosani, F.; Possanza, F.; Ciotta, E.; Pepi, F.; Salvitti, C.; Tagliatesta, P.; Pizzoferrato, R. Synthesis and characterization of two new triads with ferrocene and C60 connected by triple bonds to the beta-positions of meso-tetraphenylporphyrin. J. Porphyr. Phthalocyanines 2017, 21, 364–370. [Google Scholar] [CrossRef]
- Possanza, F.; Limosani, F.; Tagliatesta, P.; Zanoni, R.; Scarselli, M.; Ciotta, E.; Pizzoferrato, R. Functionalization of carbon spheres with a porphyrin-ferrocene diad. Chem. Phys. Chem. 2018, 19, 2243–2249. [Google Scholar] [CrossRef]
- Scarselli, M.; Limosani, F.; Passacantando, M.; D’Orazio, F.; Nardone, M.; Cacciotti, I.; Arduini, F.; Gautron, E.; De Crescenzi, M. Influence of iron catalyst in the carbon spheres synthesis for energy and electrochemical applications. Adv. Mater. Interfaces 2018, 5, 1800070. [Google Scholar] [CrossRef]
- Cinti, S.; Limosani, F.; Scarselli, M.; Arduini, F. Magnetic carbon spheres and their derivatives combined with printed electrochemical sensors. Electrochim. Acta 2018, 282, 247–254. [Google Scholar] [CrossRef]
- Kaur, R.; Possanza, F.; Limosani, F.; Bauroth, S.; Zanoni, R.; Clark, T.; Arrigoni, G.; Tagliatesta, P.; Guldi, D.M.J. Understanding and controlling short- and long-range electron/charge transfer processes in electron donor-acceptor conjugates. J. Am. Chem. Soc. 2020, 142, 7898–7911. [Google Scholar] [CrossRef]
- Limosani, F.; Kaur, R.; Cataldo, A.; Bellucci, S.; Micciulla, F.; Zanoni, R.; Lembo, A.; Wang, B.; Pizzoferrato, R.; Guldi, D.M.; et al. Designing cascades of electron transfer processes in multicomponent graphene conjugates. Angew. Chem. Int. Ed. 2020, 59, 23706–23715. [Google Scholar] [CrossRef]
- Tagliatesta, P.; Lembo, A.; Leoni, A. Synthesis and characterization of eight new tetraphenylporphyrins bearing one or two ferrocene on the β-pyrrole positions. New J. Chem. 2013, 37, 3416–3419. [Google Scholar] [CrossRef][Green Version]
- Willets, A.; Rice, J.E.; Burland, D.M.; Shelton, D.P.J. Problems in the comparison of theoretical and experimental hyperpolarizabilities. Chem. Phys. 1992, 97, 7590–7599. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Ernzerhof, M.; Scuseria, G. Assessment of the Perdew-Burke-Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The Mo6 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four Mo6-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215−241. [Google Scholar] [CrossRef]
- Johnson, L.E.; Dalton, L.R.; Robinson, B.H. Optimizing calculations of electronic excitations and relative hyperpolarizabilities of electrooptic chromophores. Acc. Chem. Res. 2014, 47, 3258−3265. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, H.A.; Dudis, D. Quantum mechanical methods for predicting nonlinear optical properties. Rev. Comput. Chem. 2007, 12, 241−279. [Google Scholar] [CrossRef]
- Pielak, K.; Tonnelé, C.; Sanguinet, L.; Cariati, E.; Righetto, S.; Muccioli, L.; Castet, F.; Champagne, B. Dynamical behaviour and second harmonic generation responses in acido-triggered molecular switches. J. Phys. Chem. C 2018, 122, 26160−26168. [Google Scholar] [CrossRef]
- Xiao, X.; Nagahara, L.A.; Rawlett, A.M.; Tao, N. Electrochemical gate-controlled conductance of single oligo(phenylene ethynylene)s. J. Am. Chem. Soc. 2005, 127, 9235–9240. [Google Scholar] [CrossRef]
- Lewis, P.A.; Inman, C.E.; Maya, F.; Tour, J.M.; Hutchinson, J.E.; Weiss, P.S. Molecular engineering of the polarity and interactions of molecular electronic switches. J. Am. Chem. Soc. 2005, 127, 17421–17426. [Google Scholar] [CrossRef]
- Huber, R.; Gonzalez, M.T.; Wu, S.; Langer, M.; Grunder, S.; Horhoiu, V.; Mayor, M.; Bryce, M.R.; Wang, C.; Jitchati, R.; et al. Electrical conductance of conjugated oligomers at the single molecule level. J. Am. Chem. Soc. 2008, 130, 1080–1084. [Google Scholar] [CrossRef]
- Di Carlo, G.; Orbelli Biroli, A.; Pizzotti, M.; Tessore, F.; Trifiletti, V.; Ruffo, R.; Abbotto, A.; Amat, A.; De Angelis, F.; Mussini, P.R. Tetraaryl ZnII porphyrinates substituted at β-pyrrolic positions as sensitizers in dye-sensitized solar cells: A comparison with meso-disubstituted push-pull Zn(II) porphyrinates. Chem. Eur. J. 2013, 19, 10723–10740. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, Y.-X.; Li, J.-H. Modified palladium-catalyzed sonogashira cross-coupling reactions under copper-, amine-, and Solvent-Free Conditions. J. Org. Chem. 2005, 71, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, J.S.; Prataphan, S.; Johnson, T.E.; Wagner, R.W. Porphyrin building blocks for modular construction of bioorganic model systems. Tetrahedron 1994, 50, 8941–8968. [Google Scholar] [CrossRef]
- Wagner, R.W.; Johnson, T.E.; Lindsey, J.S. Soluble synthetic multiporphyrin arrays. 1. modular design and synthesis J. Am. Chem. Soc. 1996, 118, 11166–11180. [Google Scholar] [CrossRef]
- Wagner, R.W.; Johnson, T.E.; Li, F.; Lindsey, J.S. Synthesis of ethyne-linked or butadiyne-linked porphyrin arrays using mild, copper-free, Pd-mediated coupling reactions. J. Org. Chem. 1995, 60, 5266–5273. [Google Scholar] [CrossRef]
- Maggini, M.; Scorrano, G.; Prato, M. Addition of azomethine ylides to C60: Synthesis, characterization, and functionalization of fullerene pyrrolidines. J. Am. Chem. Soc. 1993, 115, 9798–9799. [Google Scholar] [CrossRef]
- Gouterman, M. Spectra of porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. [Google Scholar] [CrossRef]
- Kumar, S.; Acharyya, J.N.; Banerjee, D.; Soma, V.R.; Prakash, G.V.; Sankar, M. Strong two-photon absorption and ultrafast dynamics of meso-functionalized “push-pull” trans-A2BC porphyrins. Dalton Trans. 2021, 50, 6256–6272. [Google Scholar] [CrossRef] [PubMed]
- Orbelli Biroli, A.; Tessore, F.; Vece, V.; Di Carlo, G.; Mussini, P.R.; Trifiletti, V.; De Marco, L.; Giannuzzi, R.; Manca, M.; Pizzotti, M. Highly improved performance of ZnII tetraarylporphyrinates in DSSCs by the presence of octyloxy chains in the aryl rings. J. Mater Chem. A 2015, 3, 2954–2959. [Google Scholar] [CrossRef]
- Prato, M.; Soombar, C.; Vazquez, E.; Niziol, J.; Gondek, E.; Rau, I.; Kajar, F. Synthesis and spectroscopic properties of porphyrin derivatives of C60. Mol. Cryst. Liq. Cryst. 2010, 521, 253–264. [Google Scholar] [CrossRef]
- Di Carlo, G.; Orbelli Biroli, A.; Tessore, F.; Rizzato, S.; Forni, A.; Magnano, G.; Pizzotti, M. Light-induced regiospecific bromination of meso-tetra(3,5-di-tert-butylphenyl)porphyrin on 2, 12 β-pyrrolic positons. J. Org. Chem. 2015, 80, 4973–4980. [Google Scholar] [CrossRef] [PubMed]
- Oudar, J.L.; Chemla, D.S. Hyperpolarizabilities of the nitroanilines and their relations to the excited state dipole moment. J. Chem. Phys. 1977, 66, 2664–2668. [Google Scholar] [CrossRef]
- Oudar, J.L. Optical nonlinearities of conjugated molecules. Stilbene derivatives and highly polar aromatic compounds. J. Chem. Phys. 1977, 67, 446–457. [Google Scholar] [CrossRef]
- Kanis, D.R.; Lacroix, P.G.; Ratner, M.A.; Marks, T.J. Electronic structure and quadratic hyperpolarizabilities in organotransition-metal chromophores having weakly coupled p-networks. Unusual mechanism for second-order response. J. Am. Chem. Soc. 1994, 116, 10089–10102. [Google Scholar] [CrossRef]
- Dragonetti, C.; Colombo, A.; Fontani, M.; Marinotto, D.; Nisic, F.; Righetto, S.; Roberto, D.; Tintori, F.; Fantacci, S. Novel fullerene alkynyl complexes with high second-order nonlinear optical properties as a springboard for NLO-active polymer films. Organometallics 2016, 35, 1015–1021. [Google Scholar] [CrossRef]
- Pizzotti, M.; Ugo, R.; Annoni, E.; Quici, S.; Ledoux-Rak, I.; Zerbi, G.; Del Zoppo, M.; Fantucci, P.; Invernizzi, I. A critical evaluation of EFISH and THG non-linear optical responses of asymmetrically substituted meso-tetraphenyl porphyrins and their metal complexes. Inorg. Chim. Acta 2002, 340, 70−80. [Google Scholar] [CrossRef]
- De La Torre, G.; Vazquez, P.; Agullo-Lopez, F.; Torres, T. Role of structural factors in the nonlinear optical properties of phtalocyanines and related Compounds. Chem. Rev. 2004, 104, 3723–3750. [Google Scholar] [CrossRef]
- Belviso, S.; Santoro, E.; Penconi, M.; Righetto, S.; Tessore, F. Thioethylporphyrazines: Attractive chromophores for second order nonlinear optics and DSSCs. J. Phys. Chem. C 2019, 123, 13074–13082. [Google Scholar] [CrossRef]
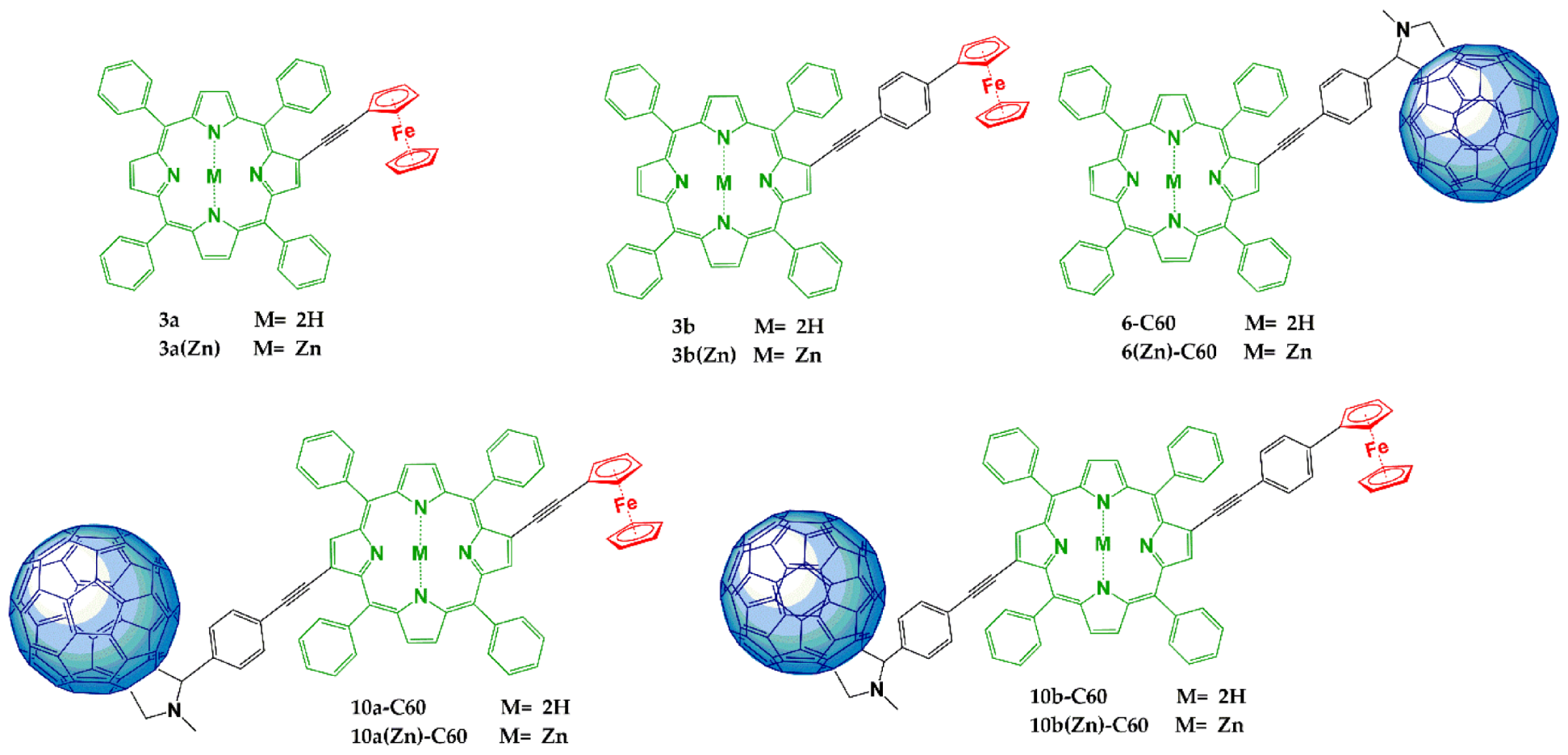
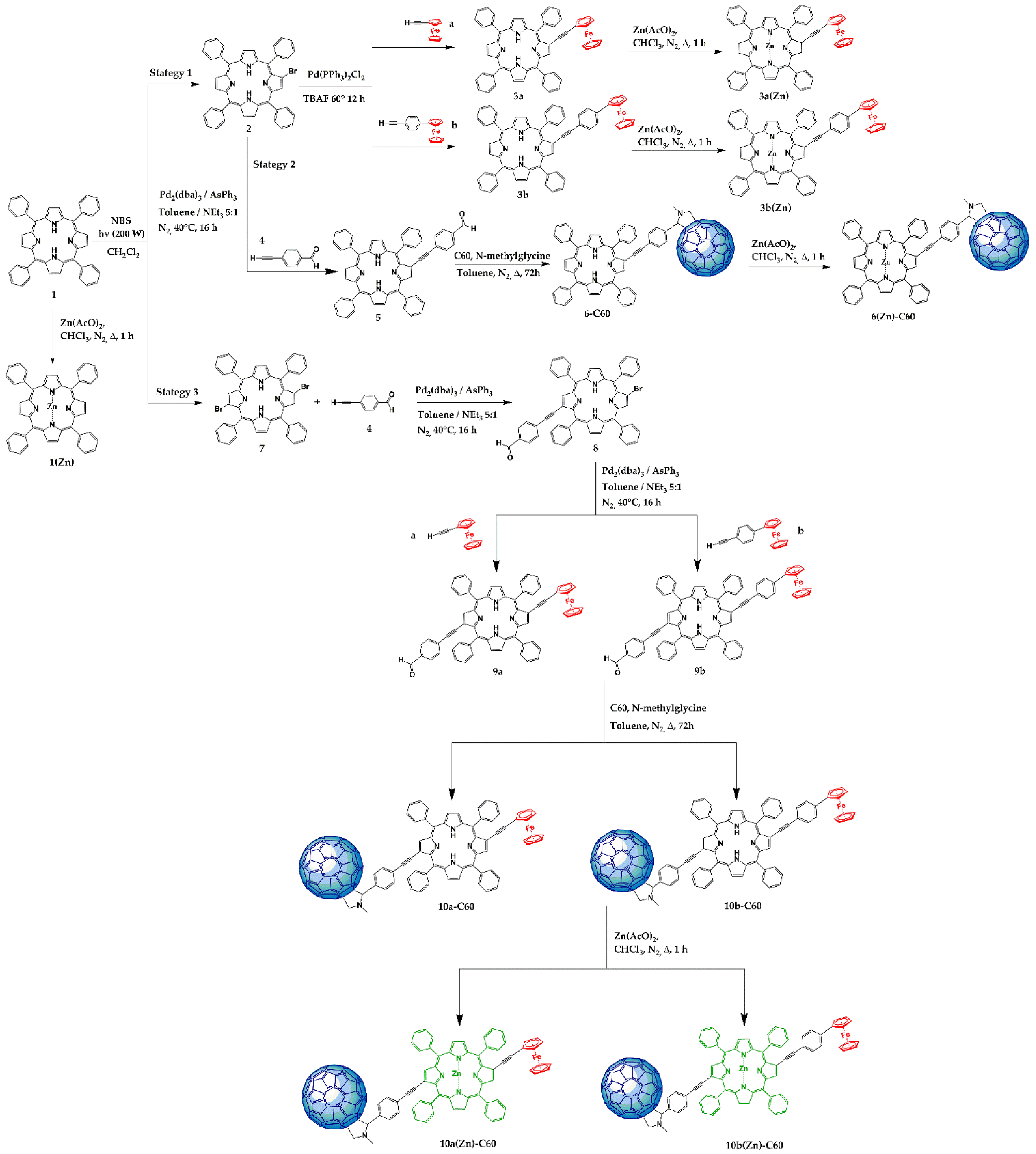
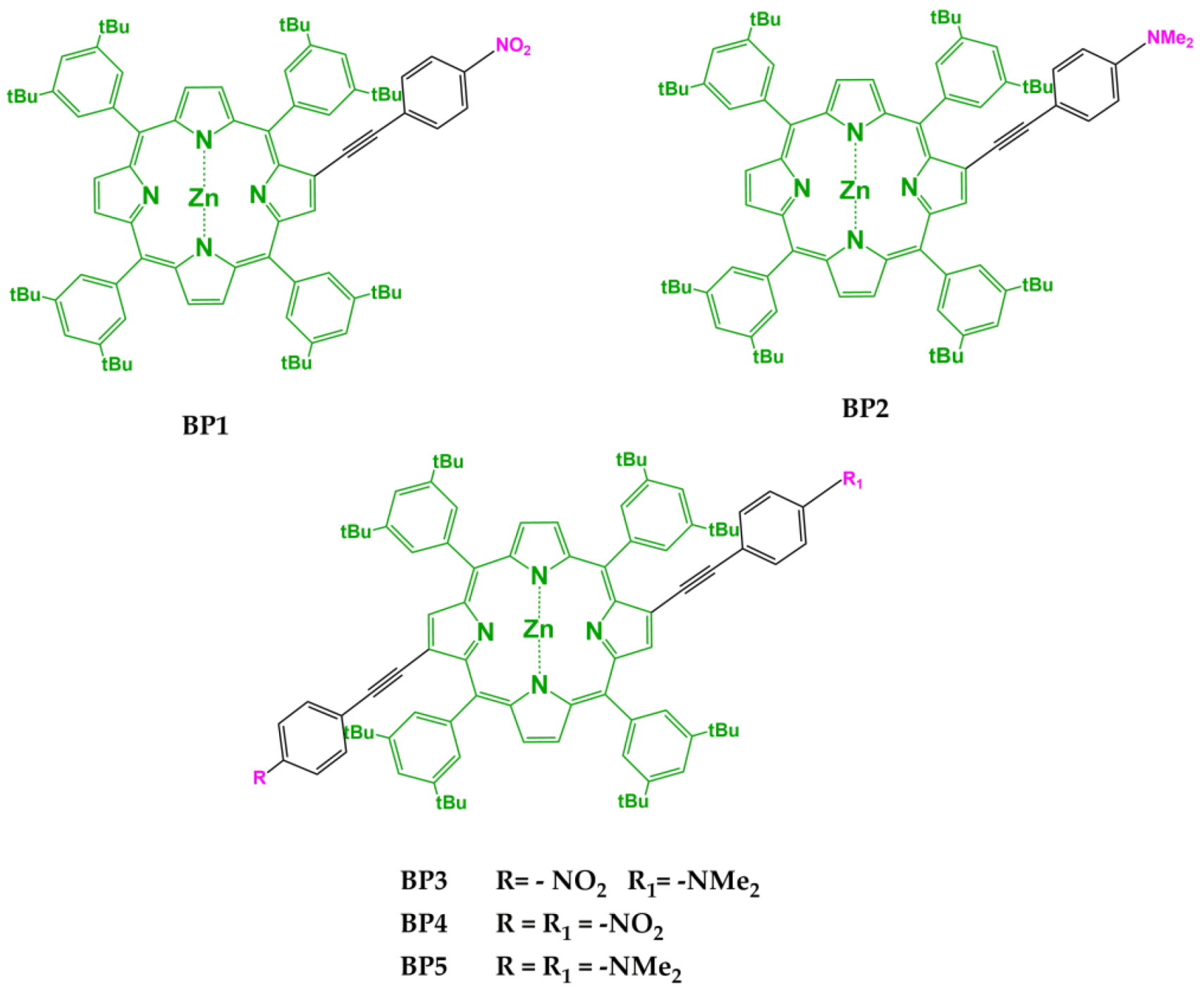
| Compound | Soret Band λmax (nm) (logε) | QIV Band λmax (nm) (logε) | QIII Band λmax (nm) (logε) | QII Band λmax (nm) (logε) | QI Band λmax (nm) (logε) |
|---|---|---|---|---|---|
| 3a | 426 (5.30) | 526 (4.22) | 566 (3.93) | 602 (3.95) | 660 (3.56) |
| 3a(Zn) | 432 (4.50) | 566 (4.08) | 602 (3.83) | ||
| 3b | 427 (5.28) | 526 (4.29) | 563 (3.94) | 601 (3.79) | 658 (3.51) |
| 3b(Zn) | 436 (5.43) | 565 (4.37) | 601 (4.06) | ||
| 6-C60 | 427 (5.31) | 522 (4.27) | 558 (3.83) | 599 (3.76) | 656 (3.42) |
| 6(Zn)-C60 | 434 (5.28) | 560 (4.15) | 598 (4.00) | ||
| 10a-C60 | 434 (5.15) | 527 (4.59) | 580 (3.81) | 616 (3.61) | 670 (3.71) |
| 10a(Zn)-C60 | 449 (5.36) | 574 (4.37) | 613 (4.34) | ||
| 10b-C60 | 435 (5.23) | 532 (4.37) | 574 (4.21) | 609 (4.00) | 666 (3.68) |
| 10b(Zn)-C60 | 438 (5.20) | 570 (3.76) | 612 (3.61) | ||
| 1 | 417 (5.58) | 515 (4.19) | 550 (3.83) | 591 (3.68) | 647 (3.61) |
| 1(Zn) | 420 (5.78) | 548 (4.41) | 589 (3.76) |
| Compound | μ (D) | γEFISH (x × 10−33 esu) | µβ1907 (x × 10−48 esu) |
|---|---|---|---|
| 3a | 0.09 | −1.54 | −320 |
| 3a(Zn) | 0.19 | −3.11 | −640 |
| 3b | 0.49 | −1.88 | −390 |
| 3b(Zn) | 0.28 | −2.85 | −595 |
| 6-C60 | 4.21 | −2.84 | −590 |
| 6(Zn)-C60 | 4.77 | −3.47 | −720 |
| 10a-C60 | 3.82 | −7.12 | −1495 |
| 10a(Zn)-C60 | 4.37 | −8.08 | −1670 |
| 10b-C60 | 3.82 | −5.19 | −1075 |
| 10b(Zn)-C60 | 4.14 | −6.41 | −1330 |
| Compound | β|| (x × 10−30 esu) | μβ||/5kT (x × 10−36 esu) | γ|| (x × 10−36 esu) |
|---|---|---|---|
| 3b(Zn) | −21 | −29 | −1820 |
| 6(Zn)-C60 | 30 | 696 | −1543 |
| 10b(Zn)-C60 | 42 | 845 | −3225 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limosani, F.; Tessore, F.; Di Carlo, G.; Forni, A.; Tagliatesta, P. Nonlinear Optical Properties of Porphyrin, Fullerene and Ferrocene Hybrid Materials. Materials 2021, 14, 4404. https://doi.org/10.3390/ma14164404
Limosani F, Tessore F, Di Carlo G, Forni A, Tagliatesta P. Nonlinear Optical Properties of Porphyrin, Fullerene and Ferrocene Hybrid Materials. Materials. 2021; 14(16):4404. https://doi.org/10.3390/ma14164404
Chicago/Turabian StyleLimosani, Francesca, Francesca Tessore, Gabriele Di Carlo, Alessandra Forni, and Pietro Tagliatesta. 2021. "Nonlinear Optical Properties of Porphyrin, Fullerene and Ferrocene Hybrid Materials" Materials 14, no. 16: 4404. https://doi.org/10.3390/ma14164404
APA StyleLimosani, F., Tessore, F., Di Carlo, G., Forni, A., & Tagliatesta, P. (2021). Nonlinear Optical Properties of Porphyrin, Fullerene and Ferrocene Hybrid Materials. Materials, 14(16), 4404. https://doi.org/10.3390/ma14164404










