Impact of Cross-Linking of Collagen Matrices on Tissue Regeneration in a Rabbit Calvarial Bone Defect
Abstract
:1. Introduction
2. Materials and Methods
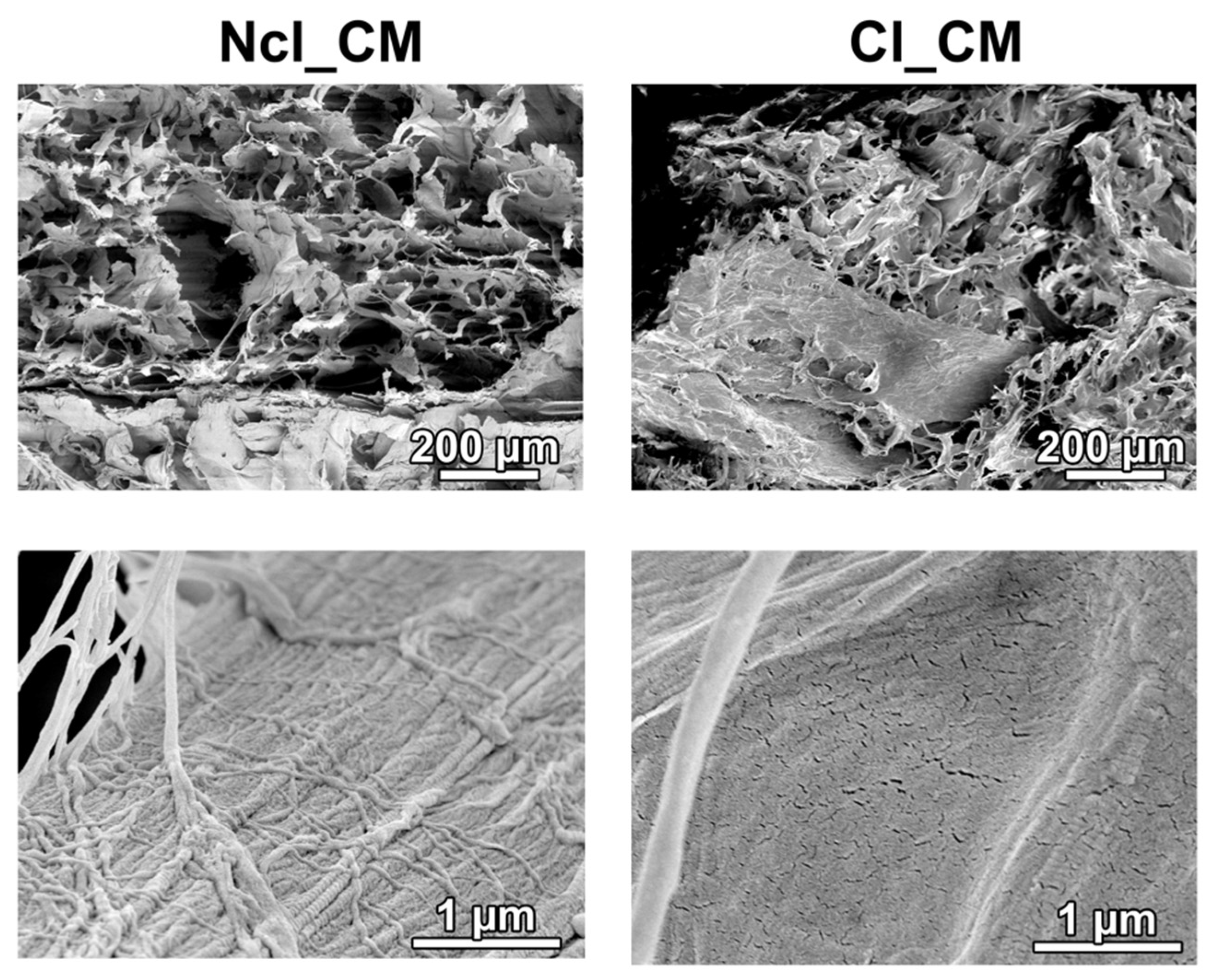
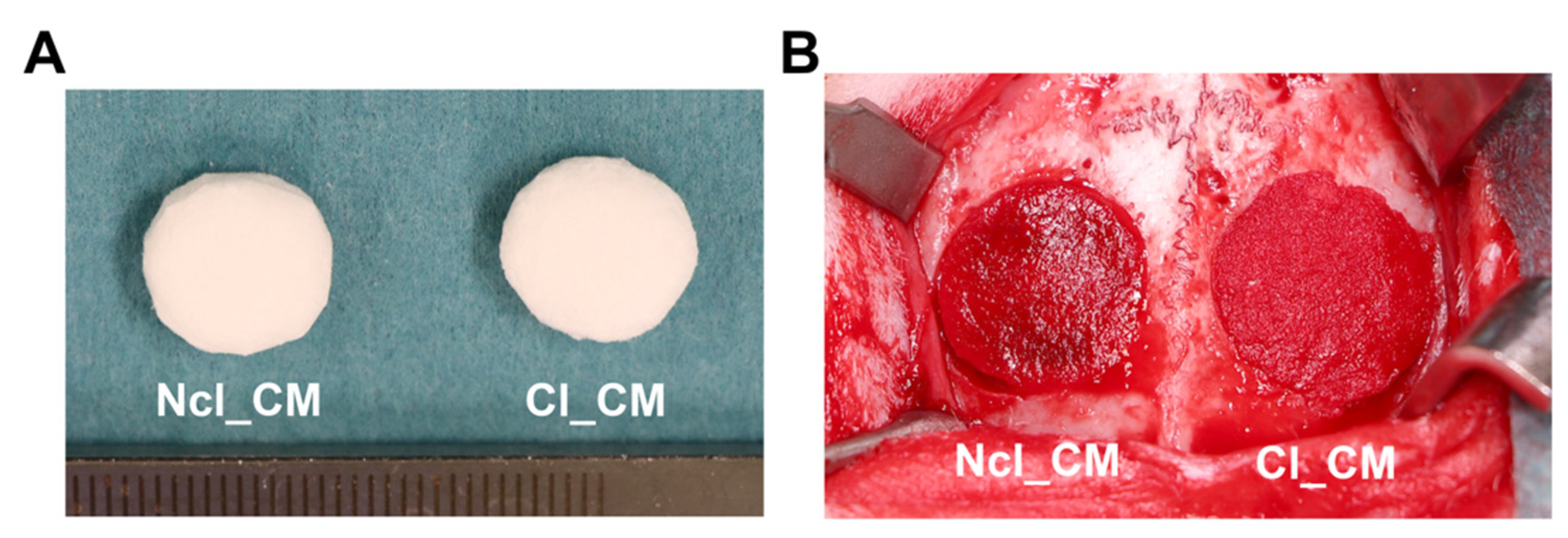
2.1. Materials
2.2. Animals
2.3. Anesthesia
2.4. Surgical Procedures
2.5. Postoperative Procedures
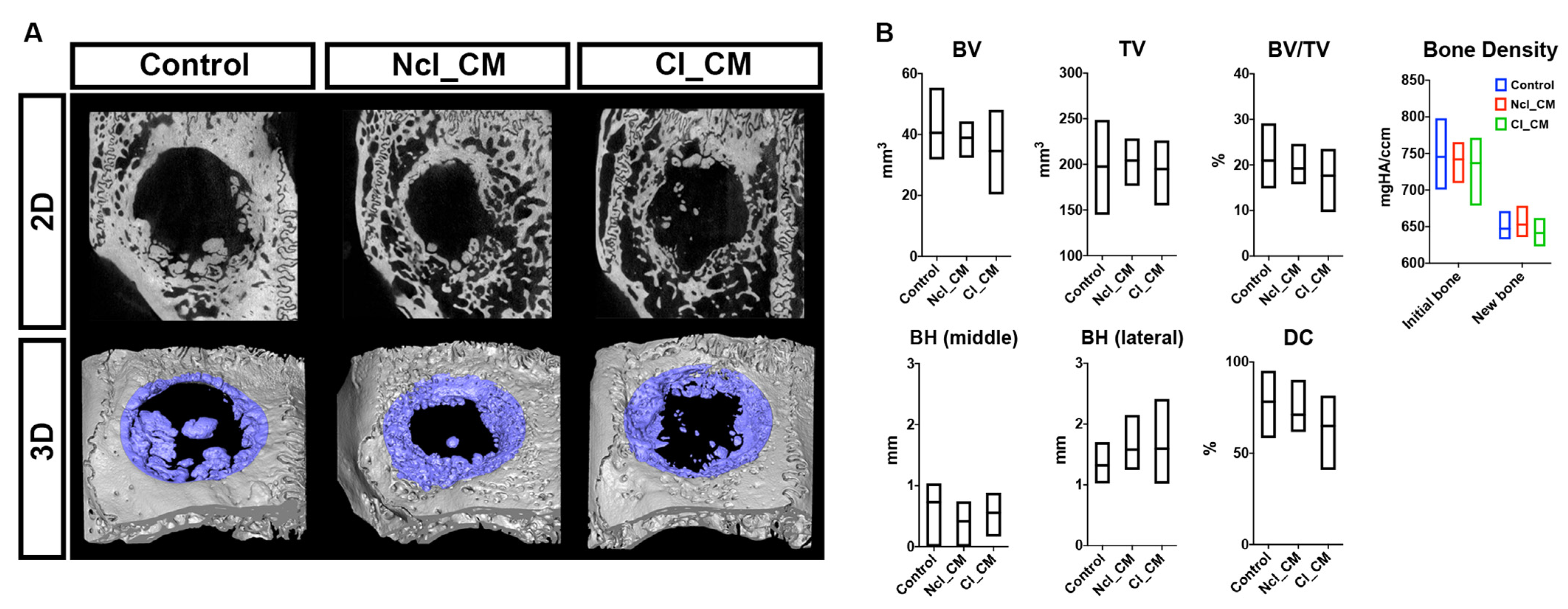
2.6. Micro-CT Analysis
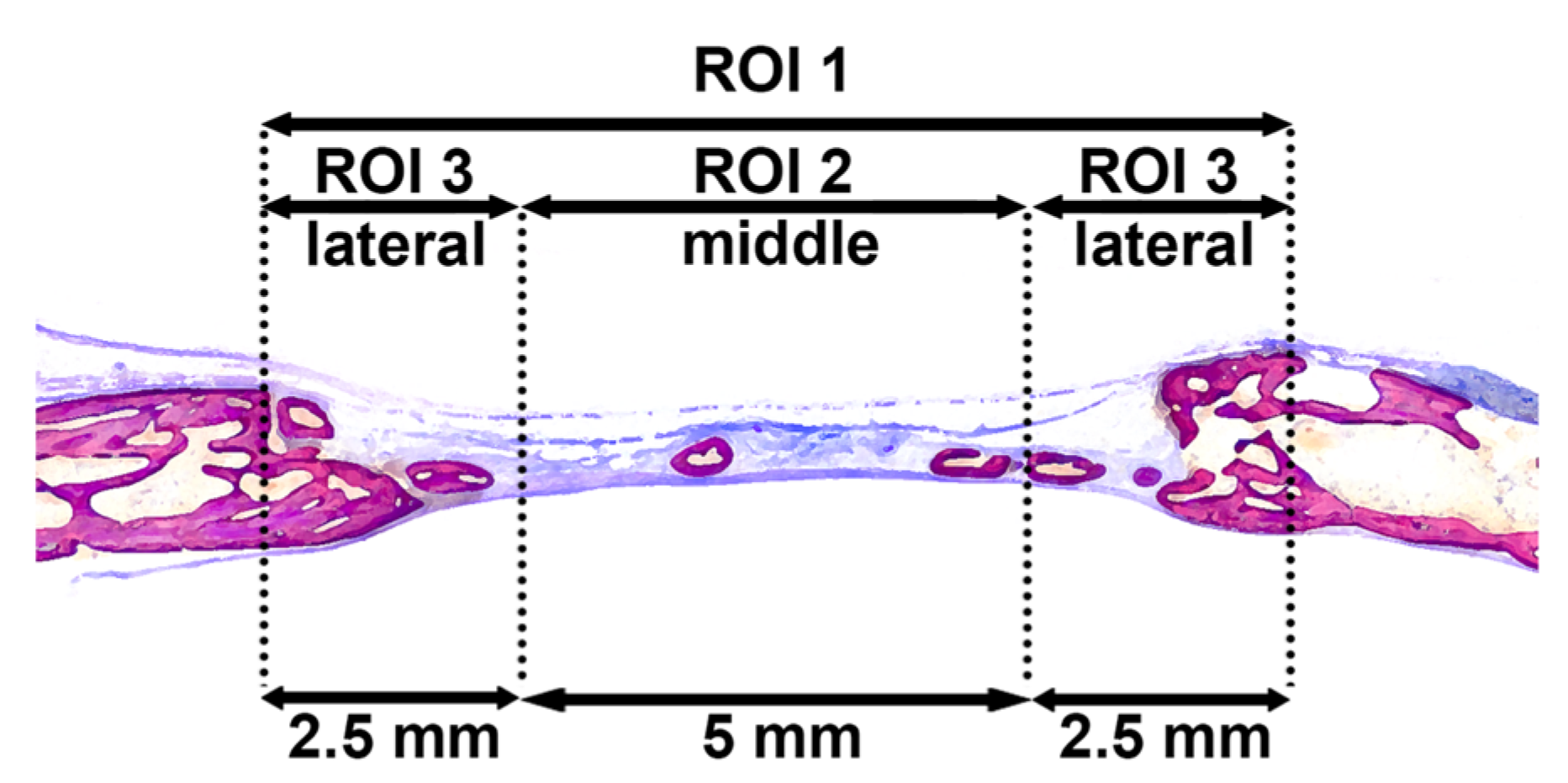
2.7. Histological Processing and Histomorphometric Analysis
2.8. Statistical Analysis
3. Results
3.1. General Observation during and Post-Surgery
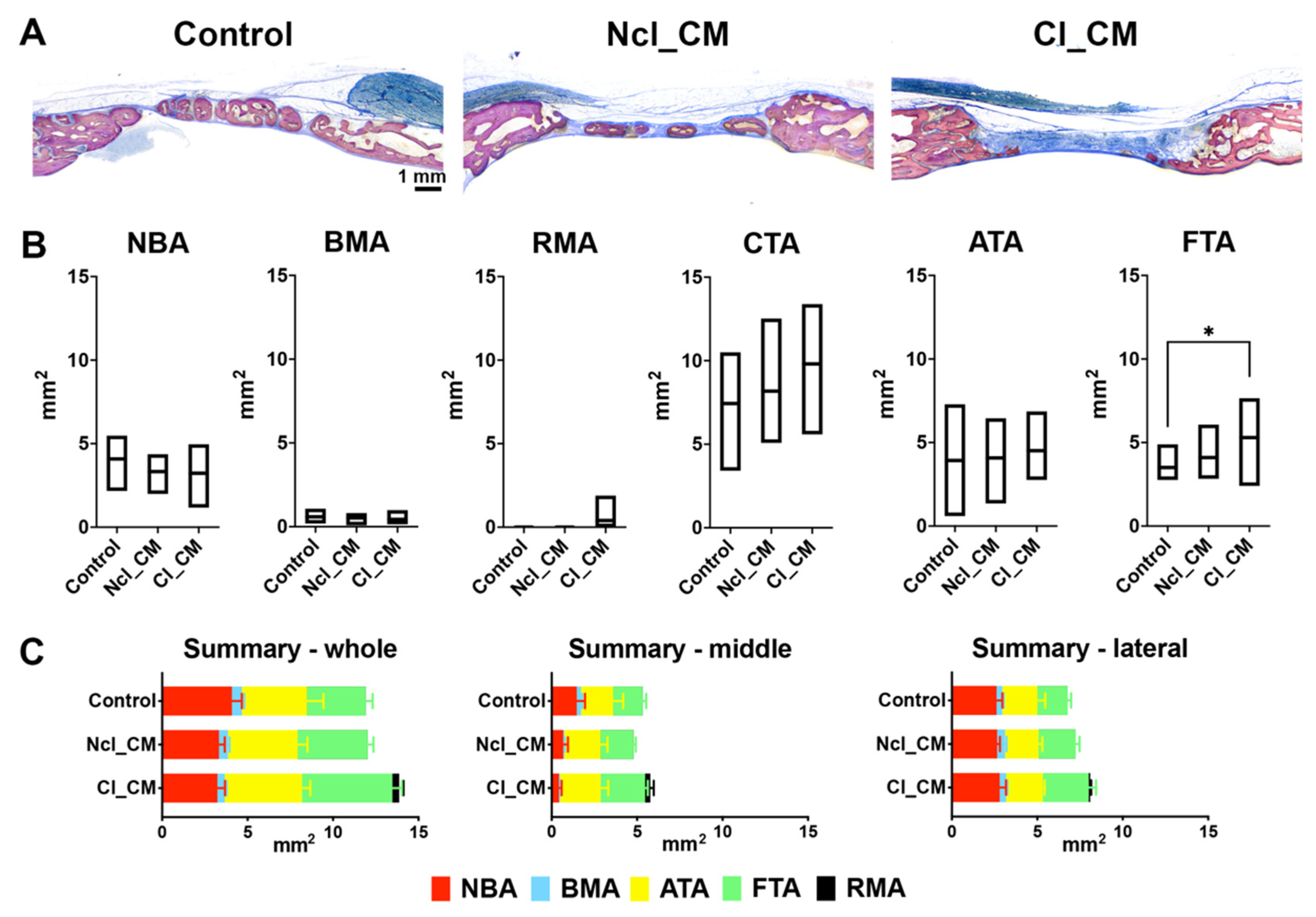
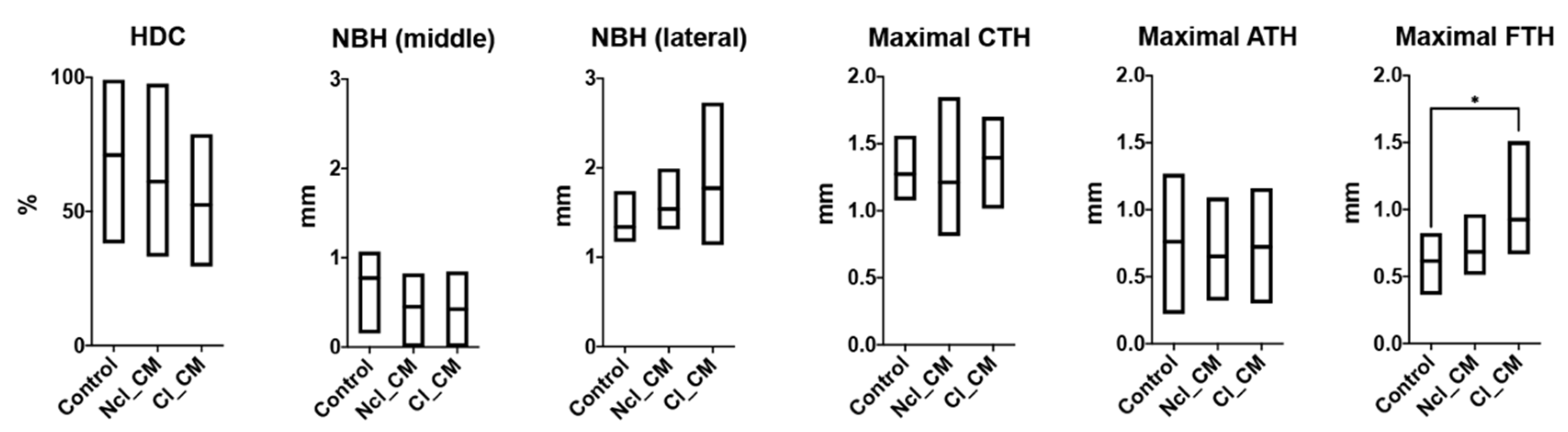
3.2. Micro-CT Analysis
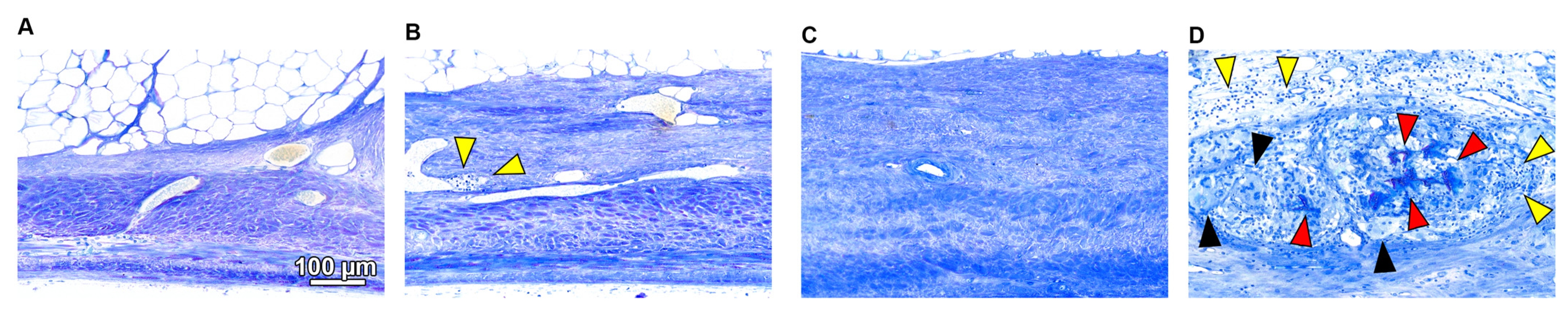
3.3. Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thoma, D.S.; Naenni, N.; Benic, G.I.; Hämmerle, C.H.; Jung, R.E. Soft tissue volume augmentation at dental implant sites using a volume stable three–dimensional collagen matrix–histological outcomes of a preclinical study. J. Clin. Periodontol. 2017, 44, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herford, A.S.; Akin, L.; Cicciu, M.; Maiorana, C.; Boyne, P.J. Use of a porcine collagen matrix as an alternative to autogenous tissue for grafting oral soft tissue defects. J. Oral Maxillofac. Surg. 2010, 68, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Zeltner, M.; Hilbe, M.; Hämmerle, C.H.; Hüsler, J.; Jung, R.E. Randomized controlled clinical study evaluating effectiveness and safety of a volume-stable collagen matrix compared to autogenous connective tissue grafts for soft tissue augmentation at implant sites. J. Clin. Periodontol. 2016, 43, 874–885. [Google Scholar] [CrossRef]
- Schmitt, C.M.; Matta, R.E.; Moest, T.; Humann, J.; Gammel, L.; Neukam, F.W.; Schlegel, K.A. Soft tissue volume alterations after connective tissue grafting at teeth: The subepithelial autologous connective tissue graft versus a porcine collagen matrix—A pre-clinical volumetric analysis. J. Clin. Periodontol. 2016, 43, 609–617. [Google Scholar] [CrossRef]
- Schmitt, C.M.; Moest, T.; Lutz, R.; Wehrhan, F.; Neukam, F.W.; Schlegel, K.A. Long-term outcomes after vestibuloplasty with a porcine collagen matrix (Mucograft®) versus the free gingival graft: A comparative prospective clinical trial. Clin. Oral Implants Res. 2016, 27, e125–e133. [Google Scholar] [CrossRef] [PubMed]
- Herford, A.S.; Nguyen, K.; Miller, M.; Tandon, R.; Signorino, F. Evaluation of the Safety and Efficacy of Soft Tissue Augmentation With a Compressive-Resistant Collagen Matrix in a Nonhuman Primate Model. J. Oral Maxillofac. Surg. 2019, 77, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.; Zeltner, M.; Hämmerle, C.H.; Jung, R.E.; Thoma, D.S. Non-interventional 1-year follow-up study of peri-implant soft tissues following previous soft tissue augmentation and crown insertion in single-tooth gaps. J. Clin. Periodontol. 2018, 45, 504–512. [Google Scholar] [CrossRef] [Green Version]
- Rothamel, D.; Benner, M.; Fienitz, T.; Happe, A.; Kreppel, M.; Nickenig, H.-J.; Zöller, J.E. Biodegradation pattern and tissue integration of native and cross-linked porcine collagen soft tissue augmentation matrices—An experimental study in the rat. Head Face Med. 2014, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Ardakani, M.R.T.; Hajizadeh, F.; Yadegari, Z. Comparison of attachment and proliferation of human gingival fibroblasts on different collagen membranes. Ann. Maxillofac. Surg. 2018, 8, 218. [Google Scholar]
- Fujioka-Kobayashi, M.; Ülgür, I.I.; Katagiri, H.; Vuignier, S.; Schaller, B. In Vitro observation of macrophage polarization and gingival fibroblast behavior on three-dimensional xenogeneic collagen matrixes. J. Biomed. Mater. Res. Part A 2020, 108, 1408–1418. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, X.; Sun, L.; She, Z.; Tan, R.; Wang, W. Immune response of bovine sourced cross-linked collagen sponge for hemostasis. J. Biomater. Appl. 2018, 32, 920–931. [Google Scholar] [CrossRef]
- Thoma, D.S.; Villar, C.C.; Cochran, D.L.; Hammerle, C.H.; Jung, R.E. Tissue integration of collagen-based matrices: An experimental study in mice. Clin. Oral Implant. Res. 2012, 23, 1333–1339. [Google Scholar] [CrossRef]
- Ferrantino, L.; Bosshardt, D.; Nevins, M.; Santoro, G.; Simion, M.; Kim, D. Tissue Integration of a Volume-Stable Collagen Matrix in an Experimental Soft Tissue Augmentation Model. Int. J. Periodontics Restor. Dent. 2016, 36, 807–815. [Google Scholar] [CrossRef]
- Zeltner, M.; Jung, R.E.; Hämmerle, C.H.; Hüsler, J.; Thoma, D.S. Randomized controlled clinical study comparing a volume-stable collagen matrix to autogenous connective tissue grafts for soft tissue augmentation at implant sites: Linear volumetric soft tissue changes up to 3 months. J. Clin. Periodontol. 2017, 44, 446–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caballe-Serrano, J.; Zhang, S.; Ferrantino, L.; Simion, M.; Chappuis, V.; Bosshardt, D.D. Tissue Response to a Porous Collagen Matrix Used for Soft Tissue Augmentation. Material 2019, 12, 3721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Galil, K.; Loukota, R. Fractures of the mandibular condyle: Evidence base and current concepts of management. Br. J. Oral Maxillofac. Surg. 2010, 48, 520–526. [Google Scholar] [CrossRef]
- Imber, J.C.; Bosshardt, D.D.; Stähli, A.; Saulacic, N.; Deschner, J.; Sculean, A. Pre-clinical evaluation of the effect of a volume-stable collagen matrix on periodontal regeneration in two-wall intrabony defects. J. Clin. Periodontol. 2021, 48, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Shahim, K.; Buser, R.; Koller, E.; Joda, T.; Reyes, M.; Buser, D. Novel collagen matrix to increase tissue thickness simultaneous with guided bone regeneration and implant placement in esthetic implant sites: A feasibility study. Int. J. Periodontics Restor. Dent. 2018, 38, 575–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdfelder, E.; Faul, F.; Buchner, A. GPOWER: A general power analysis program. Behav. Res. Methods Instrum. Comput. 1996, 28, 1–11. [Google Scholar] [CrossRef]
- Schaller, B.; Fujioka-Kobayashi, M.; Zihlmann, C.; Schuler, V.C.; Katagiri, H.; Lang, N.P.; Saulacic, N. Effects of additional collagen in biphasic calcium phosphates: A study in a rabbit calvaria. Clin. Oral Investig. 2020, 24, 3093–3103. [Google Scholar] [CrossRef]
- Saulacic, N.; Fujioka-Kobayashi, M.; Kimura, Y.; Bracher, A.I.; Zihlmann, C.; Lang, N.P. The effect of synthetic bone graft substitutes on bone formation in rabbit calvarial defects. J. Mater. Sci. Mater. Med. 2021, 32, 14. [Google Scholar] [CrossRef]
- Bax, D.V.; Davidenko, N.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Impact of UV- and carbodiimide-based crosslinking on the integrin-binding properties of collagen-based materials. Acta Biomater. 2019, 100, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.; Berlanga, O.; Best, D.; Frampton, J. Update on collagen receptor interactions in platelets: Is the two-state model still valid? Platelets 2000, 11, 252–258. [Google Scholar] [PubMed]
- El-Jawhari, J.; Moisley, K.; Jones, E.; Giannoudis, P. A crosslinked collagen membrane versus a non-crosslinked bilayer collagen membrane for supporting osteogenic functions of human bone marrow-multipotent stromal cells. ECells Mater. J. 2019, 37, 292–309. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Romanos, G.E. Critical size defects for bone regeneration experiments in rabbit calvariae: Systematic review and quality evaluation using ARRIVE guidelines. Clin. Oral Implants Res. 2015, 26, 915–930. [Google Scholar] [CrossRef]
- Thoma, D.S.; Hämmerle, C.H.; Cochran, D.L.; Jones, A.A.; Görlach, C.; Uebersax, L.; Mathes, S.; Graf-Hausner, U.; Jung, R.E. Soft tissue volume augmentation by the use of collagen-based matrices in the dog mandible—A histological analysis. J. Clin. Periodontol. 2011, 38, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.M.; Bosshardt, D.; Buser, D. Effect of two different bioabsorbable collagen membranes on guided bone regeneration: A comparative histomorphometric study in the dog mandible. J. Periodontol. 2007, 78, 1943–1953. [Google Scholar] [CrossRef]
- Tal, H.; Kozlovsky, A.; Artzi, Z.; Nemcovsky, C.E.; Moses, O. Cross-linked and non-cross-linked collagen barrier membranes disintegrate following surgical exposure to the oral environment: A histological study in the cat. Clin. Oral Implants Res. 2008, 19, 760–766. [Google Scholar] [CrossRef]
- Delgado, L.M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng. Part B Rev. 2015, 21, 298–313. [Google Scholar] [CrossRef] [Green Version]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef] [Green Version]
- Alibardi, L. Immunocalization of telomerase in cells of lizard tail after amputation suggests cell activation for tail regeneration. Tissue Cell 2016, 48, 63–71. [Google Scholar] [CrossRef]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Harmsen, M.C.; van Luyn, M.J.; Bank, R.A. The relationship between collagen scaffold cross-linking agents and neutrophils in the foreign body reaction. Biomaterials 2010, 31, 9192–9201. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M. Resorbable biomaterials as bone graft substitutes. Mater. Today 2010, 13, 24–30. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujioka-Kobayashi, M.; Andrejova, E.; Katagiri, H.; Schaller, B.; Sculean, A.; Imber, J.-C.; Lang, N.P.; Saulacic, N. Impact of Cross-Linking of Collagen Matrices on Tissue Regeneration in a Rabbit Calvarial Bone Defect. Materials 2021, 14, 3740. https://doi.org/10.3390/ma14133740
Fujioka-Kobayashi M, Andrejova E, Katagiri H, Schaller B, Sculean A, Imber J-C, Lang NP, Saulacic N. Impact of Cross-Linking of Collagen Matrices on Tissue Regeneration in a Rabbit Calvarial Bone Defect. Materials. 2021; 14(13):3740. https://doi.org/10.3390/ma14133740
Chicago/Turabian StyleFujioka-Kobayashi, Masako, Elena Andrejova, Hiroki Katagiri, Benoit Schaller, Anton Sculean, Jean-Claude Imber, Niklaus P. Lang, and Nikola Saulacic. 2021. "Impact of Cross-Linking of Collagen Matrices on Tissue Regeneration in a Rabbit Calvarial Bone Defect" Materials 14, no. 13: 3740. https://doi.org/10.3390/ma14133740
APA StyleFujioka-Kobayashi, M., Andrejova, E., Katagiri, H., Schaller, B., Sculean, A., Imber, J.-C., Lang, N. P., & Saulacic, N. (2021). Impact of Cross-Linking of Collagen Matrices on Tissue Regeneration in a Rabbit Calvarial Bone Defect. Materials, 14(13), 3740. https://doi.org/10.3390/ma14133740






