Synthesis of Highly Energetic PolyNitrogen by Nanosecond-Pulsed Plasma in Liquid Nitrogen
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- Nanosecond-pulsed spark plasma treatment of liquid nitrogen results in generation of fluorescent material, which closely resembles the Raman spectra of amorphous nitrogen previously obtained using high pressure compression of molecular nitrogen. The fluorescence disappears at a temperature around −150 °C. The material exhibits unstable properties when heated, but explosions are poorly reproducible.
- The addition of adsorbents with characteristic pore sizes of >5 nm (indicating that only relatively large molecules are adsorbed) allows the reproducible recovery of the produced material and its explosion upon heating. The fluorescence of the Raman spectra of the physisorbed material completely disappears at around −120 °C.
- Similar to our previous study with nanosecond-pulsed corona treatment of liquid nitrogen [8], FTIR measurements of the sample decomposition via evaporation compared to its explosion in air reveal the generation of only ozone and nitrous oxide (while no other NOx species were detected), which are believed to be generated via electronically excited nitrogen produced during the polynitrogen decomposition.
- Shadow imaging of the shock wave propagation from the exploded material allowed us to estimate its energy density to be about 13.3 ± 3.5 kJ/g (3.2 ± 0.8 in TNT equivalent), close to the predicted number for the polymeric nitrogen network of ~11.3 kJ/g.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eremets, M.I.; Gavriliuk, A.G.; Trojan, I.A. Single-crystalline polymeric nitrogen. Appl. Phys. Lett. 2007, 90, 171904. [Google Scholar] [CrossRef]
- Zarko, V.E. Searching for Ways to Create Energetic Materials Based on Polynitrogen Compounds (Review). Combust. Explos. Shock. Waves 2010, 46, 121–131. [Google Scholar] [CrossRef]
- Steele, B.A.; Stavrou, E.; Crowhurst, J.C.; Zaug, J.M.; Prakapenka, V.B.; Oleynik, I.I. High-Pressure Synthesis of a Pentazolate Salt. Chem. Mater. 2017, 29, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Feng, X.; Liu, H.; Hao, J.; Redfern, S.A.T.; Lei, W.; Liu, D.; Ma, Y. Route to high-energy density polymeric nitrogen t-N via He−N compounds. Nat. Commun. 2018, 9, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klapötke, T.M.; Martin, F.A.; Stierstorfer, J. C2N14: An Energetic and Highly Sensitive Binary Azidotetrazole. Angew. Chem. Int. Ed. 2011, 50, 4227–4229. [Google Scholar] [CrossRef] [PubMed]
- Eremets, M.I.; Gavriliuk, A.G.; Trojan, I.A.; Dzivenko, D.A.; Boehler, R. Single-bonded cubic form of nitrogen. Nat. Mater. 2004, 3, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Eremets, M.I.; Eremets, R.J.; Mao, H.-K. Semiconducting non-molecular nitrogen up to 240 GPa and its low-pressure stability. Nature 2001, 411, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Dobrynin, D.; Rakhmanov, R.; Fridman, A. Nanosecond-pulsed discharge in liquid nitrogen: Optical characterization and production of an energetic non-molecular form of nitrogen-rich material. J. Phys. D Appl. Phys. 2019, 52, 39LT01. [Google Scholar] [CrossRef]
- Dobrynin, D.; Rakhmanov, R.; Fridman, A. Nanosecond-pulsed spark discharge plasma in liquid nitrogen: Synthesis of polynitrogen from NaN3. J. Phys. D Appl. Phys. 2019, 52, 455502. [Google Scholar] [CrossRef]
- Touya, G.; Reess, T.; Pecastaing, L.; Gibert, A.; Domens, P. Development of subsonic electrical discharges in water and measurements of the associated pressure waves. J. Phys. D Appl. Phys. 2006, 39, 5236. [Google Scholar] [CrossRef]
- Šimek, M.; Pongrác, B.; Babický, V.; Člupek, M.; Lukeš, P. Luminous phase of nanosecond discharge in deionized water: Morphology, propagation velocity and optical emission. Plasma Sources Sci. Technol. 2017, 26, 07LT01. [Google Scholar] [CrossRef]
- Marinov, I.; Starikovskaia, S.; Rousseau, A. Dynamics of plasma evolution in a nanosecond underwater discharge. J. Phys. Appl. Phys. 2014, 47, 224017. [Google Scholar] [CrossRef]
- Pongrác, B.; Šimek, M.; Člupek, M.; Babický, V.; Lukeš, P. Spectroscopic characteristics of H α /OI atomic lines generated by nanosecond pulsed corona-like discharge in deionized water. J. Phys. D Appl. Phys. 2018, 51, 124001. [Google Scholar] [CrossRef]
- Marinov, I.; Guaitella, O.; Rousseau, A.; Starikovskaia, S.M. Modes of underwater discharge propagation in a series of nanosecond successive pulses. J. Phys. D Appl. Phys. 2013, 46, 464013. [Google Scholar] [CrossRef]
- Dobrynin, D.; Seepersad, Y.; Pekker, M.; Shneider, M.; Friedman, G.; Fridman, A. Non-equilibrium nanosecond-pulsed plasma generation in the liquid phase (water, PDMS) without bubbles: Fast imaging, spectroscopy and leader-type model. J. Phys. D Appl. Phys. 2013, 46, 105201. [Google Scholar] [CrossRef]
- Lipp, M.J.; Park Klepeis, J.; Baer, B.J.; Cynn, H.; Evans, W.J.; Iota, V.; Yoo, C.-S. Transformation of molecular nitrogen to nonmolecular phases at megabar pressures by direct laser heating. Phys. Rev. B 2007, 76, 014113. [Google Scholar] [CrossRef]
- Kossyi, I.A.; Kostinsky, A.Y.; Matveyev, A.A.; Silakov, V.P. Kinetic scheme of the non-equilibrium discharge in nitrogen-oxygen mixtures. Plasma Sources Sci. Technol. 1992, 1, 207. [Google Scholar] [CrossRef]
- Zel’dovich, Y.B.; Raizer, Y.P. Physics of Shock Waves and High-Temperature Hydrodynamic Phenomena; Dover: New York, NY, USA, 2002; p. 944. [Google Scholar]
- Bykov, M.; Bykova, E.; Aprilis, G.; Glazyrin, K.; Koemets, E.; Chuvashova, I.; Kupenko, I.; McCammon, C.; Mezouar, M.; Prakapenka, V.; et al. Fe-N system at high pressure reveals a compound featuring polymeric nitrogen chains. Nat. Commun. 2018, 9, 2756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
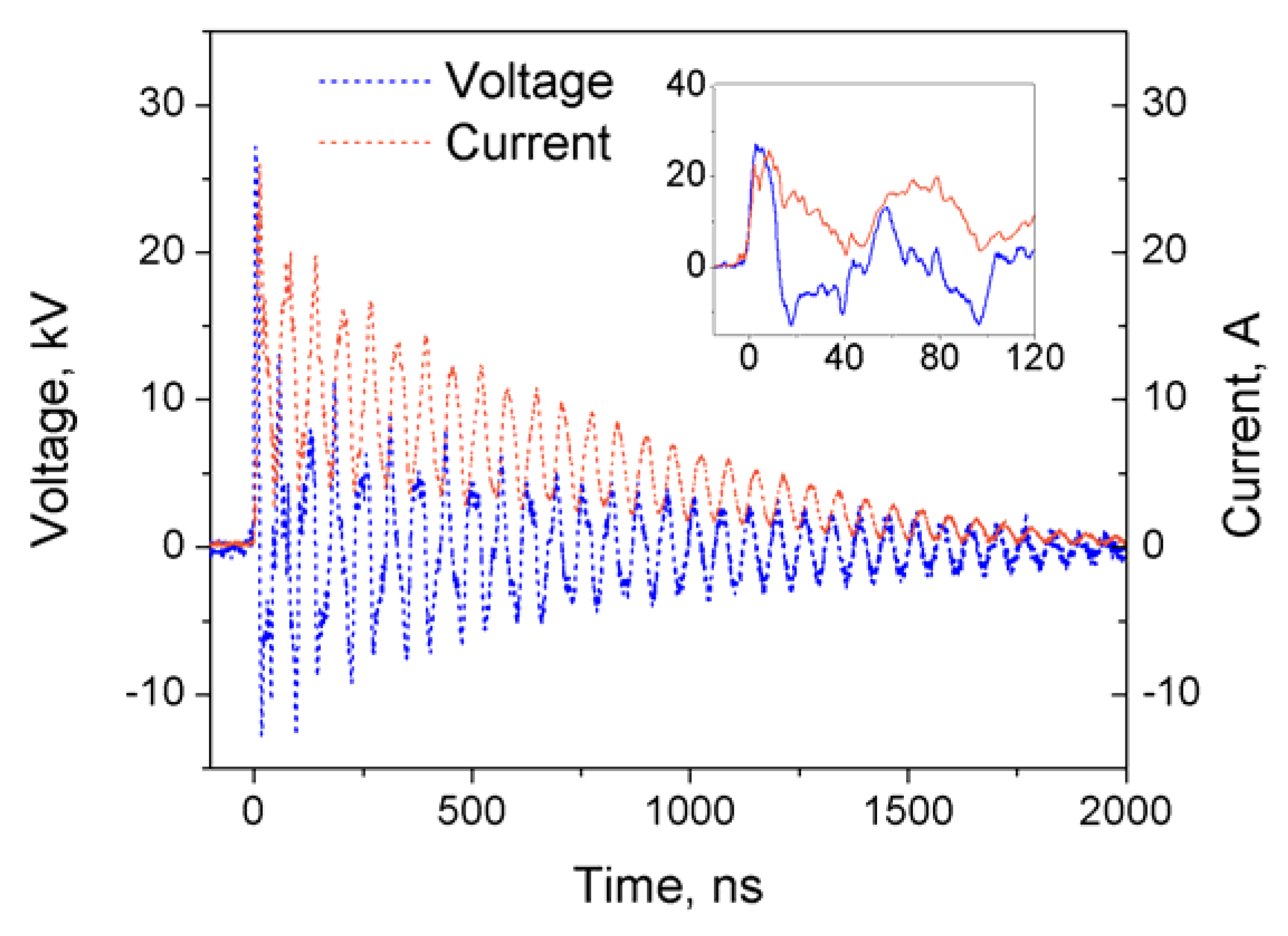
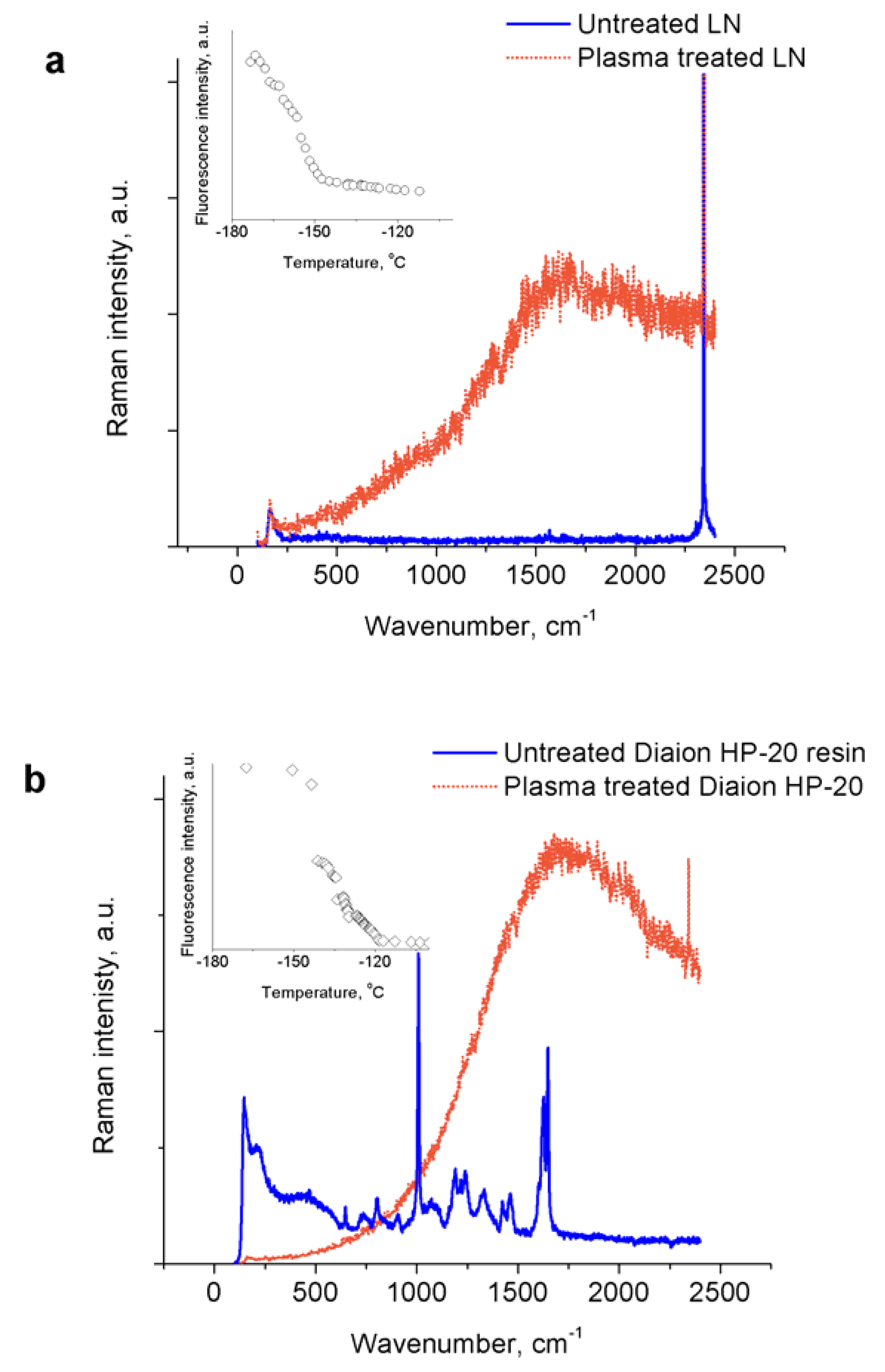

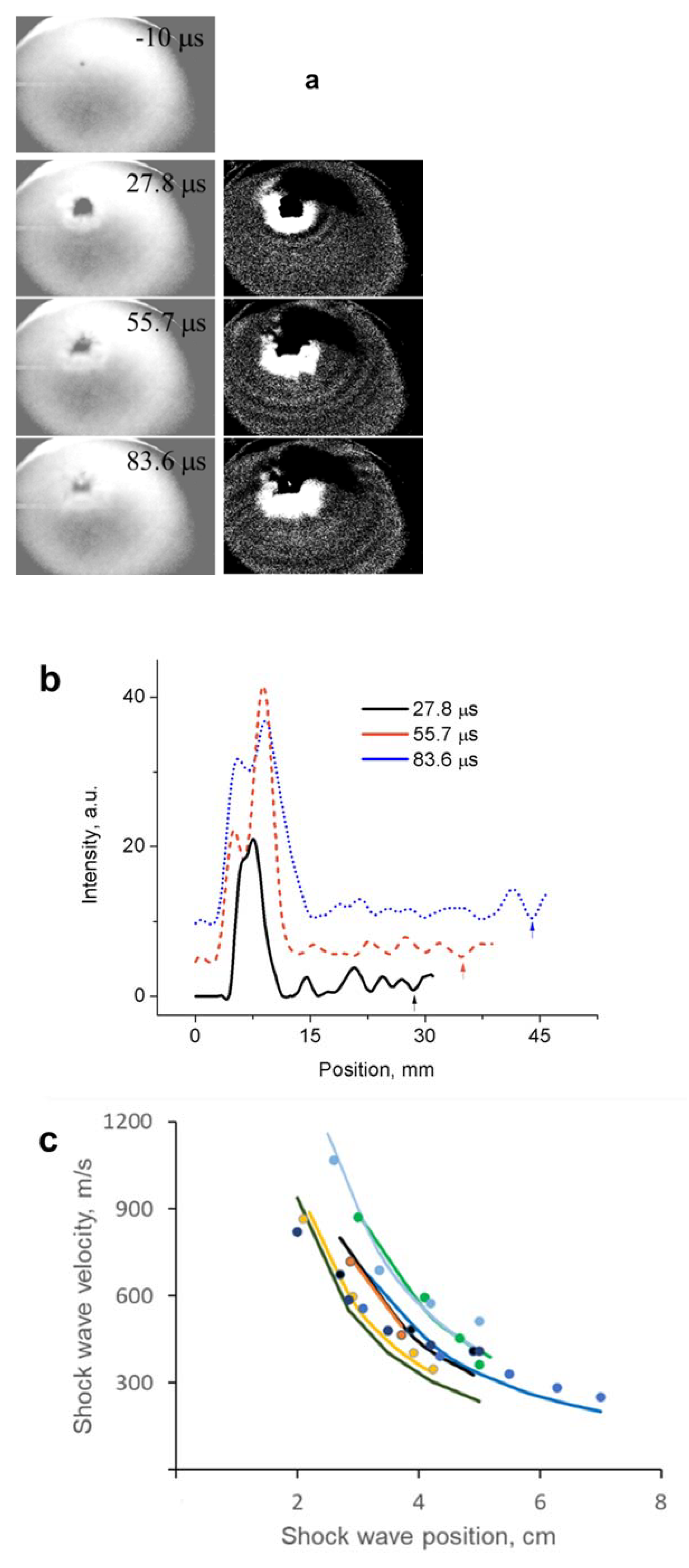
| Adsorbent | Pore Size, nm | Particle Size, μm |
|---|---|---|
| Zeolite (Sigma-Aldrich) | 0.1–1 | <20 |
| Aluminum oxide, mesoporous (Sigma-Aldrich) | 3.8 | 5.65 μm |
| Aluminosilicate, mesostructured (Sigma-Aldrich) | 2–4 | - |
| AmberSep OPTIPORE L-493 (Supelco) | 4.6 | 300–800 |
| Diaion HP-2MG methacrylic ester copolymer (Supelco) | 17 | 300–750 |
| Diaion HP-20 (Supelco) | 26 | 250–850 |
| Crushed granulated activated carbon | >50 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrynin, D.; Song, Z.; Fridman, A. Synthesis of Highly Energetic PolyNitrogen by Nanosecond-Pulsed Plasma in Liquid Nitrogen. Materials 2021, 14, 4292. https://doi.org/10.3390/ma14154292
Dobrynin D, Song Z, Fridman A. Synthesis of Highly Energetic PolyNitrogen by Nanosecond-Pulsed Plasma in Liquid Nitrogen. Materials. 2021; 14(15):4292. https://doi.org/10.3390/ma14154292
Chicago/Turabian StyleDobrynin, Danil, Zhiheng Song, and Alexander Fridman. 2021. "Synthesis of Highly Energetic PolyNitrogen by Nanosecond-Pulsed Plasma in Liquid Nitrogen" Materials 14, no. 15: 4292. https://doi.org/10.3390/ma14154292
APA StyleDobrynin, D., Song, Z., & Fridman, A. (2021). Synthesis of Highly Energetic PolyNitrogen by Nanosecond-Pulsed Plasma in Liquid Nitrogen. Materials, 14(15), 4292. https://doi.org/10.3390/ma14154292





