Facile Synthesis of Uniform Mesoporous Nb2O5 Micro-Flowers for Enhancing Photodegradation of Methyl Orange
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nb2O5
2.2. Characterization Methods
2.3. Photocatalytic Activity Tests
3. Results and Discussion
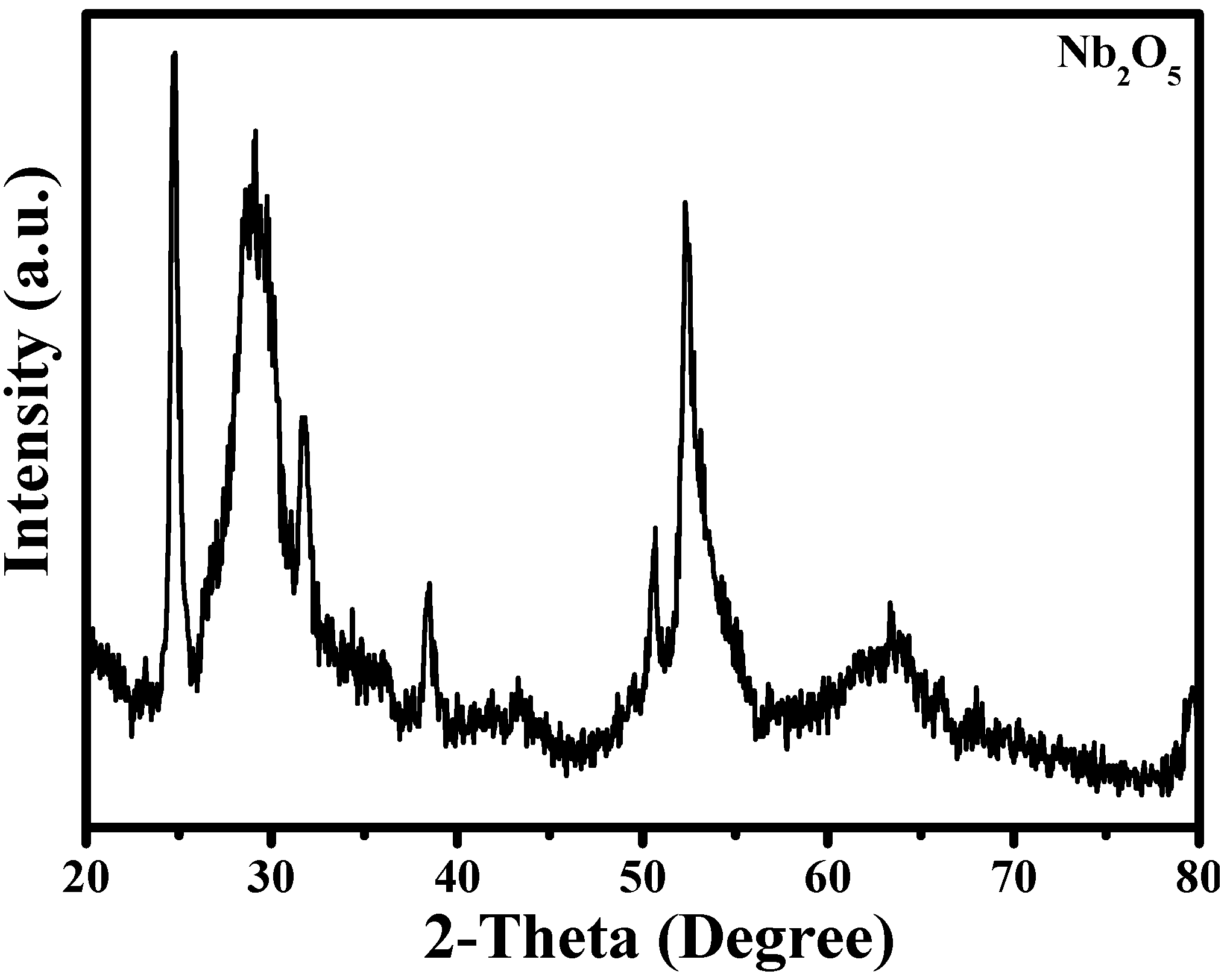
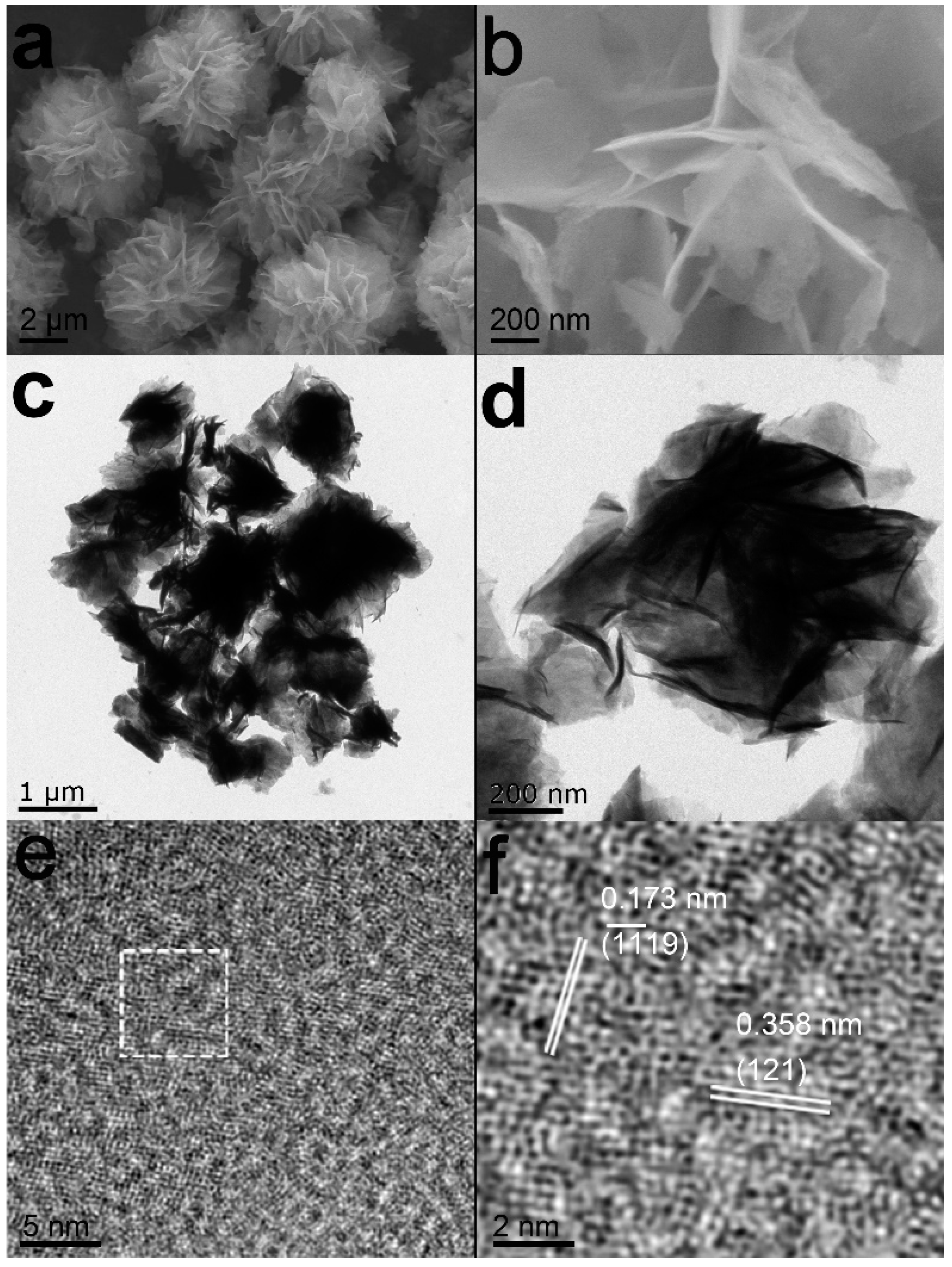
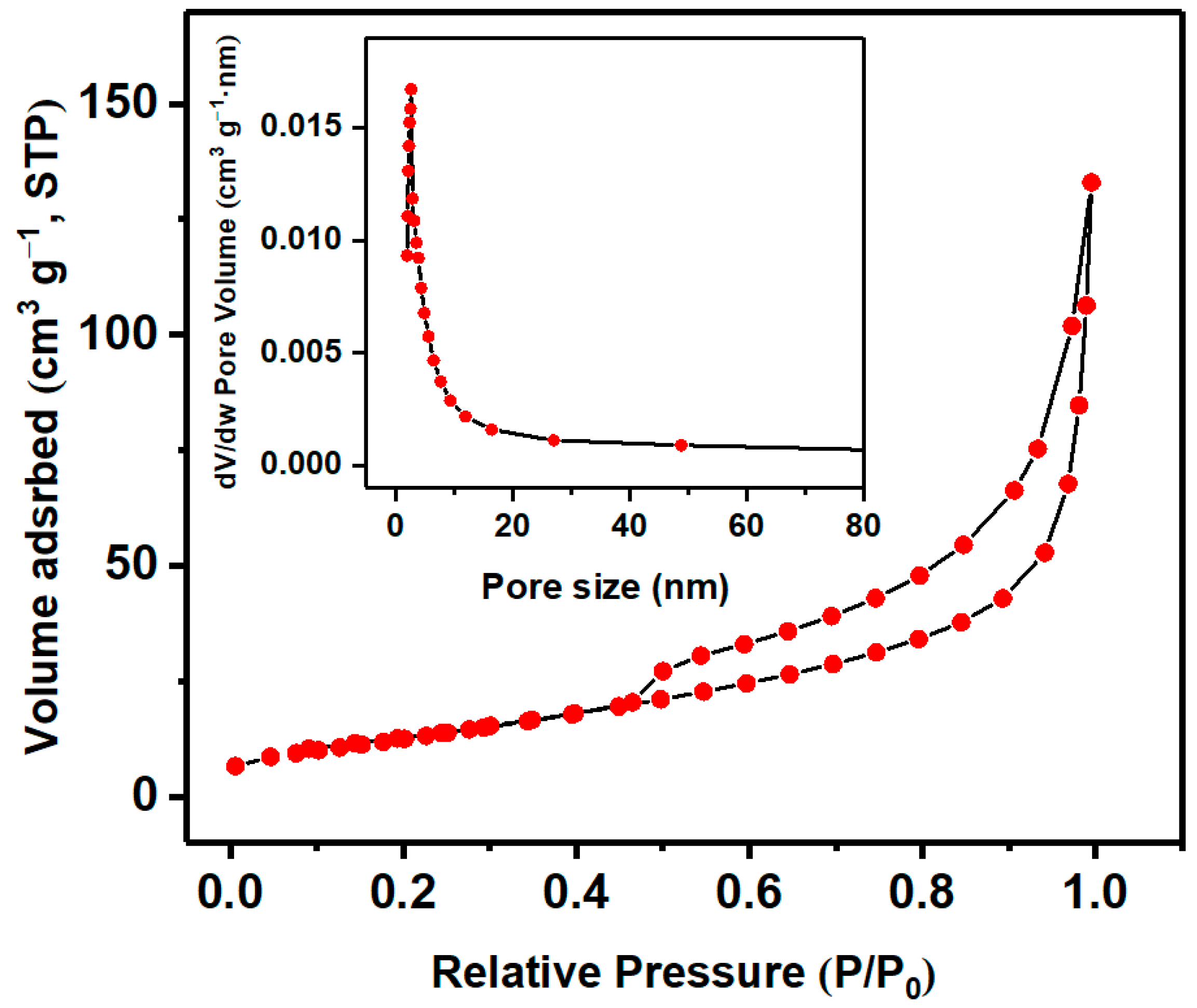
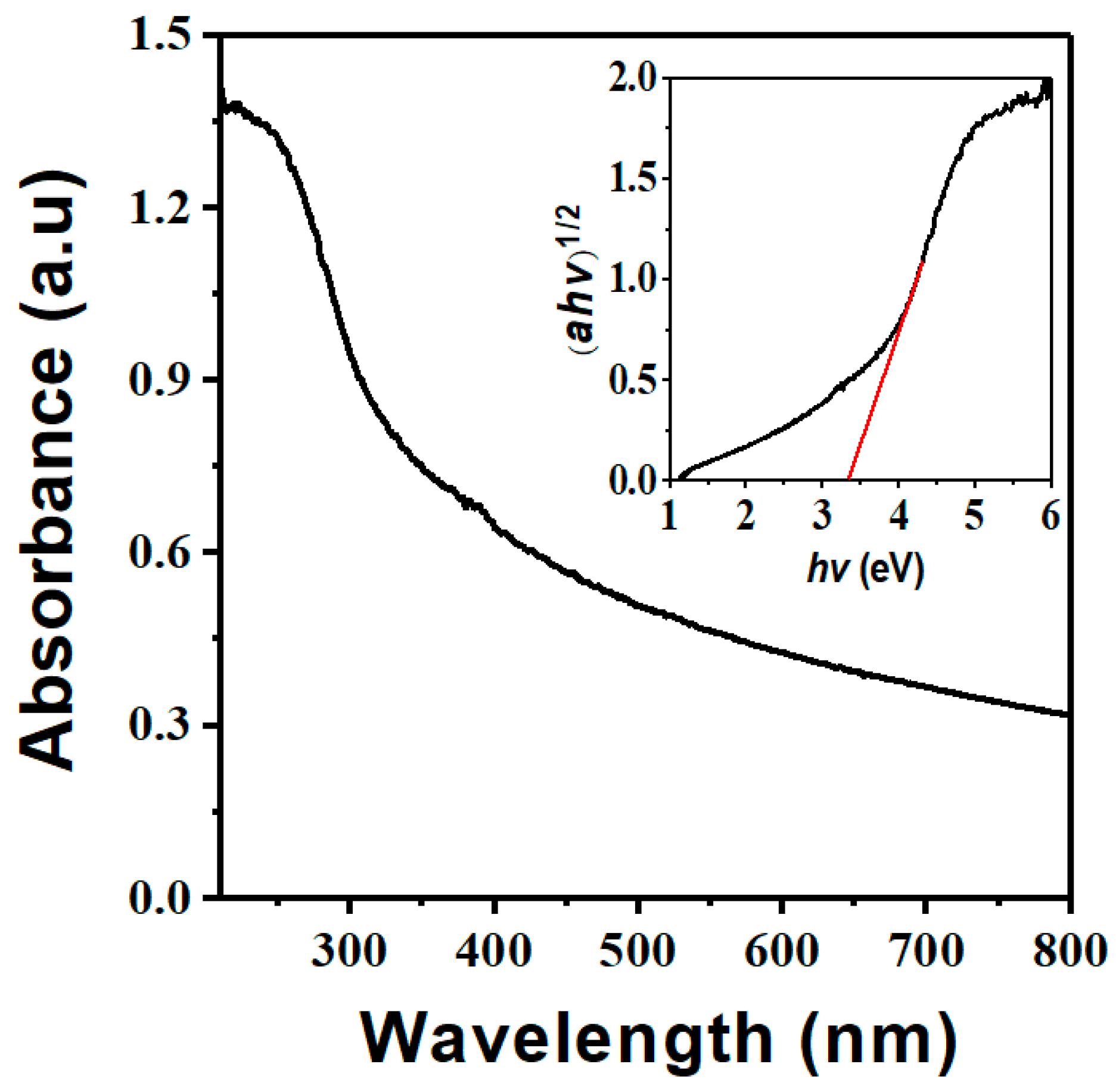
3.1. Characterization of Nb2O5 Micro-flowers
3.2. Photoactivity Evaluation of As-Prepared Materials
| Catalysts | Surface Area (m2 g−1) | Dye | Reaction Time (min) | Efficiency | Reference |
|---|---|---|---|---|---|
| Rod-like Nb2O5 | / | Methylene Blue | 150 | 95% | [25] |
| Nb2O5 (N-100) | 2.445 | Methylene Blue | 270 | 55% | [29] |
| H-Nb2O5 hollow fibers | 32.8 | Methylene orange | 50 | 93% | [28] |
| Sphere-like Nb2O5 | 104.3 | Methylene blue | 50 | 87% | [22] |
| Rose bengal | 180 | 62% | |||
| Hexagonal-like Nb2O5 nanoplates | 31.7 | Methylene blue | 60 | 92% | [23] |
| Rhdamine B | 60 | 98% | |||
| Micro-flowers Nb2O5 (this work) | 48.6 | Methylene orange | 20 | 99.9% | This work |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandes, J.V.; Rodrigues, A.M.; Menezes, R.R.; Neves, G.d.A. Adsorption of anionic dye on the acid-functionalized bentonite. Materials 2020, 13, 3600. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Moreirinha, C.; Almeida, A.; Silvestre, A.J.D.; Freire, C.S.R. Zwitterionic nanocellulose-based membranes for organic dye removal. Materials 2019, 12, 1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Shi, Z.H.; Peng, L.; He, W.M.; Li, B.L.; Li, K.Z. Preparation of carbon foam-loaded nano-TiO2 photocatalyst and its degradation on methyl orange. Surf. Interface 2017, 7, 116–124. [Google Scholar] [CrossRef]
- Arslan, I. Treatability of a simulated disperse dye-bath by ferrous iron coagulation, ozonation, and ferrous iron-catalyzed ozonation. J. Hazard. Mater. 2001, 85, 229–241. [Google Scholar] [CrossRef]
- Sun, T.; Gao, J.; Shi, H.Y.; Han, D.; Zangeneh, M.M.; Liu, N.; Liu, H.; Guo, Y.H.; Liu, X.Q. Decorated Au NPs on agar modified Fe3O4 NPs: Investigation of its catalytic performance in the degradation of methylene orange, and anti-human breast carcinoma properties. Int. J. Biol. Macromol. 2020, 165, 787–795. [Google Scholar] [CrossRef]
- Bai, L.M.; Wang, S.; Wang, Z.Y.; Hong, E.L.; Wang, Y.; Xia, C.H.; Wang, B.Q. Kinetics and mechanism of photocatalytic degradation of methyl orange in water by mesoporous Nd-TiO2-SBA-15 nanocatalyst. Environ. Pollut. 2019, 248, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Z.; Chen, J.; Huang, Y.N.; Zhang, H.J.; Qiu, H.D. Enhanced photocatalytic degradation of methyl orange by porous graphene/ZnO nanocomposite. Environ. Pollut. 2019, 249, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.J.; Lin, Y.; Pan, B.L.; Fan, W.J.; Huang, Y.C. Photocatalytic degradation of methyl orange by BiOI/Bi4O5I2 microspheres under visible light irradiation. Inorg. Chem. Commun. 2018, 93, 65–68. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wu, Q.; Guo, C.; Wu, Y.; Wu, T.H. Photocatalytic selective oxidation of 5-Hydroxymethylfurfural to 2,5-Diformylfuran over Nb2O5 under visible Light. ACS Sustain. Chem. Eng. 2017, 5, 3517–3523. [Google Scholar] [CrossRef]
- Pi, Y.H.; Li, X.Y.; Xia, Q.B.; Wu, J.L.; Li, Y.W.; Xiao, J.; Li, Z. Adsorptive and photocatalytic removal of persistent organic pollutants (POPs) in water by metal-organic frameworks (MOFs). Chem. Eng. J. 2018, 337, 351–371. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, Y.; Liu, P.; Chou, S.L.; Wang, J.Z.; Liu, H.W.; Wang, G.Z.; Zhao, H.J. Highly ordered single crystalline nanowire array assembled three-dimensional Nb3O7(OH) and Nb2O5 superstructures for energy storage and conversion applications. ACS Nano 2016, 10, 507–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.J.; Zhang, G.Y.; Yan, H.; Liu, Y.B.; Chen, X.B.; Feng, X.; Jin, X.; Yang, C.H. Liquid-phase epoxidation of light olefins over W and Nb nanocatalysts. ACS Sustain. Chem. Eng. 2018, 6, 4423–4452. [Google Scholar] [CrossRef]
- Liang, F.; Liu, J.X. Sintering microstructure and electricity properties of ITO targets with Bi2O3–Nb2O5 addition. Ceram. Int. 2017, 43, 5856–5861. [Google Scholar] [CrossRef]
- Zarrin, S.; Heshmatpour, F. Photocatalytic activity of TiO2/Nb2O5/PANI and TiO2/Nb2O5/RGO as new nanocomposites for degradation of organic pollutants. J. Hazard. Mater. 2018, 351, 147–159. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, T.; Zhu, W.Q.; Lian, J.B.; Huang, Y.P.; Yu, Y.Y.; Qiu, J.X.; Zhao, Y.; Yong, Y.C.; Li, H.M. Controllable synthesis of uniform mesoporous H-Nb2O5/rGO nanocomposites for advanced lithium ion hybrid supercapacitors. J. Mater. Chem. A 2019, 7, 693–703. [Google Scholar] [CrossRef]
- Lopes, O.F.; Paris, E.C.; Ribeiro, C. Synthesis of Nb2O5 nanoparticles through the oxidant peroxide method applied to organic pollutant photodegradation: A mechanistic study. Appl. Catal. B 2014, 144, 800–808. [Google Scholar] [CrossRef]
- Luo, H.L.; Song, W.J.; Hoertz, P.G.; Hanson, K.; Ghosh, R.; Rangan, S.; Brennaman, M.K.; Concepcion, J.J.; Binstead, R.A.; Bartynski, R.A.; et al. A sensitized Nb2O5 photoanode for hydrogen production in a dye-Sensitized photoelectrosynthesis cell. Chem. Mater. 2013, 25, 122–131. [Google Scholar] [CrossRef]
- de Carvalho, G.S.G.; de Siqueira, M.M.; do Nascimento, M.P.; de Oliveira, M.A.L.; Amarante, G.W. Nb2O5 supported in mixed oxides catalyzed mineralization process of methylene blue. Heliyon 2020, 6, e04128. [Google Scholar] [CrossRef]
- Liu, X.D.; Liu, G.Y.; Chen, H.; Ma, J.M.; Zhang, R.X. Facile synthesis of Nb2O5 nanobelts assembled from nanorods and their applications in lithium ion batteries. J. Phys. Chem. Solids 2017, 111, 8–11. [Google Scholar] [CrossRef]
- Ruwer, T.L.; Venturini, J.; Khan, S.; Bergmann, C.P. Quick synthesis of homogeneous Nb2O5 nanorod arrays via a microwave-assisted hydrothermal method. Mater. Lett. 2020, 265, 127429. [Google Scholar] [CrossRef]
- Wei, W.; Lee, K.Y.; Shaw, S.; Schmuki, P. Anodic formation of high aspect ratio, self-ordered Nb2O5 nanotubes. Chem. Commun. 2012, 48, 4244–4246. [Google Scholar] [CrossRef]
- Rathnasamy, R.; Thangasamy, P.; Aravindhan, V.; Sathyanarayanan, P.; Alagan, V. Facile one-pot solvothermal-assisted synthesis of uniform sphere-like Nb2O5 nanostructures for photocatalytic applications. Res. Chem. Intermed. 2019, 45, 3571–3584. [Google Scholar] [CrossRef]
- Liu, H.; Gao, N.; Liao, M.Y.; Fang, X.S. Hexagonal-like Nb2O5 nanoplates-based photodetectors and photocatalyst with high performances. Sci. Rep. 2015, 5, 7716–7724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Eley, C.; Hu, J.P.; Foord, J.S.; Ye, L.; He, H.Y.; Tsang, S.C.E. Shape-dependent acidity and photocatalytic activity of Nb2O5 nanocrystals with an active TT (001) surface. Angew. Chem. 2012, 124, 3912–3915. [Google Scholar] [CrossRef]
- Du, L.; Long, Z.Y.; Wen, H.M.; Ge, W.L.; Zhou, Y.; Wang, J. (Ionic liquid)-derived morphology control of Nb2O5 materials and their photocatalytic properties. Cryst. Eng. Comm. 2014, 16, 9096–9103. [Google Scholar] [CrossRef]
- Qi, S.S.; Zuo, R.Z.; Liu, Y.; Wang, Y. Synthesis and photocatalytic activity of electrospun niobium oxide nanofibers. Mater. Res. Bull. 2013, 48, 1213–1217. [Google Scholar] [CrossRef]
- Sayilkan, F.; Erdemoglu, S.; Asilturk, M.; Akarsu, M.; Sener, S.; Sayilkan, H.; Erdemoglu, M.; Arpac, E. Photocatalytic performance of pure anatase nanocrystallite TiO2 synthesized under low temperature hydrothermal conditions. Mater. Res. Bull. 2006, 41, 2276–2285. [Google Scholar] [CrossRef]
- Shao, R.Y.; Cao, Z.Z.; Xiao, Y.; Dong, H.J.; He, W.Y.; Gao, Y.F.; Liu, J.R. Enhancing photocatalytic activity by tuning the ratio of hexagonal and orthorhombic phase Nb2O5 hollow fibers. RSC Adv. 2014, 4, 26447–26451. [Google Scholar] [CrossRef]
- Kumari, N.; Gaurav, K.; Samdarshi, S.K.; Bhattacharyya, A.S.; Paul, S.; Rajbongshi, B.M.; Mohanty, K. Dependence of photoactivity of niobium pentoxide (Nb2O5) on crystalline phase and electrokinetic potential of the hydrocolloid. Sol. Energy Mater. Sol. Cells 2020, 208, 110408–110416. [Google Scholar] [CrossRef]
- Macedo, E.R.; Oliveira, P.S.; De Oliveira, H.P. Synthesis and characterization of branched polypyrrole/titanium dioxide photocatalysts. J. Photochem. Photobiol. A Chem. 2015, 307–308, 108–114. [Google Scholar] [CrossRef]
- de Moraes, N.P.; Silva, F.N.; da Silva, M.L.C.P.; Campos, T.M.B.; Thim, G.P.; Rodrigues, L.A. Methylene blue photodegradation employing hexagonal prism-shaped niobium oxide as heterogeneous catalyst: Effect of catalyst dosage, dye concentration, and radiation source. Mater. Chem. Phys. 2018, 214, 95–106. [Google Scholar] [CrossRef]
- Khan, S.U.; Hussain, S.; Perini, J.A.L.; Khan, H.; Khan, S.; Zanoni, M.V.B. Self-doping of Nb2O5 NC by cathodic polarization for enhanced conductivity properties and photoelectrocatalytic performance. Chemosphere 2021, 272, 129880–129887. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Yu, T.; Fan, X.X.; Zhang, H.T.; Li, Z.S.; Ye, J.H.; Zou, Z.G. Enhanced activity of mesoporous Nb2O5 for photocatalytic hydrogen production. Appl. Surf. Sci. 2007, 253, 8500–8506. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Yan, F.; Li, D.-F.; Xi, Y.-F.; Cao, R.; Zheng, J.-F.; Shao, Y.; Jin, S.; Chen, J.-Z.; Zhou, X.-S. Enhanced Gating Performance of Single-Molecule Conductance by Heterocyclic Molecules. J. Phys. Chem. Lett. 2021, 12, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.Z.; Wang, J.C.; Wang, P.P.; Yao, H.C.; Li, Z.J. In-situ growth of mesoporous Nb2O5 microspheres on g-C3N4 nanosheets for enhanced photocatalytic H2 evolution under visible light irradiation. Int. J. Hydrog. Energy 2017, 42, 6683–6694. [Google Scholar] [CrossRef]
- Hong, Y.Z.; Li, C.S.; Zhang, G.; Meng, Y.D.; Yin, B.X.; Zhao, Y.; Shi, W.D. Efficient and stable Nb2O5 modified g-C3N4 photocatalyst for removal of antibiotic pollutant. Chem. Eng. J. 2016, 299, 74–84. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.-P.; Xie, H.-Q.; Wang, Y.-H.; Yu, L.; Wang, F.-Y.; Chen, H.-S.; Fei, Z.-X.; Bian, C.-Q.; Mao, H.; Lian, J.-B. Facile Synthesis of Uniform Mesoporous Nb2O5 Micro-Flowers for Enhancing Photodegradation of Methyl Orange. Materials 2021, 14, 3783. https://doi.org/10.3390/ma14143783
Qiu J-P, Xie H-Q, Wang Y-H, Yu L, Wang F-Y, Chen H-S, Fei Z-X, Bian C-Q, Mao H, Lian J-B. Facile Synthesis of Uniform Mesoporous Nb2O5 Micro-Flowers for Enhancing Photodegradation of Methyl Orange. Materials. 2021; 14(14):3783. https://doi.org/10.3390/ma14143783
Chicago/Turabian StyleQiu, Jian-Ping, Huan-Qing Xie, Ya-Hao Wang, Lan Yu, Fang-Yuan Wang, Han-Song Chen, Zheng-Xin Fei, Chao-Qun Bian, Hui Mao, and Jia-Biao Lian. 2021. "Facile Synthesis of Uniform Mesoporous Nb2O5 Micro-Flowers for Enhancing Photodegradation of Methyl Orange" Materials 14, no. 14: 3783. https://doi.org/10.3390/ma14143783






