Ionic Liquid Electrolytes for Electrochemical Energy Storage Devices
Abstract
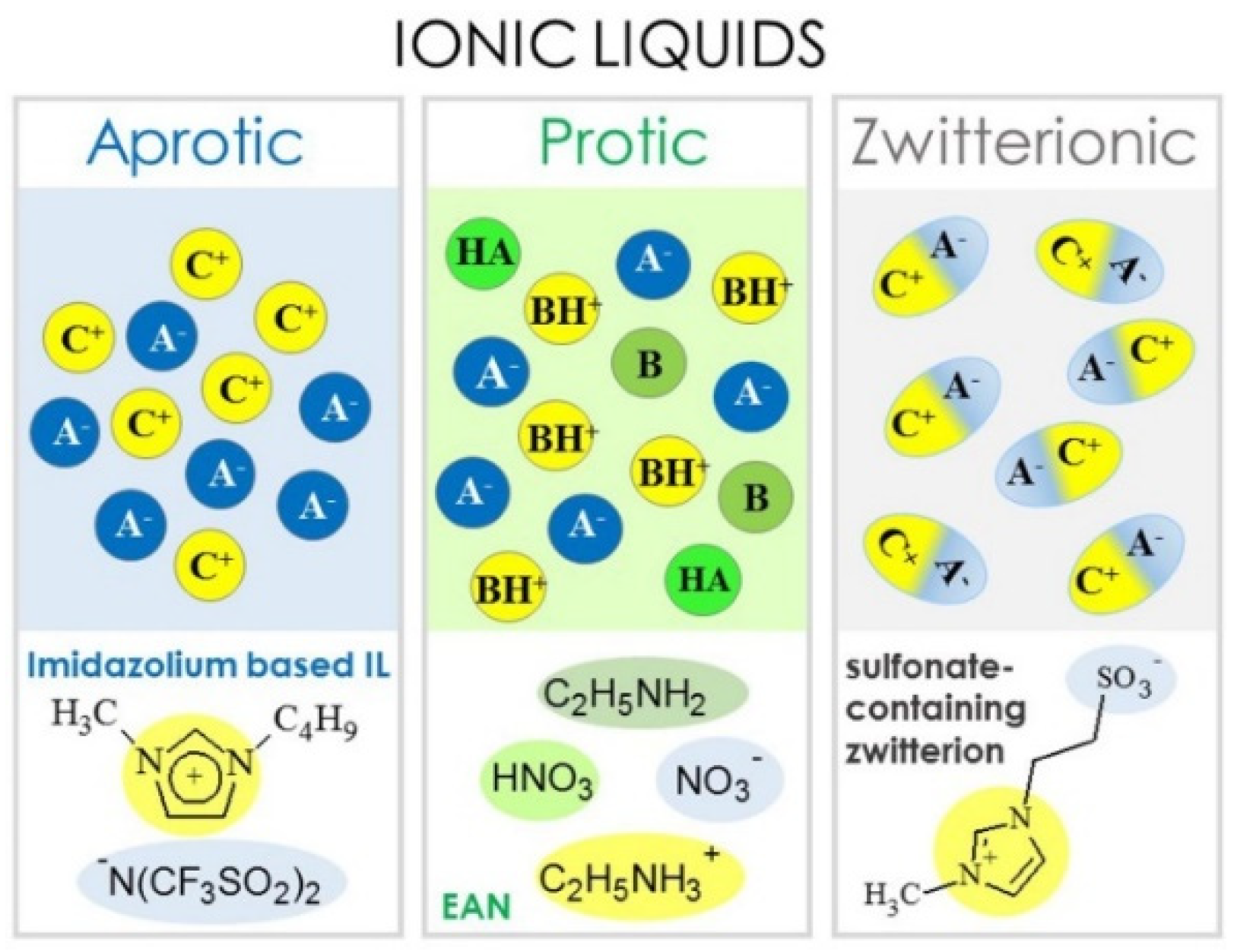
:1. Introduction
2. Ionic Liquids for Lithium-Ion Battery Electrolytes
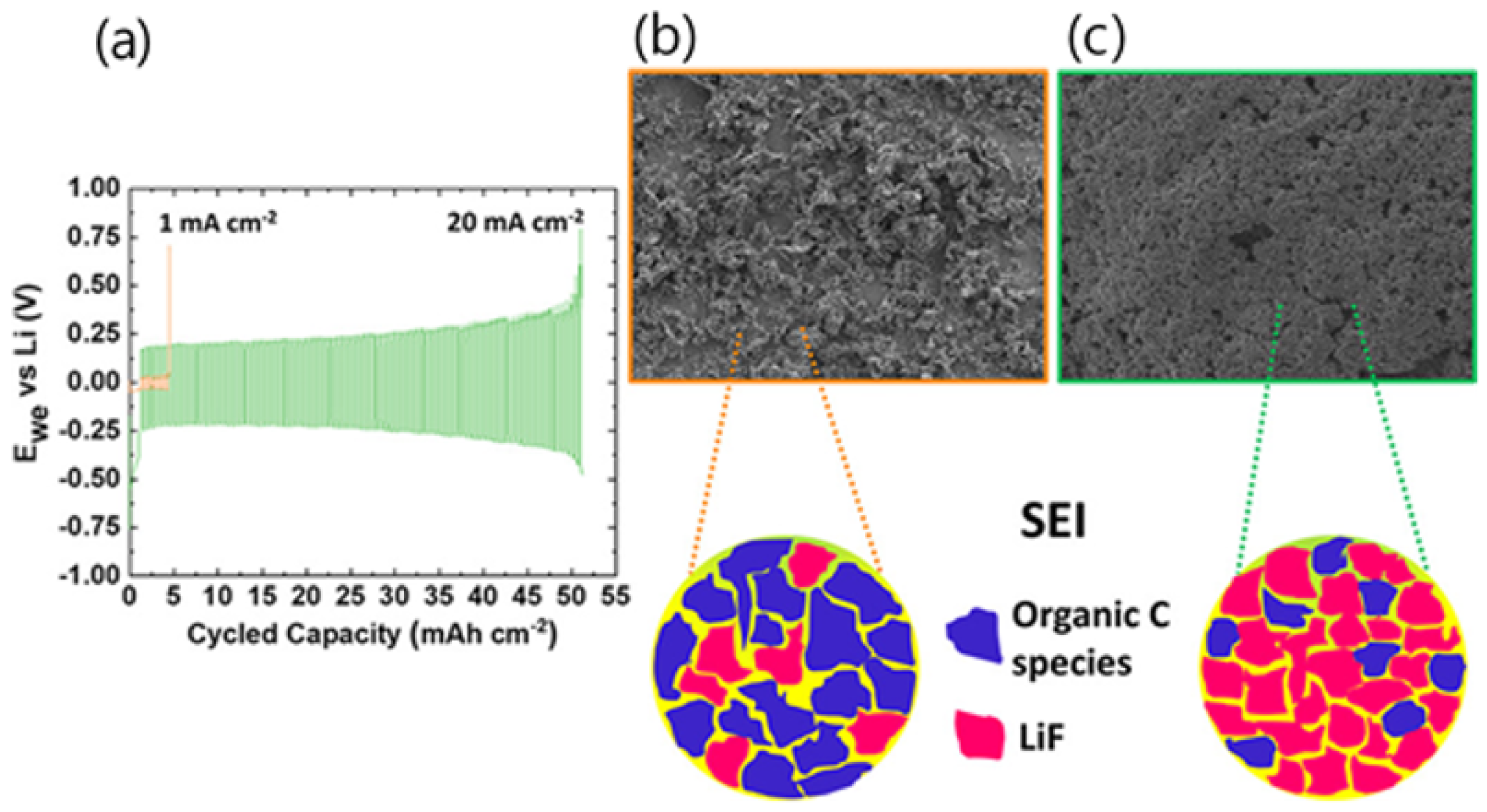
2.1. Lithium-Ion Batteries
2.2. Lithium–Sulfur Batteries
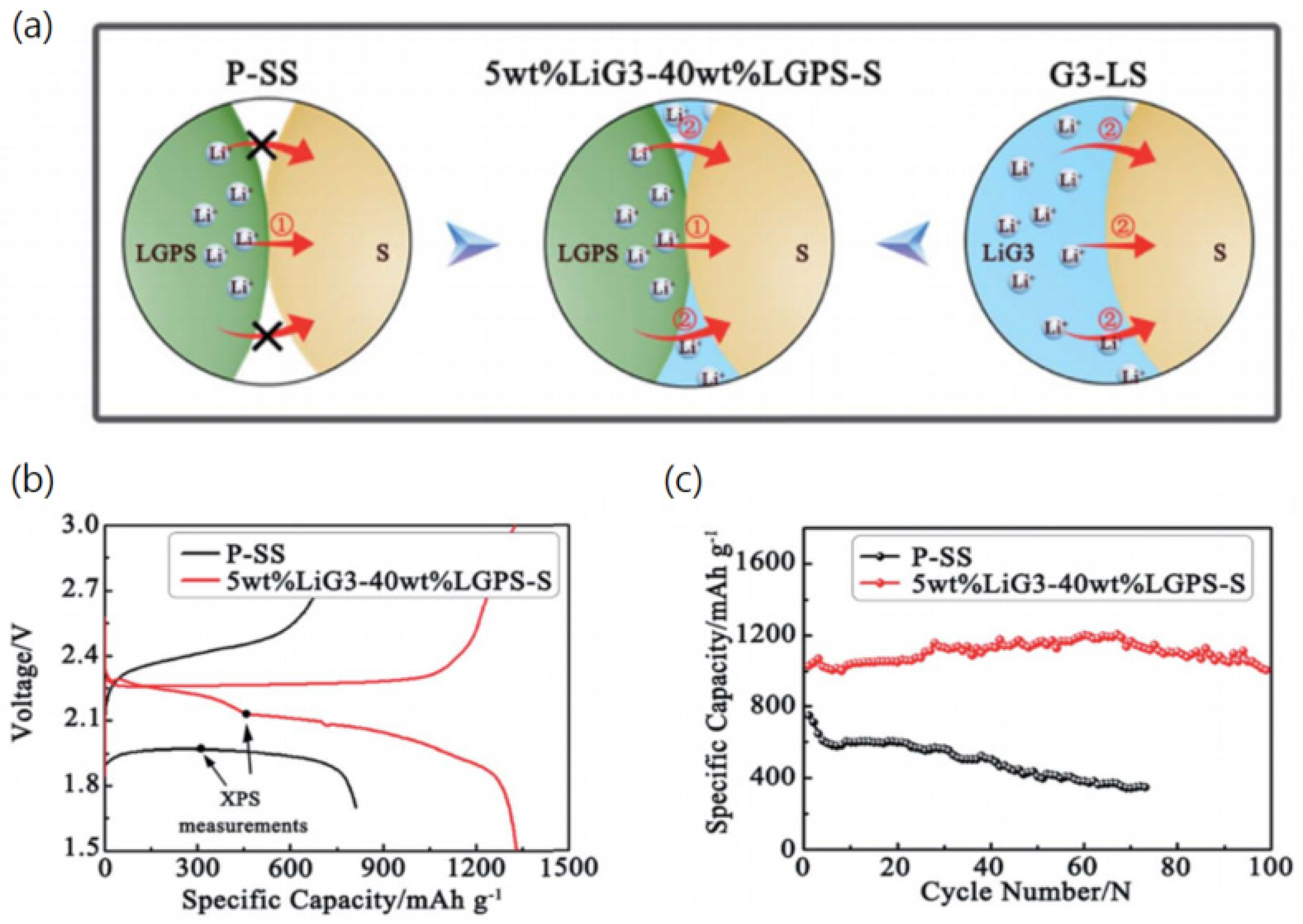
2.3. Ionic Liquids for Lithium-Ion Batteries Using Quasi-Solid- and All-Solid-State Electrolytes
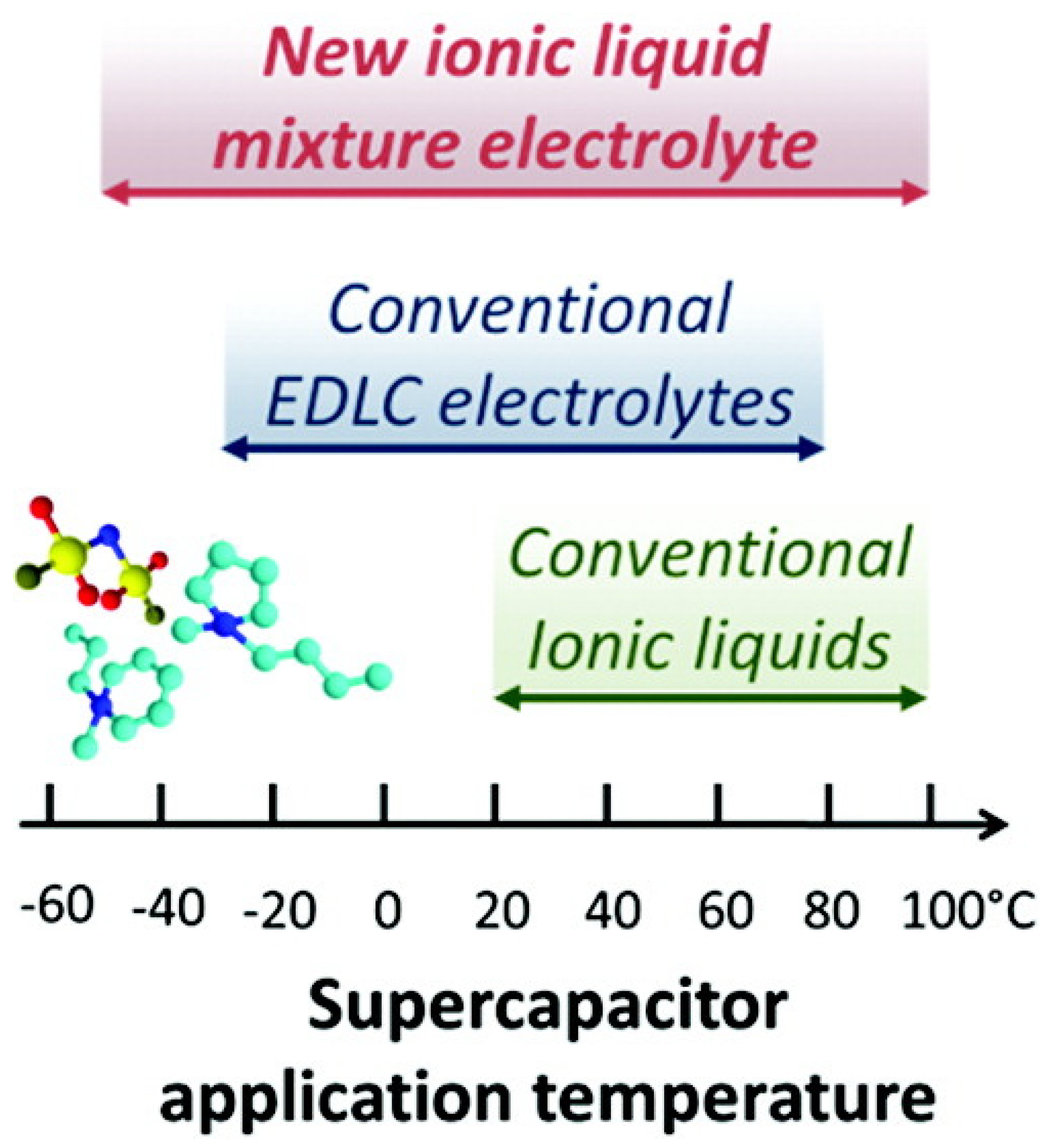
3. Ionic Liquids for Supercapacitor Electrolytes
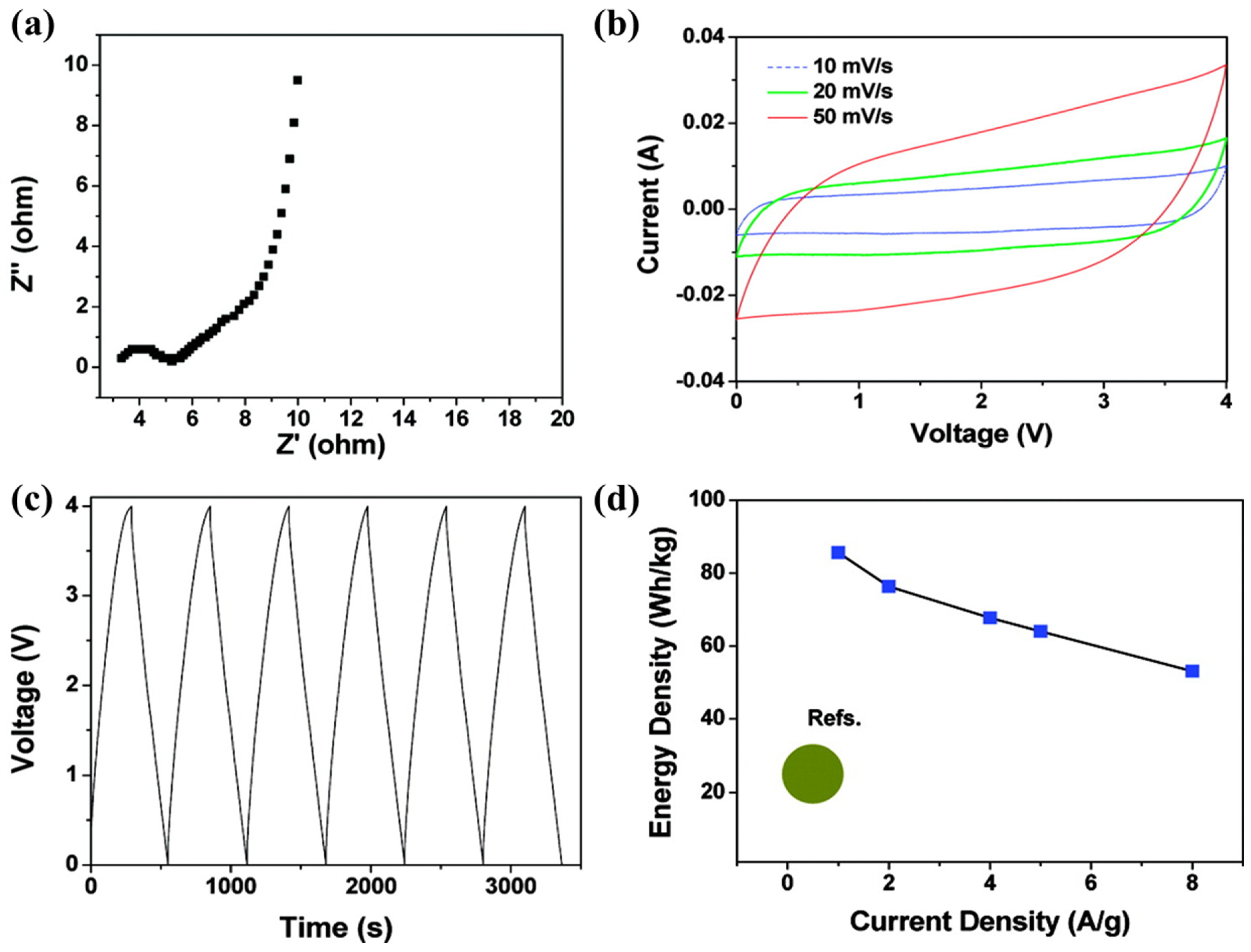
3.1. Net Ionic Liquid for Electric Double-Layer Capacitor
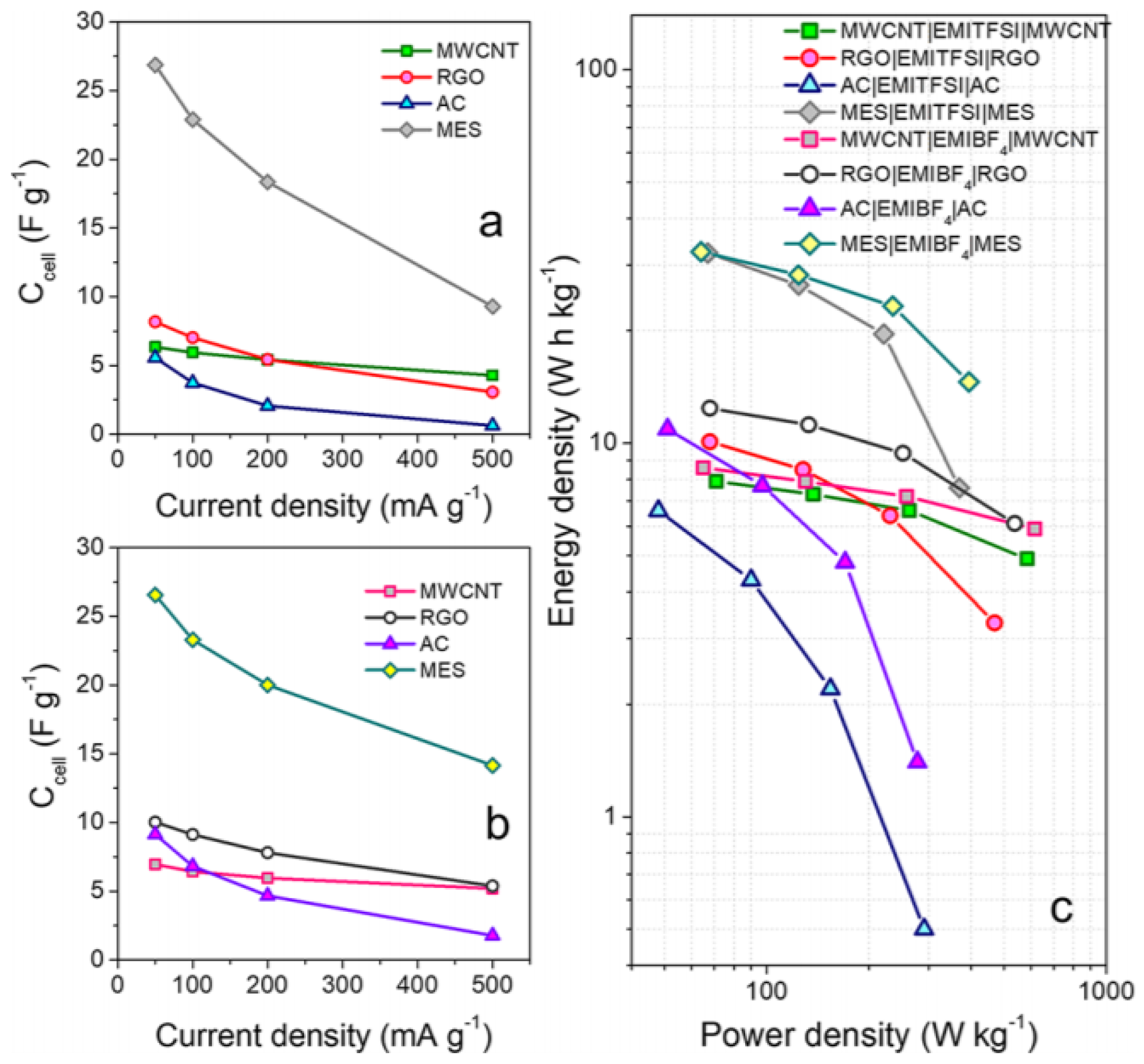
3.2. Other Approaches of Ionic Liquids as a Liquid Electrolyte
3.3. Ionic Liquid as a Quasi-Solid and All-Solid Electrolyte
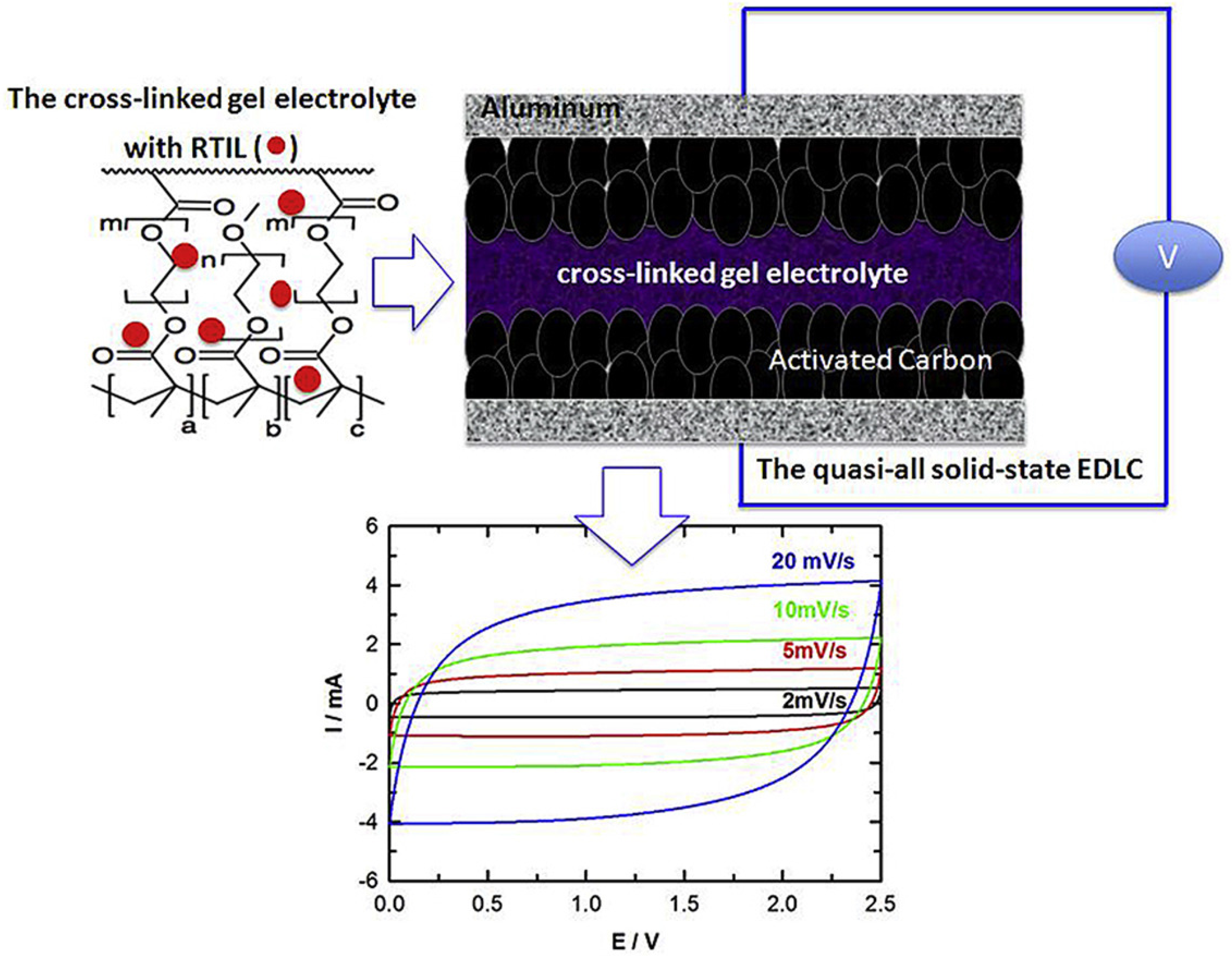
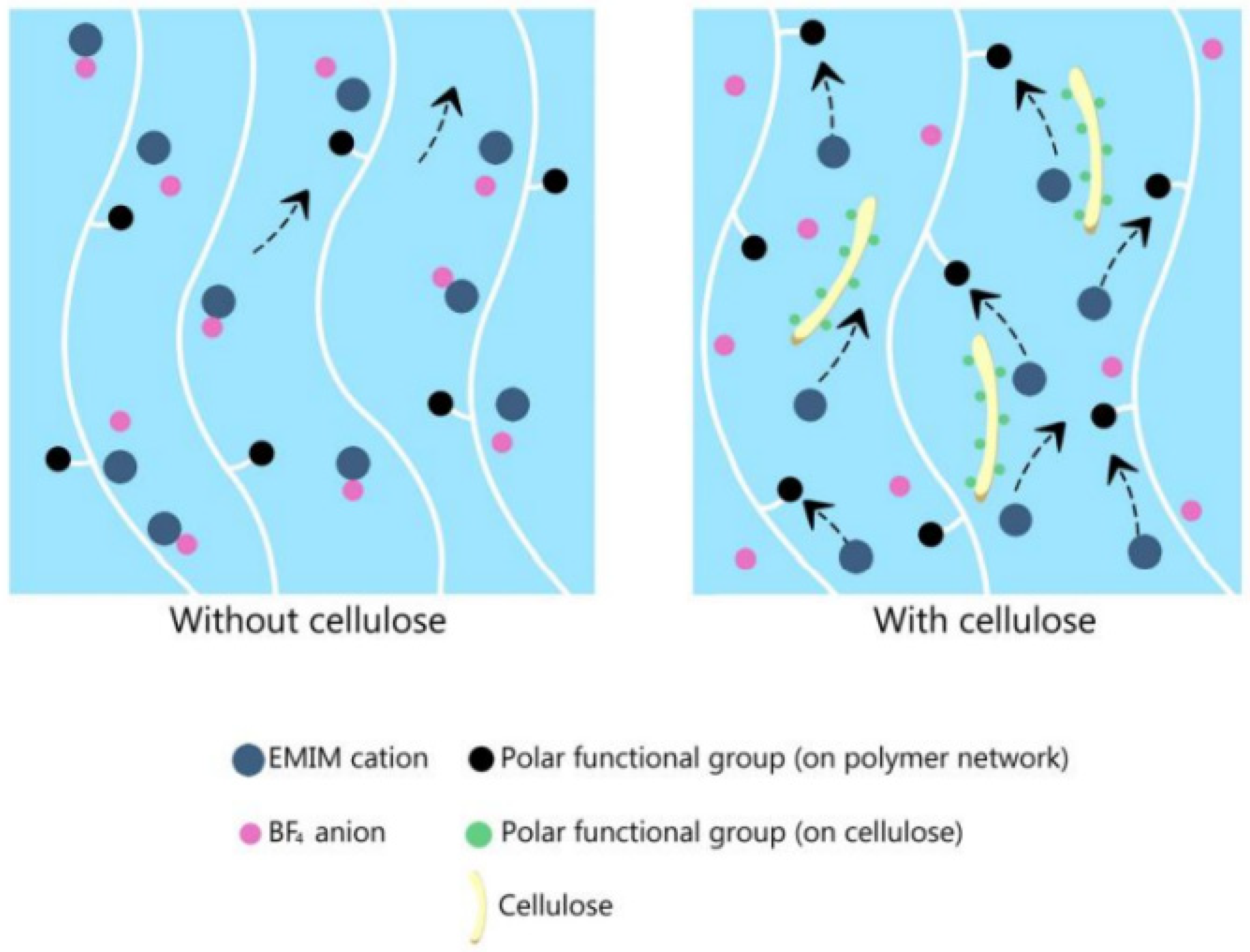
3.3.1. Ionic-Liquid-Embedded Polymer Electrolyte
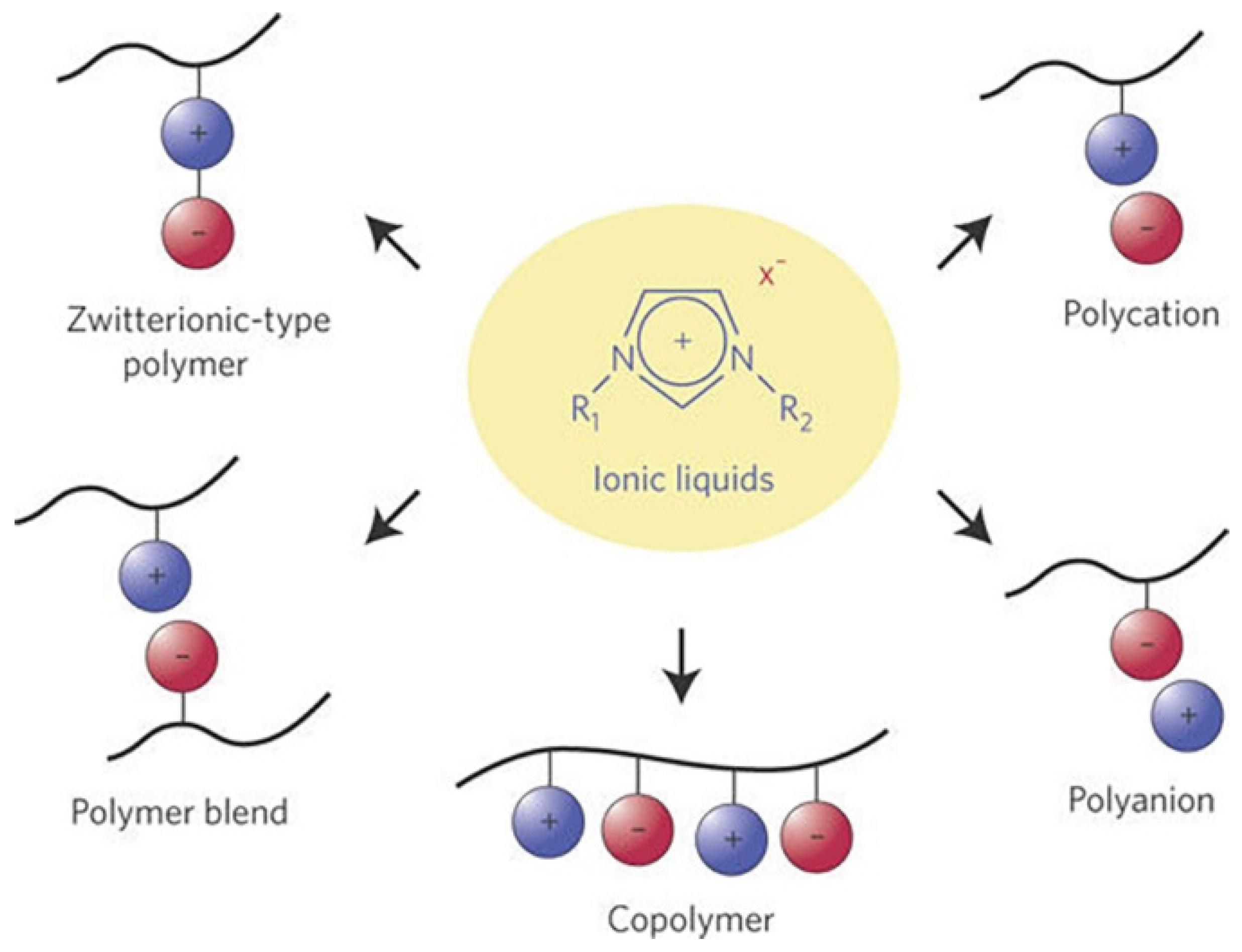
3.3.2. Poly Ionic Liquid as a Solid Electrolyte
4. Conclusions and Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Guo, M.; Zhan, J.; Jiao, X.; Chen, D.; Wang, T. A new design of an electrochromic energy storage device with high capacity, long cycle lifetime and multicolor display. J. Mater. Chem. A 2020, 8, 17098–17105. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366. [Google Scholar] [CrossRef] [Green Version]
- Macías, A.; Kandidayeni, M.; Boulon, L.; Trovão, J. Passive and Active Coupling Comparison of Fuel Cell and Supercapacitor for a Three-Wheel Electric Vehicle. Fuel Cells 2020, 20, 351–361. [Google Scholar] [CrossRef]
- Jacobson, M.Z.; Colella, W.G.; Golden, D.M. Atmospheric science: Cleaning the air and improving health with hydrogen fuel-cell vehicles. Science 2005, 308, 1901–1905. [Google Scholar] [CrossRef] [Green Version]
- Barthelemy, H.; Weber, M.; Barbier, F. Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrogen Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Tarkowski, R. Underground hydrogen storage: Characteristics and prospects. Renew. Sustain. Energy Rev. 2019, 105, 86–94. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Fan, Y. Techno-Economic Challenges of Fuel Cell Commercialization. Engineering 2018, 4, 352–360. [Google Scholar] [CrossRef]
- Lee, Y.D.; Ahn, K.Y.; Morosuk, T.; Tsatsaronis, G. Environmental impact assessment of a solid-oxide fuel-cell-based combined-heat-and-power-generation system. Energy 2015, 79, 455–466. [Google Scholar] [CrossRef]
- Elmer, T.; Worall, M.; Wu, S.; Riffat, S.B. Fuel cell technology for domestic built environment applications: State of-the-art review. Renew. Sustain. Energy Rev. 2015, 42, 913–931. [Google Scholar] [CrossRef]
- Buonomano, A.; Calise, F.; d’Accadia, M.D.; Palombo, A.; Vicidomini, M. Hybrid solid oxide fuel cells-gas turbine systems for combined heat and power: A review. Appl. Energy 2015, 156, 32–85. [Google Scholar] [CrossRef]
- Arsalis, A. A comprehensive review of fuel cell-based micro-combined-heat-and-power systems. Renew. Sustain. Energy Rev. 2019, 105, 391–414. [Google Scholar] [CrossRef]
- Ellamla, H.R.; Staffell, I.; Bujlo, P.; Pollet, B.G.; Pasupathi, S. Current status of fuel cell based combined heat and power systems for residential sector. J. Power Sources 2015, 293, 312–328. [Google Scholar] [CrossRef]
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Flamme, B.; Rodriguez Garcia, G.; Weil, M.; Haddad, M.; Phansavath, P.; Ratovelomanana-Vidal, V.; Chagnes, A. Guidelines to design organic electrolytes for lithium-ion batteries: Environmental impact, physicochemical and electrochemical properties. Green Chem. 2017, 19, 1828–1849. [Google Scholar] [CrossRef]
- Lux, S.F.; Lucas, I.T.; Pollak, E.; Passerini, S.; Winter, M.; Kostecki, R. The mechanism of HF formation in LiPF6 based organic carbonate electrolytes. Electrochem. Commun. 2012, 14, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. NPJ Comput. Mater. 2018, 4. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Wei, F.; Zhang, J.G.; Zhang, Q. A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Chen, W.; Rasim, Y.; Salman, W.; Pan, H.; Yuan, Y.; Wang, C. A high-efficiency energy regenerative shock absorber using supercapacitors for renewable energy applications in range extended electric vehicle. Appl. Energy 2016, 178, 177–188. [Google Scholar] [CrossRef]
- Redondo, E.; Fevre, L.W.L.; Fields, R.; Todd, R.; Forsyth, A.J.; Dryfe, R.A.W. Enhancing supercapacitor energy density by mass-balancing of graphene composite electrodes. Electrochim. Acta 2020, 360, 136957. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Ting, J.M. A Redox-Additive Electrolyte and Nanostructured Electrode for Enhanced Supercapacitor Energy Density. ACS Sustain. Chem. Eng. 2020, 8, 18023–18033. [Google Scholar] [CrossRef]
- Xu, B.; Wu, F.; Chen, R.; Cao, G.; Chen, S.; Zhou, Z.; Yang, Y. Highly mesoporous and high surface area carbon: A high capacitance electrode material for EDLCs with various electrolytes. Electrochem. Commun. 2008, 10, 795–797. [Google Scholar] [CrossRef]
- Shown, I.; Ganguly, A.; Chen, L.C.; Chen, K.H. Conducting polymer-based flexible supercapacitor. Energy Sci. Eng. 2015, 3, 2–26. [Google Scholar] [CrossRef]
- Mohd Abdah, M.A.A.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Mater. Des. 2020, 186, 108199. [Google Scholar] [CrossRef]
- Naseri, M.; Fotouhi, L.; Ehsani, A. Recent Progress in the Development of Conducting Polymer-Based Nanocomposites for Electrochemical Biosensors Applications: A Mini-Review. Chem. Rec. 2018, 18, 599–618. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Liu, S.; Li, L.; Zhang, C.; Liu, T. Conducting polymer composites: Material synthesis and applications in electrochemical capacitive energy storage. Mater. Chem. Front. 2017, 1, 251–268. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Selvan, R.K.; Melo, J.S. Redox additive/active electrolytes: A novel approach to enhance the performance of supercapacitors. J. Mater. Chem. A 2013, 1, 12386–12394. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Chang, J.; Zhao, D.; Wang, J.; Yang, C.; Cao, B. A Review of Redox Electrolytes for Supercapacitors. Front. Chem. 2020, 8, 1–7. [Google Scholar] [CrossRef]
- Chen, Q.; Li, X.; Zang, X.; Cao, Y.; He, Y.; Li, P.; Wang, K.; Wei, J.; Wu, D.; Zhu, H. Effect of different gel electrolytes on graphene-based solid-state supercapacitors. RSC Adv. 2014, 4, 36253–36256. [Google Scholar] [CrossRef]
- Samanta, P.; Ghosh, S.; Chandra Murmu, N.; Kuila, T. Effect of redox additive in aqueous electrolyte on the high specific capacitance of cation incorporated MnCo2O4@Ni(OH)2 electrode materials for flexible symmetric supercapacitor. Compos. Part B Eng. 2021, 215, 108755. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, J.; Zhou, Y.; Song, C.; Tian, Q.; Xu, J.; Wong, C.P. Enhancing pseudocapacitive kinetics of nanostructured MnO2 through anchoring onto biomass-derived porous carbon. Appl. Surf. Sci. 2018, 440, 1027–1036. [Google Scholar] [CrossRef]
- Mo, Y.; Meng, W.; Xia, Y.; Du, X. Redox-active gel electrolyte combined with branched polyaniline nanofibers doped with ferrous ions for ultra-high-performance flexible supercapacitors. Polymers 2019, 11, 1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Miao, J.; Quan, L.; Cai, D.; Zhan, H. Bimetallic CoNiSx nanocrystallites embedded in nitrogen-doped carbon anchored on reduced graphene oxide for high-performance supercapacitors. Nanoscale 2018, 10, 4051–4060. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.M.; Macfarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [Green Version]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef]
- Sato, T.; Masuda, G.; Takagi, K. Electrochemical properties of novel ionic liquids for electric double layer capacitor applications. Electrochim. Acta 2004, 49, 3603–3611. [Google Scholar] [CrossRef]
- Zhu, X.; Du, M.; Feng, J.; Wang, H.; Xu, Z.; Wang, L.; Zuo, S.; Wang, C.; Wang, Z.; Zhang, C.; et al. High-Efficiency Perovskite Solar Cells with Imidazolium-Based Ionic Liquid for Surface Passivation and Charge Transport. Angew. Chem. 2021, 133, 4284–4290. [Google Scholar] [CrossRef]
- Chen, S.H.; Yang, F.R.; Wang, M.T.; Wang, N.N. Synthesis, characterization, and crystal structure of several novel acidic ionic liquids based on the corresponding 1-alkylbenzimidazole with tetrafluoroboric acid. Comptes Rendus Chim. 2010, 13, 1391–1396. [Google Scholar] [CrossRef]
- Kunal, A.; Mamtani, K. Ionic Liquids Market Size, Share & Trends Analysis Report by Application (Solvents & Catalysts, Extractions & Separations, Bio-Refineries, Energy Storage), By Region, And Segment Forecasts, 2018–2025; Report ID GVR-1-68038-283-9; Global Market Insights: Lexington, MA, USA, 2015. [Google Scholar]
- Lovelock, K.R.J. Quantifying Intermolecular Interactions of Ionic Liquids Using Cohesive Energy Densities. R. Soc. Open Sci. 2017, 4, 171223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, Y.K.; Kim, B.; Ko, J.; You, D.J.; Yin, Z.; Kim, H.; Shin, D.; Cho, S.; Yoo, J.; Kim, Y.S. All solid state flexible supercapacitors operating at 4 v with a cross-linked polymer-ionic liquid electrolyte. J. Mater. Chem. A 2016, 4, 4386–4391. [Google Scholar] [CrossRef]
- Domínguez De María, P. “Nonsolvent” applications of ionic liquids in biotransformations and organocatalysis. Angew. Chemie Int. Ed. 2008, 47, 6960–6968. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Henry, K.; Turchi, C.; Pellegrino, J. Incorporating Ionic Liquid Electrolytes into Polymer Gels for Solid-State Ultracapacitors. J. Electrochem. Soc. 2008, 155, A361. [Google Scholar] [CrossRef] [Green Version]
- Kokubo, H.; Sano, R.; Murai, K.; Ishii, S.; Watanabe, M. Ionic polymer actuators using poly(ionic liquid) electrolytes. Eur. Polym. J. 2018, 106, 266–272. [Google Scholar] [CrossRef]
- Lin, B.; Yuan, W.; Xu, F.; Chen, Q.; Zhu, H.; Li, X.; Yuan, N.; Chu, F.; Ding, J. Protic ionic liquid/functionalized graphene oxide hybrid membranes for high temperature proton exchange membrane fuel cell applications. Appl. Surf. Sci. 2018, 455, 295–301. [Google Scholar] [CrossRef]
- Giffin, G.A. Ionic liquid-based electrolytes for “beyond lithium” battery technologies. J. Mater. Chem. A 2016, 4, 13378–13389. [Google Scholar] [CrossRef]
- Paduszyński, K.; Domańska, U. Viscosity of ionic liquids: An extensive database and a new group contribution model based on a feed-forward artificial neural network. J. Chem. Inf. Model. 2014, 54, 1311–1324. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Tanahashi, I.; Yoshida, A.; Nishino, A. Electrochemical Characterization of Activated Carbon-Fiber Cloth Polarizable Electrodes for Electric Double-Layer Capacitors. J. Electrochem. Soc. 1990, 137, 3052–3057. [Google Scholar] [CrossRef]
- Ue, M. Conductivities and ion association of quaternary ammonium tetrafluoroborates in propylene carbonate. Electrochim. Acta 1994, 39, 2083–2087. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, K.; Kim, S.G.; Yeu, T. The Preparation of Ionic Liquids Based on AlkylImidazolium Salts for the Electrolyte of EDLC. In ECS Meeting Abstracts; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- McEwen, A.B.; McDevitt, S.F.; Koch, V.R. Nonaqueous Electrolytes for Electrochemical Capacitors: Imidazolium Cations and Inorganic Fluorides with Organic Carbonates. J. Electrochem. Soc. 1997, 144, L84–L86. [Google Scholar] [CrossRef]
- Chernyak, Y. Dielectric constant, dipole moment, and solubility parameters of some cyclic acid esters. J. Chem. Eng. Data 2006, 51, 416–418. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Kim, H.; Kang, K.; Choi, N.S. Highly stable linear carbonate-containing electrolytes with fluoroethylene carbonate for high-performance cathodes in sodium-ion batteries. J. Power Sources 2016, 320, 49–58. [Google Scholar] [CrossRef]
- Pal, B.; Yang, S.; Ramesh, S.; Thangadurai, V.; Jose, R. Electrolyte selection for supercapacitive devices: A critical review. Nanoscale Adv. 2019, 1, 3807–3835. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, T.; Hiratsuka, K.; Sanada, Y.; Kurihara, K. Electric double-layer capacitor using organic electrolyte. J. Power Sources 1996, 60, 239–247. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Simon, P. True performance metrics in electrochemical energy storage. Science 2011, 334, 917–918. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chandrabose, R.S.; Chun, S.E.; Zhang, T.; Evanko, B.; Jian, Z.; Boettcher, S.W.; Stucky, G.D.; Ji, X. High energy density aqueous electrochemical capacitors with a KI-KOH electrolyte. ACS Appl. Mater. Interfaces 2015, 7, 19978–19985. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Wojnarowska, Z.; Paluch, M. Recent progress on dielectric properties of protic ionic liquids. J. Phys. Condens. Matter 2015, 27. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Properties and applications. Chem. Rev. 2008, 108, 206–237. [Google Scholar] [CrossRef]
- Ohno, H.; Yoshizawa-Fujita, M.; Kohno, Y. Design and properties of functional zwitterions derived from ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 10978–10991. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.S.; Rick, J.; Hwang, B.J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2013, 1, 2719–2743. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly(ionic liquid)s: Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. [Google Scholar] [CrossRef] [Green Version]
- Kasprzak, D.; Stępniak, I.; Galiński, M. Acetate- and lactate-based ionic liquids: Synthesis, characterisation and electrochemical properties. J. Mol. Liq. 2018, 264, 233–241. [Google Scholar] [CrossRef]
- Naushad, M.; ALOthman, Z.A.; Khan, A.B.; Ali, M. Effect of ionic liquid on activity, stability, and structure of enzymes: A review. Int. J. Biol. Macromol. 2012, 51, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Shiflett, M.B.; Drew, D.W.; Cantini, R.A.; Yokozeki, A. Carbon dioxide capture using ionic liquid 1-butyl-3-methylimidazolium acetate. Energy Fuels 2010, 24, 5781–5789. [Google Scholar] [CrossRef]
- Shinde, P.A.; Lokhande, V.C.; Chodankar, N.R.; Ji, T.; Kim, J.H.; Lokhande, C.D. Enhanced electrochemical performance of monoclinic WO3 thin film with redox additive aqueous electrolyte. J. Colloid Interface Sci. 2016, 483, 261–267. [Google Scholar] [CrossRef]
- Ye, B.; Gong, C.; Huang, M.; Tu, Y.; Zheng, X.; Fan, L.; Lin, J.; Wu, J. Improved performance of a CoTe//AC asymmetric supercapacitor using a redox additive aqueous electrolyte. RSC Adv. 2018, 8, 7997–8006. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, J.C.; Correia, D.M.; Gonçalves, R.; de Zea Bermudez, V.; Silva, M.M.; Lanceros-Mendez, S.; Costa, C.M. Enhanced ionic conductivity in poly(vinylidene fluoride) electrospun separator membranes blended with different ionic liquids for lithium ion batteries. J. Colloid Interface Sci. 2021, 582, 376–386. [Google Scholar] [CrossRef]
- Zou, K.; Deng, W.; Cai, P.; Deng, X.; Wang, B.; Liu, C.; Li, J.; Hou, H.; Zou, G.; Ji, X. Prelithiation/Presodiation Techniques for Advanced Electrochemical Energy Storage Systems: Concepts, Applications, and Perspectives. Adv. Funct. Mater. 2021, 31. [Google Scholar] [CrossRef]
- Guerfi, A.; Dontigny, M.; Charest, P.; Petitclerc, M.; Lagacé, M.; Vijh, A.; Zaghib, K. Improved electrolytes for Li-ion batteries: Mixtures of ionic liquid and organic electrolyte with enhanced safety and electrochemical performance. J. Power Sources 2010, 195, 845–852. [Google Scholar] [CrossRef]
- Xiang, H.F.; Yin, B.; Wang, H.; Lin, H.W.; Ge, X.W.; Xie, S.; Chen, C.H. Improving electrochemical properties of room temperature ionic liquid (RTIL) based electrolyte for Li-ion batteries. Electrochim. Acta 2010, 55, 5204–5209. [Google Scholar] [CrossRef]
- Hoffknecht, J.P.; Drews, M.; He, X.; Paillard, E. Investigation of the N-butyl-N-methyl pyrrolidinium trifluoromethanesulfonyl-N-cyanoamide (PYR14TFSAM) ionic liquid as electrolyte for Li-ion battery. Electrochim. Acta 2017, 250, 25–34. [Google Scholar] [CrossRef]
- Fang, S.; Jin, Y.; Yang, L.; Hirano, S.I.; Tachibana, K.; Katayama, S. Functionalized ionic liquids based on quaternary ammonium cations with three or four ether groups as new electrolytes for lithium battery. Electrochim. Acta 2011, 56, 4663–4671. [Google Scholar] [CrossRef]
- Sano, H.; Kitta, M.; Shikano, M.; Matsumoto, H. Effect of Temperature on Li Electrodeposition Behavior in Room-Temperature Ionic Liquids Comprising Quaternary Ammonium Cation. J. Electrochem. Soc. 2019, 166, A2973–A2979. [Google Scholar] [CrossRef]
- Liu, Q.; Hsu, C.W.; Dzwiniel, T.L.; Pupek, K.Z.; Zhang, Z. A fluorine-substituted pyrrolidinium-based ionic liquid for high-voltage Li-ion batteries. Chem. Commun. 2020, 56, 7317–7320. [Google Scholar] [CrossRef]
- Periyapperuma, K.; Arca, E.; Harvey, S.; Pathirana, T.; Ban, C.; Burrell, A.; Pozo-Gonzalo, C.; Howlett, P.C. High Current Cycling in a Superconcentrated Ionic Liquid Electrolyte to Promote Uniform Li Morphology and a Uniform LiF-Rich Solid Electrolyte Interphase. ACS Appl. Mater. Interfaces 2020, 12, 42236–42247. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J.; Yuan, S.; Wang, Y.; Xia, Y. To mitigate self-discharge of lithium-sulfur batteries by optimizing ionic liquid electrolytes. Energy Environ. Sci. 2016, 9, 224–231. [Google Scholar] [CrossRef]
- Cao, Y.; Zuo, P.; Lou, S.; Sun, Z.; Li, Q.; Huo, H.; Ma, Y.; Du, C.; Gao, Y.; Yin, G. A quasi-solid-state Li-S battery with high energy density, superior stability and safety. J. Mater. Chem. A 2019, 7, 6533–6542. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, Z.; An, X.; Yoshida, A.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Bifunctional ionic liquid and conducting ceramic co-assisted solid polymer electrolyte membrane for quasi-solid-state lithium metal batteries. J. Memb. Sci. 2019, 586, 122–129. [Google Scholar] [CrossRef]
- Zhai, W.; Zhu, H.J.; Wang, L.; Liu, X.M.; Yang, H. Study of PVDF-HFP/PMMA blended micro-porous gel polymer electrolyte incorporating ionic liquid [BMIM]BF4 for Lithium ion batteries. Electrochim. Acta 2014, 133, 623–630. [Google Scholar] [CrossRef]
- Li, Q.; Ardebili, H. Flexible thin-film battery based on solid-like ionic liquid-polymer electrolyte. J. Power Sources 2016, 303, 17–21. [Google Scholar] [CrossRef]
- Xu, P.; Chen, H.; Zhou, X.; Xiang, H. Gel polymer electrolyte based on PVDF-HFP matrix composited with rGO-PEG-NH2 for high-performance lithium ion battery. J. Memb. Sci. 2021, 617, 118660. [Google Scholar] [CrossRef]
- Fuller, J.; Carlin, R.T.; Osteryoung, R.A. The Room Temperature Ionic Liquid 1-Ethyl-3-methylimidazolium Tetrafluoroborate: Electrochemical Couples and Physical Properties. J. Electrochem. Soc. 1997, 144, 3881–3886. [Google Scholar] [CrossRef]
- Handa, N.; Sugimoto, T.; Yamagata, M.; Kikuta, M.; Kono, M.; Ishikawa, M. A neat ionic liquid electrolyte based on FSI anion for electric double layer capacitor. J. Power Sources 2008, 185, 1585–1588. [Google Scholar] [CrossRef]
- Balducci, A.; Dugas, R.; Taberna, P.L.; Simon, P.; Plée, D.; Mastragostino, M.; Passerini, S. High temperature carbon-carbon supercapacitor using ionic liquid as electrolyte. J. Power Sources 2007, 165, 922–927. [Google Scholar] [CrossRef] [Green Version]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef]
- Lin, R.; Taberna, P.-L.; Fantini, S.; Presser, V.; Pérez, C.R.; Malbosc, F.; Rupesinghe, N.L.; Teo, K.B.K.; Gogotsi, Y.; Simon, P. Capacitive Energy Storage from −50 to 100 °C Using an Ionic Liquid Electrolyte. J. Phys. Chem. Lett. 2011, 2, 2396–2401. [Google Scholar] [CrossRef] [Green Version]
- Timperman, L.; Vigeant, A.; Anouti, M. Eutectic mixture of Protic Ionic Liquids as an Electrolyte for Activated Carbon-Based Supercapacitors. Electrochim. Acta 2015, 155, 164–173. [Google Scholar] [CrossRef]
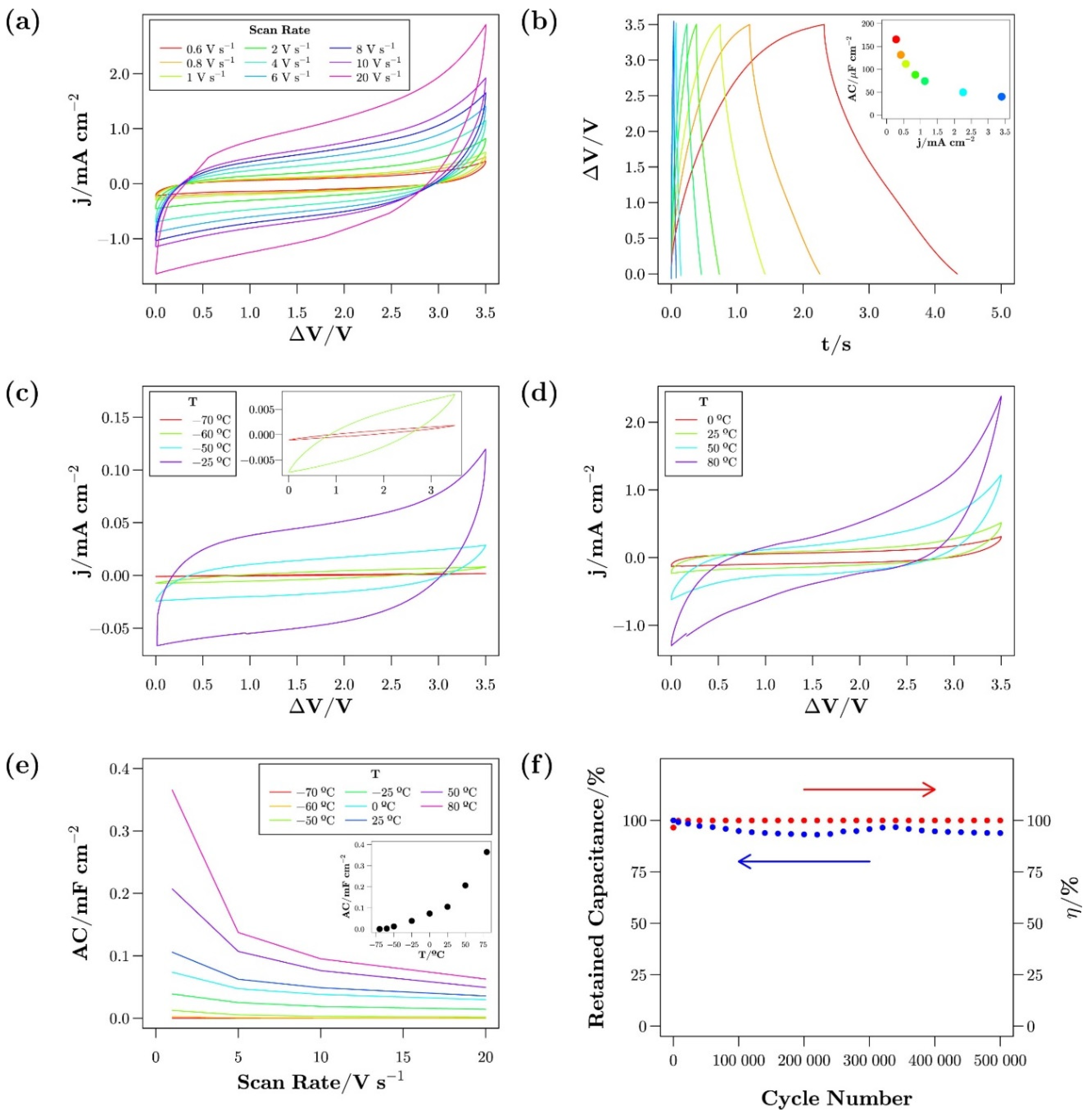
- Newell, R.; Faure-Vincent, J.; Iliev, B.; Schubert, T.; Aradilla, D. A new high performance ionic liquid mixture electrolyte for large temperature range supercapacitor applications (−70 °C to 80 °C) operating at 3.5V cell voltage. Electrochim. Acta 2018, 267, 15–19. [Google Scholar] [CrossRef]
- Fletcher, S.; Kirkpatrick, I.; Thring, R.; Dring, R.; Tate, J.L.; Geary, H.R.M.; Black, V.J. Ternary Mixtures of Sulfolanes and Ionic Liquids for Use in High-Temperature Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 2612–2620. [Google Scholar] [CrossRef] [Green Version]
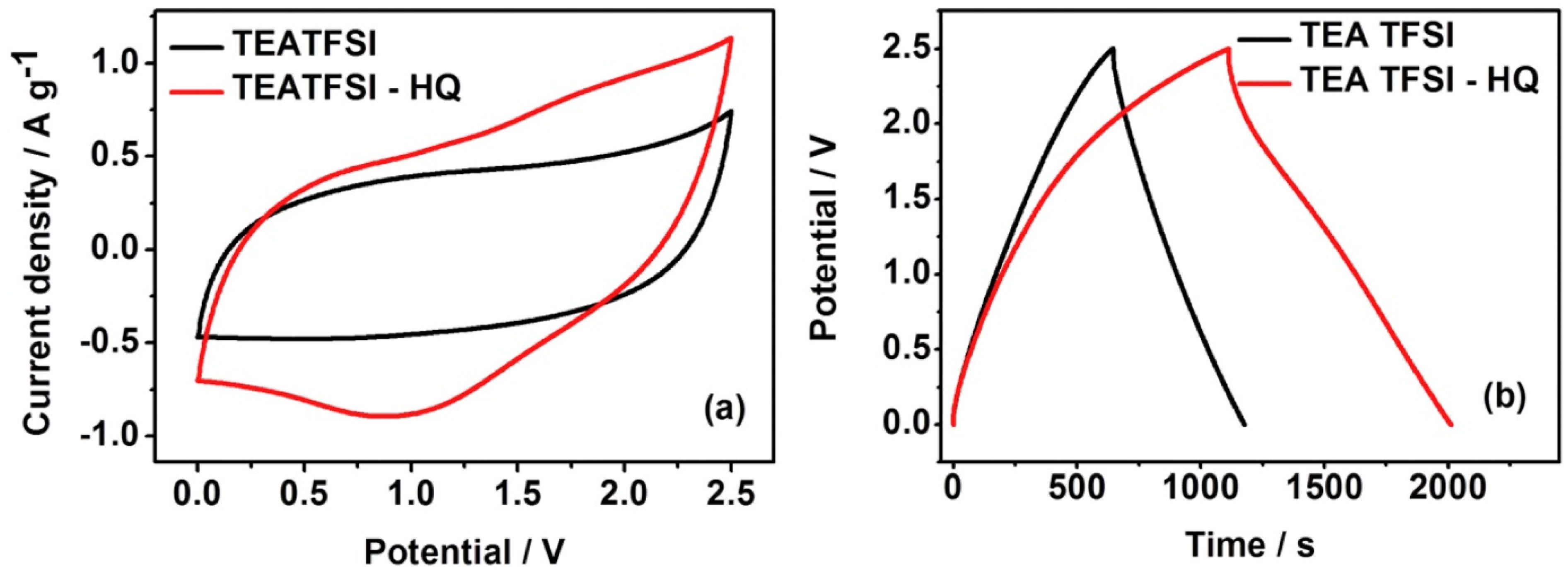
- Xie, H.J.; Gélinas, B.; Rochefort, D. Redox-active electrolyte supercapacitors using electroactive ionic liquids. Electrochem. Commun. 2016, 66, 42–45. [Google Scholar] [CrossRef]
- Kim, D.; Kannan, P.K.; Chung, C.H. High-Performance Flexible Supercapacitors Based on Ionogel Electrolyte with an Enhanced Ionic Conductivity. ChemistrySelect 2018, 3, 2190–2195. [Google Scholar] [CrossRef]
- Tu, Q.M.; Fan, L.Q.; Pan, F.; Huang, J.L.; Gu, Y.; Lin, J.M.; Huang, M.L.; Huang, Y.F.; Wu, J.H. Design of a novel redox-active gel polymer electrolyte with a dual-role ionic liquid for flexible supercapacitors. Electrochim. Acta 2018, 268, 562–568. [Google Scholar] [CrossRef]
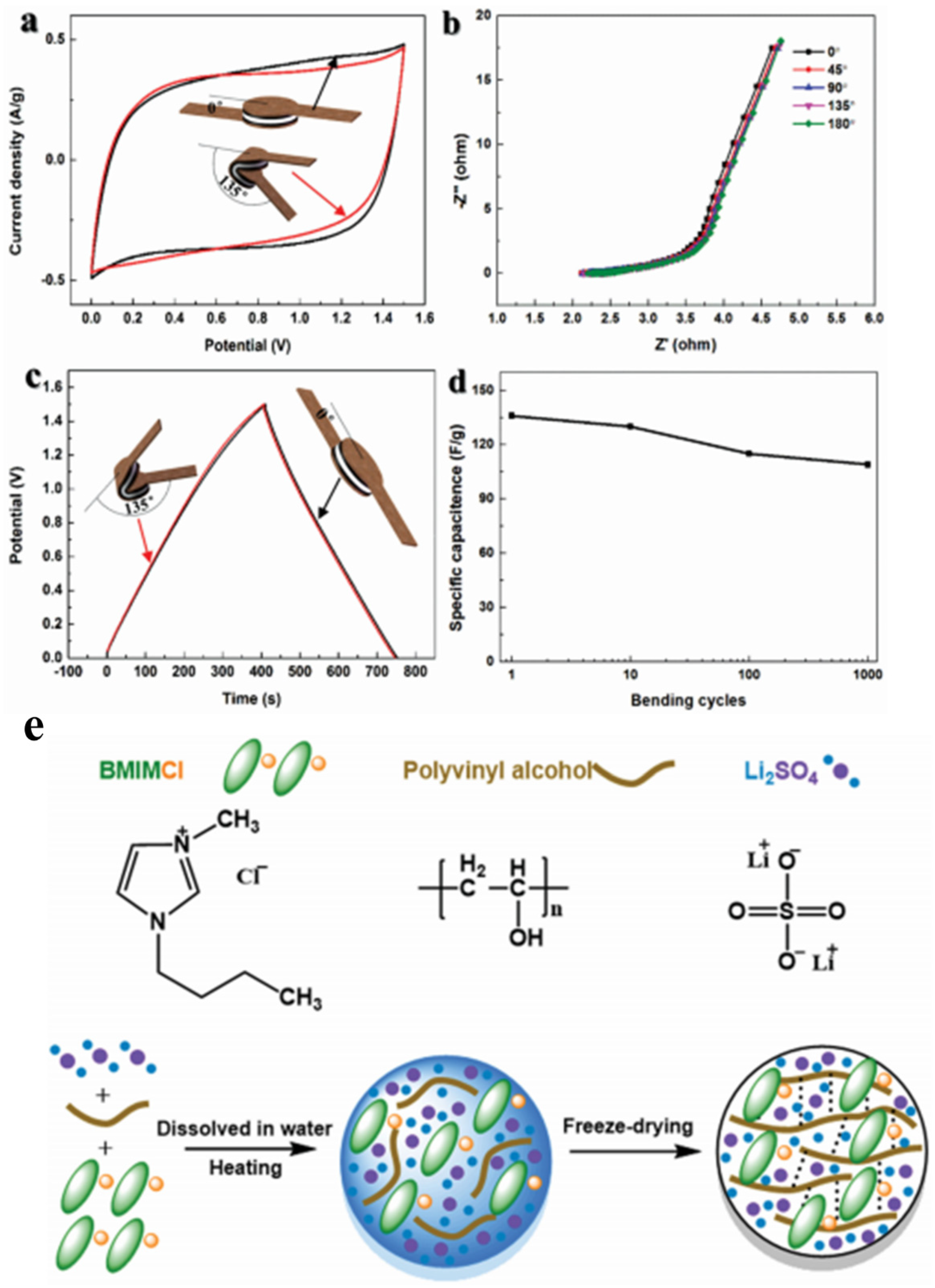
- Zhang, X.; Wang, L.; Peng, J.; Cao, P.; Cai, X.; Li, J.; Zhai, M. A Flexible Ionic Liquid Gelled PVA-Li2SO4 Polymer Electrolyte for Semi-Solid-State Supercapacitors. Adv. Mater. Interfaces 2015, 2, 1–9. [Google Scholar] [CrossRef]
- Cho, K.G.; Kim, H.S.; Jang, S.S.; Kyung, H.; Kang, M.S.; Lee, K.H.; Yoo, W.C. Optimizing Electrochemically Active Surfaces of Carbonaceous Electrodes for Ionogel Based Supercapacitors. Adv. Funct. Mater. 2020, 30, 1–11. [Google Scholar] [CrossRef]
- Han, J.; Choi, Y.; Lee, J.; Pyo, S.; Jo, S.; Yoo, J. UV curable ionogel for all-solid-state supercapacitor. Chem. Eng. J. 2021, 416, 129089. [Google Scholar] [CrossRef]
- Tiruye, G.A.; Muñoz-Torrero, D.; Palma, J.; Anderson, M.; Marcilla, R. Performance of solid state supercapacitors based on polymer electrolytes containing different ionic liquids. J. Power Sources 2016, 326, 560–568. [Google Scholar] [CrossRef]
- De Oliveira, P.S.C.; Alexandre, S.A.; Silva, G.G.; Trigueiro, J.P.C.; Lavall, R.L. PIL/IL gel polymer electrolytes: The influence of the IL ions on the properties of solid-state supercapacitors. Eur. Polym. J. 2018, 108, 452–460. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef]
- Han, D.; Row, K.H. Recent applications of ionic liquids in separation technology. Molecules 2010, 15, 2405–2426. [Google Scholar] [CrossRef] [Green Version]
- Neale, A.R.; Schütter, C.; Wilde, P.; Goodrich, P.; Hardacre, C.; Passerini, S.; Balducci, A.; Jacquemin, J. Physical-Chemical Characterization of Binary Mixtures of 1-Butyl-1-methylpyrrolidinium Bis{(trifluoromethyl)sulfonyl}imide and Aliphatic Nitrile Solvents as Potential Electrolytes for Electrochemical Energy Storage Applications. J. Chem. Eng. Data 2017, 62, 376–390. [Google Scholar] [CrossRef]
- Lee, M.T.; Tsai, W.T.; Cheng, H.F.; Sun, I.W.; Chang, J.K. Effect of electrolyte temperature on the pseudo-capacitive behavior of manganese oxide in N-butyl-N-methylpyrrolidinium-dicyanamide ionic liquid. J. Power Sources 2013, 233, 28–33. [Google Scholar] [CrossRef]
- Mai, Y.J.; Luo, H.; Zhao, X.Y.; Wang, J.L.; Davis, J.; Lyons, L.J.; Zhang, L.Z. Organosilicon functionalized quaternary ammonium ionic liquids as electrolytes for lithium-ion batteries. Ionics 2014, 20, 1207–1215. [Google Scholar] [CrossRef]
- Yang, B.; Li, C.; Zhou, J.; Liu, J.; Zhang, Q. Pyrrolidinium-based ionic liquid electrolyte with organic additive and LiTFSI for high-safety lithium-ion batteries. Electrochim. Acta 2014, 148, 39–45. [Google Scholar] [CrossRef]
- Beltrop, K.; Qi, X.; Hering, T.; Röser, S.; Winter, M.; Placke, T. Enabling bis(fluorosulfonyl)imide-based ionic liquid electrolytes for application in dual-ion batteries. J. Power Sources 2018, 373, 193–202. [Google Scholar] [CrossRef]
- Xu, F.; Liu, C.; Feng, W.; Nie, J.; Li, H.; Huang, X.; Zhou, Z. Molten salt of lithium bis(fluorosulfonyl)imide (LiFSI)-potassium bis(fluorosulfonyl)imide (KFSI) as electrolyte for the natural graphite/LiFePO4 lithium-ion cell. Electrochim. Acta 2014, 135, 217–223. [Google Scholar] [CrossRef]
- Kärnä, M.; Lahtinen, M.; Valkonen, J. Preparation and characterization of new low melting ammonium-based ionic liquids with ether functionality. J. Mol. Struct. 2009, 922, 64–76. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable lithium-sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide Shuttle Study in the Li/S Battery System. J. Electrochem. Soc. 2004, 151, A1969. [Google Scholar] [CrossRef]
- Gao, T.; Li, X.; Wang, X.; Hu, J.; Han, F.; Fan, X.; Suo, L.; Pearse, A.J.; Lee, S.B.; Rubloff, G.W.; et al. A Rechargeable Al/S Battery with an Ionic-Liquid Electrolyte. Angew. Chem. 2016, 128, 10052–10055. [Google Scholar] [CrossRef]
- Yuan, L.X.; Feng, J.K.; Ai, X.P.; Cao, Y.L.; Chen, S.L.; Yang, H.X. Improved dischargeability and reversibility of sulfur cathode in a novel ionic liquid electrolyte. Electrochem. Commun. 2006, 8, 610–614. [Google Scholar] [CrossRef]
- Barghamadi, M.; Best, A.S.; Bhatt, A.I.; Hollenkamp, A.F.; Mahon, P.J.; Musameh, M.; Rüther, T. Effect of LiNO3 additive and pyrrolidinium ionic liquid on the solid electrolyte interphase in the lithium-sulfur battery. J. Power Sources 2015, 295, 212–220. [Google Scholar] [CrossRef]
- Schaefer, J.L.; Lu, Y.; Moganty, S.S.; Agarwal, P.; Jayaprakash, N.; Archer, L.A. Electrolytes for high-energy lithium batteries. Appl. Nanosci. 2012, 2, 91–109. [Google Scholar] [CrossRef] [Green Version]
- Polu, A.R.; Rhee, H.W. Ionic liquid doped PEO-based solid polymer electrolytes for lithium-ion polymer batteries. Int. J. Hydrogen Energy 2017, 42, 7212–7219. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, P.; Fang, Q.; Jing, M.; Shen, X.; Yang, L. A novel solid PEO/LLTO-nanowires polymer composite electrolyte for solid-state lithium-ion battery. Electrochim. Acta 2018, 292, 718–726. [Google Scholar] [CrossRef]
- Lewandowski, A.; Galinski, M. Practical and theoretical limits for electrochemical double-layer capacitors. J. Power Sources 2007, 173, 822–828. [Google Scholar] [CrossRef]
- Lockett, V.; Sedev, R.; Ralston, J.; Horne, M.; Rodopoulos, T. Differential capacitance of the electrical double layer in imidazolium-based ionic liquids: Influence of potential, cation size, and temperature. J. Phys. Chem. C 2008, 112, 7486–7495. [Google Scholar] [CrossRef]
- Bettini, L.G.; Galluzzi, M.; Podestà, A.; Milani, P.; Piseri, P. Planar thin film supercapacitor based on cluster-assembled nanostructured carbon and ionic liquid electrolyte. Carbon N. Y. 2013, 59, 212–220. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor devices based on graphene materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 6–10. [Google Scholar] [CrossRef]
- Kakaei, K.; Esrafili, M.D.; Ehsani, A. Graphene-Based Electrochemical Supercapacitors. Interface Sci. Technol. 2019, 27, 339–386. [Google Scholar] [CrossRef]
- Lv, W.; Tang, D.M.; He, Y.B.; You, C.H.; Shi, Z.Q.; Chen, X.C.; Chen, C.M.; Hou, P.X.; Liu, C.; Yang, Q.H. Low-temperature exfoliated graphenes: Vacuum-promoted exfoliation and electrochemical energy storage. ACS Nano 2009, 3, 3730–3736. [Google Scholar] [CrossRef] [PubMed]
- Largeot, C.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Microporous carbon-based electrical double layer capacitor operating at high temperature in ionic liquid electrolyte. Electrochem. Solid State Lett. 2011, 14, 174–177. [Google Scholar] [CrossRef]
- Hoffman, E.N.; Yushin, G.; Barsoum, M.W.; Gogotsi, Y. Synthesis of carbide-derived carbon by chlorination of Ti2AlC. Chem. Mater. 2005, 17, 2317–2322. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Nikitin, A.; Ye, H.; Zhou, W.; Fischer, J.E.; Yi, B.; Foley, H.C.; Barsoum, M.W. Nanoporous carbide-derived carbon with tunable pore size. Nat. Mater. 2003, 2, 591–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimovski, S.; Nikitin, A.; Ye, H.; Gogotsi, Y. Synthesis of graphite by chlorination of iron carbide at moderate temperatures. J. Mater. Chem. 2004, 14, 238–243. [Google Scholar] [CrossRef]
- Tallo, I.; Thomberg, T.; Kontturi, K.; Jänes, A.; Lust, E. Nanostructured carbide-derived carbon synthesized by chlorination of tungsten carbide. Carbon N. Y. 2011, 49, 4427–4433. [Google Scholar] [CrossRef]
- Cambaz, Z.G.; Yushin, G.N.; Gogotsi, Y.; Vyshnyakova, K.L.; Pereselentseva, L.N. Formation of carbide-derived carbon on β-silicon carbide whiskers. J. Am. Ceram. Soc. 2006, 89, 509–514. [Google Scholar] [CrossRef]
- Ortega, P.F.R.; Santos, G.A.D.; Trigueiro, J.P.C.; Silva, G.G.; Quintanal, N.; Blanco, C.; Lavall, R.L.; Santamaría, R. Insights on the Behavior of Imidazolium Ionic Liquids as Electrolytes in Carbon-Based Supercapacitors: An Applied Electrochemical Approach. J. Phys. Chem. C 2020, 124, 15818–15830. [Google Scholar] [CrossRef]
- Lazzari, M.; Mastragostino, M.; Pandolfo, A.G.; Ruiz, V.; Soavi, F. Role of Carbon Porosity and Ion Size in the Development of Ionic Liquid Based Supercapacitors. J. Electrochem. Soc. 2011, 158, A22. [Google Scholar] [CrossRef]
- Cooper, E.R.; Andrews, C.D.; Wheatley, P.S.; Webb, P.B.; Wormald, P.; Morris, R.E. Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature 2004, 430, 1012–1016. [Google Scholar] [CrossRef]
- Kunze, M.; Jeong, S.; Paillard, E.; Winter, M.; Passerini, S. Melting behavior of pyrrolidinium-based ionic liquids and their binary mixtures. J. Phys. Chem. C 2010, 114, 12364–12369. [Google Scholar] [CrossRef]
- Kunze, M.; Montanino, M.; Appetecchi, G.B.; Jeong, S.; Schönhoff, M.; Winter, M.; Passerini, S. Melting behavior and ionic conductivity in hydrophobic ionic liquids. J. Phys. Chem. A 2010, 114, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, G.; Yan, Z.; Kang, L.; Xu, H.; Shi, F.; Lei, Z.; Liu, Z.H. High capacitive property for supercapacitor using Fe3+/Fe2+ redox couple additive electrolyte. Electrochim. Acta 2017, 231, 705–712. [Google Scholar] [CrossRef]
- Lee, J.; Krüner, B.; Tolosa, A.; Sathyamoorthi, S.; Kim, D.; Choudhury, S.; Seo, K.H.; Presser, V. Tin/vanadium redox electrolyte for battery-like energy storage capacity combined with supercapacitor-like power handling. Energy Environ. Sci. 2016, 9, 3392–3398. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Yoo, Y.E.; Mai, L.; Kim, W. Rational Design of a Redox-Active Nonaqueous Electrolyte for a High-Energy-Density Supercapacitor Based on Carbon Nanotubes. ACS Sustain. Chem. Eng. 2019, 7, 7728–7735. [Google Scholar] [CrossRef]
- Park, J.; Kim, B.; Yoo, Y.E.; Chung, H.; Kim, W. Energy-density enhancement of carbon-nanotube-based supercapacitors with redox couple in organic electrolyte. ACS Appl. Mater. Interfaces 2014, 6, 19499–19503. [Google Scholar] [CrossRef] [PubMed]
- Tooming, T.; Thomberg, T.; Siinor, L.; Tõnurist, K.; Jänes, A.; Lust, E. A Type High Capacitance Supercapacitor Based on Mixed Room Temperature Ionic Liquids Containing Specifically Adsorbed Iodide Anions. J. Electrochem. Soc. 2014, 161, A222–A227. [Google Scholar] [CrossRef]
- Yamazaki, S.; Ito, T.; Yamagata, M.; Ishikawa, M. Non-aqueous electrochemical capacitor utilizing electrolytic redox reactions of bromide species in ionic liquid. Electrochim. Acta 2012, 86, 294–297. [Google Scholar] [CrossRef]
- You, D.J.; Yin, Z.; Ahn, Y.K.; Lee, S.H.; Yoo, J.; Kim, Y.S. Redox-active ionic liquid electrolyte with multi energy storage mechanism for high energy density supercapacitor. RSC Adv. 2017, 7, 55702–55708. [Google Scholar] [CrossRef] [Green Version]
- Sathyamoorthi, S.; Suryanarayanan, V.; Velayutham, D. Organo-redox shuttle promoted protic ionic liquid electrolyte for supercapacitor. J. Power Sources 2015, 274, 1135–1139. [Google Scholar] [CrossRef]
- Deng, J.; Li, J.; Song, S.; Zhou, Y.; Li, L. Electrolyte-dependent supercapacitor performance on nitrogen-doped porous bio-carbon from gelatin. Nanomaterials 2020, 10, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Song, H.; Wang, Z.; Zhang, J.; Zhang, J.; Ba, X. Stretchable, self-healable, and reprocessable chemical cross-linked ionogels electrolytes based on gelatin for flexible supercapacitors. J. Mater. Sci. 2020, 55, 3991–4004. [Google Scholar] [CrossRef]
- Yadav, N.; Hashmi, S.A. Energy enhancement of quasi-solid-state supercapacitors based on a non-aqueous gel polymer electrolyteviaa synergistic effect of dual redox additives diphenylamine and potassium iodide. J. Mater. Chem. A 2020, 8, 18266–18279. [Google Scholar] [CrossRef]
- Pandey, G.P.; Liu, T.; Hancock, C.; Li, Y.; Sun, X.S.; Li, J. Thermostable gel polymer electrolyte based on succinonitrile and ionic liquid for high-performance solid-state supercapacitors. J. Power Sources 2016, 328, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Austen Angell, C.; Ansari, Y.; Zhao, Z. Ionic Liquids: Past, present and future. Faraday Discuss. 2012, 154, 9–27. [Google Scholar] [CrossRef]
- Liu, X.; Taiwo, O.O.; Yin, C.; Ouyang, M.; Chowdhury, R.; Wang, B.; Wang, H.; Wu, B.; Brandon, N.P.; Wang, Q.; et al. Aligned Ionogel Electrolytes for High-Temperature Supercapacitors. Adv. Sci. 2019, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Senokos, E.; Reguero, V.; Cabana, L.; Palma, J.; Marcilla, R.; Vilatela, J.J. Large-Area, All-Solid, and Flexible Electric Double Layer Capacitors Based on CNT Fiber Electrodes and Polymer Electrolytes. Adv. Mater. Technol. 2017, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Qian, W.; Texter, J.; Yan, F. Frontiers in poly(io147nic liquid)s: Syntheses and applications. Chem. Soc. Rev. 2017, 46, 1124–1159. [Google Scholar] [CrossRef]
- Alexandre, S.A.; Silva, G.G.; Santamaría, R.; Trigueiro, J.P.C.; Lavall, R.L. A highly adhesive PIL/IL gel polymer electrolyte for use in flexible solid state supercapacitors. Electrochim. Acta 2019, 299, 789–799. [Google Scholar] [CrossRef]
- Kim, D.W.; Jung, S.M.; Jung, H.Y. A super-thermostable, flexible supercapacitor for ultralight and high performance devices. J. Mater. Chem. A 2020, 8, 532–542. [Google Scholar] [CrossRef]
- Al-Zohbi, F.; Jacquemin, J.; Ghamouss, F.; Schmaltz, B.; Abarbri, M.; Cherry, K.; Tabcheh, M.F.; Tran-Van, F. Impact of the aqueous pyrrolidinium hydrogen sulfate electrolyte formulation on transport properties and electrochemical performances for polyaniline-based supercapacitor. J. Power Sources 2019, 431, 162–169. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhuang, Q.; Zhang, M.; Wang, H.; Gao, Z.; Sun, J.K.; Yuan, J. Poly(ionic liquid) composites. Chem. Soc. Rev. 2020, 49, 1726–1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcilla, R.; Blazquez, J.A.; Fernandez, R.; Grande, H.; Pomposo, J.A.; Mecerreyes, D. Synthesis of novel polycations using the chemistry of ionic liquids. Macromol. Chem. Phys. 2005, 206, 299–304. [Google Scholar] [CrossRef]


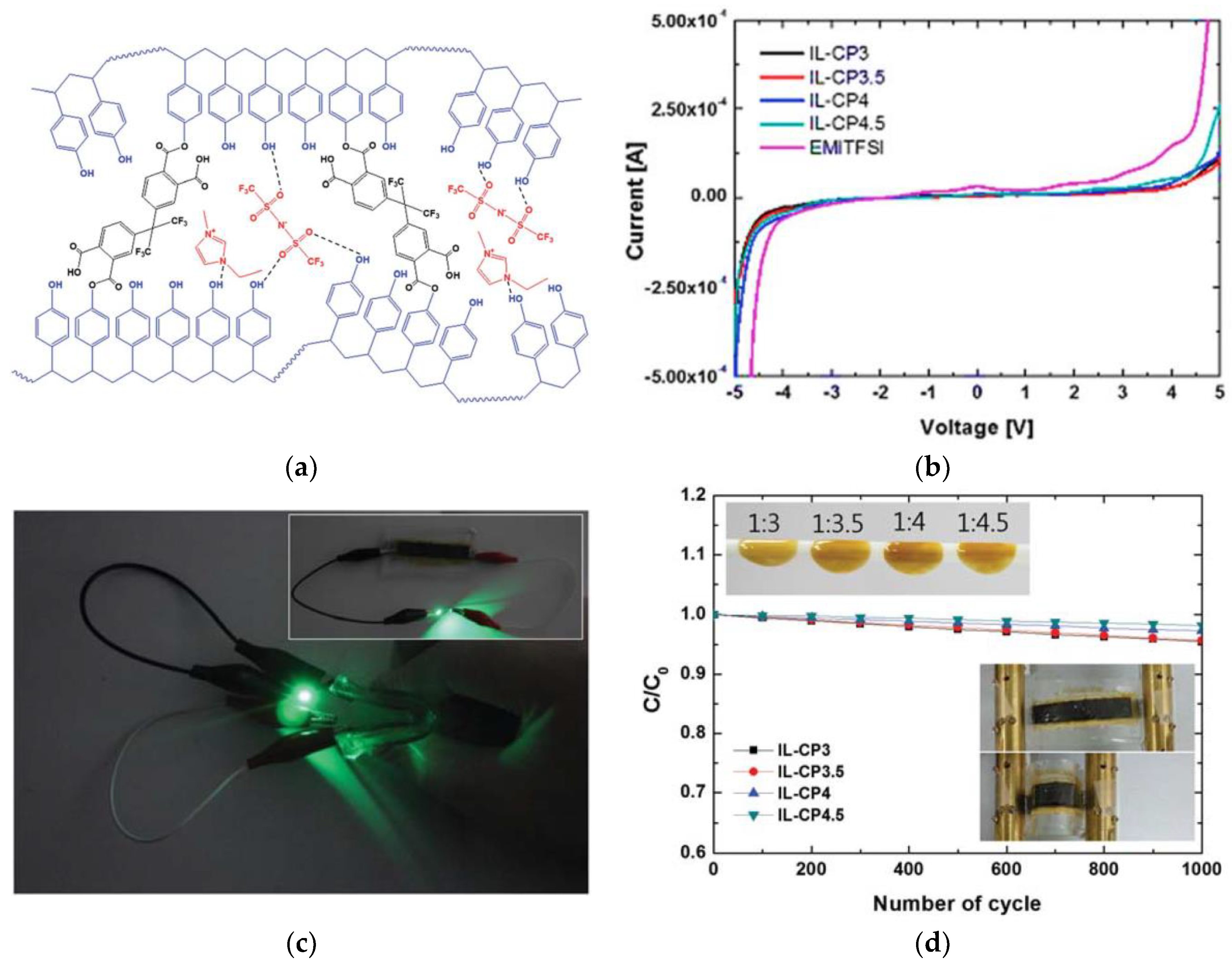
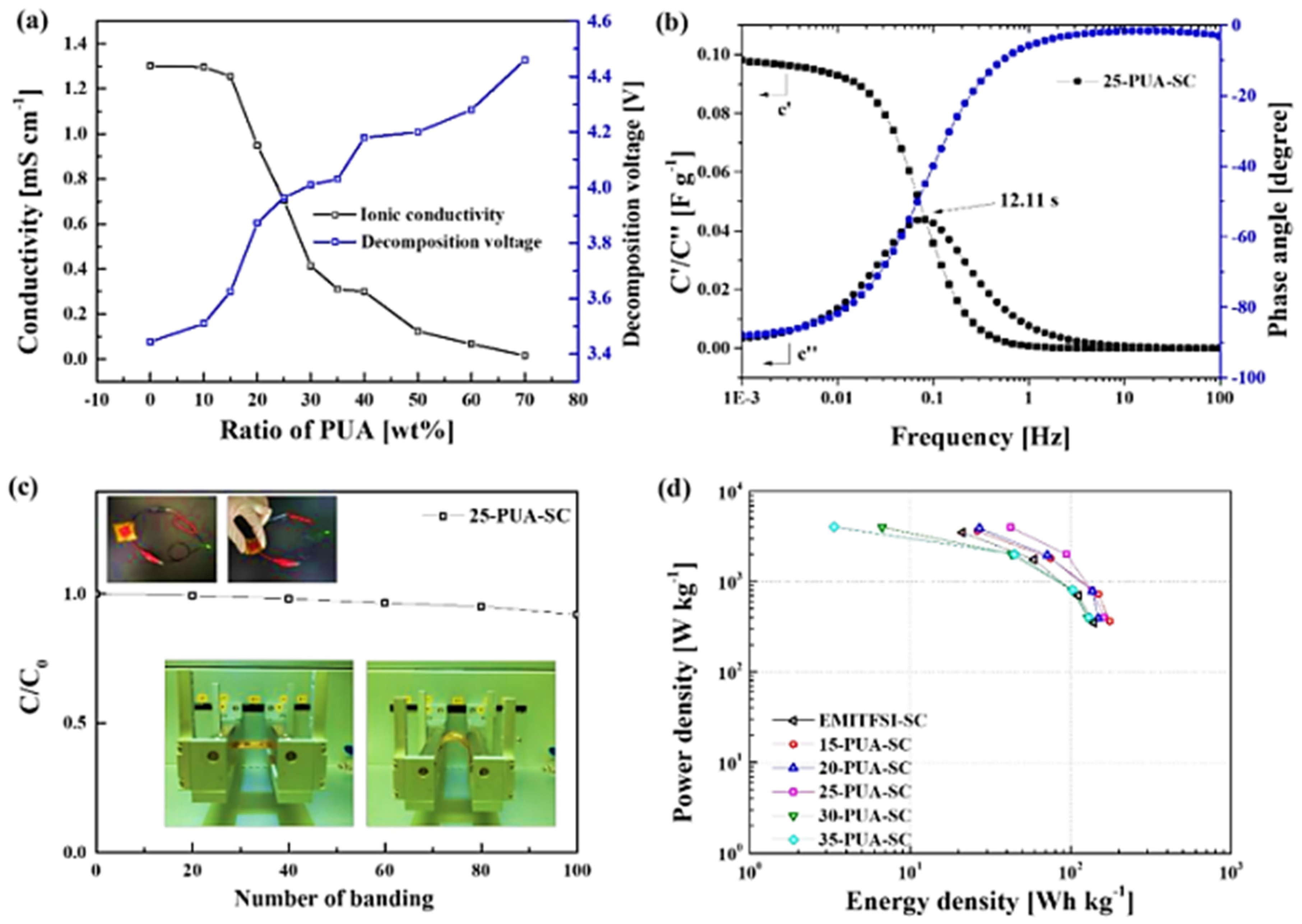
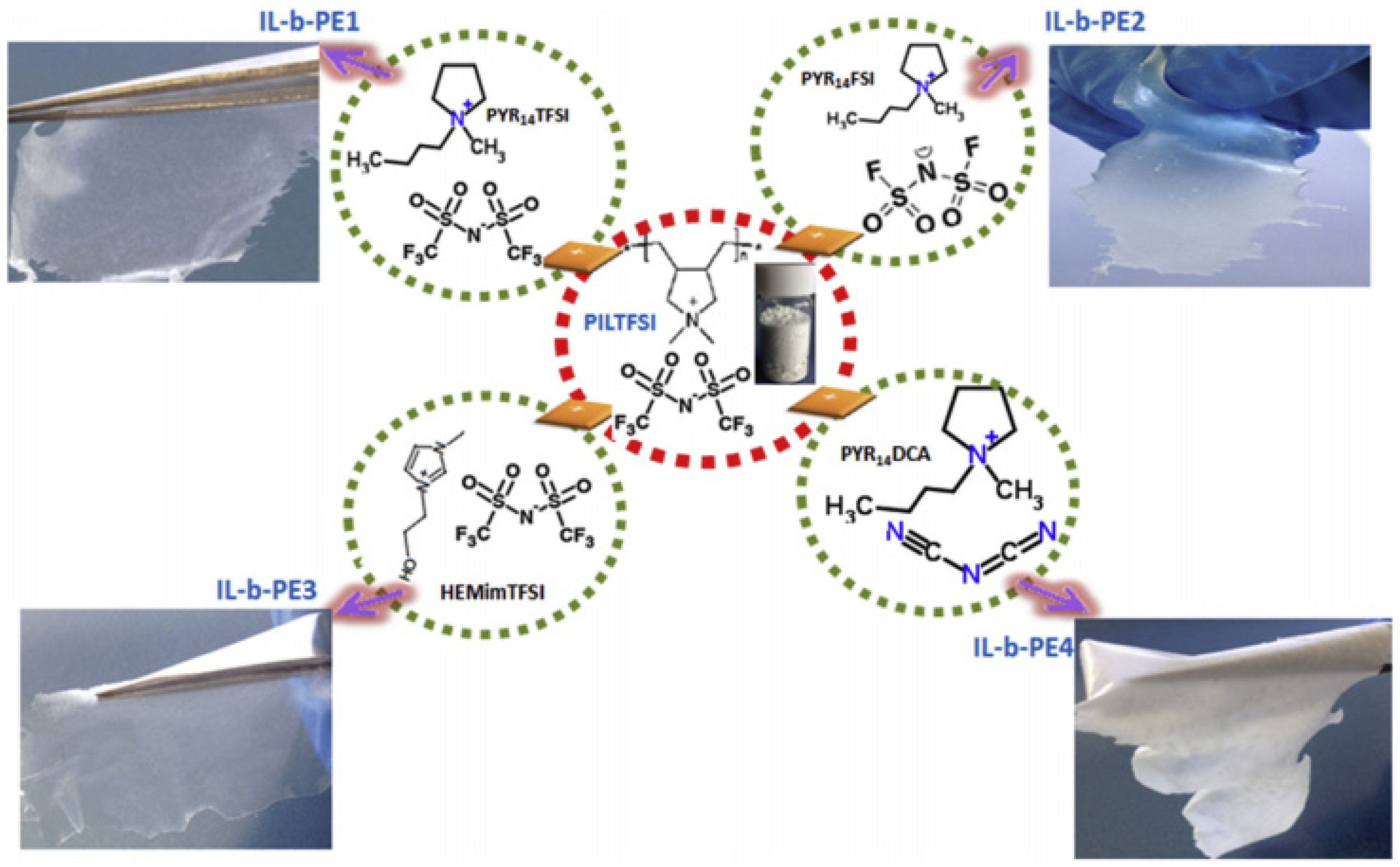

| Classification | Acronyms | Ionic Liquid | Reference(s) |
|---|---|---|---|
| Li-ion batteries | [PMI][TFSI] | 1-propyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | [70] |
| [BMI][TFSI] | 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | [70] | |
| [EMI][TFSI] | 1-ethyl-2,3trimethyleneimidazolium bis(trifluoromethane sulfonyl)imide | [72] | |
| [PP13][TFSI] | N-methyl-N-propylpiperidinium Bis(trifluoromethanesulfonyl)imide | [73] | |
| [Pyr14][DCA] | N-butyl-N-methyl pyrrolidinium-dicyanamide | [74] | |
| [Pyr14][TFSI] | N-butyl-N-methyl pyrrolidinium bis(trifluoromethylsulfonyl)imide | [74] | |
| [Pyr14][TFSAM] | N-butyl-N-methyl pyrrolidinium bis(trifluoromethylsulfonyl)-N-cyanoamide | [74] | |
| [N2(2o1)3][TFSI] | N-ethyl-N,N,N-tri-(2-methoxyethyl)ammonium bis(trifluoromethanesulfonyl)imide | [75] | |
| [(N2(2o1)2(2o2)][TFSI] | N-ethyl-N,N-di-(2-methoxyethyl)-N-2-ethoxyethylammonium bis(trifluoromethanesulfonyl)imide | [75] | |
| [(N3(2o1)3)][TFSI] | N-propyl-N,N,N-tri-(2-methoxyethyl)ammonium bis(trifluoromethanesulfonyl)imide | [75] | |
| [(N4(2o1)3)][TFSI] | N-butyl-N,N,N-tri-(2-methoxyethyl)ammonium bis(trifluoromethanesulfonyl)imide | [75] | |
| [C4mpyr][TFSI] | N-methyl-N-butyl-pyrrolidinium bis(trifluoromethanesulfonyl)imide | [76] | |
| [PfMpyr][FSI] | 1-Methyl-1-propyl-3-fluoropyrrolidinium bis(fluorosulfonyl)-imide | [77] | |
| [C3mpyr][FSI] | N-methyl-N-propyl-pyrrolidinium (fluorosulfonyl) imide | [78] | |
| LiS batteries | [PP13][TFSI] | N-methyl-N-propylpiperidinium bis(trifluoromethanesulfonyl)imide | [79] |
| [LiG3][TFSI] | Li(triglyme) bis(trifluoromethylsulfonyl)imide | [80] | |
| PEO, LiTFSI-[TBP][HP] | poly(ethyleneoxide)-lithium bis(trifluoromethylsulfonyl)imide-tetrabutylphosphonium 2-hydroxypyridine | [81] | |
| PVdF-HFP/PMMA/[BMI][BF4] | (poly(vinylidene fluoride-co-hexafluoropropylene)/poly(methyl methacrylate)- 1-butyl-3-methylimidazolium tetrafluoroborate)) | [82] | |
| PVdF-HFP/[EMI][DCA] | (poly(vinylidene fluoride-co-hexafluoropropylene)/1-ethyl-3-methylimidazolium dicyanamide)) | [83] | |
| PVdF-HFP/[EMI][TFSI]/rGO-PEG-NH2 | (poly(vinylidene fluoride-co-hexafluoropropylene)/ (covalent linked 2,2″-(ethylenedioxy) bis (ethylamine) to reduced graphene oxide)) | [84] | |
| Supercapacitors | [EMI][BF4] | 1-ethyl-3-methylimidazolium tetrafluoroborate | [85] |
| [EMI][FSI] | 1-ethyl-3-methyleneimidazolium (fluorosulfonyl) imide | [86] | |
| [Pyr14][TFSI] | N-butyl-N-methyl pyrrolidinium bis(trifluoromethylsulfonyl)imide | [87] | |
| [EMI][TFSI] | 1-ethyl-3-methyleneimidazolium bis(trifluoromethane sulfonyl)imide | [88] | |
| [Pyr14][FSI] | N-ethyl-N-methylpyrrolidinium (fluorosulfonyl) imide | [89] | |
| [PIP13][FSI] | N-methyl-N-propylpiperidinium (fluorosulfonyl)imide | [89] | |
| [Pyr][TFSI] | N-butyl-N-methyl pyrrolidinium bis(trifluoromethylsulfonyl)imide | [90] | |
| [PMPyrr][TFSI] | 1-Methyl-1propylpyrrolidinium bis(trifluoromeyhanesulfonyl)imide | [91] | |
| [BTM][TFSI] | Butyltrimethylammonium- bis(trifluoromethylsulfonyl)imide | [92] | |
| [TEA][TFSI] | Trimethylamine- bis(trifluoromethylsulfonyl)imide | [93] | |
| Quasi-solid-state supercapacitors | PHEMA-co-PEGDMA/[EMI][BF4] | poly(2-hydroxyethyl methacrylate) and poly(ethylene glycol) diacrylate/1-ethyl-3-methylimidazolium tetrafluoroborate | [94] |
| [BMI][I] | 1-butyl-3-methylimidazolium iodide | [95] | |
| [BMI][Cl] | 1-butyl-3-methylimidazolium chloride | [96] | |
| PVdF-HFP/[EMI][BF4] | (poly(vinylidene fluoride-co-hexafluoropropylene) /- 1-ethyl-2,3methylimidazolium tetrafluoroborate)) | [97] | |
| PUA/[EMI][TFSI] | (polyurethane acrylate-(1-ethyl-3-methylimidazolium- bis(trifluoromethylsulfonyl)imide) | [98] | |
| All-solid-state supercapacitors | [Pyr14][TFSI] | 1-butyl-1-methylpyrrolidinium bis (trifluoromethanesulfonyl)) imide | [99] |
| [Pyr14][DCA] | 1-butyl-1-methylpyrrolidinium dish Amide | [99] | |
| [PIL][TFSI] | poly(diallyldi-methylammonium) bis (trifluoromethylsulfonyl) imide | [100] | |
| [EMI][FSI] | 1-ethyl-3-methyleneimidazolium (fluorosulfonyl) imide | [100] | |
| [MBI][FSI] | 1-methyl-3-butylimidazolium (fluorosulfonyl) imide | [100] | |
| [DPI][TFSI] | 1,2-dimethyl-3-propylimidazolium bis(tri-fluoromethylsulfonyl) imide | [100] |
| Type of Cation | Ionic Liquid | Melting Point (°C) | Density (g mL−1) at 25 °C (lit.) | Cnductivity (mS cm−1) | Electrochemical Stability Window (V) | Reference(s) |
|---|---|---|---|---|---|---|
| Imidazolium | [EMI][BF4] | 15 | 1.294 | 13–15 | 4 (ref. elecrode: carbon) | [101,102] |
| [EMI][TFSI] | −15 | 1.53 | 8–10 | 3.5–3.7 (ref. elecrode: carbon) | [91] | |
| [BMI][TFSI] | 1 | 1.44 | 3.9 | 4.5–5 (ref. elecrode: carbon) | [70] | |
| Pyrrolidinum | [Pyr14][TFSI] | −6 | 1.4216 | 2.5–3 | 3.5 (ref. elecrode: carbon) | [103] |
| [Pyr14][DCA] | −55 | 0.95 | 10.8 | 3 (ref. elecrode: carbon) | [104] | |
| Piperidinium | [PP13][TFSI] | 12 | 151 | 1.4 | 5–6 (ref. electrode: Li) | [73] |
| [PP13][FSI] | 95 | 3.7 | 5–6 (ref. electrode: Li) | [73] |
| Chlorination Temperature ( °C) | BET SSA (m2 g−1) | Pore Volume (cc g−1) | Average Pore Widt (nm) | Maximum Pore Width a (nm) |
|---|---|---|---|---|
| 400 | 1113 | 0.51 | 0.65 | 1.12 |
| 500 | 1140 | 0.50 | 0.68 | 1.18 |
| 550 | 1202 | 0.51 | 0.72 | 1.29 |
| 600 | 1269 | 0.60 | 0.74 | 1.23 |
| 700 | 1401 | 0.66 | 0.76 | 1.41 |
| 800 | 1595 | 0.79 | 0.81 | 1.54 |
| 1000 | 1625 | 0.81 | 1.10 | 2.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Han, J.; Ryu, S.; Choi, Y.; Yoo, J. Ionic Liquid Electrolytes for Electrochemical Energy Storage Devices. Materials 2021, 14, 4000. https://doi.org/10.3390/ma14144000
Kim E, Han J, Ryu S, Choi Y, Yoo J. Ionic Liquid Electrolytes for Electrochemical Energy Storage Devices. Materials. 2021; 14(14):4000. https://doi.org/10.3390/ma14144000
Chicago/Turabian StyleKim, Eunhwan, Juyeon Han, Seokgyu Ryu, Youngkyu Choi, and Jeeyoung Yoo. 2021. "Ionic Liquid Electrolytes for Electrochemical Energy Storage Devices" Materials 14, no. 14: 4000. https://doi.org/10.3390/ma14144000
APA StyleKim, E., Han, J., Ryu, S., Choi, Y., & Yoo, J. (2021). Ionic Liquid Electrolytes for Electrochemical Energy Storage Devices. Materials, 14(14), 4000. https://doi.org/10.3390/ma14144000






