Sintering, Microstructure, and Dielectric Properties of Copper Borates for High Frequency LTCC Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
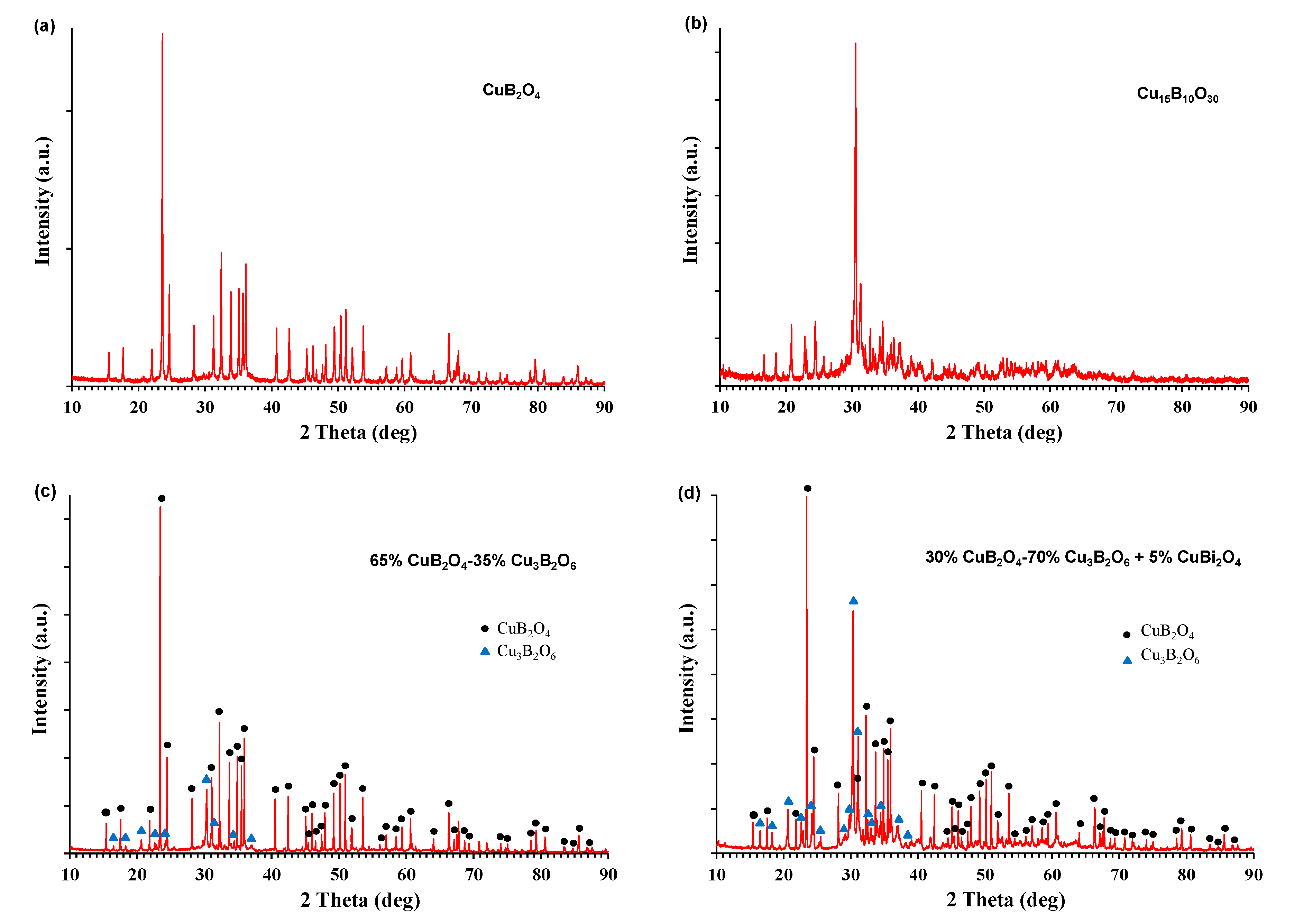
3.1. Phase Composition
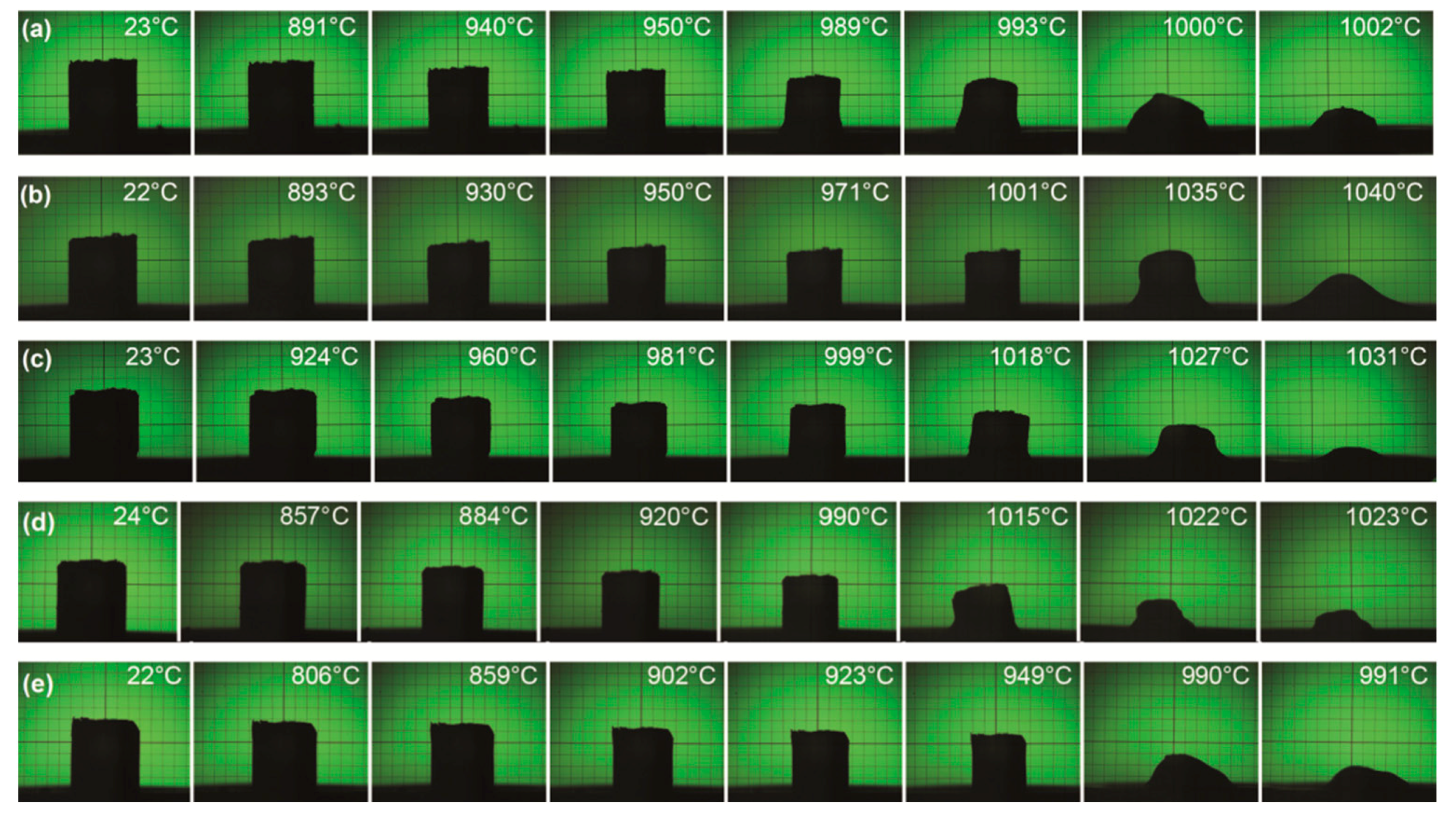
3.2. Heating Microscope Studies
3.3. Microstructural Studies
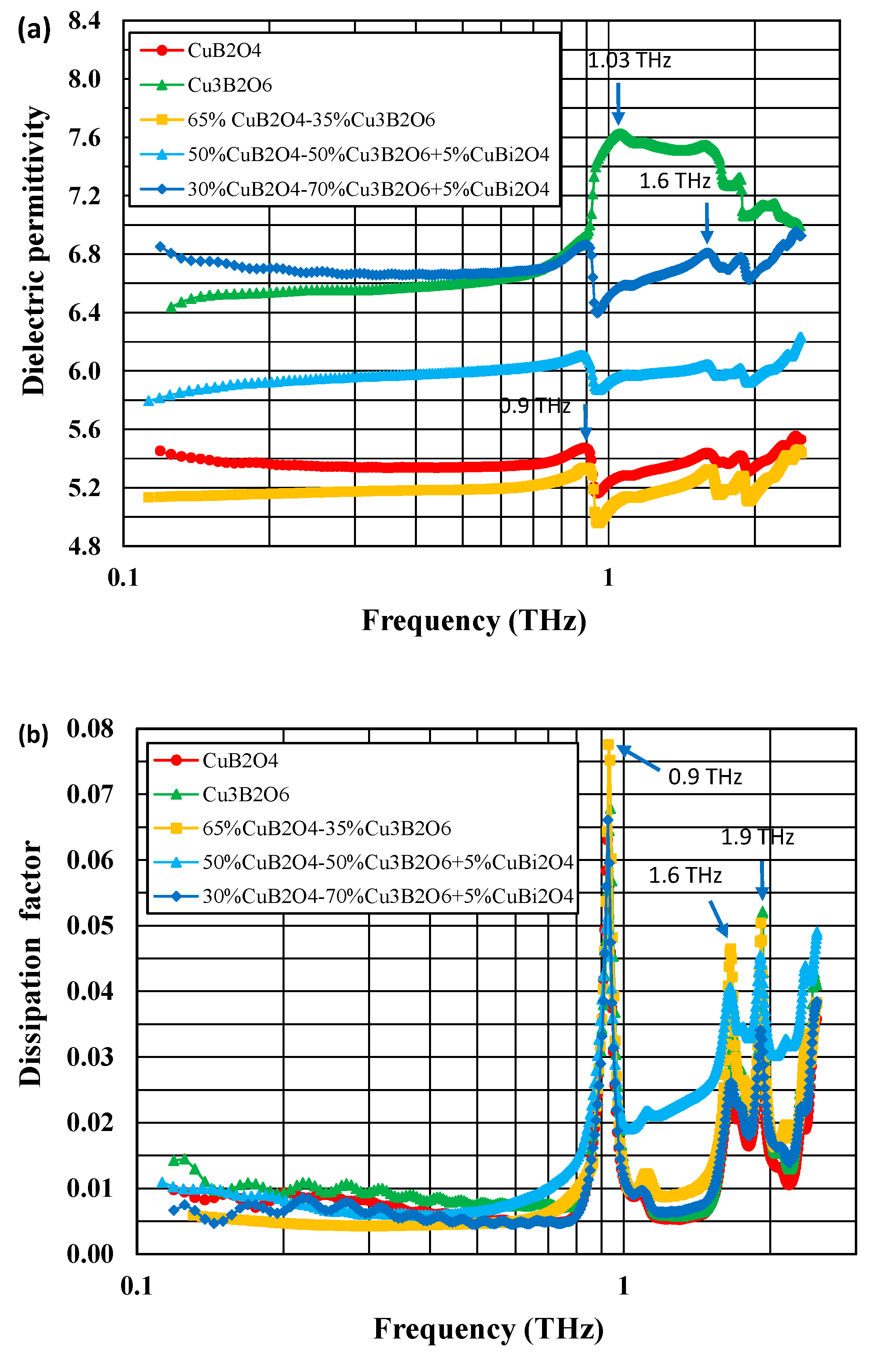
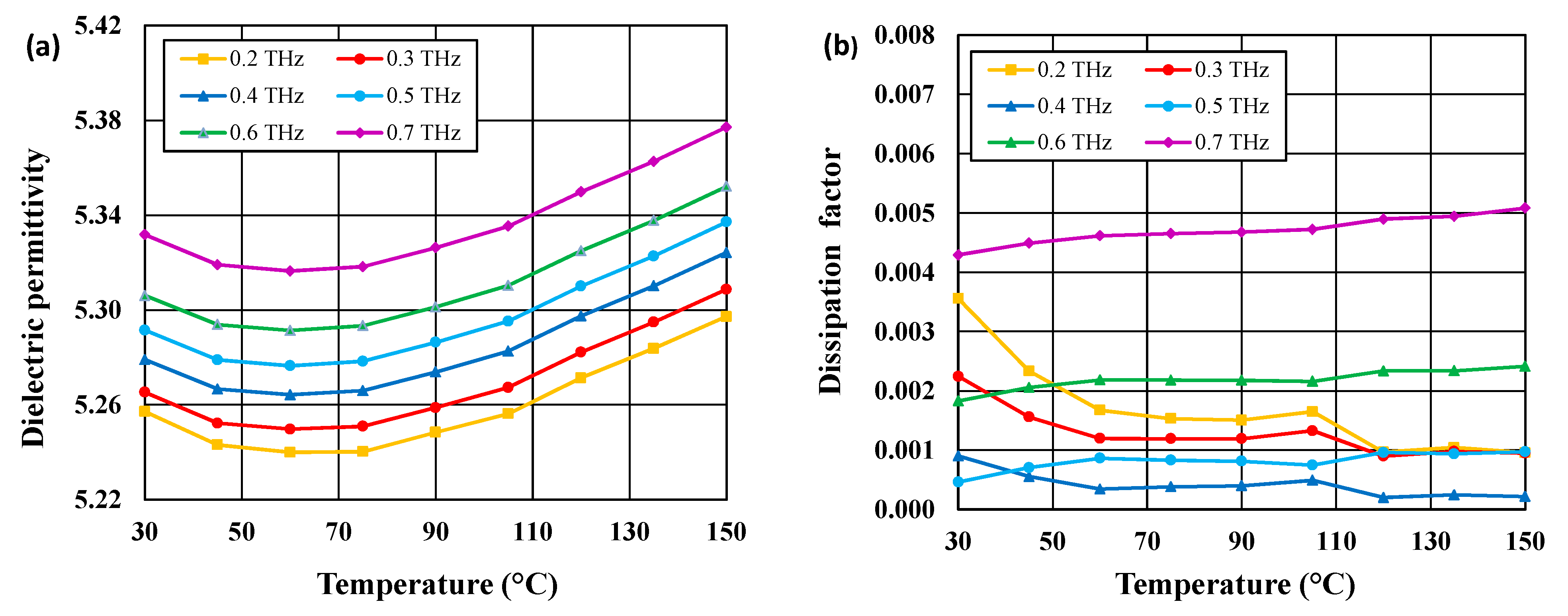
3.4. Dielectric Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muhammad, R.; Iqbal, Y.; Rambo, C.R.; Khan, H. Research trends in microwave dielectrics and factors affecting their properties: A review. Int. J. Mater. Res. 2014, 105, 431–439. [Google Scholar] [CrossRef]
- Raveendran, R.A.; Sebastian, M.T.; Raman, S. Applications of microwave materials: A review. J. Electron. Mater. 2019, 48, 2601–2634. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, M.T.; Ubic, R.; Jantunen, H. Low-loss dielectric ceramic materials and their properties. Int. Mater. Rev. 2015, 60, 392–412. [Google Scholar] [CrossRef]
- Song, H.J. Packages for terahertz electronics. Proc. IEEE 2017, 105, 1121–1138. [Google Scholar] [CrossRef] [Green Version]
- Rappaport, T.S.; Sun, S.; Mayzus, R.; Zhao, H.; Azar, Y.; Wang, K.; Wong, G.N.; Schulz, J.K.; Samimi, M.; Gutierrez, F. Millimeter wave mobile communications for 5G cellular: It will work! IEEE Access 2013, 1, 335–349. [Google Scholar] [CrossRef]
- Tsunooka, T.; Ando, M.; Suzuki, S.; Yasufuku, Y.; Ohsato, H. Research & developments for millimeter-wave dielectric forsterite with low dielectric constant, high Q, and zero temperature coefficient of resonant frequency. Jpn. J. Appl. Phys. 2013, 52, 09KH02. [Google Scholar] [CrossRef]
- Bafrooei, H.B.; Liu, B.; Su, W.; Song, K.X. Ca3MgSi2O8: Novel low-permittivity microwave dielectric ceramics for 5G application. Mater. Lett. 2020, 263, 127248. [Google Scholar] [CrossRef]
- Szwagierczak, D.; Synkiewicz, B.; Kulawik, J. Low dielectric constant composites based on B2O3 and SiO2 rich glasses, cordierite and mullite. Ceram. Int. 2018, 44, 14495–14501. [Google Scholar] [CrossRef]
- Lan, X.K.; Li, J.; Wang, F.; Wang, X.; Lu, W.Z.; Hu, M.Z.; Lei, W. A novel low permittivity LiAl0.98(Zn0.5Si0.5)0.02O2 based microwave dielectric ceramics for LTCC application. Int. J. Appl. Ceram. Tech. 2020, 17, 745–750. [Google Scholar] [CrossRef]
- Weng, Z.Z.; Song, C.X.; Xiong, Z.X.; Xue, H.; Sun, W.F.; Zhang, Y.; Yang, B.; Reece, M.J.; Yan, H.X. Microstructure and broadband dielectric properties of Zn2SiO4 ceramics with nano-sized TiO2 addition. Ceram. Int. 2019, 45, 13251–13256. [Google Scholar] [CrossRef]
- Hu, X.; Huang, X.J.; Chen, Y.H.; Li, Y.; Ling, Z.Y. Phase evolution and microwave dielectric properties of SrTiO3 added ZnAl2O4-Zn2SiO4-SiO2 ceramics. Ceram. Int. 2020, 46, 7050–7054. [Google Scholar] [CrossRef]
- Synkiewicz-Musialska, B.; Szwagierczak, D.; Kulawik, J.; Pałka, N.; Bajurko, P.R. Impact of additives and processing on microstructure and dielectric properties of willemite ceramics for LTCC terahertz applications. J. Eur. Ceram. Soc. 2020, 40, 362–370. [Google Scholar] [CrossRef]
- Sasidharanpillai, A.; Kim, C.H.; Lee, C.H.; Sebastian, M.T.; Kim, H.T. Environmental friendly approach for the development of ultra-low firing Li2WO4 ceramic tapes. ACS Sustain. Chem. Eng. 2018, 6, 6849–6855. [Google Scholar] [CrossRef]
- Yin, C.; Li, C.; Yang, G.; Fang, L.; Yuan, Y.; Shu, L.; Khaliq, J. NaCa4V5O17: A low-firing microwave dielectric ceramic with low permittivity and chemical compatibility with silver for LTCC applications. J. Eur. Ceram. Soc. 2020, 40, 386–390. [Google Scholar] [CrossRef]
- Oliveira, R.G.M.; Silva, R.A.; de Morais, J.E.V.; Batista, G.S.; Silva, M.A.S.; Goes, J.C.E.; de Andrade, H.D.; Queiroz Júnior, I.S.; Singh, C.; Sombra, A.S.B. Effects of CaTiO3 addition on the microwave dielectric properties and antenna properties of BiVO4 ceramics. Compos. Part B Eng. 2019, 175, 107122. [Google Scholar] [CrossRef]
- Joseph, N.; Varghese, J.; Teirikangas, M.; Sebastian, M.T.; Jantunen, H. Ultra-low sintering temperature ceramic composites of CuMoO4 through Ag2O addition for microwave applications. Compos. Part B Eng. 2018, 141, 214–220. [Google Scholar] [CrossRef]
- Faouri, S.S.; Mostaed, A.; Dean, J.S.; Wang, D.; Sinclair, D.C.; Zhang, S.; Whittow, W.G.; Vardaxoglou, Y.; Reaney, I.M. High quality factor cold sintered Li2MoO4-BaFe12O19 composites for microwave applications. Acta Mater. 2019, 166, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Zhang, S.; Wang, G.; Vardaxoglou, Y.; Whittow, W.; Cadman, D.; Zhou, D.; Song, K. Cold sintered CaTiO3-K2MoO4 microwave dielectric ceramics for integrated microstrip patch antennas. Appl. Mater. Today 2020, 18, 100519. [Google Scholar] [CrossRef]
- Dou, G.; Guo, M.; Li, Y.; Lin, J. The effect of LMBS glass on the microwave dielectric properties of the Mg3B2O6 for LTCC. J. Mater. Sci. Mater. Electron. 2015, 26, 4207–4211. [Google Scholar] [CrossRef]
- Zhou, D.; Pang, L.X.; Wang, D.W.; Qi, Z.M.; Reaney, I.M. High quality factor, ultralow sintering temperature Li6B4O9 microwave dielectric ceramics with ultralow density for antenna substrates. ACS Sustain. Chem. Eng. 2018, 6, 11138–11143. [Google Scholar] [CrossRef] [Green Version]
- Szwagierczak, D.; Synkiewicz-Musialska, B.; Kulawik, J.; Pałka, N. LTCC and Bulk Zn4B6O13–Zn2SiO4 Composites for Submillimeter Wave Applications. Materials 2021, 14, 1014. [Google Scholar] [CrossRef]
- Xi, J.; Shang, F.; Liu, F.; Xu, J.; Chen, G. A facile preparation of temperature–Stable borate ultra-low permittivity microwave ceramics for LTCC applicatons. Ceram. Int. 2020, 46, 19650–19653. [Google Scholar] [CrossRef]
- Szwagierczak, D.; Synkiewicz-Musialska, B.; Kulawik, J.; Czerwińska, E.; Pałka, N.; Bajurko, P.R. Low temperature sintering of Zn4B6O13 based substrates, their microstructure and dielectric properties up to the THz range. J. Alloys Compd. 2020, 819, 153025. [Google Scholar] [CrossRef]
- Peng, R.; Li, Y.; Su, H.; Lu, Y.; Yun, Y.; Zhang, Q. A detailed study of the substitution mechanism for improved zinc-borate: High-performance and its crystal structure variation. J. Mater. Res. Technol. 2021, 12, 1360–1367. [Google Scholar] [CrossRef]
- Martinez-Ripoll, M.; Martínez-Carrera, S.; García-Blanco, S. The crystal structure of copper metaborate, CuB2O4. Acta Cryst. 1971, 27, 677–681. [Google Scholar] [CrossRef]
- Pisarev, R.V.; Boldyrev, K.N.; Popova, M.N.; Smirnov, A.N.; Davydov, V.Y.; Bezmaternykh, L.N.; Smirnov, M.B.; Kazimirov, V.Y. Lattice dynamics of piezoelectric copper metaborate CuB2O4. Phys. Rev. B 2013, 88, 024301. [Google Scholar] [CrossRef]
- Imasaka, K.; Pisarev, R.V.; Bezmaternykh, L.N.; Shimura, T.; Kalashnikova, A.M.; Satoh, T. Excitation of multiple phonon modes in copper metaborate CuB2O4 via nonresonant impulsive stimulated Raman scattering. Phys. Rev. B 2018, 98, 054303. [Google Scholar] [CrossRef] [Green Version]
- Molchanova, A.D.; Prosnikov, M.A.; Dubrovin, R.M.; Davydov, V.Y.; Smirnov, A.N.; Pisarev, R.V.; Boldyrev, K.N.; Popova, M.N. Lattice dynamics and electronic transitions in a structurally complex layered copper borate Cu3(BO3)2. Phys. Rev. B 2017, 96, 174305. [Google Scholar] [CrossRef] [Green Version]
- Mero, R.D.; Lai, C.H.; Du, C.H.; Liu, H.L. Spectroscopic signature of spin–charge–lattice coupling in CuB2O4. J. Phys. Chem. C 2021, 125, 4322–4329. [Google Scholar] [CrossRef]
- Saito, M.; Taniguchi, K.; Arima, T.H. Gigantic optical magnetoelectric effect in CuB2O4. J. Phys. Soc. Jpn. 2008, 77, 013705. [Google Scholar] [CrossRef] [Green Version]
- Petrakovskii, G.A.; Sablina, K.A.; Velikanov, D.A.; Vorotynov, A.M.; Volkov, N.V.; Bovina, A.F. Synthesis and magnetic properties of copper metaborate single crystals CuB2O4. Crystallogr. Rep. 2000, 45, 853–856. [Google Scholar] [CrossRef]
- Pisarev, R.V.; Kalashnikova, A.M.; Schöps, O.; Bezmaternykh, L.N. Electronic transitions and genuine crystal-field parameters in copper metaborate CuB2O4. Phys. Rev. B 2011, 84, 075160. [Google Scholar] [CrossRef]
- Kawamata, T.; Sugawara, N.; Haidar, S.M.; Adachi, T.; Noji, T.; Kudo, K.; Kobayashi, N.; Fujii, Y.; Kikuchi, H.; Chiba, M.; et al. Thermal conductivity and magnetic phase diagram of CuB2O4. J. Phys. Soc. Jpn. 2019, 88, 114708. [Google Scholar] [CrossRef]
- Toyod, S.; Abe, N.; Arima, T. Gigantic directional asymmetry of luminescence in multiferroic CuB2O4. Phys. Rev. B 2016, 93, 2011091. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, R.; Nii, Y.; Onose, Y. Surface acoustic wave coupled to magnetic resonance on a multiferroic CuB2O4. Phys. Rev. B 2019, 99, 014418. [Google Scholar] [CrossRef] [Green Version]
- Kudlacik, D.; Ivanov, V.Y.; Yakovlev, D.R.; Sapega, V.F.; Schindler, J.J.; Debus, J.; Bayer, M.; Pisarev, R.V. Exciton and exciton-magnon photoluminescence in the antiferromagnet CuB2O4. Phys. Rev. B 2020, 102, 035128. [Google Scholar] [CrossRef]
- Ursu, D.; Dabici, A.; Miclau, M.; Miclau, N. Low-temperature hydrothermal synthesis of hierarchical flower-like CuB2O4 superstructures. Process. Appl. Ceram. 2020, 14, 113–118. [Google Scholar] [CrossRef]
- Liu, J.; Wen, S.; Zou, X.; Feng, P. Visible-light-responsive copper(II) borate photocatalysts with intrinsic midgap states for water splitting. J. Mater. Chem. A 2013, 1, 1553–1556. [Google Scholar] [CrossRef]
- Luo, S.; Wang, F.; Yu, K.; Shao, J.; Peng, L.; Zeng, Q. Enhancement of visible-light photocatalytic activity of Cu3B2O6 hybridized with g-C3N4. Coll. Surf. A 2017, 520, 409–419. [Google Scholar] [CrossRef]
- Shannon, R.D. Dielectric polarizabilities of ions in oxides and fluorides. J. Appl. Phys. 1993, 73, 348–366. [Google Scholar] [CrossRef]
- Qin, J.; Liu, Z.; Ma, M.; Li, Y. Machine learning approaches for permittivity prediction and rational design of microwave dielectric ceramics. J. Mater. 2021, Article in press. [Google Scholar] [CrossRef]
- Kim, E.S.; Chun, B.S.; Freer, R.; Cernik, R.J. Effects of packing fraction and bond valence on microwave dielectric properties of A2+B6+O4 (A2+: Ca, Pb, Ba; B6+: Mo, W) ceramics. J. Eur. Ceram. Soc. 2010, 30, 1731–1736. [Google Scholar] [CrossRef]
- Li, J.; Han, Y.; Qiu, T.; Jin, C. Effect of bond valence on microwave dielectric properties of (1-x)CaTiO3-x(Li0.5La0.5)TiO3 ceramics. Mater. Res. Bull. 2012, 47, 2375–2379. [Google Scholar] [CrossRef]
- Kamba, S.; Petzelt, J.; Buixaderas, E.; Haubrich, D.; Vanek, P.; Kuzel, P.; Jawahar, I.N.; Sebastian, M.T.; Mohanan, P. High frequency dielectric properties of A5B4O15 microwave ceramics. J. Appl. Phys. 2001, 89, 3900–3906. [Google Scholar] [CrossRef]
- Ma, M.; Wang, Y.; Navarro-Cia, M.; Liu, F.; Zhang, F.; Liu, Z.; Li, Y.; Hanham, S.M.; Hao, Z. The dielectric properties of some ceramic substrate materials at terahertz frequencies. J. Eur. Ceram. Soc. 2019, 39, 4424–4428. [Google Scholar] [CrossRef]


| Element | at. % | ||||
|---|---|---|---|---|---|
| Point 1 | Point 2 | Point 3 | Point 4 | Point 5 | |
| B | 45.76 | 26.06 | 28.20 | 31.63 | 26.31 |
| O | 31.40 | 16.75 | 25.15 | 26.65 | 17.11 |
| Bi | 0.20 | 0.62 | 0.92 | 0.47 | 0.74 |
| Cu | 22.64 | 56.57 | 45.73 | 41.25 | 55.84 |
| Cu/B | 0.49 | 2.17 | 1.62 | 1.30 | 2.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwagierczak, D.; Synkiewicz-Musialska, B.; Kulawik, J.; Pałka, N. Sintering, Microstructure, and Dielectric Properties of Copper Borates for High Frequency LTCC Applications. Materials 2021, 14, 4017. https://doi.org/10.3390/ma14144017
Szwagierczak D, Synkiewicz-Musialska B, Kulawik J, Pałka N. Sintering, Microstructure, and Dielectric Properties of Copper Borates for High Frequency LTCC Applications. Materials. 2021; 14(14):4017. https://doi.org/10.3390/ma14144017
Chicago/Turabian StyleSzwagierczak, Dorota, Beata Synkiewicz-Musialska, Jan Kulawik, and Norbert Pałka. 2021. "Sintering, Microstructure, and Dielectric Properties of Copper Borates for High Frequency LTCC Applications" Materials 14, no. 14: 4017. https://doi.org/10.3390/ma14144017
APA StyleSzwagierczak, D., Synkiewicz-Musialska, B., Kulawik, J., & Pałka, N. (2021). Sintering, Microstructure, and Dielectric Properties of Copper Borates for High Frequency LTCC Applications. Materials, 14(14), 4017. https://doi.org/10.3390/ma14144017






