ZrN Phase Formation, Hardening and Nitrogen Diffusion Kinetics in Plasma Nitrided Zircaloy-4
Abstract
:1. Introduction
2. Materials and Methods
3. Results
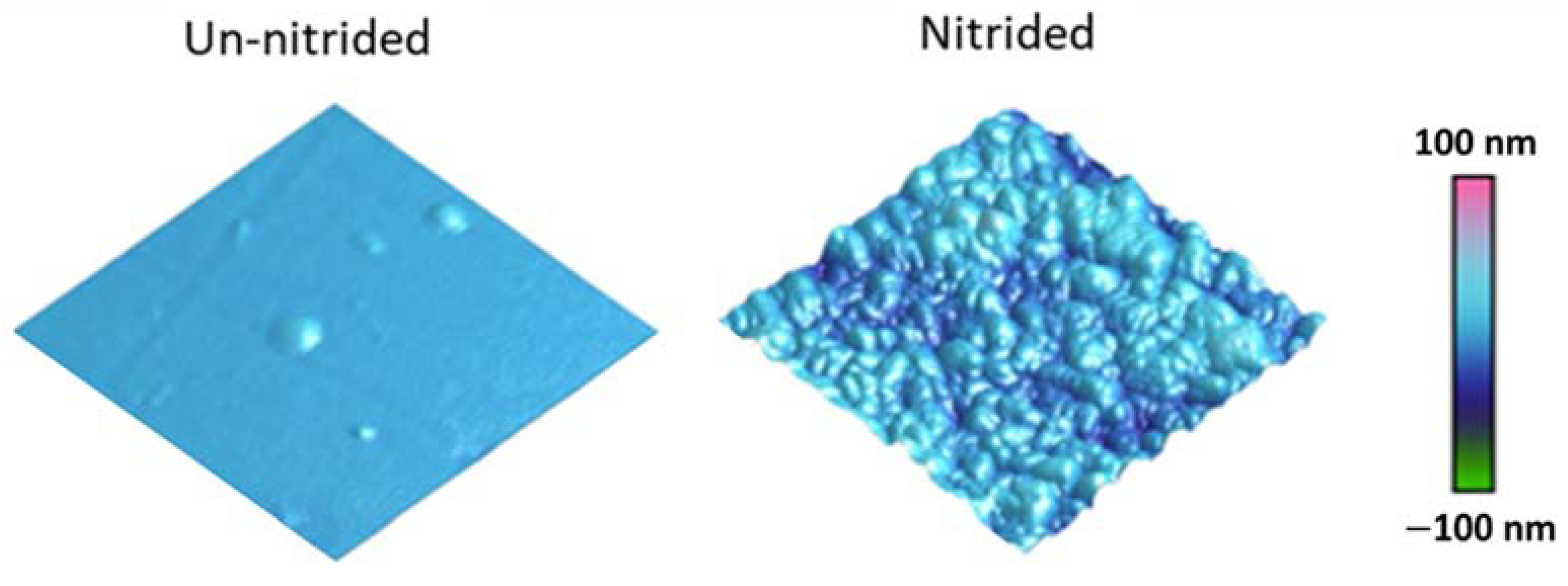
3.1. Atomic Force Microscopy Characterization
3.2. Raman Characterization
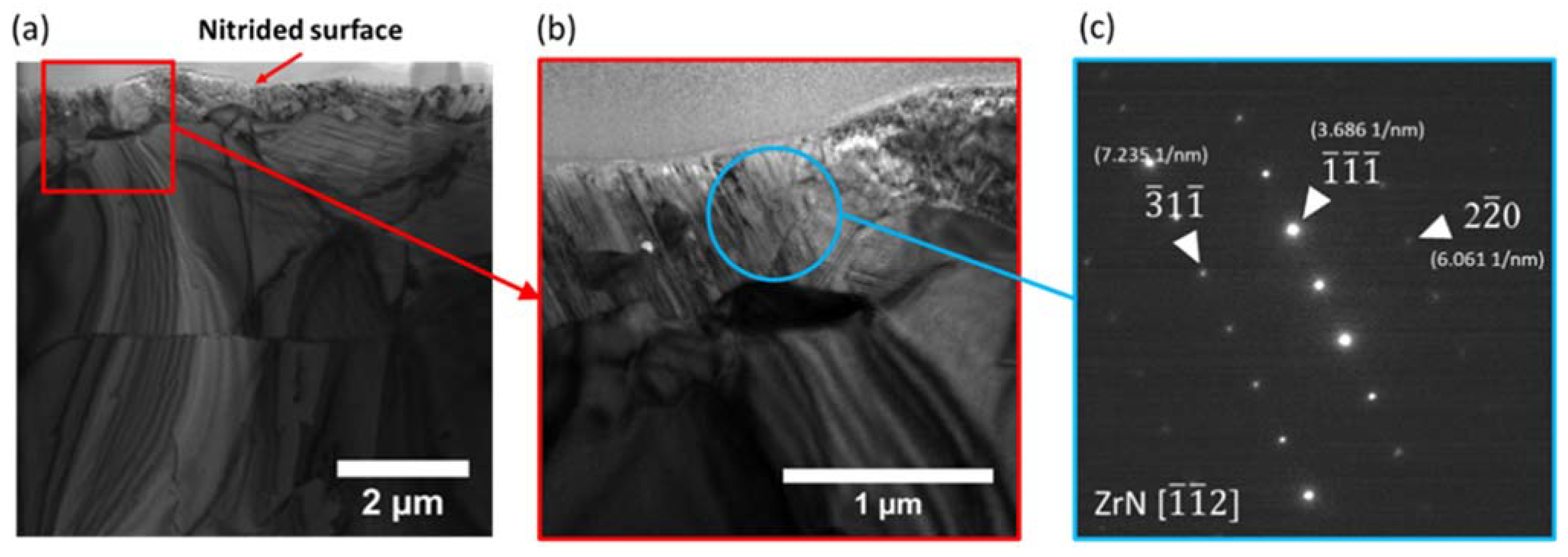
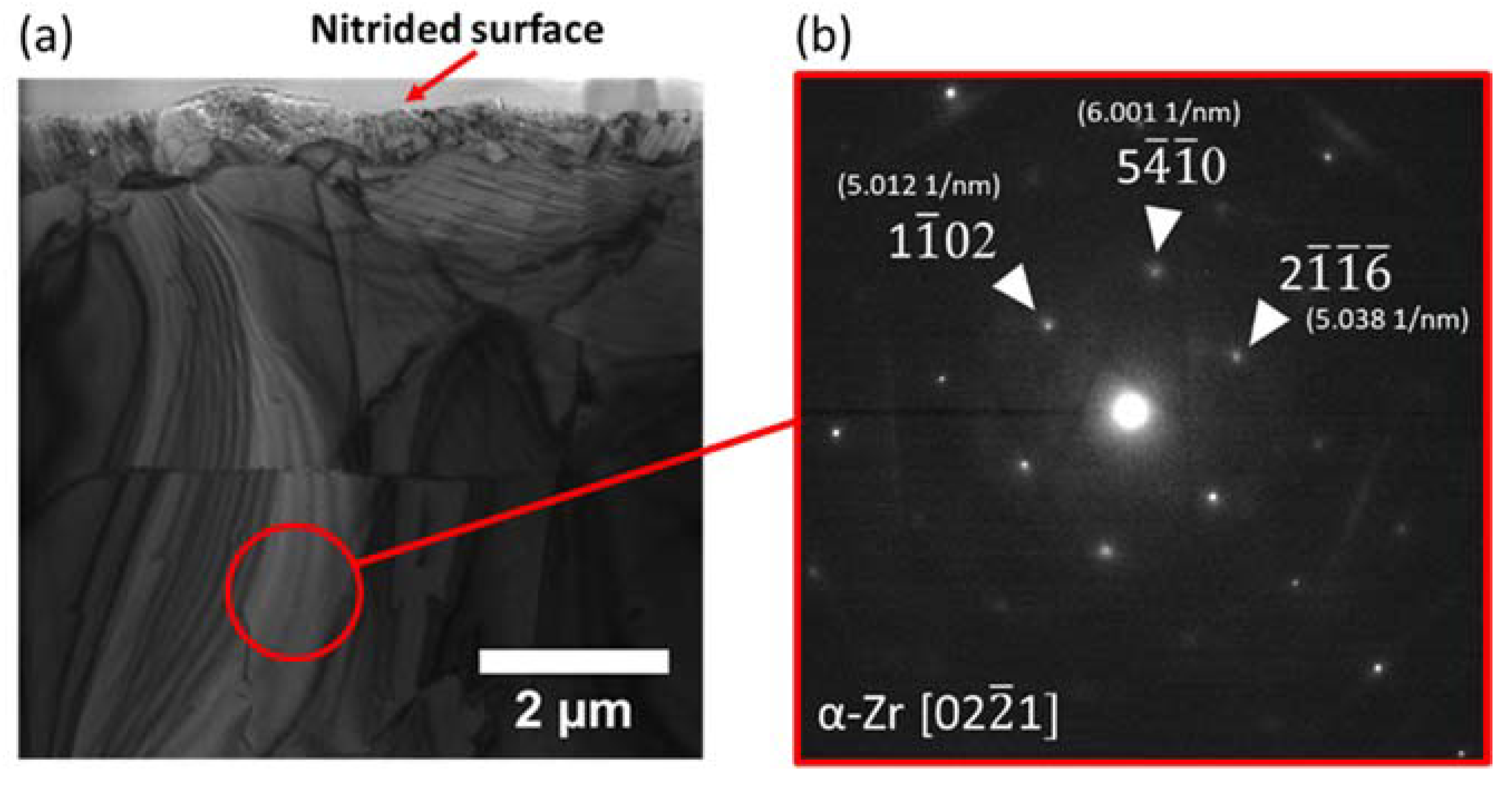
3.3. Transmission Electron Microscopy Characterization
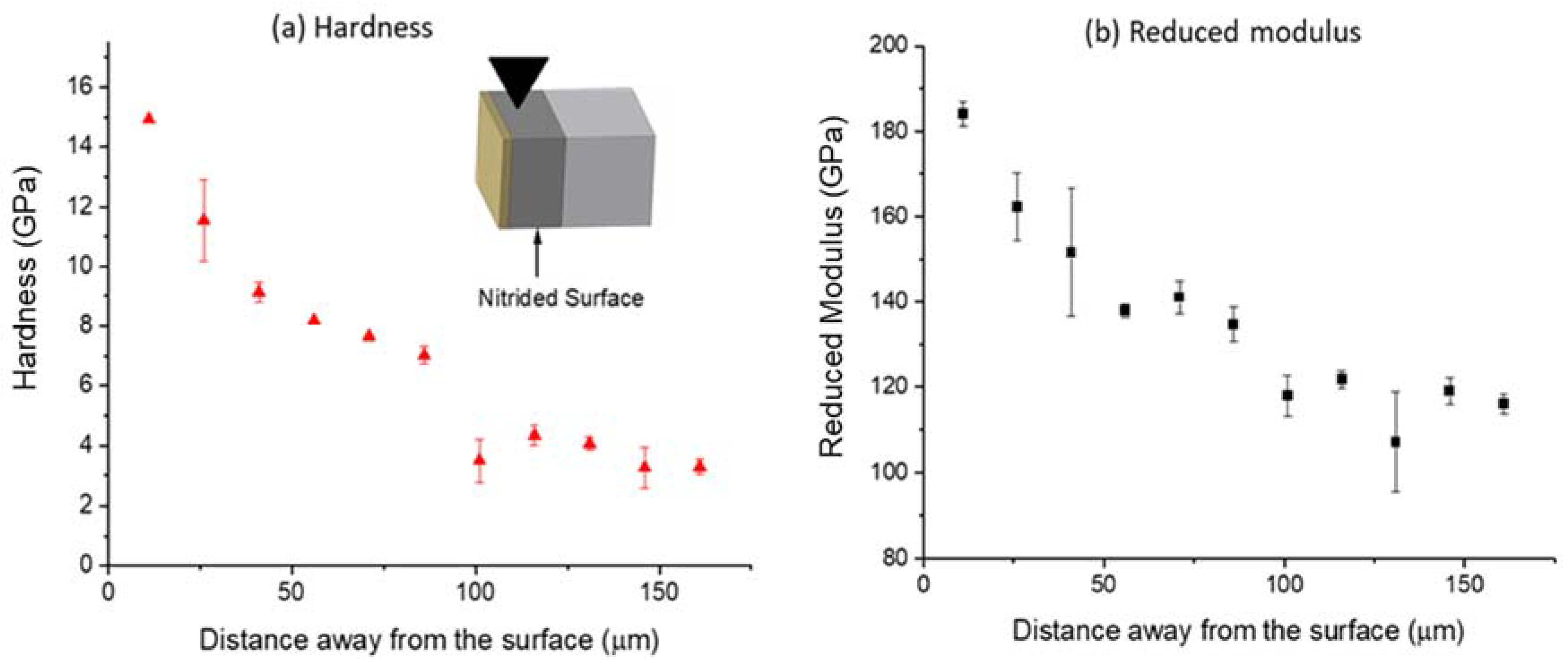
3.4. Nanoindentation
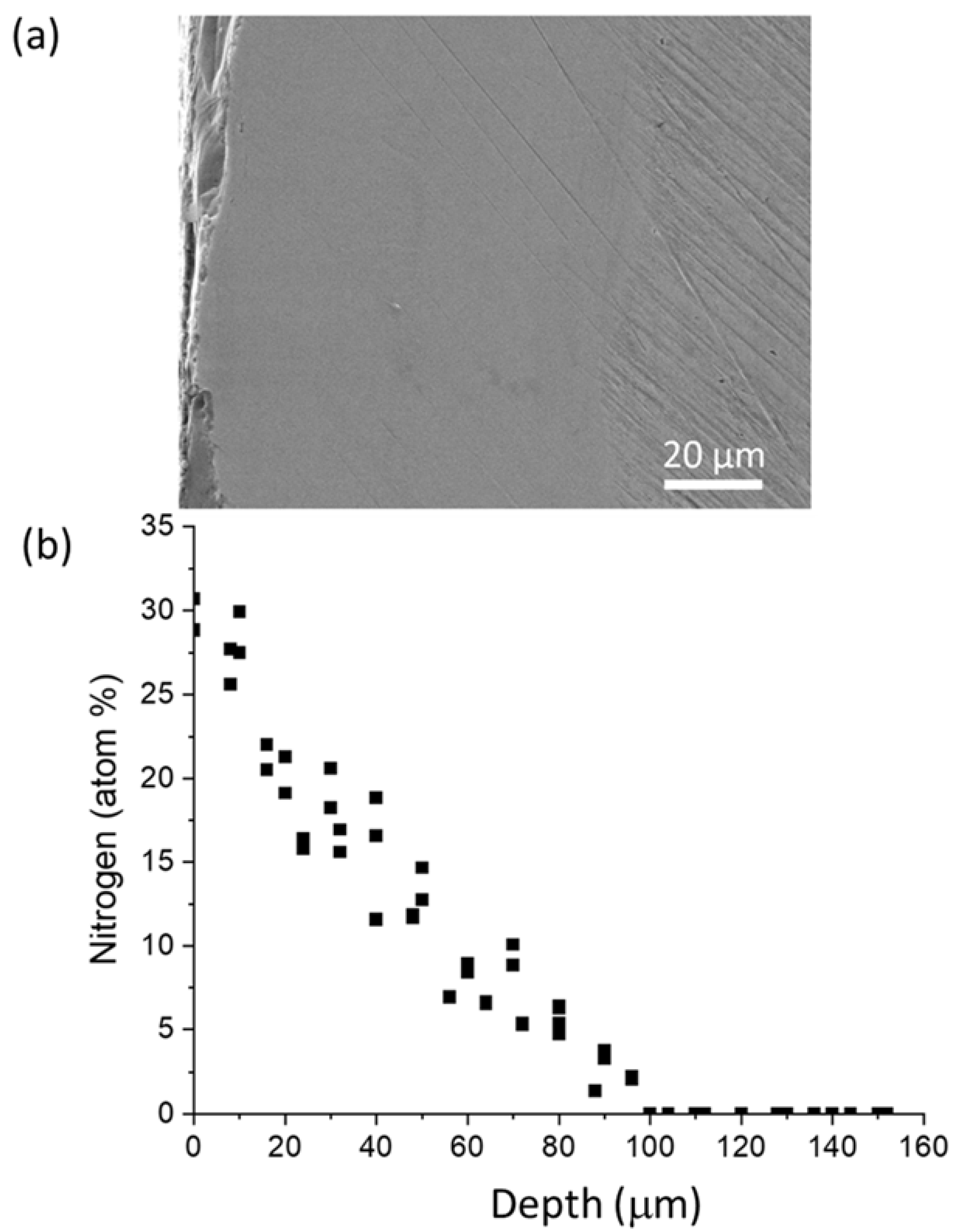
3.5. Nitrogen Profiling
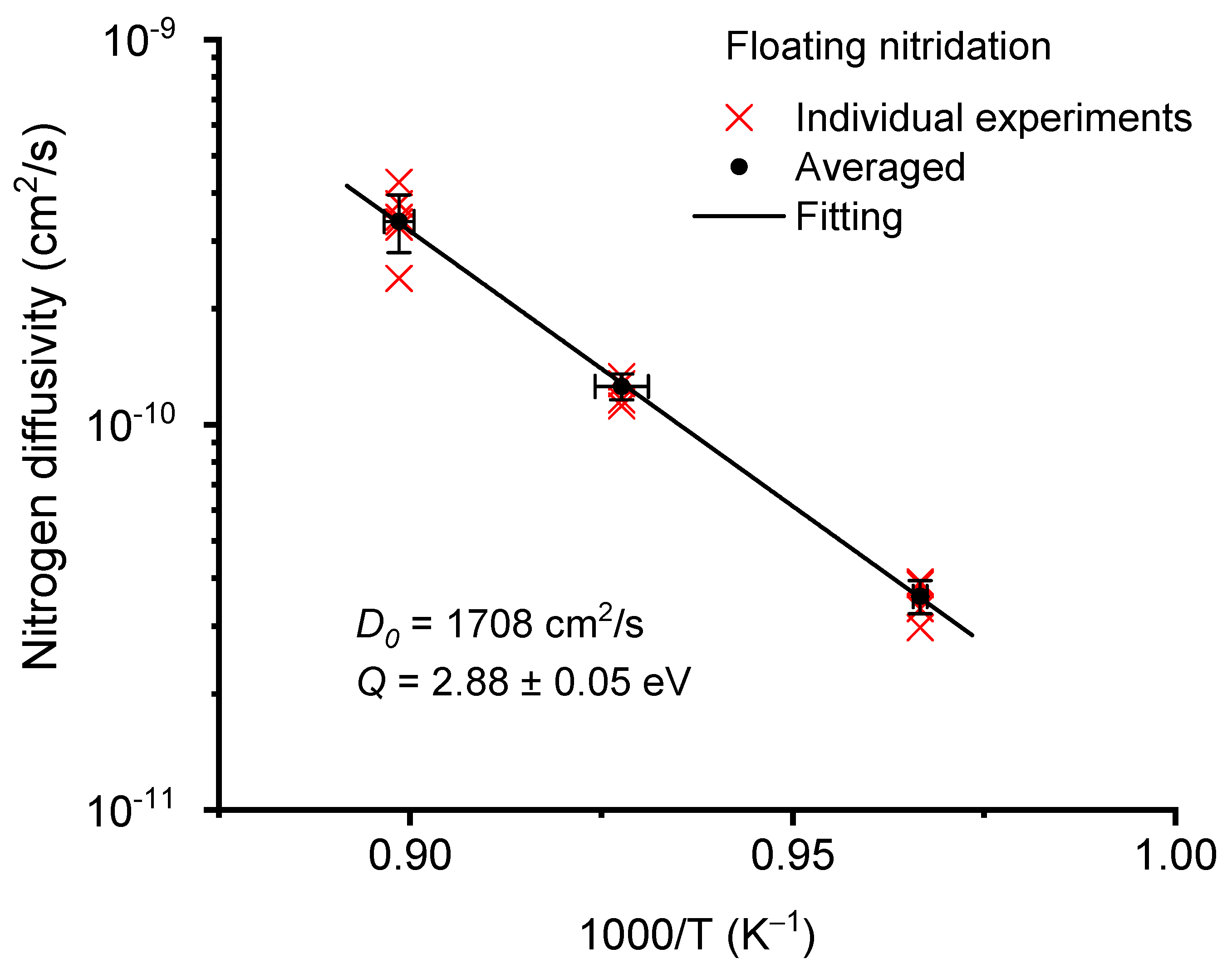
3.6. Nitrogen Diffusion Kinetics
4. Discussion
5. Conclusions
- The formation of ZrN phase was confirmed by both TEM and Raman spectroscopy.
- Through a combination of surface indentation and cross-sectional indentation, the study showed that the ZrN layer has a hardness up to 23 GPa, while the nitrogen diffusion zone has a hardness of about 7 to 15 GPa. In comparison, the bulk hardness is about 3 GPa.
- The hardness-increased zones match the nitrogen profiles.
- The nitrogen diffusivities were measured under various nitridation conditions. The study showed that a single Arrhenius equation can be used to predict the width changes in nitrogen hardened zones. A diffusion activation energy of 2.88 eV was extracted.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Bragg-Sitton, S. Development of advanced accident tolerant fuels for commercial LWRs. Nucl. News 2014, 57, 83–91. [Google Scholar]
- Engkvist, J.; Bexell, U.; Grehk, M.; Olsson, M. High temperature oxidation of FeCrAl alloys-influence of Al-concentration on oxide layer characterisitics. Mater Corr. 2009, 60, 876–881. [Google Scholar]
- Ševeček, M.; Gurgen, A.; Seshadri, A.; Che, Y.; Wagih, M.; Phillips, B.; Champagne, V.; Shirvan, K. Development of Cr cold spray-coated fuel cladding with enhanced accident tolerance. Nucl. Eng. Tech. 2018, 50, 229–236. [Google Scholar] [CrossRef]
- Basu, S.; Obando, N.; Gowdy, A.; Karaman, I.; Radovic, M. Long-term oxidation of Ti2AlC in air and water vapor at 1000–1300 °C temperature range. J. Electrochem. Soc. 2012, 159, C90–C96. [Google Scholar] [CrossRef]
- Ryabikovskaya, E.; French, A.; Gabriel, A.; Kim, H.; Wang, T.; Shirvan, K.; Garner, F.A.; Shao, L. Irradiation-induced swelling of pure chromium with 5 MeV Fe ions in the temperature range 450–650 °C. J. Nucl. Mat. 2021, 543, 152585. [Google Scholar] [CrossRef]
- Gigax, J.G.; Kennas, M.; Kim, H.; Wang, T.; Maier, B.R.; Yeom, H.; Johnson, G.O.; Sridharan, K.; Shao, L. Radiation response of Ti2AlC MAX phase coated Zircaloy-4 for accident tolerant fule cladding. J. Nucl. Mat. 2019, 523, 26–32. [Google Scholar] [CrossRef]
- Kennas, M.; Kim, H.; Gigax, J.G.; Wang, T.; Maier, B.R.; Yeom, H.; Johnson, G.O.; Dabney, T.; Sridharan, K.; Peddicord, K.L.; et al. Radiation response of FeCrAl-coated Zircaloy-4. J. Nucl. Mat. 2020, 536, 152175. [Google Scholar] [CrossRef]
- Maier, B.R.; Garcia-Diaz, B.L.; Hauch, B.; Olson, L.C.; Sindelar, R.L.; Sridharan, K. Cold spray deposition of Ti2AlC coatings for improved nuclear fuel cladding. J. Nucl. Mater. 2015, 466, 712–717. [Google Scholar] [CrossRef]
- BeauxII, M.F.; Vodnik, D.R.; Peterson, R.J.; Bennett, B.L.; Salazar, J.J.; Holesinger, T.G.; King, G.; Maloy, S.A.; Devlin, D.J.; Usov, I.O. Chemical vapor deposition of Mo tubes for fuel cladding applications. Surf. Coat. Technol. 2018, 337, 510–515. [Google Scholar] [CrossRef]
- Kim, H.-G.; Kim, I.-H.; Jung, Y.-I.; Park, D.-J.; Park, J.-H.; Park, J.-Y.; Koo, Y.-H. Oxidation behavior and mechanical property of Cr-coated zirconium cladding prepared by 3D laser coating. In Proceedings of the 2014 Water Reactor Fuel Performance Meeting (WRFPM 2014), Sendai, Japan, 14–17 September 2014. [Google Scholar]
- Dong, H. S-phase surface engineering of Fe-Cr, Co-Cr and Ni-Cr alloys. Int. Mater. Rev. 2010, 55, 65–98. [Google Scholar] [CrossRef]
- Alves, C.; de Araújo, F.O.; Ribeiro, K.J.B.; da Costa, J.A.P.; Sousa, R.R.M.; de Sousa, R.S. Use of cathodic cage in plasma nitriding. Surf. Coat. Technol. 2006, 201, 2450–2454. [Google Scholar] [CrossRef]
- Sousa, R.; de Araújo, F.; Da Costa, J.; Brandim, A.; Brito, R.; Alves, C. Cathodic cage plasma nitriding: An innovative technique. J. Metall. 2012, 2012, 385963. [Google Scholar] [CrossRef] [Green Version]
- Morell-Pacheco, A.; Kim, H.; Wang, T.; Shiau, C.-H.; Balerio, R.; Gabriel, A.; Shao, L. Ni coating on 316L stainless steel using cage plasma treatment: Feasibility and swelling studies. J. Nucl. Mat. 2020, 540, 152385. [Google Scholar] [CrossRef]
- Ma, X.; Li, C.; Bai, K.; Wu, P.; Zhang, W. Thermodynamic assessment of the Zr-N system. J. Alloys Compd. 2004, 373, 194–201. [Google Scholar] [CrossRef]
- Gribaudo, L.; Arias, A.; Abriata, J. The N-Zr (nitrogen-zirconium) system. J. Phase Equilib. 1994, 15, 441–449. [Google Scholar] [CrossRef]
- Zerr, A.; Miehe, G.; Riedel, R. Synthesis of cubic zirconium and hafnium nitride having Th3P4 structure. Nat. Mater. 2003, 2, 185–189. [Google Scholar] [CrossRef]
- Ching, W.Y.; Xu, Y.-N.; Ouyang, L. Electronic and dielectric properties of insulating Zr3N4. Phys. Rev. B 2002, 66, 235106. [Google Scholar] [CrossRef]
- Khidirov, V.T.E.; Karimov, I.; Loryan, V.E. The ordered phase in the supersaturated solution of nitrogen in α-zirconium. Phys. Met. Metallogr. 1987, 64, 174–176. [Google Scholar]
- Juza, R.; Gabel, A.; Rabenau, H.; Klose, W. On the blue zirconium nitride. Z Anorg. Allg. Chem. 1964, 329, 136–145. (In German) [Google Scholar] [CrossRef]
- Chen, X.-J.; Struzhkin, V.V.; Kung, S.; Mao, H.-K.; Hemley, R.J. Pressure-induced phonon frequency shifts in transition-metal nitrides. Phys. Rev. B 2004, 70, 014501. [Google Scholar] [CrossRef] [Green Version]
- Anttile, A.; Raisanen, J.; Keinonen, J. Diffusion of nitrogen in α-Zr and α-Hf. J. Less Common Met. 1984, 96, 257–262. [Google Scholar] [CrossRef]
- Chhowalla, M.; Unalan, H.E. Thin films of hard cubic Zr3N4 stabilized by stress. Nat. Mater. 2005, 4, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Adachi, J.; Kurosaki, K.; Uno, M.; Yamanaka, S. Porosity influence on the mechanical property of polycrystalline zirconium nitride ceramics. J. Nucl. Mat. 2006, 358, 106–110. [Google Scholar] [CrossRef]
- Shiau, C.-H.; Balerio, R.; Kim, H.; Morell-Pacheco, A.; Hawkins, L.; Shao, L. Formation of hardened hyperstoichiometry ZrNx on pure Zr using nitrogen plasma treatment. Unpublished work (under preparation).
- Yan, C.; Wang, R.; Wang, Y.; Wang, X.; Lin, Z.; Bai, G.; Zhang, Y. Size effects in indentation measurements of Zr-1Nb-0.05Cu alloy. Mat. Sci. Eng. A 2015, 628, 50–55. [Google Scholar] [CrossRef]
- Lu, Z.; Chernatynskiy, A.; Noordhoek, M.J.; Sinnott, S.B.; Phillpot, R.R. Nanoindentation of Zr by molecular dynamics simulation. J. Nucl. Mat. 2015, 467, 742–757. [Google Scholar]
- Rieck, G.D.; Bruning, H.A.C.M. Thermal diffusion of oxygen and nitrogen in Zirconium. Nature 1961, 190, 1181–1182. [Google Scholar] [CrossRef]
- Bakosi, J.; Christon, M.A.; Lowrie, R.B.; Pritchett-Sheats, L.A.; Nourgaliev, R.R. Large-eddy simulations of turbulent flow for grid-to-rod fretting in nuclear reactors. Nucl. Eng. Des. 2013, 262, 544–561. [Google Scholar] [CrossRef] [Green Version]
- Organisation for Economic Co-Operation and Development (OECD). Very High Burn-Ups in Light Water Reactors; Nuclear Energy Agency (NEA): Paris, France, 2006. [Google Scholar]
- Pelletier, J.; Lacoste, A.; Arnel, Y.; Lagarde, T.; Lincot, C.; Hertz, D. New trends in DECR plasma technology: Applications to novel duplex treatments and process combinations with extreme plasma specifications. Surf. Coat. Technol. 2001, 139, 222–232. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Mo, K.; Mei, Z.; Seidman, D.; Stepnik, B.; Pellin, M.J.; Yacout, A.M. Improving stability of ALD ZrN thin film coatingg sover U-Mo dispersion fuel. Appl. Surf. Sci. 2020, 533, 147378. [Google Scholar] [CrossRef]
- Leenaers, A.; Van den Berghe, S.; Koonen, E.; Kuzminova, V. Fuel swelling and in-teraction layer formation in the SELENIUM Si and ZrN coated U(Mo) dispersion fuel plates irradiated at high power in BR2. J. Nucl. Mater. 2015, 458, 380–393. [Google Scholar] [CrossRef]
- Sudderth, L.; Perez-Nunez, D.; Keiser, D.; McDeavitt, S. Fabrication of ZrN barrier coatings for U-Mo microspheres via fluidized bed chemical vapor deposition using a metalorganic pre-cursor. Nucl. Technol. 2018, 202, 81–93. [Google Scholar] [CrossRef]
- Ningshen, S.; Gupta, R.K.; Kamal, S.; Chawla, V.; Chandra, R.; Mudali, U.K. Corrosion study of ZrN deposited on 304L stainless steel. Surf. Eng. 2013, 29, 264–270. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balerio, R.; Kim, H.; Morell-Pacheco, A.; Hawkins, L.; Shiau, C.-H.; Shao, L. ZrN Phase Formation, Hardening and Nitrogen Diffusion Kinetics in Plasma Nitrided Zircaloy-4. Materials 2021, 14, 3572. https://doi.org/10.3390/ma14133572
Balerio R, Kim H, Morell-Pacheco A, Hawkins L, Shiau C-H, Shao L. ZrN Phase Formation, Hardening and Nitrogen Diffusion Kinetics in Plasma Nitrided Zircaloy-4. Materials. 2021; 14(13):3572. https://doi.org/10.3390/ma14133572
Chicago/Turabian StyleBalerio, Robert, Hyosim Kim, Andres Morell-Pacheco, Laura Hawkins, Ching-Heng Shiau, and Lin Shao. 2021. "ZrN Phase Formation, Hardening and Nitrogen Diffusion Kinetics in Plasma Nitrided Zircaloy-4" Materials 14, no. 13: 3572. https://doi.org/10.3390/ma14133572
APA StyleBalerio, R., Kim, H., Morell-Pacheco, A., Hawkins, L., Shiau, C.-H., & Shao, L. (2021). ZrN Phase Formation, Hardening and Nitrogen Diffusion Kinetics in Plasma Nitrided Zircaloy-4. Materials, 14(13), 3572. https://doi.org/10.3390/ma14133572






