Polyethylene Migration from Food Packaging on Cheese Detected by Raman and Infrared (ATR/FT-IR) Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
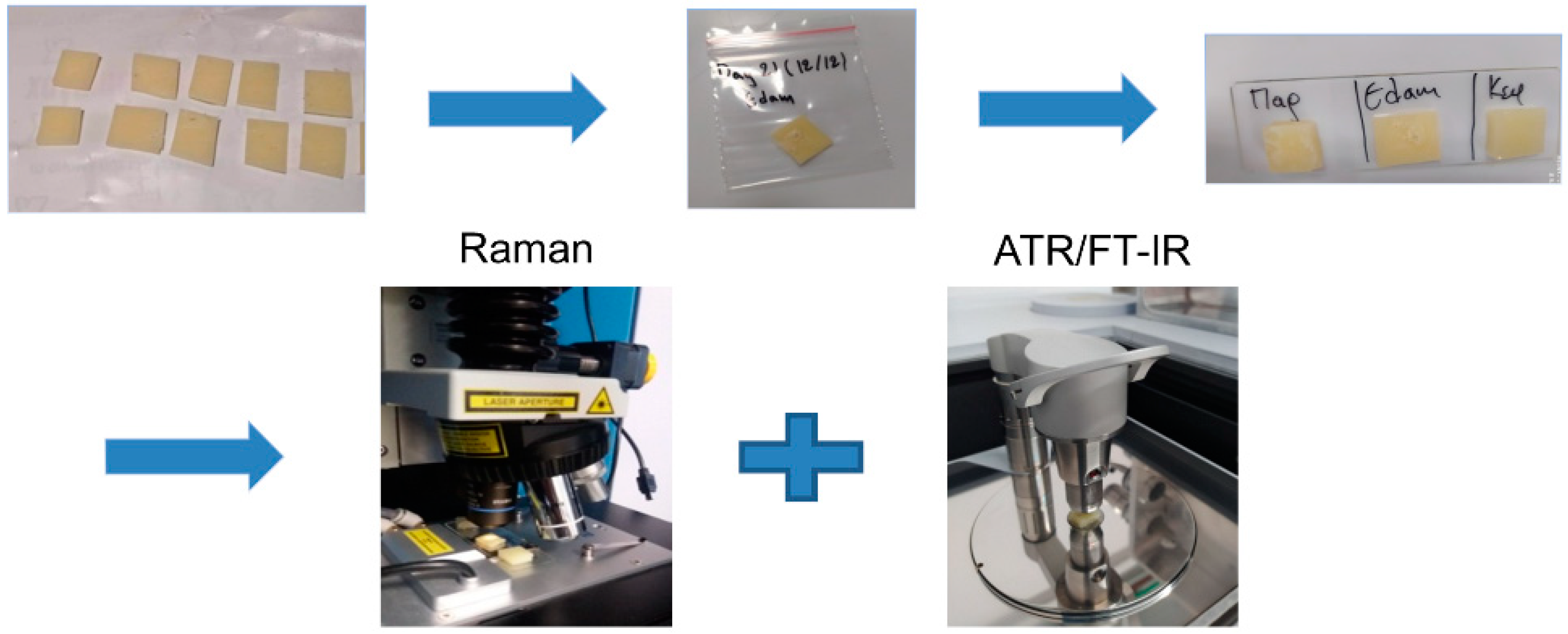
2.1. Samples and Experiment Preparation
2.2. Data Acquisition
2.2.1. Instrument Description and Acquisition Settings
LabRAM HR Raman Microscope
ATR/FT-IR
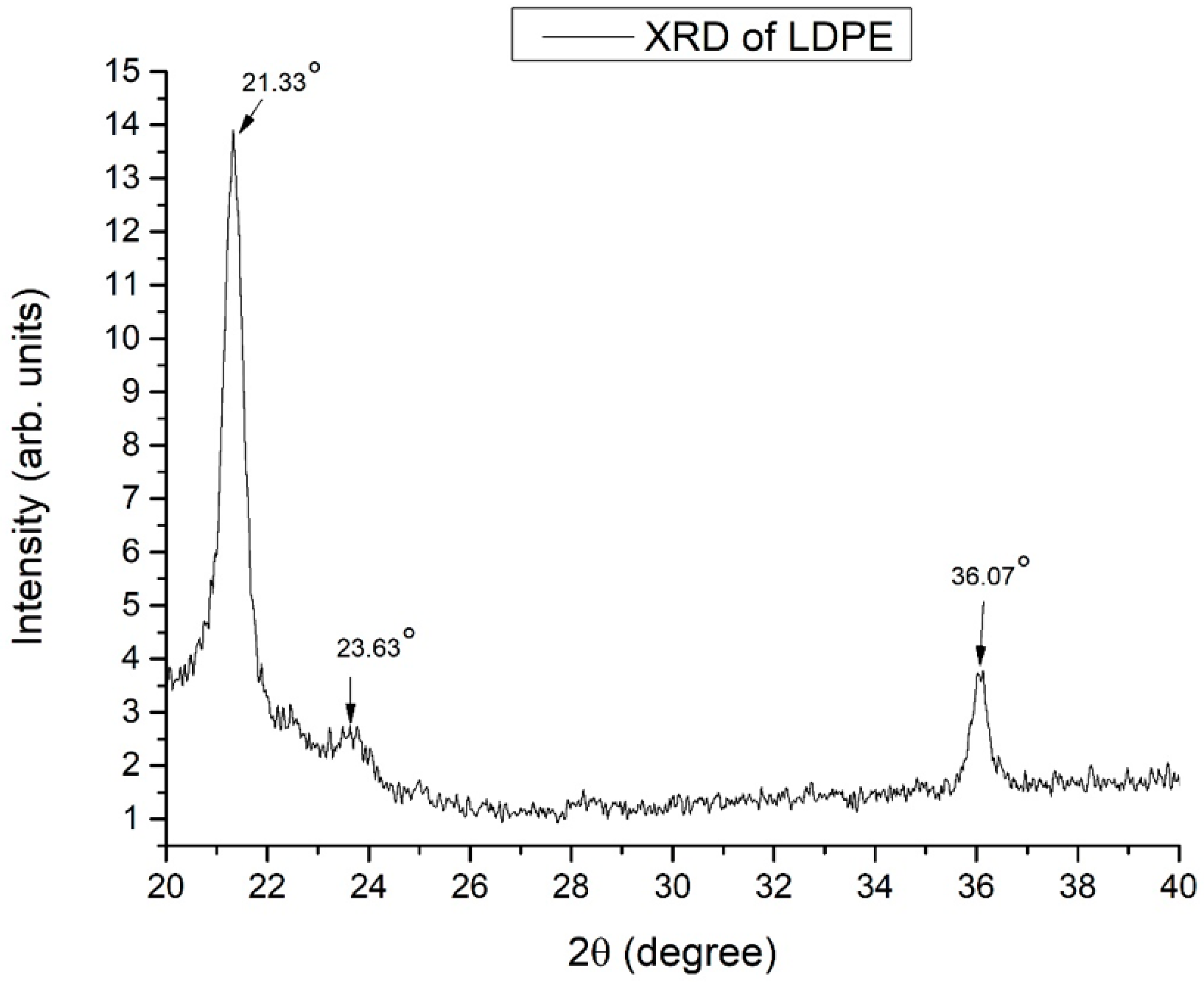
XRD
2.3. Data Processing and Analysis
2.3.1. Spectral References
Raman Spectral References
ATR/FT-IR Spectral References
XRD Spectral References
2.3.2. Spectra Processing and Analysis
Raman Spectra Processing and Analysis
ATR/FT-IR Spectra Processing and Analysis
3. Results and Discussion
3.1. Spectroscopic Analysis (Raman and ATR/FT-IR Spectroscopy)
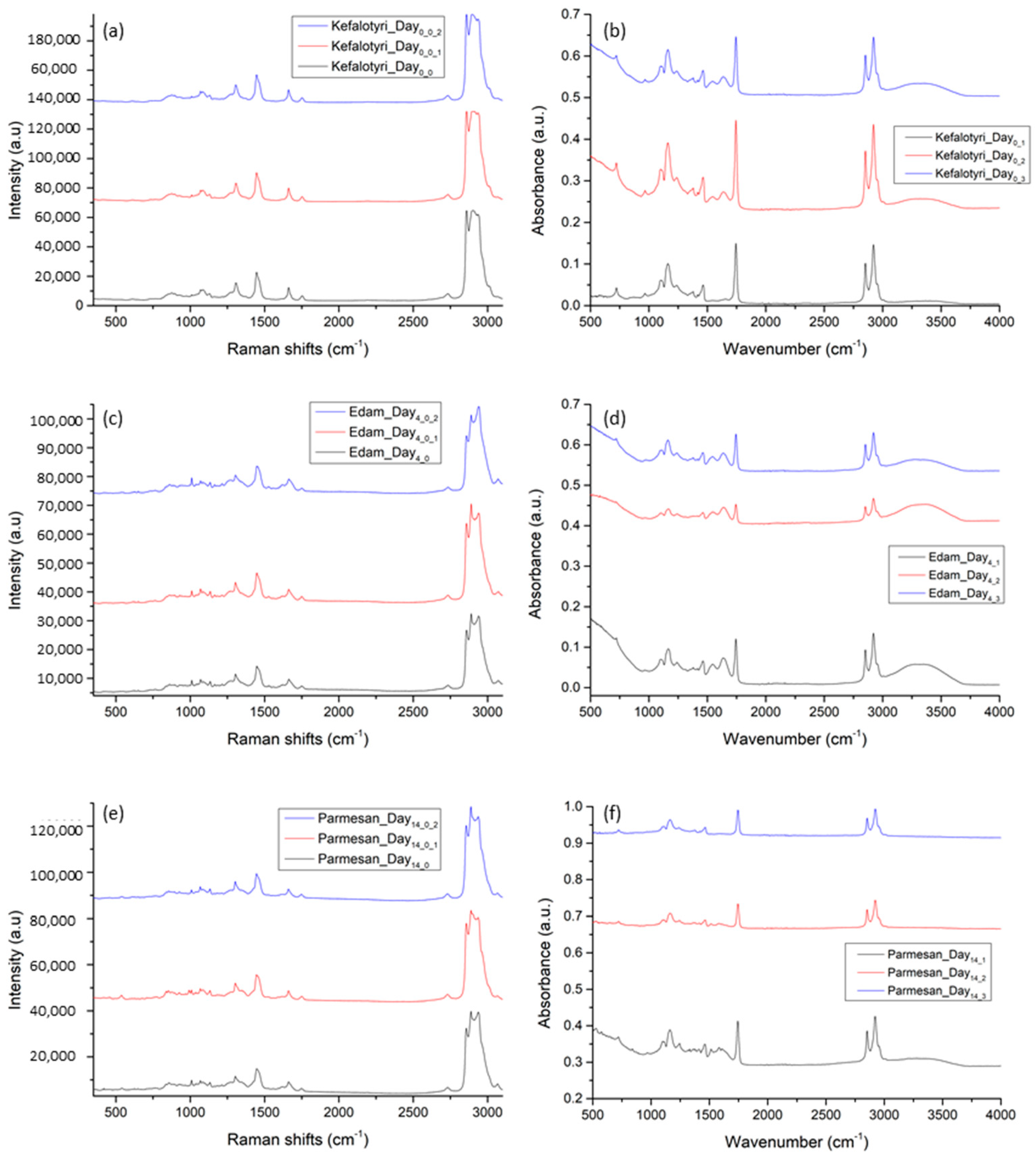
3.1.1. Consistency/Repeatability Tests
3.1.2. LDPE Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arvanitoyannis, I.S.; Bosnea, L. Migration of Substances from Food Packaging Materials to Foods. Crit. Rev. Food Sci. Nutr. 2004, 44, 63–76. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging Principles and Practice, 2nd ed.; CRC Press: Raton, FL, USA; Taylor and Francis Group: Raton, FL, USA, 2006. [Google Scholar]
- Bhunia, K.; Sablani, S.S.; Tang, J.; A Rasco, B. Migration of Chemical Compounds from Packaging Polymers during Microwave, Conventional Heat Treatment, and Storage. Compr. Rev. Food Sci. Food Saf. 2013, 12, 523–545. [Google Scholar] [CrossRef] [PubMed]
- Crompton, T. Additive Migration from Plastics into Food, 1st ed.; Elsevier: Oxford, UK, 2007. [Google Scholar]
- Mauricio-Iglesias, M.; Guillard, V.; Gontard, N.; Peyron, S. Application of FTIR and Raman microspectroscopy to the study of food/packaging interactions. Food Addit. Contam. Part A 2009, 26, 1515–1523. [Google Scholar] [CrossRef]
- Begley T, C.L.; Feigenbaum, A.; Franz, R.; Hinrichs, K.; Lickly, T.; Mercea, P.; Milana, M.; O’Brien, A.; Rebre, S.; Rijk, R.; et al. Evaluation of migration models that might be used in support of regulations for food contact plastics. Food Addit. Contam. 2005, 22, 73–90. [Google Scholar] [CrossRef] [Green Version]
- John, N.; Hahladakisa, C.A.; Velis, A.; Roland, W.; Eleni, I.; Phil, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Mater. 2018, 344, 179–199. [Google Scholar]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A.; Soltanzadeh, M.; Peressini, D. Migration analysis, antioxidant, and mechanical characterization of polypropylene-based active food packaging films loaded with BHA, BHT, and TBHQ. J. Food Sci. 2020, 85, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- Pax, A.; Ong, L.; Vongsvivut, J.; Tobin, M.J.; Kentish, S.E.; Gras, S.L. The characterisation of Mozzarella cheese microstructure using high resolution synchrotron transmission and ATR-FTIR microspectroscopy. Food Chem. 2019, 291, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, V.A.G.; De Quirós, A.R.B.; Losada, P.P.; Sendón, R. Identification of intentionally and non-intentionally added substances in plastic packaging materials and their migration into food products. Anal. Bioanal. Chem. 2018, 410, 3789–3803. [Google Scholar] [CrossRef] [PubMed]
- El-Ziney, M.G. Migration Levels of Monostyrene from Polystyrene Containers to Dairy Products. MOJ Food Process. Technol. 2016, 3. [Google Scholar] [CrossRef] [Green Version]
- Maia, J.; De Quirós, A.R.-B.; Sendón, R.; Cruz, J.M.; Seiler, A.; Franz, R.; Simoneau, C.; Castle, L.; Driffield, M.; Mercea, P.; et al. The Determination of Key Diffusion and Partition Parameters and Their Use in Migration Modelling of Benzophenone from Low Density Polyethylene (LDPE) into different Foodstuffs. Food Addit. Contam. Part A 2016, 33, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Burdikova, Z.; Svindrych, Z.; Hickey, C.; Wilkinson, M.G.; Auty, M.A.; Samek, O.; Bernatova, S.; Krzyzanek, V.; Periasamy, A.; Sheehan, J.J. Application of advanced light microscopic techniques to gain deeper insights into cheese matrix physico-chemistry. Dairy Sci. Technol. 2015, 95, 687–700. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.P.; Holroyd, S.E.; Reid, D.C.; Gordon, K.C.; Geoffrey, P.S.; Smith, S.E.H.; David, C.W.R.; Gordona, K.C. Raman imaging processed cheese and its components. J. Raman Spectrosc. 2016, 48, 374–383. [Google Scholar] [CrossRef]
- D’Incecco, P.; Limbo, S.; Faoro, F.; Hogenboom, J.; Rosi, V.; Morandi, S.; Pellegrino, L.M. New insight on crystal and spot development in hard and extra-hard cheeses: Association of spots with incomplete aggregation of curd granules. J. Dairy Sci. 2016, 99, 6144–6156. [Google Scholar] [CrossRef]
- AMECA. Chapter 10 Identifying Sensory Attributes for A Functional “Ideal Beverage” for Cirrhotic Patients. In Book of Proceedings Latin Food 2018; 8th Food Science, Biotechnology & Safety Congress: Puerto Vallarta, Jalisco, Mexico, 2018. [Google Scholar]
- de Sá Oliveira, K.; de Souza Callegaro, L.; Stephani, R.; Almeida, M.R.; de Oliveira, L.F.C. Analysis of spreadable cheese by Raman spectroscopy and chemometric tools. Food Chem. 2016, 194, 441–446. [Google Scholar] [CrossRef]
- Talari, A.C.S.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2015, 50, 46–111. [Google Scholar] [CrossRef]
- Lin, J.-H.; Pan, Y.-J.; Liu, C.-F.; Huang, C.-L.; Hsieh, C.-T.; Chen, C.-K.; Lin, Z.-I.; Lou, C.-W. Preparation and Compatibility Evaluation of Polypropylene/High Density Polyethylene Polyblends. Materials 2015, 8, 8850–8859. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, W.C.a.S. NIR, DSC, and FTlR as Quantitative Methods for Compositional Analysis of Blends of Polymers Obtained from Recycled Mixed Plastic Waste. Polym. Eng. Sci. 2001, 41, 1626–1635. [Google Scholar]
- Martin-del-Campoa, S.T.; Cosio-Ramirezb, D.P.R.; Corrieua, G. Middle infrared spectroscopy characterization of ripening stages of Camembert-type cheeses. Int. Dairy J. 2007, 17, 835–845. [Google Scholar] [CrossRef]
- Koca, N.; Rodriguez-Saona, L.; Harper, W.; Alvarez, V. Application of Fourier Transform Infrared Spectroscopy for Monitoring Short-Chain Free Fatty Acids in Swiss Cheese. J. Dairy Sci. 2007, 90, 3596–3603. [Google Scholar] [CrossRef]
- Nguyen, V.G.; Thai, H.; Mai, D.H.; Tran, H.T.; Tran, D.L.; Vu, M.T. Effect of titanium dioxide on the properties of polyethylene/TiO2 nanocomposites. Compos. Part B Eng. 2013, 45, 1192–1198. [Google Scholar] [CrossRef]
- Madani, M. Structure, optical and thermal decomposition characters of LDPE graft copolymers synthesized by gamma irradiation. Curr. Appl. Phys. 2011, 11, 70–76. [Google Scholar] [CrossRef]
- Marinkovic, F.S.; Popovic, D.M.; Jovanovic, J.D.; Stankovic, B.S.; Adnadjevic, B.K. Methods for quantitative determination of filler weight fraction and filler dispersion degree in polymer composites: Example of low-density polyethylene and NaA zeolite composite. Appl. Phys. A 2019, 125, 611. [Google Scholar] [CrossRef]
- Alsaygh, A.A.; Al-Hamidi, J.; Alsewailem, F.D.; Al-Najjar, I.M.; Kuznetsov, V.L. Characterization of polyethylene synthesized by zirconium single site catalysts. Appl. Petrochem. Res. 2014, 4, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Cooper, I.; Tice, P.A. Migration studies on fatty acid amide slip additives from plastics into food simulants. Food Addit. Contam. 1995, 12, 235–244. [Google Scholar] [CrossRef]
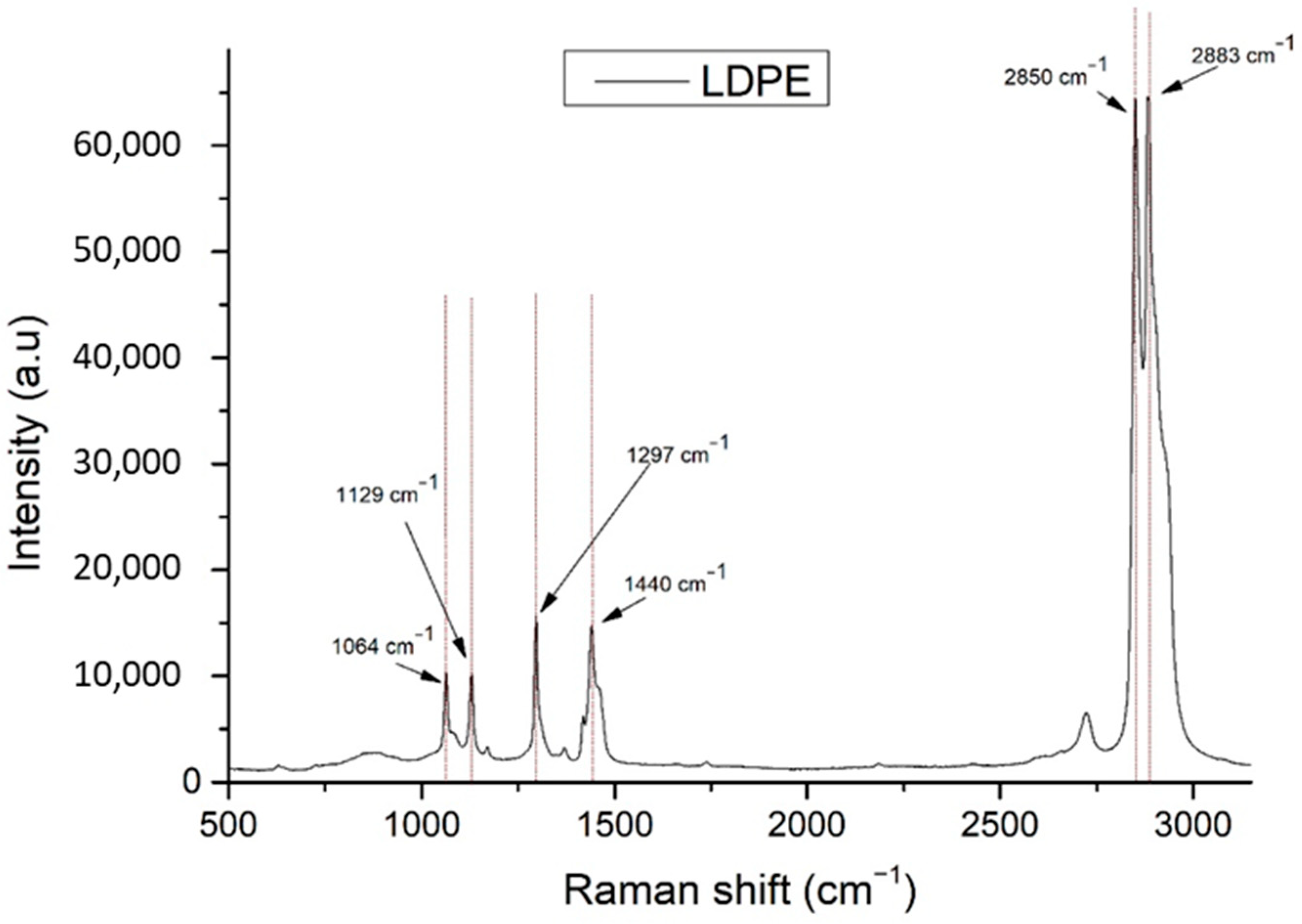
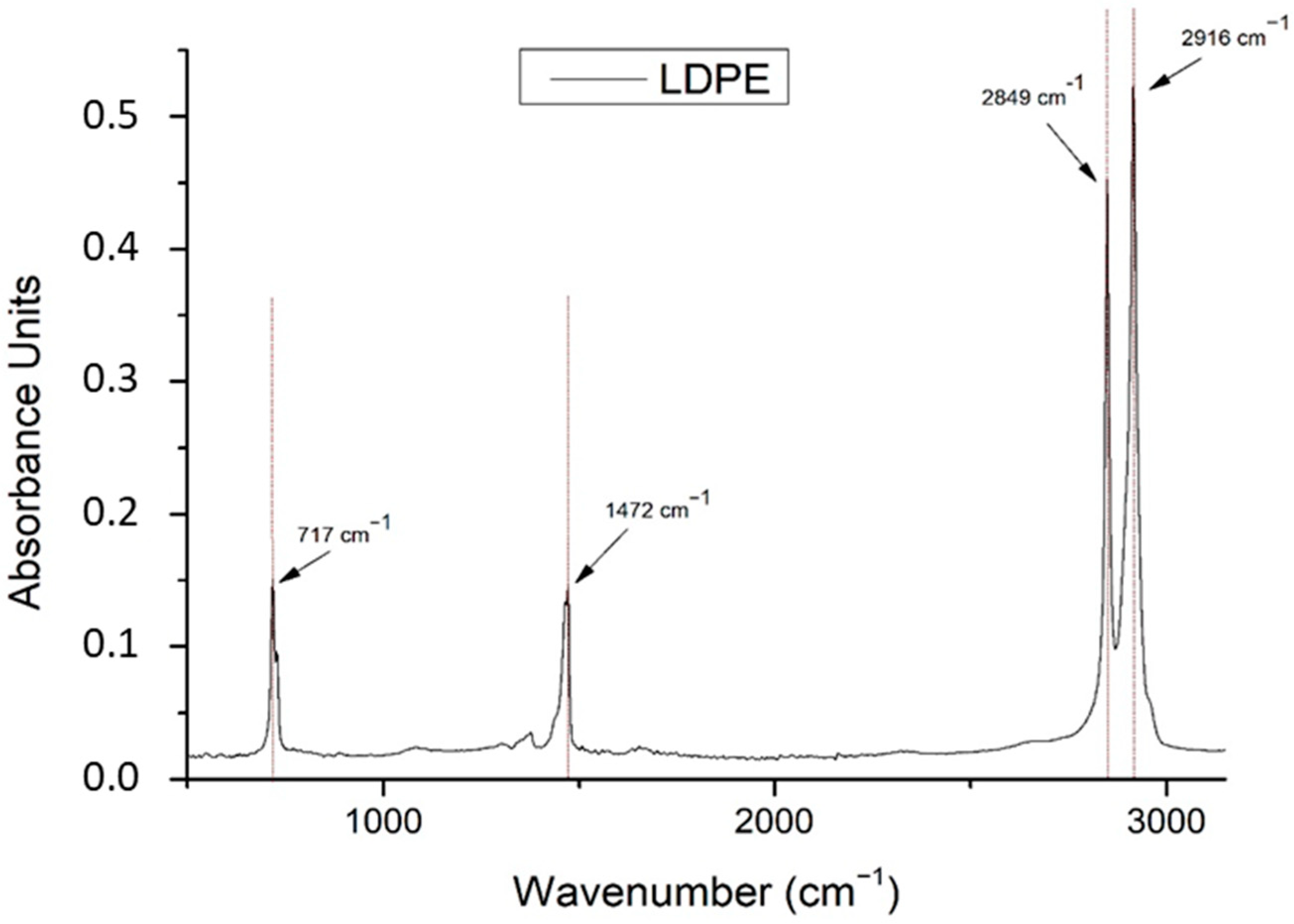
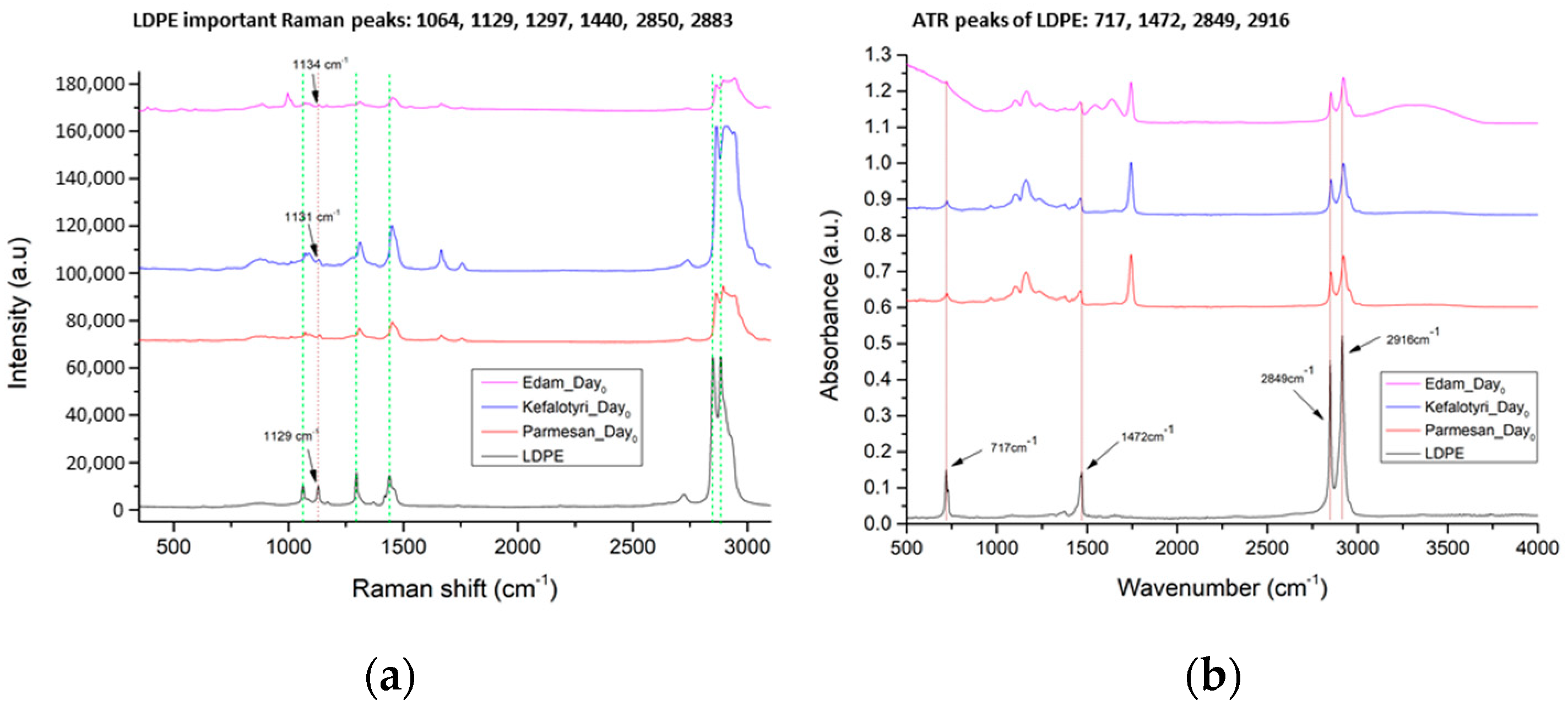
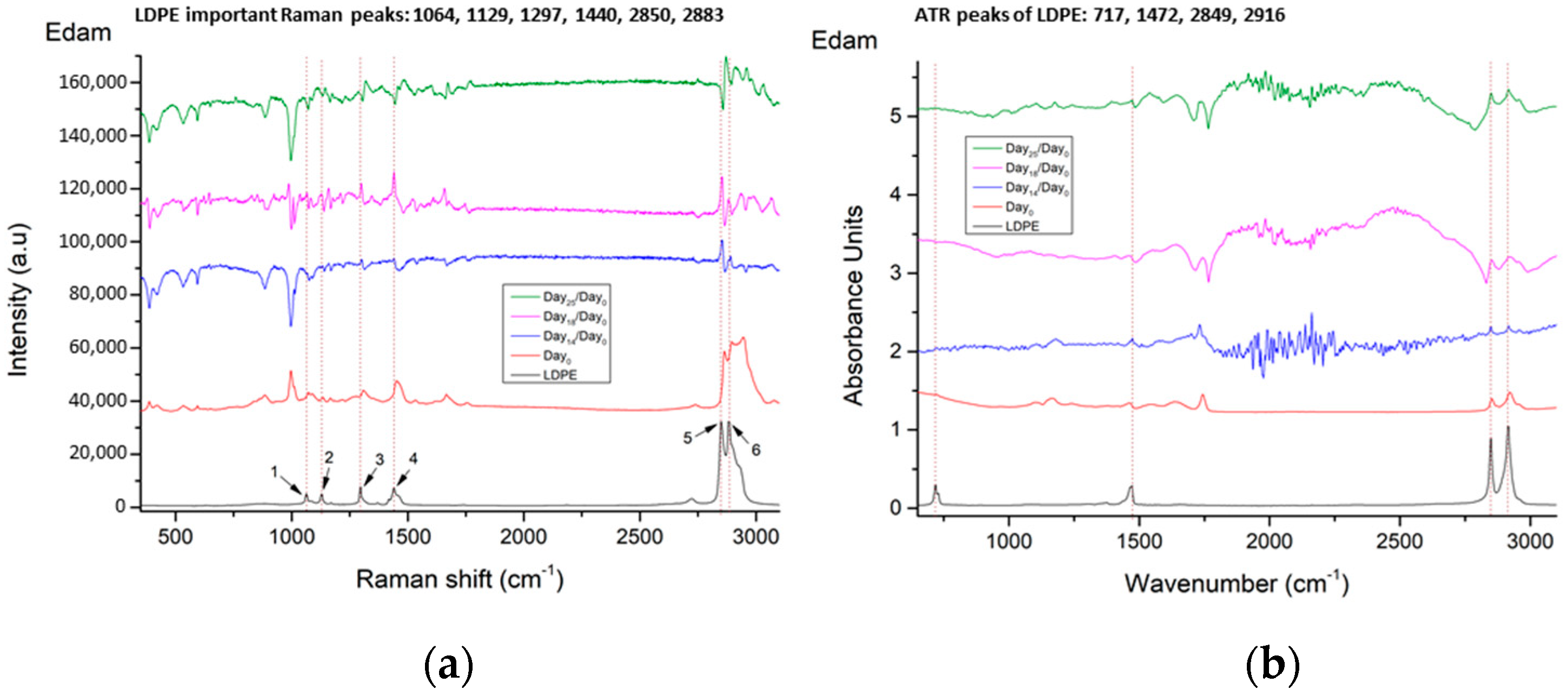
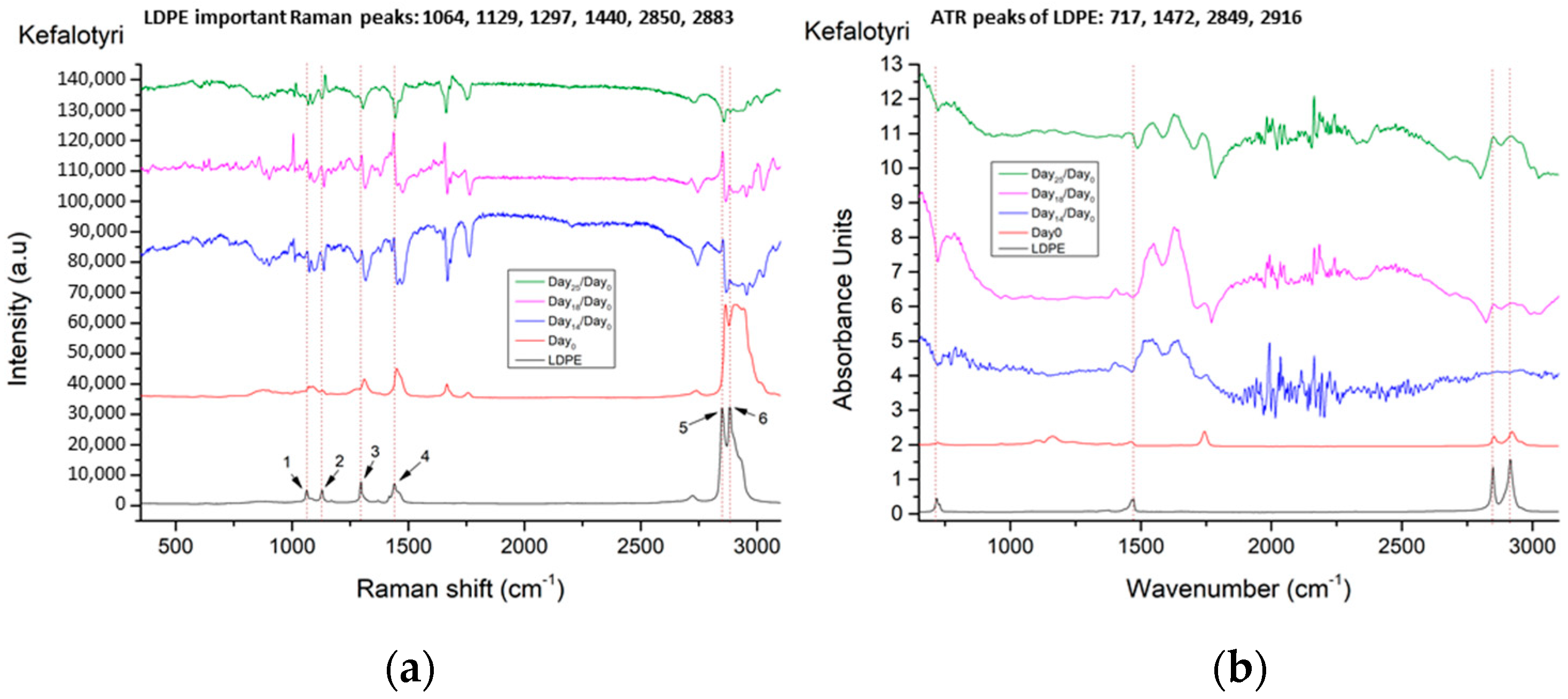
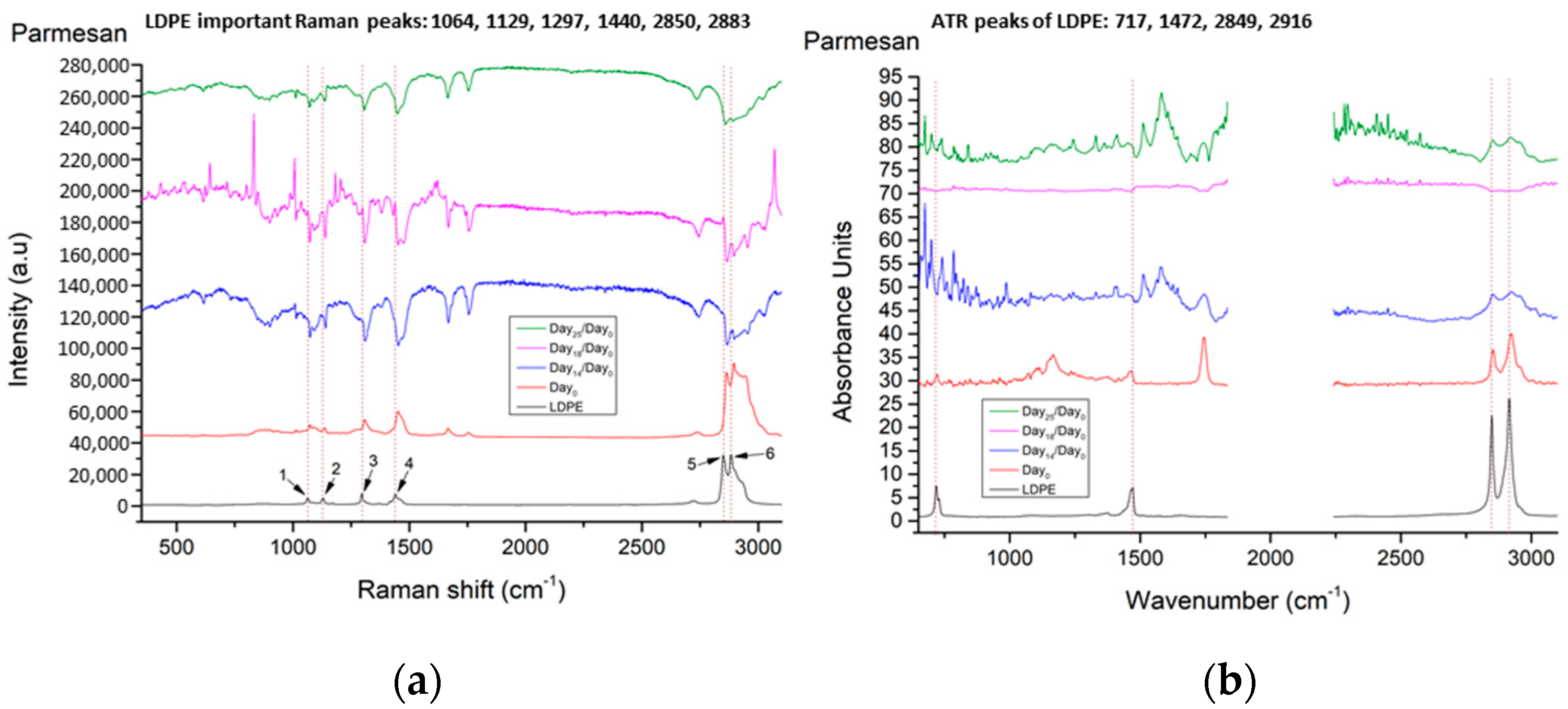
| No. | LDPE Major Raman Peaks (cm−1) | Raman Peak Assignments Found in Cheese Studies (cm−1) |
|---|---|---|
| 1 | 1064 | 1060 ➔ C-C skeletal stretching vibration out-of-plane [13] 1065 ➔ v (C-C) in fatty acids [14] |
| 2 | 1129 | 1125 ➔ C-C skeletal stretching vibration trans chain conformation in plane [13] 1127 ➔ v (C-C) of carotenoids [14] 1132 ➔ rocking of NH3+ in leucine [15] |
| 3 | 1297 | 1296 ➔ CH2 twist [13] 1301 ➔ CH2 twisting mode of phospholipids (phosphatidylcholine, phosphatidylinositol, phosphatidylserine) [14] 1303 ➔ fat band [14], amide III (protein structures) [16] |
| 4 | 1440 | 1440 ➔ deformation modes of the CH2 group (in lipids, carbohydrates, amino acids) [17] 1440,1441 ➔ large fat globule, fat band [14] 1442 ➔ CH2 scissoring from cholesterol [14] 1444 ➔ lipids [16] 1445 ➔ CH2 bend scissoring deformation, amide III [13] |
| 5 | 2850 | 2850 ➔ νs CH2, lipids, fatty acids, CH2 symmetric (especially for biological tissues) [18] 2852 ➔ vibrational modes of lipids [17] |
| 6 | 2883 | 2883 ➔ CH2 asymmetric stretch of lipids and proteins (especially for biological tissues) [18] 2880 ➔ vibrational modes of lipids [17] |
| No. | LDPE Major ATR/FT-IR Peaks (cm−1) [19,20] | ATR/FT-IR Peak Assignments in Cheese Bonds (cm−1) |
|---|---|---|
| 1 | 717 ➔ rocking vibration of -CH2 in LDPE | Does not exist in cheese samples |
| 2 | 1472 ➔ bending vibrations of -CH2 and -CH3 in LDPE | 950–1490 ➔ carbohydrates (lactose and monosaccharides) [21] 1199–1474 ➔ O-C-H, C-C-H, C-OH vibrational modes from sugars and organic acids [22] |
| 3 | 2849 ➔ stretching vibration of -CH in LDPE | 2850 ➔ C-H stretching of methylene groups [22] 2851 ➔ methylene band (CH2 in fat) [21] |
| 4 | 2916 ➔ stretching vibration of -CH2 in LDPE | 2920 ➔ C-H stretching of methylene groups [22] 2920 ➔ methylene band (CH2 in fat) [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsara, K.; Kenanakis, G.; Viskadourakis, Z.; Papadakis, V.M. Polyethylene Migration from Food Packaging on Cheese Detected by Raman and Infrared (ATR/FT-IR) Spectroscopy. Materials 2021, 14, 3872. https://doi.org/10.3390/ma14143872
Katsara K, Kenanakis G, Viskadourakis Z, Papadakis VM. Polyethylene Migration from Food Packaging on Cheese Detected by Raman and Infrared (ATR/FT-IR) Spectroscopy. Materials. 2021; 14(14):3872. https://doi.org/10.3390/ma14143872
Chicago/Turabian StyleKatsara, Klytaimnistra, George Kenanakis, Zacharias Viskadourakis, and Vassilis M. Papadakis. 2021. "Polyethylene Migration from Food Packaging on Cheese Detected by Raman and Infrared (ATR/FT-IR) Spectroscopy" Materials 14, no. 14: 3872. https://doi.org/10.3390/ma14143872
APA StyleKatsara, K., Kenanakis, G., Viskadourakis, Z., & Papadakis, V. M. (2021). Polyethylene Migration from Food Packaging on Cheese Detected by Raman and Infrared (ATR/FT-IR) Spectroscopy. Materials, 14(14), 3872. https://doi.org/10.3390/ma14143872









