Durability Study on High-Performance Fiber-Reinforced Mortar under Simulated Wastewater Pipeline Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Curing Environments
2.3. Test Procedures
3. Results and Discussion
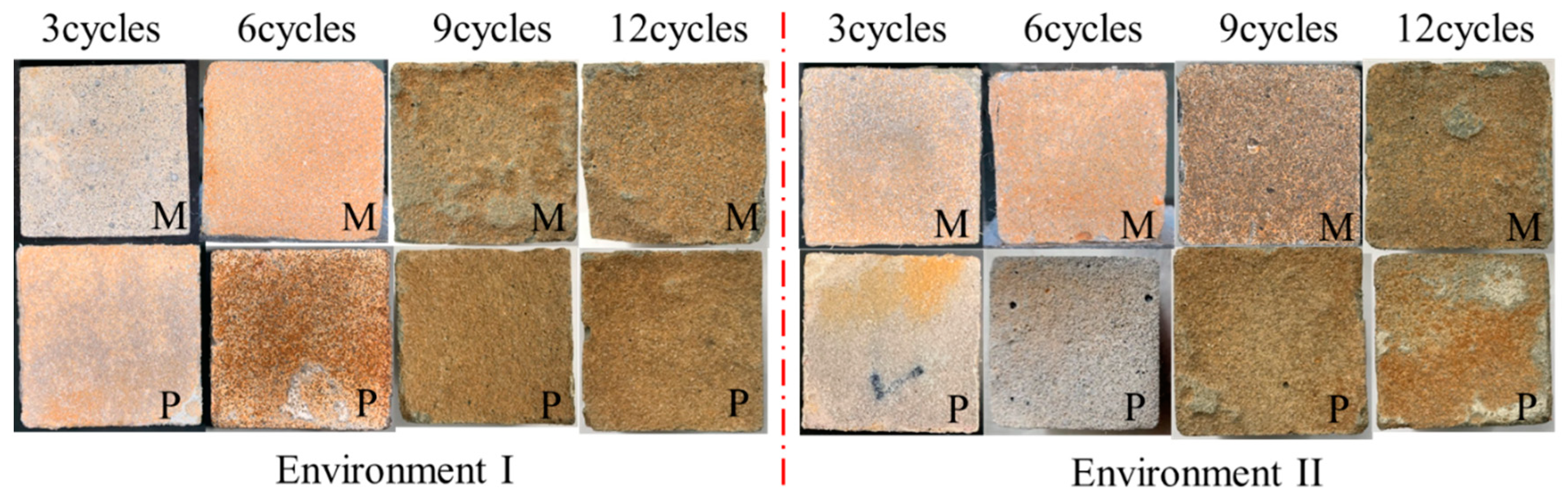
3.1. Visual Appearance and Height Loss
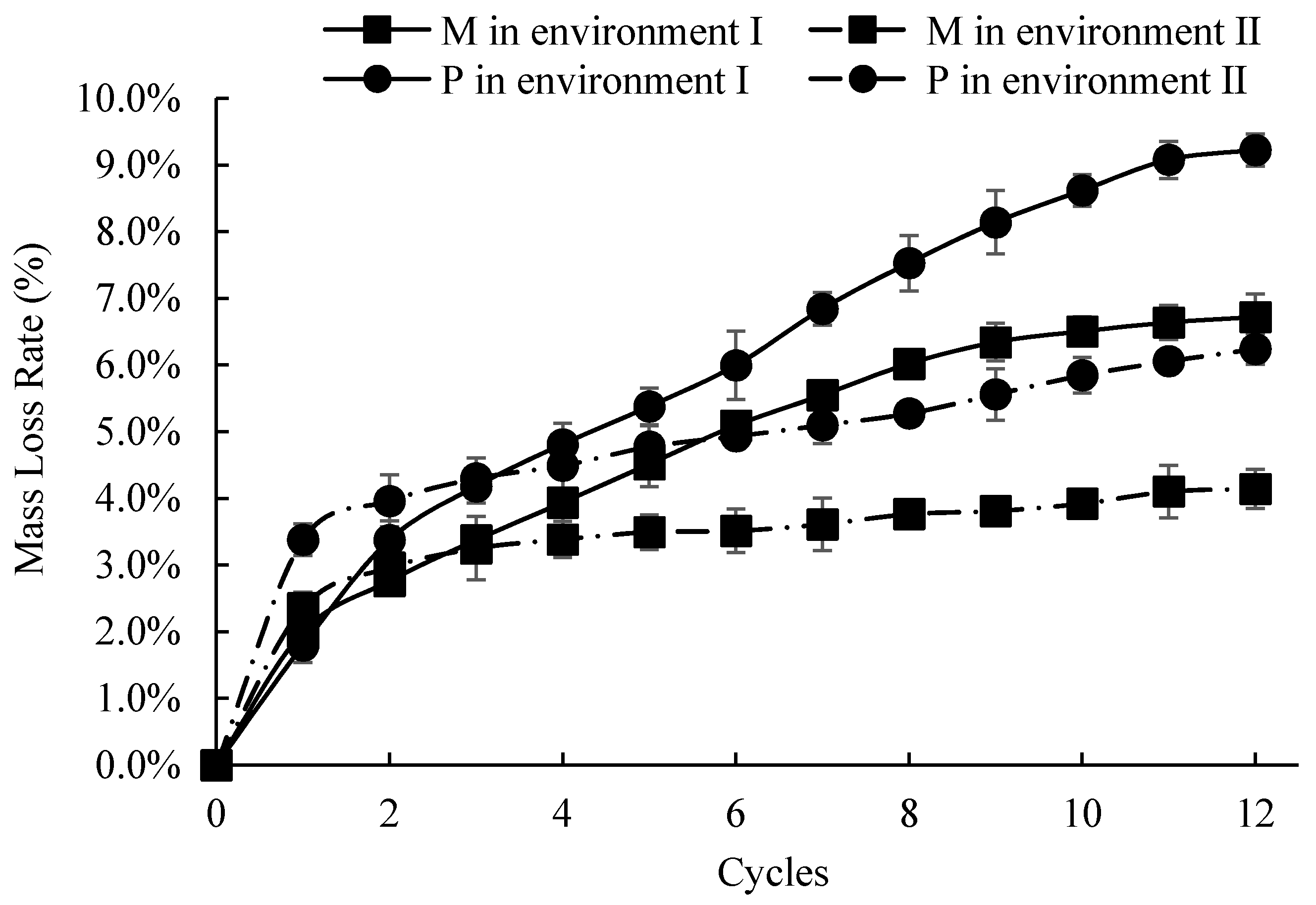
3.2. Mass Loss Ratio
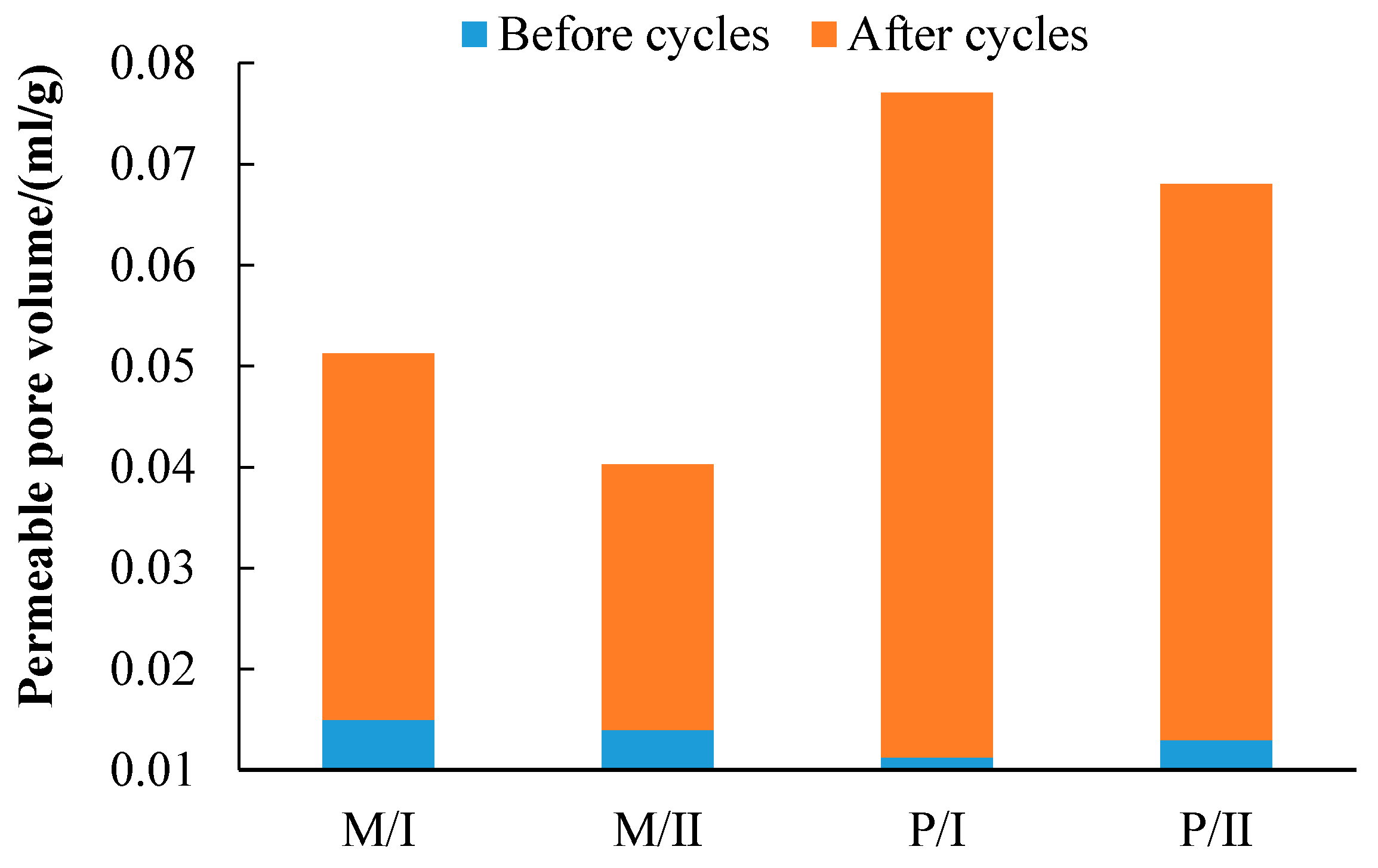
3.3. The Permeable Pore Volume
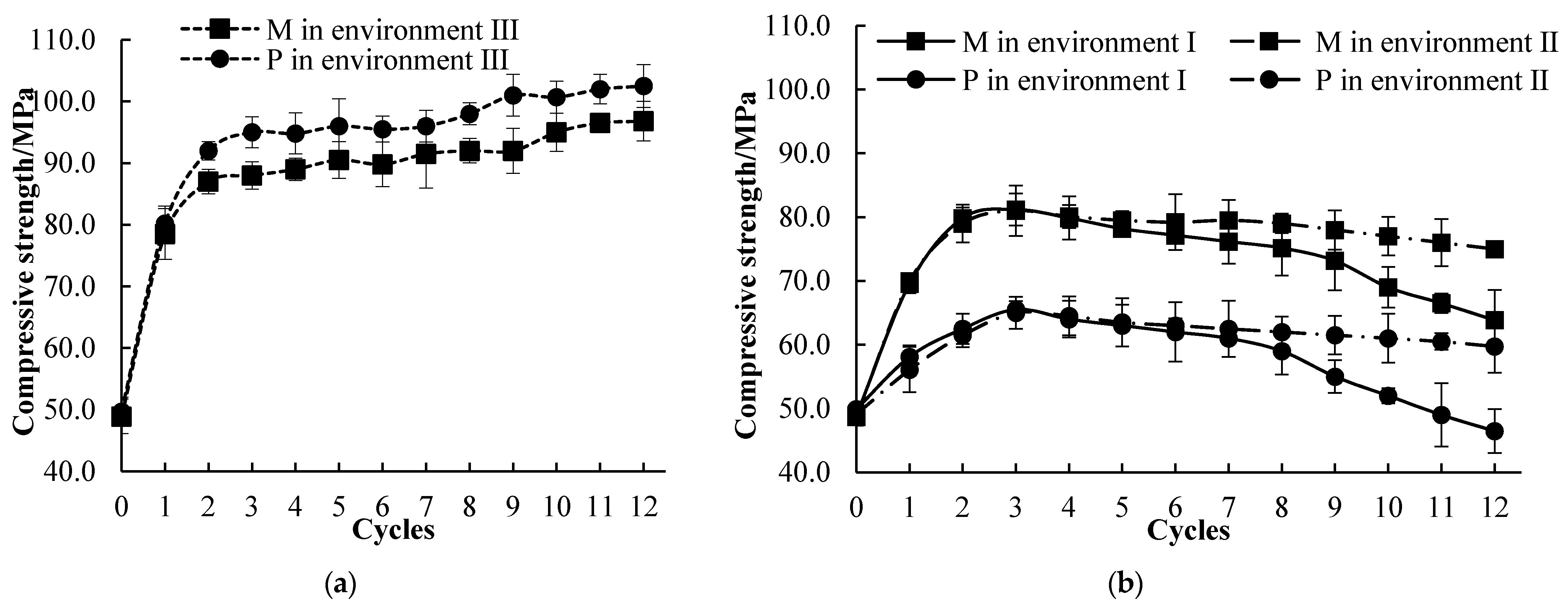
3.4. Compressive Strength
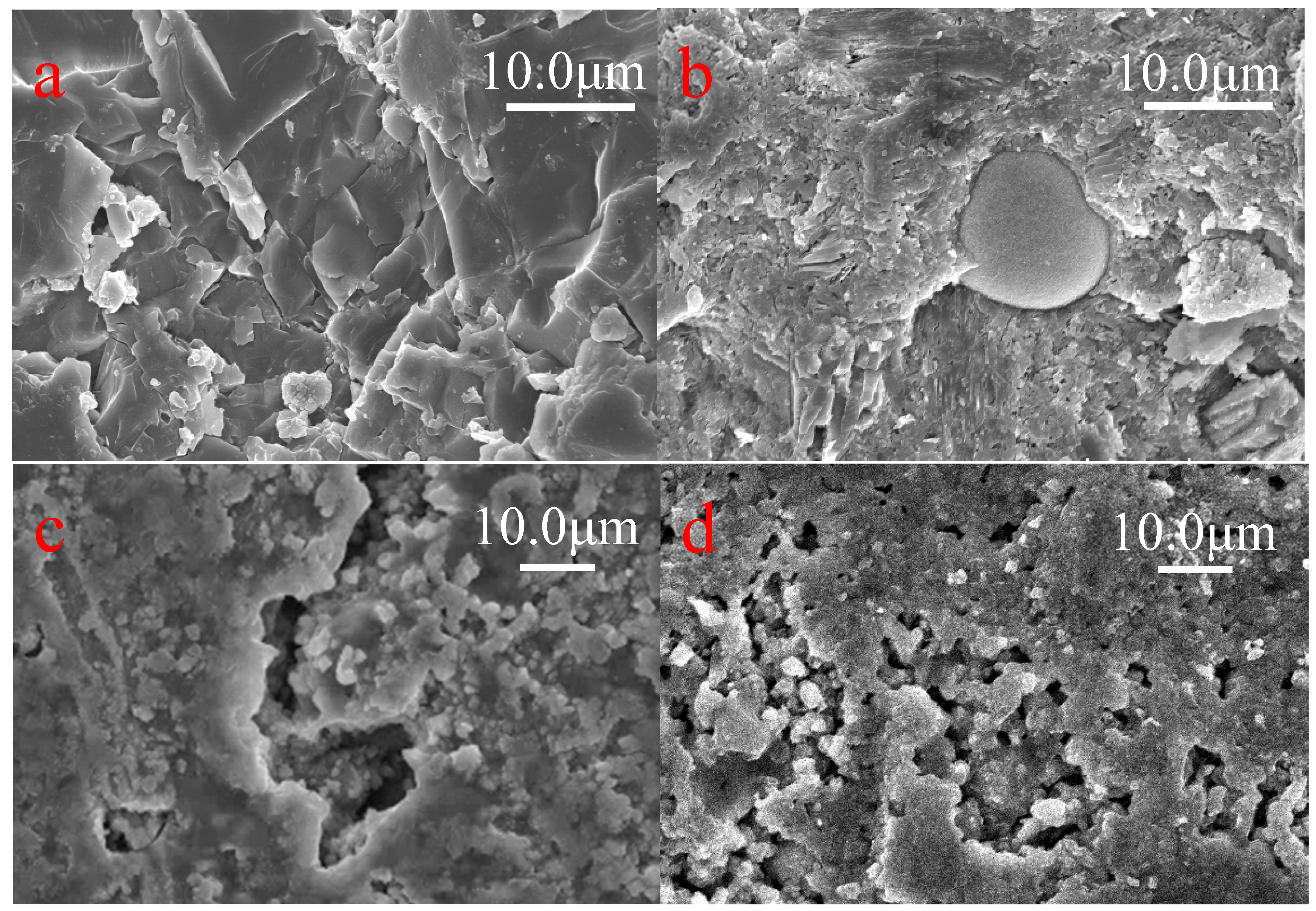
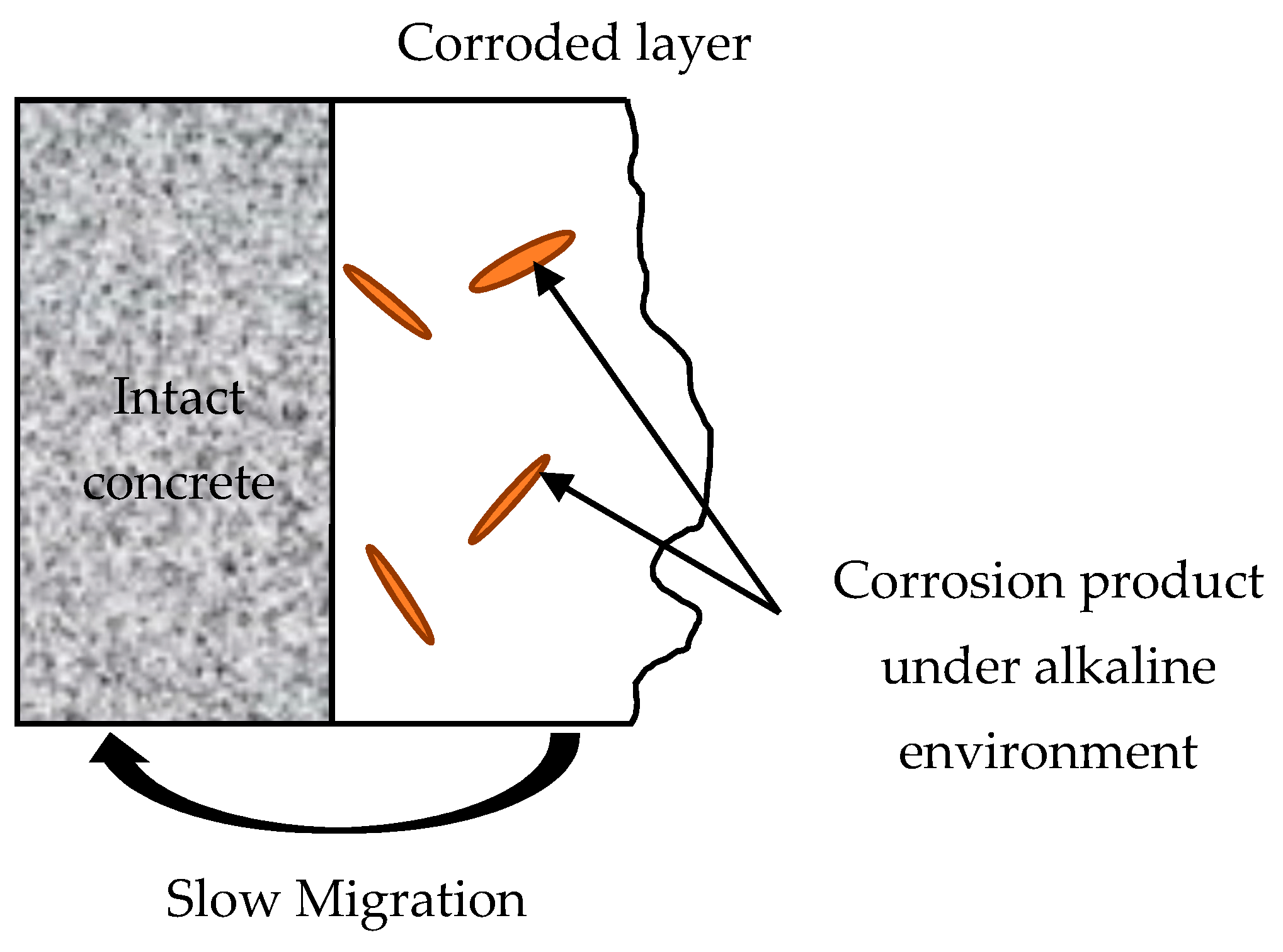
3.5. XRD and SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broere, W. Urban underground space: Solving the problems of today’s cities. Tunn. Undergr. Space Technol. 2016, 55, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhao, J. Assessment and planning of underground space use in Singapore. Tunn. Undergr. Space Technol. 2016, 55, 249–256. [Google Scholar] [CrossRef]
- Jaw, S.W.; Van Son, R.; Soon, V.K.H.; Schrotter, G.; Kiah, R.L.W.; Ni, S.T.S.; Yan, J. The need for a reliable map of utility networks for planning underground spaces. In Proceedings of the 2018 17th International Conference on Ground Penetrating Radar (GPR), Rapperswil, Switzerland, 18–21 June 2018. [Google Scholar]
- Wang, T.; Tan, L.; Xie, S.; Ma, B. Development and applications of common utility tunnels in China. Tunn. Undergr. Space Technol. 2018, 76, 92–106. [Google Scholar] [CrossRef]
- Ma, B. Trenchless Pipeline Rehabilitation and Renewal Technology; China Communications Press: Beijing, China, 2014. [Google Scholar]
- ASCE American’s Infrastructure Report Card 2021: America’s Infrastructure Scores a C. Available online: https://infrastructurereportcard.org (accessed on 5 March 2021).
- Zeng, C.; Ma, B. Analytic Mode for Predicting the Failure risk of pipeline System. In Proceedings of the ICPTT 2009: Advances and Experiences with Pipelines and Trenchless Technology for Water, Sewer, Gas, and Oil Applications, Shanghai, China, 18–21 October 2009; International Conference on Pipelines and Trenchless Technology (ICPTT): Shanghai, China, 2009; Volume 89, pp. 17–27. [Google Scholar]
- Ma, B.; Najafi, M. Development and applications of trenchless technology in China. Tunn. Undergr. Space Technol. 2008, 23, 476–480. [Google Scholar] [CrossRef]
- Sand, W. Importance of hydrogen sulfide, thiosulfate, and methylmercaptan for growth of thiobacilli during simulation of concrete corrosion. Appl. Environ. Microbiol. 1987, 53, 1645–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, C.; Falk, C.; Hetzel, F.; Pinnekamp, J.; Roder, S.; Ruppelt, J. State of the Sewer System in Germany-Results of the DWA Survey 2015. KA Korresp. Abwasser Abfall 2016, 63, 15–17. [Google Scholar]
- Berger, C.; Lohaus, J. Zustand der Kanalisation in Deutschland–Ergebnisse der DWA-Umfrage 2004. Korresp. Abwasser 2005, 52, 528–539. [Google Scholar]
- Sand, W.; Bock, E. Concrete corrosion in the Hamburg sewer system. Environ. Technol. 1984, 5, 517–528. [Google Scholar] [CrossRef]
- Wu, L.; Hu, C.; Liu, W.V. The sustainability of concrete in Sewer tunnel—A narrative review of acid corrosion in the city of Edmonton, Canada. Sustainability 2018, 10, 517. [Google Scholar] [CrossRef] [Green Version]
- Grengg, C.; Mittermayr, F.; Ukrainczyk, N.; Koraimann, G.; Kienesberger, S.; Dietzel, M. Advances in concrete materials for sewer systems affected by microbial induced concrete corrosion: A review. Water Res. 2018, 134, 341–352. [Google Scholar] [CrossRef]
- Zhang, L.; De Schryver, P.; De Gusseme, B.; De Muynck, W.; Boon, N.; Verstraete, W. Chemical and biological technologies for hydrogen sulfide emission control in sewer systems: A review. Water Res. 2008, 42, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zeng, C. Study of Cement-Based Superhydrophobic Composite Coating: New Option for Water Drainage Pipeline Rehabilitation. Materials 2020, 13, 5004. [Google Scholar] [CrossRef] [PubMed]
- Bahtli, T.; Ozbay, N.S. Corrosion resistances of C30 concrete: Effect of finely ground bronze sawdust waste. Mater. Chem. Phys. 2020, 243, 122588. [Google Scholar] [CrossRef]
- Attiogbe, E.K.; Rizkalla, S.H. Response of concrete to sulfuric acid attack. ACI Mater. J. 1988, 85, 481–488. [Google Scholar]
- Gutiérrez-Padilla, M.G.D.; Bielefeldt, A.; Ovtchinnikov, S.; Hernandez, M.; Silverstein, J. Biogenic sulfuric acid attack on different types of commercially produced concrete sewer pipes. Cem. Concr. Res. 2010, 40, 293–301. [Google Scholar] [CrossRef]
- Islander, R.L.; Devinny, J.S.; Mansfeld, F.; Postyn, A.; Shih, H. Microbial ecology of crown corrosion in sewers. J. Environ. Eng. 1991, 117, 751–770. [Google Scholar] [CrossRef]
- Jiang, G.; Zhou, M.; Chiu, T.H.; Sun, X.; Keller, J.; Bond, P.L. Wastewater-enhanced microbial corrosion of concrete sewers. Environ. Sci. Technol. 2016, 50, 8084–8092. [Google Scholar] [CrossRef]
- Kaushal, V.; Najafi, M.; Love, J. Qualitative investigation of microbially induced corrosion of concrete in sanitary sewer pipe and manholes. Proc. ASCE Pipelines 2018, 2018, 768–775. [Google Scholar]
- Huber, B.; Hilbig, H.; Mago, M.M.; Drewes, J.E.; Müller, E. Comparative analysis of biogenic and chemical sulfuric acid attack on hardened cement paste using laser ablation-ICP-MS. Cem. Concr. Res. 2016, 87, 14–21. [Google Scholar] [CrossRef]
- He, R.; Zheng, S.; Gan, V.J.; Wang, Z.; Fang, J.; Shao, Y. Damage mechanism and interfacial transition zone characteristics of concrete under sulfate erosion and Dry-Wet cycles. Constr. Build. Mater. 2020, 255, 119340. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Zhang, P.; Ma, Y.; Zhao, T.; Wang, H.; Zhang, Z. Water absorption and chloride diffusivity of concrete under the coupling effect of uniaxial compressive load and freeze-thaw cycles. Constr. Build. Mater. 2019, 209, 566–576. [Google Scholar] [CrossRef]
- Han, T.; Wang, X.; Li, D.; Han, N.; Xing, F. Damage and degradation mechanism for single intermittent cracked mortar specimens under a combination of chemical solutions and dry-wet cycles. Constr. Build. Mater. 2019, 213, 567–581. [Google Scholar] [CrossRef]
- RashCid, S.S.; Liu, Y.Q. Assessing environmental impacts of large centralized wastewater treatment plants with combined or separate sewer systems in dry/wet seasons by using LCA. Environ. Sci. Pollut. Res. 2020, 27, 1–17. [Google Scholar]
- Liang, X.; Lu, Y.; Li, Z.; Yang, C.; Niu, C.; Su, X. Bentonite/carbon composite as highly recyclable adsorbents for alkaline wastewater treatment and organic dye removal. Microporous Mesoporous Mater. 2017, 241, 107–114. [Google Scholar] [CrossRef]
- Roghanian, N.; Banthia, N. Development of a sustainable coating and repair material to prevent bio-corrosion in concrete sewer and waste-water pipes. Cem. Concr. Compos. 2019, 100, 99–107. [Google Scholar] [CrossRef]
- Mori, T.; Nonaka, T.; Tazaki, K.; Koga, M.; Hikosaka, Y.; Noda, S. Interactions of nutrients, moisture and pH on microbial corrosion of concrete sewer pipes. Water Res. 1992, 26, 29–37. [Google Scholar] [CrossRef]
- Monteny, J.; De Belie, N.; Vincke, E.; Verstraete, W.; Taerwe, L. Chemical and microbiological tests to simulate sulfuric acid corrosion of polymer-modified concrete. Cem. Concr. Res. 2001, 31, 1359–1365. [Google Scholar] [CrossRef]
- Li, B.; Wang, F.; Fang, H.; Yang, K.; Zhang, X.; Ji, Y. Experimental and numerical study on polymer grouting pretreatment technology in void and corroded concrete pipes. Tunn. Undergr. Space Technol. 2021, 113, 103842. [Google Scholar] [CrossRef]
- De Belie, N.; Monteny, J.; Beeldens, A.; Vincke, E.; Van Gemert, D.; Verstraete, W. Experimental research and prediction of the effect of chemical and biogenic sulfuric acid on different types of commercially produced concrete sewer pipes. Cem. Concr. Res. 2004, 34, 2223–2236. [Google Scholar] [CrossRef]
- Li, J.; Wu, Z.; Shi, C.; Yuan, Q.; Zhang, Z. Durability of ultra-high performance concrete—A review. Constr. Build. Mater. 2020, 255, 119296. [Google Scholar] [CrossRef]
- Richard, P.; Cheyrezy, M. Reactive powder concretes with high ductility and 200–800 MPa compressive strength. Symp. Pap. 1994, 144, 507–518. [Google Scholar]
- Richard, P.; Cheyrezy, M. Composition of reactive powder concretes. Cem. Concr. Res. 1995, 25, 1501–1511. [Google Scholar] [CrossRef]
- Zhou, W.; Kong, Y. Centrifugal spraying rehabilitation technology has been used in the world’s largest trenchless rehabilitation project for manholes. China Trenchless Technol. 2015, 3, 51–54. [Google Scholar]
- Zhang, P.; Li, Q.F. Effect of polypropylene fiber on durability of concrete composite containing fly ash and silica fume. Compos. Part B Eng. 2013, 45, 1587–1594. [Google Scholar] [CrossRef]
- Mahdavi, M.; Abolmaali, A.; Ghahremannejad, M. The effects of pH and temperature on compressive strength of synthetic fiber-reinforced concrete cylinders exposed to sulfuric acid. Adv. Civ. Eng. Mater. 2018, 7, 403–413. [Google Scholar]
- AQSIQ. SAC Common Portland Cement, GB 175-2007; China Standard Press: Beijing, China, 2007. [Google Scholar]
- GB/T 14684-2011, Sand for Construction. Ministry of Housing and Urban-Rural Development of the People’s Republic of China; Standards Press of China: Beijing, China, 2011. [Google Scholar]
- Barakan, S.; Aghazadeh, V. Structural modification of nano bentonite by aluminum, iron pillarization and 3D growth of silica mesoporous framework for arsenic removal from gold mine wastewater. J. Hazard. Mater. 2019, 378, 120779. [Google Scholar] [CrossRef]
- Sabour, M.; Dezvareh, G.; Bazzazzadeh, R. Corrosion prediction using the weight loss model in the sewer pipes made from sulfur and cement concretes and Response Surface Methodology (RSM). Constr. Build. Mater. 2019, 199, 40–49. [Google Scholar] [CrossRef]
- Huber, B.; Hilbig, H.; Drewes, J.E.; Müller, E. Evaluation of concrete corrosion after short-and long-term exposure to chemically and microbially generated sulfuric acid. Cem. Concr. Res. 2017, 94, 36–48. [Google Scholar] [CrossRef]
- ASTM C642-13. Standard Test Method for Density, Absorption, and Voids in Hardened Concrete; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Gutberlet, T.; Hilbig, H.; Beddoe, R.E. Acid attack on hydrated cement—Effect of mineral acids on the degradation process. Cem. Concr. Res. 2015, 74, 35–43. [Google Scholar] [CrossRef]
- Min, H.; Song, Z. Investigation on the sulfuric acid corrosion mechanism for concrete in soaking environment. Adv. Mater. Sci. Eng. 2018, 2018, 3258123. [Google Scholar] [CrossRef] [Green Version]
- Bertron, A.; Duchesne, J.; Escadeillas, G. Accelerated tests of hardened cement pastes alteration by organic acids: Analysis of the pH effect. Cem. Concr. Res. 2005, 35, 155–166. [Google Scholar] [CrossRef]
- Khan, M.; Ali, M. Improvement in concrete behavior with fly ash, silica-fume and coconut fibres. Constr. Build. Mater. 2019, 203, 174–187. [Google Scholar] [CrossRef]
- Gökçe, H.S.; Hatungimana, D.; Ramyar, K. Effect of fly ash and silica fume on hardened properties of foam concrete. Constr. Build. Mater. 2019, 194, 1–11. [Google Scholar] [CrossRef]
- Pala, M.; Özbay, E.; Öztaş, A.; Yuce, M.I. Appraisal of long-term effects of fly ash and silica fume on compressive strength of concrete by neural networks. Constr. Build. Mater. 2007, 21, 384–394. [Google Scholar] [CrossRef]
- Yazıcı, H. The effect of silica fume and high-volume Class C fly ash on mechanical properties, chloride penetration and freeze–thaw resistance of self-compacting concrete. Constr. Build. Mater. 2008, 22, 456–462. [Google Scholar] [CrossRef]
- Wu, M.; Wang, T.; Wu, K.; Kan, L. Microbiologically induced corrosion of concrete in sewer structures: A review of the mechanisms and phenomena. Constr. Build. Mater. 2020, 239, 117813. [Google Scholar] [CrossRef]
- Wu, H.L.; Zhang, D.; Du, Y.J.; Li, V.C. Durability of engineered cementitious composite exposed to acid mine drainage. Cem. Concr. Compos. 2020, 108, 103550. [Google Scholar] [CrossRef]
- Mounanga, P.; Khelidj, A.; Loukili, A.; Baroghel-Bouny, V. Predicting Ca(OH)2 content and chemical shrinkage of hydrating cement pastes using analytical approach. Cem. Concr. Res. 2004, 34, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Hewlett, P.; Liska, M. (Eds.) Lea’s Chemistry of Cement and Concrete; Butterworth-Heinemann: Oxford, UK, 2003; pp. 1–845. [Google Scholar]
- Zhang, D.; Ghouleh, Z.; Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- Supit, S.W.M.; Shaikh, F.U.A. Durability properties of high volume fly ash concrete containing nano-silica. Mater. Struct. 2015, 48, 2431–2445. [Google Scholar] [CrossRef]
- Tang, W.C.; Wang, Z.; Liu, Y.; Cui, H.Z. Influence of red mud on fresh and hardened properties of self-compacting concrete. Constr. Build. Mater. 2018, 178, 288–300. [Google Scholar] [CrossRef]





| Mixture ID | M | P |
|---|---|---|
| PC (Portland cement) | 1 | 1 |
| FA (fly ash) | 0.19 | - |
| SF (silica fume) | 0.08 | - |
| SS (silica sand) | 1.4 | 1.4 |
| PVA (% by volume) (polyvinyl alcohol) | 1.5 | - |
| Water/cement ratio | 0.26 | 0.26 |
| SP (% by weight) (superplasticizer) | 0.12 | 0.12 |
| No. | Curing Environment | The 10-Day Cycle | ||
|---|---|---|---|---|
| 4.5 Days | 24 h | 4.5 Days | ||
| Ⅰ | Acid/Alkaline cycle | PH = 1 acid environment | Dry at 20 °C | PH = 13 alkaline environment |
| Ⅱ | Acid-only | PH = 1 acid environment | ||
| Ⅲ | Tap water | Tap water (PH around 7) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Zhao, Y.; Ma, B.; Zeng, C. Durability Study on High-Performance Fiber-Reinforced Mortar under Simulated Wastewater Pipeline Environment. Materials 2021, 14, 3781. https://doi.org/10.3390/ma14143781
Wang T, Zhao Y, Ma B, Zeng C. Durability Study on High-Performance Fiber-Reinforced Mortar under Simulated Wastewater Pipeline Environment. Materials. 2021; 14(14):3781. https://doi.org/10.3390/ma14143781
Chicago/Turabian StyleWang, Tianyu, Yahong Zhao, Baosong Ma, and Cong Zeng. 2021. "Durability Study on High-Performance Fiber-Reinforced Mortar under Simulated Wastewater Pipeline Environment" Materials 14, no. 14: 3781. https://doi.org/10.3390/ma14143781
APA StyleWang, T., Zhao, Y., Ma, B., & Zeng, C. (2021). Durability Study on High-Performance Fiber-Reinforced Mortar under Simulated Wastewater Pipeline Environment. Materials, 14(14), 3781. https://doi.org/10.3390/ma14143781







