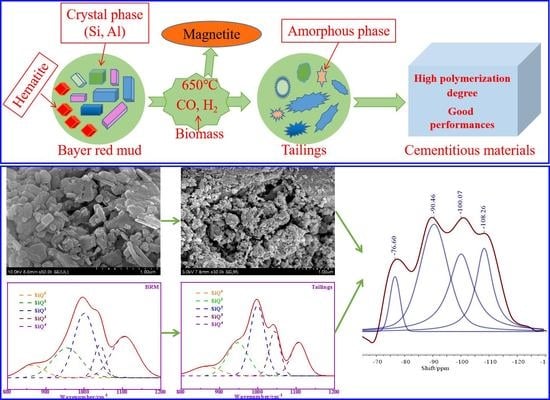
3.1. Mechanical Properties and Pozzolanic Activity
The compressive and flexural strength of BRM-cement, CBRM-cement, and Tailings-cement at 3, 7, and 28 d are shown in
Figure 2 and
Figure 3. Here, it can be seen that the compressive and flexural strength of all the samples increase with the increase of hydration time. The compressive strength of Tailings-cement is slightly higher than that of BRM-cement and CBRM-cement. The compressive strength of Tailings-cement at 3, 7, and 28 d is 23.7, 28.8, and 44.3 MPa, respectively, which meet the requirements of level 42.5 of the ordinary Portland cement. In addition, the change trend of flexural strength of all the samples is the same as that of compressive strength. The flexural strength of Tailings-cement at 3, 7, and 28 d is 6.44, 8.37, and 9.01 MPa, respectively, which meet the requirements of level 42.5 of the ordinary Portland cement. It is worth noting that the different compressive strength values of BRM-cement, CBRM-cement, and Tailings-cement indicates that they have different pozzolanic activities.
According to the GB/T12957-2005 “Test method for activity of industrial waste slag used as addition to cement”, the pozzolanic activity index of BRM, CBRM, and Tailings is calculated, as shown in
Figure 4. Here, it can be seen that the pozzolanic activity index of BRM, CBRM, and Tailings is 74%, 86%, and 91%, respectively. Next, the XRD, TG-DTG, and FTIR of BRM, CBRM, and Tailings were analyzed to reveal the mechanism of enhancing the pozzolanic activity of Tailings compared with BRM and CBRM.
3.2. XRD, TG, and FTIR Analysis of BRM, CBRM, and Tailings
The phase composition has an important influence on the pozzolanic activity of BRM. The amorphous phase is the most active phase in BRM. Most of the crystalline components of BRM are considered to be inert and hardly participate in the hydration reaction. The XRD patterns of BRM are shown in
Figure 5a. The main mineral compositions of BRM are Ca
3Al
2(SiO
4)(OH)
8 (Katoite), Fe
2O
3 (Hematite), Ca(Si
6Al
2)O
16·4H
2O (Yugawaralite), CaCO
3 (Calcite), AlO(OH) (Diaspore), and Na
6Ca
2Al
6Si
6O
24(CO
3)
2·2H
2O (Cancrinite).
Figure 5b shows the XRD patterns of CBRM, where the main mineral compositions of CBRM are Fe
2O
3 (Hematite), CaAl
2SiO
4(OH)
4 (Chantalite), CaCO
3 (Calcite), CaAl
2Si
2O
7(OH)
2·H
2O (Lawsonite), CaO (Lime), and Na
6Ca
2Al
6Si
6O
24(CO
3)
2·2H
2O (Cancrinite). The results show that Chantalite, lime, and lawsonite are the newly formed phase, and the phase of Fe
2O
3 remains unchanged. Compared with
Figure 5a, the diffraction peak intensity of CBRM crystals decrease gradually, especially for cancrinite and calcite. The decrease of the peak intensity of the crystals reveal the appearance of amorphous substances, and the amorphous peaks of 15° to 40° become larger. It shows that some aluminosilicate crystals are decomposed and transformed into active substances of silicon and aluminum, which may be the reason why the pozzolanic activity of CBRM is higher than that of BRM.
Figure 5c shows the XRD patterns of Tailings, where the main mineral compositions of Tailings are CaAl
2Si
2O
7(OH)
2·H
2O (Lawsonite), Na
6Ca
2Al
6Si
6O
24(CO
3)
2·2H
2O (Cancrinite), CaO (Lime) and Ca
3Al
2(Si
3O
4, CO
3, OH)
3 (Grossular). Compared with CBRM, Fe
2O
3 is almost completely transformed into Fe
3O
4 and separated by magnetic separation, and the diffraction peak intensity of all the crystals in Tailings decrease gradually. The amorphous peaks at 15° to 40° become significantly larger, which indicate that some aluminosilicate phases are transformed into active substances of silicon and aluminum under the action of biomass. This may be the reason why the pozzolanic activity of Tailings is higher than that of CBRM. The results showed that the amorphization degree of each crystal phase in BRM, CBRM, and Tailings is different, and its contribution to the pozzolanic activity is also different. The existence of bamboo powder is conducive to the improvement of amorphization degree of all crystal phases in BRM.
The TG-DTG curves of BRM are shown in
Figure 6. Here, it can be seen that the continuous mass loss of BRM occurs at 0–1000 °C and the mass loss rate is 14.63%. The mass loss rate in the range of 0–225 °C and 700–1000 °C is slower than that in the range of 225–700 °C. The DTG curve shows that BRM mainly contains two obvious mass loss stages in the heating process. The first stage is 30–500 °C, which is mainly the removal of physically adsorbed water and chemically bound water in BRM. A large amount of physically adsorbed water has been removed during the drying process as the BRM was dried at 100 °C before the experiment, only 0.26% of the physically adsorbed water is removed within the range of 30 °C to 105 °C. The weight loss rate is 7.92% in the range of 105–500 °C, which is mainly the removal of chemically bound water. The second stage is located in the temperature range of 500 to 770 °C, and the weight loss rate is 4.86%, which is mainly caused by the decomposition of aluminosilicate-carbonate in the BRM to release CO
2.
The FTIR spectra of BRM, CBRM, and Tailings are shown in
Figure 7. The absorption peak of 3641 cm
−1 corresponds to the telescopic vibration of free -OH. The absorption peak of 3493 cm
−1 corresponds to the telescopic vibration of associated -OH. After calcination at 650 °C, the free -OH in the BRM is converted to an associated -OH, which indicates a certain amount of hydrogen bond. The absorption peak of 1624 cm
−1 corresponds to the telescopic vibration of C-O, and about 1450 cm
−1 corresponds to the antisymmetric telescopic vibration of C-O. The absorption peak in the range of 800–1200 cm
−1 is asymmetric tensile vibration of Si-O-Si or Si-O-Al connected with tetrahedron of [SiO
4] or [AlO
4]
− [
18]. The peak at 1090 cm
−1 is caused by the tensile vibration of O-Si-(Si). The peak at 996 cm
−1 is caused by the tensile vibration of Si-O (Al). The peak at 865 cm
−1 is caused by the tensile vibration of Si-O-. The absorption peak in the range of 800–600 cm
−1 is symmetrical to telescopic vibrations between Si-O-(Si, Al) in tetrahedron of [SiO
4] or [AlO
4]
−. The absorption peak of 575 cm
−1 may be related to the rings of silicon oxygen and aluminum oxygen. The peak in the range of 500–400 cm
−1 is caused by the bending vibration of Si-O-Si or Si-O-Al. It is worth noting that the FTIR spectra at 800–1200 cm
−1 of BRM, CBRM, and Tailings are similar, but the area of peak has some differences. Based on the existing research results, the characteristic peaks of SiQ
0, SiQ
1, SiQ
2, SiQ
3, and SiQ
4 are 840–900 cm
−1, 900–950 cm
−1, 950–1030 cm
−1, 1030–1100 cm
−1, and 1100–1200 cm
−1, respectively. The Origin software was used to separate and fit the peaks of 800–1200 cm
−1, whereas the peaks area and RBO were calculated. The related peaks information are shown in
Figure 7 and
Table 4. It can be seen from
Table 4 that the content of SiQ
4 in BRM is higher than that in CBRM and Tailings. Combined with the XRD results, SiQ
4 in BRM represents the structure with more Si-O-Si (Al), which indicates that its crystal has a high degree of polymerization and stable chemical properties. The SiQ
4 of CBRM is lower than that of BRM, which indicates that some stable SiQ
4 structures in BRM are depolymerized and low degrees of polymerization substances are formed at 650 °C. It is worth noting that SiQ
4 of Tailings is significantly lower than that of CBRM, which indicates that bamboo powder promotes the depolymerization of SiQ
4 structure at 650 °C. The alkali system of cement hydration reaction is conducive to the dissolution of silicon and aluminum components in Tailings, which makes the pozzolanic activity of Tailings higher than that of BRM and CBRM. The results showed that the Si-O-Si (Al) bond of aluminosilicate in BRM is destroyed after being reduced by biomass, and the polymerization degree of aluminosilicate decreases, which leads to the increase of the active substance of silicon and aluminum in Tailings.
In summary, the diaspore in BRM will decompose into Al2O3 at about 500 °C, there is no corresponding characteristic peak in XRD of CBRM and Tailings, which indicates that alumina may be amorphous. In the range of 300–600 °C, Katoite and yugawaralite begin to remove part of the -OH and gradually transform into Chantalite and lawsonite. The free -OH of aluminosilicates in BRM is removed, which is conducive to the depolymerization of aluminosilicates and the reduction of polymerization degree of CRBM. Cancrinite in BRM begins to decompose into lime and amorphous aluminosilicate at about 650 °C. More importantly, the reduction gases of lime and H2 produced by pyrolysis of bamboo powder can transform all hematite in BRM into magnetite, and also promote the decomposition of aluminosilicate phase (cancrinite), which produces CaO and active silicon and aluminum. At the same time, the FTIR spectra of Tailings show that the Si-O bond and Al-O bond are broken, which indicate that the polymerization degree of aluminosilicate is reduced, and the pozzolanic activity of Tailings is improved.